Abstract
Several clinical studies have reported the analgesic effect of curcumin (Curc) in various situations such as rheumatoid arthritis, osteoarthritis, and postsurgical pain. Therefore, in this work, Curc-loaded electrospun nanofibers (NFs) are designed to evaluate their sustained release on analgesic effect duration in rats after epidural placement via repeated formalin and tail-flick tests. The Curc-loaded polycaprolactone/gelatin NFs (Curc-PCL/GEL NFs) are prepared through an electrospinning technique and introduced to the rat’s epidural space after laminectomy. The physicochemical and morphology features of the prepared Curc-PCL/GEL NFs were characterized via FE-SEM, FTIR, and degradation assay. The in vitro and in vivo concentrations of Curc were measured to evaluate the analgesic efficacy of the drug-loaded NFs. Rat nociceptive responses are investigated through repeated formalin and tail-flick tests for 5 weeks after the placement of NFs. Curc had a sustained release from the NFs for 5 weeks, and its local pharmaceutical concentrations were much greater than plasma concentrations. Rat’s pain scores in both early and late phases of the formalin test were remarkably decreased in the experimental period. Rat’s tail-flick latency was remarkably enhanced and remained constant for up to 4 weeks. Our findings show that the Curc-PCL/GEL NFs can supply controlled release of Curc to induce extended analgesia after laminectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laminectomy is considered a common procedure to decompress the spinal canal in cases of narrowing of the canal (spinal stenosis) to different circumstances such as degenerative stenosis, fracture, spinal tumors, abscess, and deformity [1]. This surgery generates space through removing bone spurs and tissues related to spine arthritis. Laminectomy typically leads to elimination of a small piece of the back part (lamina) in the small bones of the spine (vertebrae). It enlarges the spinal canal to mitigate pressure on the nerve tissues or spinal cord [2].
The intraoperative injury to the posterior supporting structure of the lumbar spine leads to moderate-to-severe postoperative back pain. Providing postoperative analgesia is essential in relieving or minimalizing distress and pain. Though opioids or non-narcotics (oral analgesics) and intramuscular/intravenous analgesics are the first-line therapies typically applied in these conditions, they usually do not provide efficient postoperative analgesia. Epidural injection of opioids is also effective in relieving pain after laminectomy and can be administered easily. Epidural or peridural narcotics can give a desirable effect immediately after posterior spinal surgery for discectomy, decompression, and/or spinal fusion [3,4,5]. In prior investigations, the application of local analgesics such as lidocaine, bupivacaine, morphine, and opioids used topically to the dura effectively relieved pain after laminectomy [5]. However, the effective duration of these local anesthetics was limited and ranged from a few hours to a few days postoperatively. In addition, adverse effects on the gastrointestinal, cardiovascular, hepatic, and renal systems commonly occur by applying the current analgesics. Opioids have the extra issue of misuse and abuse potential. Therefore, more analgesic choices are required [6].
A better analgesic should be able to provide pain relief and enhance overall life quality of patients without causing severe side effects or the potential for abuse. Several reports propose that the compounds applied in traditional medicine, such as curcumin (Curc), a phenolic compound of turmeric, might be a safe and efficient alternative analgesic. The analgesic effect of Curc is reported on various pain types, such as inflammatory, neuropathic, postoperative, burn pain, and wound healing [7, 8]. The evident analgesic features of Curc can be ascribed to recognized pain-modulatory mechanisms, particularly its ability to decrease inflammation through inhibiting pro-inflammatory cytokines. Clinical trial reports showed that turmeric can be efficient in relieving spontaneous pain and sensitization, providing these analgesic advantages while maintaining a favorable safety profile [6].
On the other hand, incorporating therapeutic molecules in electrospun nanofibers (NFs) has been recently applied to make a controlled and sustained release of various drugs [7, 9]. NFs are very useful for drug delivery due to their high surface area-to-volume ratio, high porosity, and 3D open porous structure [10,11,12]. Using NFs for localized delivery of analgesics provides site specificity and needs a lower overall drug dosage with minor adverse side effects. Analgesic-eluting NFs offer further advantages in avoiding wound adhesion and scar formation [13]. Tseng and coworkers could fabricate lidocaine-embedded poly ([d, l]-lactide-co-glycolide) (PLGA) biodegradable NFs and exhibited a sustained delivery of lidocaine into the epidural space in rats after laminectomy [5]. Yosefifard and Hassanpour-Ezatti indicated that the neostigmine-loaded polyvinyl alcohol (PVA) NFs could provide sustained release of neostigmine to induce a extended analgesia following epidural administration [14].
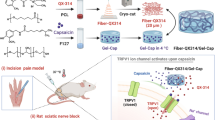
Therefore, this work was aimed to fabricate the biodegradable electrospun poly (caprolactone)/gelatin (PCL/GEL) NFs are loaded with Curc molecules to evaluate the analgesic effect duration after laminectomy in rats through repeated formalin and tail-flick tests.
Materials and Methods
Materials
Polycaprolactone (PCL, MW 80,000), gelatin (type A), acetic acid (99.7%), formic acid (88%), curcumin, dimethyl sulfoxide, Tween 80, and (3,4,5-dimethyl thiazol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS), DMEM, penicillin G, streptomycin, and Trypsin–EDTA were all provided from Gibco (Invitrogen, Paisley, UK). All other chemicals and reagents were mainly of analytic grade from commercial sources and were used without further purification.
Fabrication of Curc-PCL/GEL NFs
In total, 1.0 g of PCL/GEL (70:30, wt/wt%) mix was dissolved into 5.0 ml of a mixed solvent, including acetic acid/formic acid (50:50, v/v%), to produce the PCL/GEL electrospinnable solution with 20% (w/v%) concentration. A total of 20% (wt/wt%) Curc-PCL/GEL electrospinnable solution was prepared via dissolving 1.0 g of PCL/GEL and 200 mg of Curc into the 5.0 ml of the mixed solvent. To homogenize the Curc molecules in the polymeric solution, the mixed solution was stirred for 8 h. The mixture was shifted to a 5-ml syringe with a 22-gauge stainless-steel needle. A voltage range of 18–21 kV was applied for the electrospinning process. The distance between the aluminum foil-wrapped collector and needle tip was 200 mm. The solution flow rate was adjusted to 1 ml/h. The prepared electrospun NFs were placed in a vacuum oven for 24 h to eliminate the residual solvent before further usage [15].
Characterization of NFs
Morphological analysis of electrospun NFs was carried out via FE-SEM (MIRA3 TESCAN, Czech Republic). The average thickness of NFs and their distribution were calculated from the FE-SEM images using an image processing program (ImageJ, National Institutes of Health, USA).
A Fourier transform infrared spectroscopy (FTIR; Shimadzu 8400 S, Kyoto, Japan) in the range of 400–4000 cm−1 was utilized to reveal the presence of functional groups in NFs.
The drug encapsulation efficiency was determined as follows. A piece of the membrane (3.0 × 10.0 mm, 2.0 mg) was dissolved in 1 ml of acetic acid/formic acid (50:50, v/v%). Then, the solution was added dropwise to 20 ml of methanol, in which the polymer was precipitated, and Curc was dissolved. After centrifugation of the methanol solution, the liquid supernatant was detected by a UV–vis spectrometer (PerkinElmer Fremont, CA, USA) at λmax = 427 nm. The amount of Curc was obtained from the calibration curve of Curc. The following equation calculated the encapsulation efficiency (EE):
The degradation behavior of NFs assessed by placing them in a 24-well plate containing 1 ml PBS (pH 7.4) in each well and incubating for various time intervals at 37 °C. The weight loss percentages were measured from the below equation.
where Wt loss% = the percentage of fiber weight loss after time t, W0 = fiber weight at the beginning of the degradation assay, Wt = fiber weight after time t.
In Vitro Cytotoxicity Assay
PCL/GEL NFs and Curc-PCL/GEL NFs (2.0 mg) were sterilized under UV radiation overnight for both top and bottom surfaces in a laminar flow hood, washed thrice with PBS to remove any residual solvent, and subsequently immersed in DMEM overnight before cell seeding to facilitate protein adsorption and cell attachment on the fiber surface. A Fischer rat fibroblast 3T3-like line RAT-1 was cultured in DMEM supplemented with 10% FBS and 1% antibiotics at 37 °C and 5% CO2. When the cells reached 80% confluency, they were trypsinized and seeded onto the top of the nanofibrous matrices dropwise at a cell density of 106 cells per well and incubated at 37 °C and 5% CO2. The cells’ viability that adhered to the surface of nanofibrous mats was assessed using the MTT assay on days 1 and 3 of culture.
In Vitro Release of Curc
To determine the in vitro release of Curc from NFs, pieces of Curc-PCL/GEL NFs (3.0 × 10.0 mm, 2.0 mg) were cut and immersed in PBS containing 0.5% (w/v) Tween 80 (2 ml, pH = 7.4), and incubated with shaking at 37 °C. Thereafter, at specified time intervals, 1.0 ml of the PBS solution was substituted with 1.0 ml of fresh release media for capacity adjustment. The concentration of Curc was determined through a HPLC Instrument. The HPLC analysis was performed on a Hitachi L-2200® multi solvent delivery system. Chromatographic separations were carried out on a reversed-phase C-18 column (4.6 cm × 150 mm HPLC column (Waters)). The mobile phase included 0.01 mol ammonium formate and methanol (20/80 v/v) with a flow rate of 1 ml/min. The absorbency of column effluent was measured at 210 nm.
Laminectomy and Fiber Implantation
Twenty-four healthy Wistar adult male rats weighing 250 and 300 g were obtained from the Razi Institute of Iran. All animal experiments were conducted using protocols approved by the Institutional Animal Care and Use Committee of Tabriz University of Medical Sciences. The rats were divided randomly into four groups (n = 6), including the control group that has not received any surgery or treatment; the rats that received laminectomy without treatment; the rats implanted with neat PCL/GEL nanofibrous membranes after laminectomy; and the rats implanted with Curc-PCL/GEL nanofibrous membrane after laminectomy.
The 6% chloral hydrate was used to anesthetize the rats with an intraperitoneal injection. A laminectomy was carried out at the L5–L6 intervertebral space. After local hemostasis, biodegradable PCL/GEL NFs containing Curc, with a size of 3.0 × 10.0 mm (2.0 mg), were implanted in the epidural region (Fig. 1). Blood and epidural tissue fluid samples were taken on days 1, 3, 7, 10, and 14 from the rats.
In Vivo Release of Curc
To determine the in vivo release of Curc, blood samples were obtained from the rats at 1, 3, 7, 10, and 14 days. Peridural fluid was tapped from tissue fluid in the epidural region by micropipe. The plasma was collected after the centrifuge of specimens and stored at − 80 °C. The HPLC assay was used to determine Curc concentrations of the tapped fluid. The tapped samples were analyzed after dilution with saline and were evaluated with the assay standard curve.
Behavioral Tests
Two tail-flick and formalin tests were utilized to assess the analgesic effects of Curc-loaded NFs against various pain stimuli after epidural implantation.
Tail-Flick Test
The analgesic responses of rats to a great strength thermal nociceptive stimulus after epidural treatment with Curc-loaded NFs were estimated by a repeated tail-flick method. The alterations in tail-flick latency might be explained with regard to central sensitization. Also, repeated tail-flick latency could be assumed as a chronic nociception marker [16]. Therefore, the repeated tail-flick test was performed before and on days 0, 14, 21, and 28 after their epidural treatment with Curc-loaded NFs. Tail-flick latency was defined as the time elapsed between initiation of light stimulus and response of tail-flick. Since triplicate experiments were needed, the tail was signed in three places: proximal, middle, distal. For establishing baseline latencies, the intensity of radiant heat was adjusted for 3–5 s, and it was finished after 20 s to elude tissue injuries.
Formalin Test
Formalin test is a usual method for assessing analgesic effects of drugs administered intrathecally against the great severity of chemical nociceptive stimuli. In this study, the formalin test was performed 7 days after the tail-flick test. To avoid the interaction of both techniques’ effects on the same animals, it is suggested that the formalin test be evaluated at least 7 days after the tail-flick test since the tail-flick test has no impact on the results of the formalin test after this period [14, 17]. The formalin was injected subcutaneously into the intraplantar surface of rats’ feet treated with NFs. The behavioral response in the initial 15 min of formalin injection was considered as the early phase, and induced response by formalin at 20–60 min after injection was considered as the late phase. A score of 0 to 3 was considered for the behavioral response rating: 0 = the injected paw is not favored, 1 = the injected paw has little or no weight on it, 2 = the injected paw is elevated and is not in contact with any surface, and 3 = the injected paw is licked, bitten, or shaken. The following formula was used to calculate pain scores:
where T0–T3 are seconds spent in each of the behavioral classes.
Statistical Analyses
The data were expressed as mean ± S.E.M., and statistical data analyses were done using the software Graph Pad Prism 7.01. Statistically, differences between groups were determined utilizing a two-way repeated measure analysis of variance (ANOVA).
Results
Characterization of NFs
Figure 2 shows the FE-SEM graphs of neat PCL/GEL NFs and Curc- PCL/GEL NFs. According to the FE-SEM images, both NFs were composed of directional networks with a smooth surface morphology and bead-free. The average diameters of PCL/GEL NFs and Curc-PCL/GEL NFs were 330 ± 86 and 340 ± 75 nm, respectively, showing the slight effect of loaded Curc on the diameter distribution of NFs.
The presence of Curc within the NFs was determined by FTIR (Fig. 3A). The PCL typical bands were at 2865 and 2937 cm−1, related to the symmetric and asymmetric stretching of CH2 bonds. Peaks at 1722 cm−1, 1249 cm−1, and 1156 cm−1 were attributed to C = O stretching ester bond, C–O and C–C stretching, and asymmetric and symmetric stretching of C–O–C bonds, respectively. Peaks at 1545 cm−1 and 1665 cm−1 were characteristic bands of N–H bending of amide II and C = O stretching of amide I of GEL, respectively. With the loading of Curc, new peaks were detected at 3515 cm−1, 1640 cm−1, and 1510 cm−1, which related to the phenol, carbonyl, and ethylene groups of Curc, respectively.
The drug encapsulation efficiency of PCL/GEL NFs was 92.2% due to the proper miscibility of PCL and GEL and the better interaction of Curc with the hybrid polymer matrix.
The degradation rate of the prepared NFs was measured for 5 weeks. As shown in Fig. 3B, it was found that the mass loss of both PCL/GEL NFs and Curc-PCL/GEL NFs raised as a function of time. The results showed that both fiber groups did not show significant weight loss during the first 3 days. More than 80% of PCL/GEL NFs and Curc-PCL/GEL NFs were degraded within 30 days with a relatively constant rate of weight loss. Moreover, no discrepancy between the degradation pattern of PCL/GEL NFs and Curc-PCL/GEL NFs was observed. This result was expected, as the amount of Curc in the NFs represents only a small part of their total weight.
Biocompatibility of NFs
Investigations on cell viability are essential to investigate the biocompatibility of the nanofibrous membranes for potential use as a drug-eluting implant for in vivo studies. In this work, MTT assay was conducted to assess the viability of the Rat-1 fibroblasts cultured on the PCL/GEL and Curc-PCL/GEL NFs for 24, 48, and 72 h. As presented in Fig. 4, cell proliferation is increased time-dependently in the fibroblasts seeded onto the TCP, PCL/GEL NFs, and Curc-PCL/GEL NFs. All groups had a negligible difference in cell viability after 24 h of culture. After 48 and 72 h, it was detected that cells slightly proliferated on the Curc-loaded PCL/GEL NFs as compared with the PCL/GEL. The results suggest that the cellular uptake of Curc released in a controlled and slow manner did not significantly affect the viability and proliferation of the cells seeded onto the NFs, indicating the nontoxicity and good cytocompatibility of the used Curc-PCL/PEG NFs.
In Vitro Release of Curc
The in vitro release profile for Curc is shown in Fig. 5A. The uniform distribution of Curc in NFs is shown with a small standard deviation of the curve. When Curc was released from NFs, an initial rapid release occurred on day 1, which was pursued by a gradual, sustained drug discharge through 5 weeks. After this period, nearly 90% of Curc was released from Curc-PCL/GEL NFs.
In Vivo Release of Curc
The in vivo Curc levels were calculated for 14 days postprocedure via the HPLC assay. The measured concentrations of Curc are indicated in Fig. 5B. The Curc concentration in the plasma on the first day of postprocedure was 9.5 μg/ml, while the concentration in the peridural tissue fluid was 43.2 μg/ml. As shown in Fig. 4B, local concentrations of Curc in the epidural region were all greater than plasma concentrations. No initial rapid drug discharge occurred. Curc concentration reduced slowly in the 7th to 14th days of postprocedure. The average local concentration of Curc was 65 μg/ml at the end of the 14th day, whereas its concentration in plasma remained low (3.5 μg/ml).
Formalin Test
The pain score in early (Fig. 6A) and late (Fig. 6B) phases of formalin test was reduced remarkably in rats for 4 weeks after placement of Curc-loaded NFs.
Tail-Flick Test
Figure 7 showed the analgesia duration in rats for 4 weeks after epidural placement of Curc-loaded NFs. According to the results, rats’ tail-flick latency was remarkably enhanced after placement of Curc-loaded NFs and then remained stable for 4 weeks.
Discussion
Various preclinical and clinical trials have reported the beneficial effects of Curc on pain relief, such as neuropathic pain, intervertebral disc herniation, burn pain, cancer pain, visceral pain, arthritis, osteoarthritis, delayed onset muscle soreness, and musculoskeletal pain [6, 18]. A number of investigations propose that the apparent analgesic effects of Curc can be attributed to recognized pain-modulatory mechanisms, and especially to its capability to decrease inflammation through inhibiting pro-inflammatory mediators: leukotrienes, thromboxane, cyclooxygenase, prostaglandins, lipoxygenase, hyaluronidase elastase, collagenase, MCP-1, tumor necrosis factor, IL-12, and nitric oxide [18]. All of which are recognized components of pain-attenuating or pain-transmitting pathways. Despite the promising therapeutic effects of Curc to relieve pain in different illnesses, these effects are hindered through its weak solubility, susceptibility to photodegradation and hydrolysis, weak absorption, fast metabolism, and rapid systemic removal, eventually leading to low bioavailability (< 1%) of Curc at the target site [19, 20]. To overcome these limitations, a number of various drug delivery vehicle including phospholipids, liposomes, niosomes, dendrimers, nanoparticles, and NFs have been utilized to improve the bioavailability of Curc [21,22,23]. Therefore, in the present work, as a proper localized drug delivery system, electrospun PCL/GEL NFs were used for extended delivery of Curc molecules into the epidural region of rats to increase its analgesic effect after laminectomy. The successful loading of Curc into the NFs was confirmed by FE-SEM micrographs. According to Fig. 2, no Curc crystals were observed on the surface of loaded NFs. The electrospinning method is an effective approach to preparing drug-loaded NFs [24, 25]. The two biomaterials of PCL and GEL used in this study have been FDA approved and are widely used for fabrication of drug-containing NFs [26]. PCL is a suitable choice for biomedicine applications because of its great mechanical features, and excellent biodegradability and biocompatibility, as well as relatively low cost [27]. Electrospun PCL NFs were designed to simulate the structure of extracellular matrix, but lack of cell recognition sites, hydrophobicity, and slow degradation limit their biomedical applications [28]. Numerous in vitro and in vivo examinations have been perfomed based on the state-of-the-art standards to demonstrate the safety and nontoxicity of PCL and GEL when located in contact with human fluids and tissues so that little to no negative effect was detected for medical implants made of PCL and PCL/GEL on local tissues. However, it has been found that the acidic degradation products of PCL have been a negative effect on cell culture systems because in these closed systems, the clearance of these products is prevented [29].
It has been well-demonstrated that blending GEL with PCL can increase the biomimetic and bioactivity properties of PCL [30, 31].
The therapeutic efficiency of Curc is related to its release from the carrier system. The initial burst release is related to Curc molecules distributed on the surface of the NFs with a high tendency for diffusion. It is because of the weak physiochemical interactions between polymeric matrix and Curc molecules at the surface areas. The release of the drug in this form is an effective approach for the fast alleviation of signs that improves the treatment and removes the need for repeated administration of the drug. Moreover, using the drug-loaded NFs for delivery of the drug to the spinal cord can expand the duration of drug efficacy [14].
The embedded Curc can be released to the epidural region of rats with the hydrolysis of NFs. The local concentration of Curc within the epidural region reached therapeutic concentration at 1-day postprocedure. The Curc concentration was much greater toward its therapeutic concentration throughout 14 days, while this concentration remained low in the plasma.
Moreover, the in vivo concentrations of Curc were greater toward its in vitro concentrations, which is because of less volume of tissue fluid inside the epidural region toward saline volume utilized in the in vitro experiment. Also, the metabolic rate is slower in the in vivo environment than that in the in vitro environment. Therefore, collected in vivo eluents indicated greater drug concentrations.
As mentioned, the thermal pain threshold in rats was enhanced after epidural placement of Curc-loaded NFs and continued for as long as 28 days. Reduction of rats’ pain scores in both early and late phases was shown by consecutive formalin testing, and the results also showed that this reduction remained constant for up to 35 days after placement. The early-phase response of the formalin test is hypothesized to be due to formalin’s direct effect on nociceptors, which can be moderated through cholinergic spinal inhibitory interneurons [32]. The response of late phase is related to inflammation and subsequent tissue damage after formalin injection that reflects a situation of central sensitization.
The high efficiency and sensitivity of the tail-flick test for the evaluation of pain threshold after spinal cholinergic manipulation has been confirmed [33]. Moreover, consecutive measurement of tail-flick latency can be assumed as a chronic nociception marker. Comparing the antinociceptive impact of Curc-PCL/GEL NFs in both two pain models indicated that efficient analgesic doses of Curc were selected for alleviation of pain in our studies.
It should be noted that the loading of drugs in NFs enhanced their power for penetration into tissue. Therefore, drugs can activate more inhibitory interneurons in deeper layers of the dorsal horn of the spinal cord. This feature is another benefit of using Curc-laden NFs for pain relief.
In this work, 1000 mg PCL/GEL and 200 mg of Curc were used to fabricate electrospun Curc-PCL/PEG nanofibrous scaffolds. For the in vivo study, only a small part of the scaffold (a size of 3.0 × 10.0 mm, 2 mg) was implanted to the epidural region of rats. According to the encapsulation efficacy of Curc loaded into the fibers (92.2%), 313.5 μg of Curc was finally released from the fiber into the epidural region of Wistar adult male rats weighing between 250 and 300 g, in which this amount of Curc per weight of an adult rat cannot have toxic and adverse effects, and it can be considered as the safe and therapeutic dose. The dosing of turmeric depends on its formulation. Various animal and human studies have shown that Curc is safe and could be tolerated even at very high doses without any toxic effects [34, 35]. In an acute toxicity study, there was no mortality or gross effects 72 h after oral doses of Curc up to 5 g/kg body wight of rats [36]. In another work, no mortality or clinical sign of toxicity was found after 250, 500, and 1000 mg/kg Curc administertion for 90 days (34). Hormone blood, urine and hematological, pathological, and histopathological analyses along with neurological and ocular investigation showed no evidence of toxicity due to the Curc treatment in rats. The no-observed adverse effect level was detected to be 1000 mg/kg body weight in albino Wistar rats according to this study’s observations.
Conclusion
In this investigation, PCL/GEL NFs were applied as a scaffold for the controlled release of loaded Curc. The results of FE-SEM and FTIR demonstrated the successful loading of Curc in NFs. Both chronic and acute chemical and thermal pains in rats can decrease by the lumbar epidural placement of the NFs for 5 weeks. Generally, the findings suggest that Curc-PCL/GEL NFs can supply a safe, practical, and easy way of reaching efficient post-laminectomy analgesia.
Data Availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Wang, Y., Li, L., Ma, Y., Tang, Y., Zhao, Y., Li, Z., et al. (2020). Multifunctional supramolecular hydrogel for prevention of epidural adhesion after laminectomy. ACS Nano, 14(7), 8202–8219.
Peene, L., Le Cacheux, P., Sauter, A. R., Joshi, G. P., & Beloeil, H. (2021). Pain management after laminectomy: A systematic review and procedure-specific post-operative pain management (prospect) recommendations. European Spine Journal., 30(10), 2925–2935.
Foley, P. L., Liang, H., & Crichlow, A. R. (2011). Evaluation of a sustained-release formulation of buprenorphine for analgesia in rats. Journal of the American Association for Laboratory Animal Science., 50(2), 198–204.
Mishra, L., Nath, S., Gairola, R., Verma, R., & Mohanty, S. (2004). Buprenorphine-soaked absorbable gelatin sponge: An alternative method for postlaminectomy pain relief. Journal of Neurosurgical Anesthesiology., 16(2), 115–121.
Tseng, Y.-Y., Liao, J.-Y., Chen, W.-A., Kao, Y.-C., & Liu, S.-J. (2014). Biodegradable poly ([D, L]-lactide-co-glycolide) nanofibers for the sustainable delivery of lidocaine into the epidural space after laminectomy. Nanomedicine, 9(1), 77–87.
Eke-Okoro, U., Raffa, R., Pergolizzi Jr, J., Breve, F., Taylor Jr, R., Group NR. (2018). Curcumin in turmeric: basic and clinical evidence for a potential role in analgesia. Journal of Clinical Pharmacy and Therapeutics, 43(4), 460–466.
Gulsun, T., Inal, M., Akdag, Y., Izat, N., Oner, L., & Sahin, S. (2022). The development and characterization of electrospun gelatin nanofibers containing indomethacin and curcumin for accelerated wound healing. Journal of Drug Delivery Science and Technology., 67, 103000.
Pirmoradi, S., Fathi, E., Farahzadi, R., Pilehvar-Soltanahmadi, Y., & Zarghami, N. (2018). Curcumin affects adipose tissue-derived mesenchymal stem cell aging through TERT gene expression. Drug Research., 68(04), 213–221.
Rasouli, S., Montazeri, M., Mashayekhi, S., Sadeghi-Soureh, S., Dadashpour, M., Mousazadeh, H., et al. (2020). Synergistic anticancer effects of electrospun nanofiber-mediated codelivery of curcumin and chrysin: Possible application in prevention of breast cancer local recurrence. Journal of Drug Delivery Science and Technology., 55, 101402.
Wei, W., Zarghami, N., Abasi, M., Ertas, Y. N., & Pilehvar, Y. (2022). Implantable magnetic nanofibers with ON–OFF switchable release of curcumin for possible local hyperthermic chemotherapy of melanoma. Journal of Biomedical Materials Research Part A., 110(4), 851–860.
Mohebian, Z., Babazadeh, M., Zarghami, N., & Mousazadeh, H. (2021). Anticancer efficiency of curcumin-loaded mesoporous silica nanoparticles/nanofiber composites for potential postsurgical breast cancer treatment. Journal of Drug Delivery Science and Technology., 61, 102170.
Samadzadeh, S., Babazadeh, M., Zarghami, N., Pilehvar-Soltanahmadi, Y., & Mousazadeh, H. (2021). An implantable smart hyperthermia nanofiber with switchable, controlled and sustained drug release: Possible application in prevention of cancer local recurrence. Materials Science and Engineering: C., 118, 111384.
Tseng, Y.-Y., & Liu, S.-J. (2015). Nanofibers used for the delivery of analgesics. Nanomedicine, 10(11), 1785–1800.
Yosefifard, M., & Hassanpour-Ezatti, M. (2014). Epidural administration of neostigmine-loaded nanofibers provides extended analgesia in rats. DARU Journal of Pharmaceutical Sciences., 22(1), 1–10.
Pourpirali, R., Mahmoudnezhad, A., Oroojalian, F., Zarghami, N., & Pilehvar, Y. (2021). Prolonged proliferation and delayed senescence of the adipose-derived stem cells grown on the electrospun composite nanofiber co-encapsulated with TiO2 nanoparticles and metformin-loaded mesoporous silica nanoparticles. International Journal of Pharmaceutics., 604, 120733.
Kříž, N., Yamamotova, A., Tobiáš, J., & Rokyta, R. (2006). Tail-flick latency and self-mutilation following unilateral deafferentation in rats. Physiological Research, 55, 213–220.
Afolabi, A. O., Mudashiru, S. K., & Alagbonsi, I. A. (2013). Effects of salt-loading hypertension on nociception in rats. Journal of Pain Research., 6, 387.
Sun, J., Chen, F., Braun, C., Zhou, Y.-Q., Rittner, H., Tian, Y.-K., et al. (2018). Role of curcumin in the management of pathological pain. Phytomedicine, 48, 129–140.
Mashayekhi, S., Rasoulpoor, S., Shabani, S., Esmaeilizadeh, N., Serati-Nouri, H., Sheervalilou, R., et al. (2020). Curcumin-loaded mesoporous silica nanoparticles/nanofiber composites for supporting long-term proliferation and stemness preservation of adipose-derived stem cells. International journal of pharmaceutics., 587, 119656.
Serati-Nouri, H., Mahmoudnezhad, A., Bayrami, M., Sanajou, D., Tozihi, M., Roshangar, L., et al. (2021). Sustained delivery efficiency of curcumin through ZSM-5 nanozeolites/electrospun nanofibers for counteracting senescence of human adipose-derived stem cells. Journal of Drug Delivery Science and Technology., 66, 102902.
Tavakoli, F., Jahanban-Esfahlan, R., Seidi, K., Jabbari, M., Behzadi, R., Pilehvar-Soltanahmadi, Y., et al. (2018). Effects of nano-encapsulated curcumin-chrysin on telomerase, MMPs and TIMPs gene expression in mouse B16F10 melanoma tumour model. Artificial Cells, Nanomedicine, and Biotechnology., 46(sup2), 75–86.
Montazeri, M., Pilehvar-Soltanahmadi, Y., Mohaghegh, M., Panahi, A., Khodi, S., Zarghami, N., et al. (2017). Antiproliferative and apoptotic effect of dendrosomal curcumin nanoformulation in P53 mutant and wide-type cancer cell lines. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents), 17(5), 662–73.
Khodadadi, M., Alijani, S., Montazeri, M., Esmaeilizadeh, N., Sadeghi-Soureh, S., & Pilehvar-Soltanahmadi, Y. (2020). Recent advances in electrospun nanofiber-mediated drug delivery strategies for localized cancer chemotherapy. Journal of Biomedical Materials Research Part A., 108(7), 1444–1458.
Talaei, S., Mellatyar, H., Pilehvar-Soltanahmadi, Y., Asadi, A., Akbarzadeh, A., & Zarghami, N. (2019). 17-Allylamino-17-demethoxygeldanamycin loaded PCL/PEG nanofibrous scaffold for effective growth inhibition of T47D breast cancer cells. Journal of Drug Delivery Science and Technology., 49, 162–168.
Mellatyar, H., Talaei, S., Pilehvar-Soltanahmadi, Y., Dadashpour, M., Barzegar, A., Akbarzadeh, A., et al. (2018). 17-DMAG-loaded nanofibrous scaffold for effective growth inhibition of lung cancer cells through targeting HSP90 gene expression. Biomedicine & Pharmacotherapy., 105, 1026–1032.
Mondal, D., Griffith, M., & Venkatraman, S. S. (2016). Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. International Journal of Polymeric Materials and Polymeric Biomaterials., 65(5), 255–265.
Sadeghi-Soureh, S., Jafari, R., Gholikhani-Darbroud, R., & Pilehvar-Soltanahmadi, Y. (2020). Potential of Chrysin-loaded PCL/gelatin nanofibers for modulation of macrophage functional polarity towards anti-inflammatory/pro-regenerative phenotype. Journal of Drug Delivery Science and Technology., 58, 101802.
Ahmadi, S., Pilehvar, Y., Zarghami, N., & Abri, A. (2021). Efficient osteoblastic differentiation of human adipose-derived stem cells on TiO2 nanoparticles and metformin co-embedded electrospun composite nanofibers. Journal of Drug Delivery Science and Technology., 66, 102798.
Erdal, N.B., Lando, G.A., Yadav, A., Srivastava, R.K. Hakkarainen, M. (2020) Hydrolytic degradation of porous crosslinked poly (ε-caprolactone) synthesized by high internal phase emulsion templating. Polymers, 12(8), 1849.
Nejati-Koshki, K., Pilehvar-Soltanahmadi, Y., Alizadeh, E., Ebrahimi-Kalan, A., Mortazavi, Y., & Zarghami, N. (2017). Development of Emu oil-loaded PCL/collagen bioactive nanofibers for proliferation and stemness preservation of human adipose-derived stem cells: Possible application in regenerative medicine. Drug development and industrial pharmacy., 43(12), 1978–1988.
Mohandesnezhad, S., Pilehvar-Soltanahmadi, Y., Alizadeh, E., Goodarzi, A., Davaran, S., Khatamian, M., et al. (2020). In vitro evaluation of Zeolite-nHA blended PCL/PLA nanofibers for dental tissue engineering. Materials Chemistry and Physics., 252, 123152.
Yu, D., Thakor, D. K., Han, I., Ropper, A. E., Haragopal, H., Sidman, R. L., et al. (2013). Alleviation of chronic pain following rat spinal cord compression injury with multimodal actions of huperzine A. Proceedings of the National Academy of Sciences., 110(8), E746–E755.
Lograsso, M., Nadeson, R., & Goodchild, C. S. (2002). The spinal antinociceptive effects of cholinergic drugs in rats: Receptor subtype specificity in different nociceptive tests. BMC Pharmacology., 2(1), 1–9.
Mirzaei, H., Shakeri, A., Rashidi, B., Jalili, A., Banikazemi, Z., & Sahebkar, A. (2017). Phytosomal curcumin: A review of pharmacokinetic, experimental and clinical studies. Biomedicine & Pharmacotherapy., 85, 102–112.
Alibakhshi, A., Ranjbari, J., Pilehvar-Soltanahmadi, Y., Nasiri, M., Mollazade, M., & Zarghami, N. (2016). An update on phytochemicals in molecular target therapy of cancer: Potential inhibitory effect on telomerase activity. Current medicinal chemistry., 23(22), 2380–2393.
Wahlström, B. and Blennow, G. (1978) A study on the fate of curcumin in the rat. Acta pharmacologica et toxicologica, 43, 86–92.
Acknowledgements
The authors would like to thank the “Stem Cell Research Center, Tabriz University of Medical Sciences, Tabriz, Iran” for their kind cooperation.
Author information
Authors and Affiliations
Contributions
TJ and YH: methodology, investigation, original draft preparation. NE: conceptualization, investigation, resources. AB: investigation, methodology, validation. ATJ: formal analysis, writing–review and editing. MMS: methodology, writing–review and editing. ST: project administration. YP: supervision, writing–review and editing, funding acquisition.
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed in studies involving human participants were under the ethical standards of the Ethics Committee of Tabriz University of Medical Sciences and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tingting Jiang and Yu Han are co-first authors (these authors contributed equally to this work).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, T., Han, Y., Esmaeilizadeh, N. et al. Epidural Administration of Curcumin-Loaded Polycaprolactone/Gelatin Electrospun Nanofibers for Extended Analgesia After Laminectomy in Rats. Appl Biochem Biotechnol 195, 6557–6571 (2023). https://doi.org/10.1007/s12010-023-04342-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04342-y