Abstract
The study investigated the effect of polyphenols present in Cassia auriculata (CA) leaves in enhancing the stability of the collagen protein and the wound healing potential of collagen films. The crude ethanol extract of CA was analyzed for the presence of phytochemicals and purified by column chromatography using solvents with increasing polarity. The ethanol eluted active fractions (EEAF) that precipitated gelatin was characterized using HP-TLC, FTIR spectroscopy, ESI-FT-MS/MS, and 1H NMR spectroscopy. The active compound was identified to be procyanidin B belonging to the proanthocyanidins group. The wound healing property of EEAF and collagen type I extracted from Clarias batrachus fish skin and the bovine tendon was assessed by in vitro scratch assay on L929 mice fibroblast cell lines. The EEAF-treated collagen coating enhanced in vitro wound closure in comparison with the uncoated dish. It was observed that EEAF treatment improved the physical strength of collagen films. The in vivo wound healing of the EEAF-treated collagen film was examined in male Wister rats and the wound site tissues were assessed. In vivo wound examination showed enhanced healing with EEAF incorporated collagen films. Comparatively, the EEAF-treated bovine tendon collagen films showed improved physical properties and better wound healing property than fish collagen films.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wound dressing helps in protecting the wound from infectious agents, aid in faster recovery, and accelerate healing. Hence, the selection of the wound dressing for a particular wound type and stage of healing becomes important. The healing process involves different phases, and the duration of each phase may vary depending on the health of the individual [1]. There are different classes of wound dressings available based on the functionality of the material and treatment requirements. Passive wound dressings made of non-functional synthetic materials only cover the wound to protect it from further damage. Nowadays, functionalized biomaterials like collagen, hyaluronic acid, elastin, alginate, and chitosan support wound healing self-sufficiently [2, 3] or in combination with other active compounds like antibiotics and cytokines are being used [4, 5]. These biomaterials will act as a structural scaffold to support cell attachment and proliferation. Though they are biocompatible, they can be easily degraded and hence must be applied to the wound repeatedly. The development and modification of bio-based wound dressing materials for improved stability and functionalization are active areas of research in wound management [6].
Collagen is the most abundant protein in the extracellular matrix (ECM) with essential structural and biological functions assisting in cell attachment, proliferation, and tissue formation. Tissue collagen is considered to play a central role in controlling the events of wound healing. The exposed collagen in the wound plays a hemostatic role by interacting with the blood to form clots over the wound immediately and attract the inflammatory cells by activating the growth factors. In the inflammatory phase, the collagen is degraded by proteolytic cells to form peptides that have a chemotactic effect to attract macrophages and are directed to secrete inflammatory cytokines [4, 7]. During the proliferation phase, the collagen cleavage products activate fibroblast production to produce more collagen, endothelial cells to promote angiogenesis, and keratinocytes to undergo epithelization for scar formation. The increased understanding of the biochemical events in wound healing emphasizes the need for an exogenous collagen-based wound dressing that could correct the imbalances in the wound microenvironment [8].
Collagen extracted from different sources like bovine skin, porcine skin, fish skin, rat tail, and tendons could be fabricated in different forms to be used as a dressing material [6]. Though collagen has certain advantages as a wound dressing material, it has been stabilized by chemical cross-linkers to improve stability [9]. The chemical cross-linkers such as formaldehyde and glutaraldehyde are cytotoxic [10]. Plant-based compounds could replace chemical cross-linkers taking the biocompatible and safety attributes into consideration [11, 12]. The plant polyphenols cross-link the collagen protein through hydrogen bonding, hydrophobic, and aromatic interactions to improve stability [13].
The plant extracts have been exploited for healing wounds for many years and are preferred due to their less toxic nature and effectiveness, though neither their mechanism of action nor the active components are much known. CA is an Asian herb belonging to the Caesalpiniaceae family used for medicinal purposes for ages in Siddha and Ayurveda [14]. It is also known as tanners’ senna as it is used to preserve skins [15]. The leaves and flowers are known to possess bioactivities like antioxidant and anti-diabetic properties. The active compounds so far identified in this plant extract are flavonoids, proanthocyanidins, tannins, alkaloids, anthocyanins, saponins, proteins, carbohydrates, and steroids [14, 16]. Apart from pharmacological properties, the plant polyphenols could help in stabilizing the protein [17].
Collagen films cross-linked with plant polyphenols have been studied in the present work to explore newer wound treatment strategies. This work focuses on the extraction and characterization of the active compound in CA that improved the stability and wound healing efficacy of collagen films.
Experimental
Materials
The plant CA was collected, taxonomically identified, and authenticated by the Head of the Department of Botany, Voorhees College, Vellore, Tamil Nadu, India. Cell line L929 (murine fibroblast) was sourced from National Centre for Cell Science, Pune, India. The type I collagen extracted from Clarias batrachus fish skin and bovine tendon collagen by acid hydrolysis method was used in this study. The chemicals and solvents used were of analytical grade.
Purification and Characterization of Plant Extract
The crude ethanol extract of CA leaves was purified in silica 60–120 gel column and eluted in gradient mode using solvent systems of increasing polarity with a flow rate of 2 ml/min. The different ratios of solvent combinations successively used in the elution process are given as follows; n-hexane: chloroform—50:0, 40:10, 35:15, 25:25, and 0:50; chloroform: acetone—50:0, 40:10, 35:15, 25:25, and 0:50; acetone: ethyl acetate—50:0, 40:10, 35:15, 25:25, and 0:50; ethyl acetate: ethanol—50:0, 40:10, 35:15, 25:25, and 0:50 ethanol: water—50:0, 40:10, 35:15, 25:25, and 0:50. Gelatin precipitation assay was carried out to determine the protein cross-linking property of the eluted fractions. The 20 and 50 µl of ethanol fractions were mixed with the 10 µg/ml gelatin, and absorbance was measured at 600 nm after 10 min.
Characterization of the EEAF
The EEAF was subjected to qualitative analysis using high-performance–thin-layer chromatography (HP-TLC) system (CAMAG, Muttenz, Switzerland) fitted with visionCATS software. The samples were injected and run on precoated HP-TLC silica gel 60 F254, Merck using a HPLC-grade methanol as solvent and developed using iodine. The TLC plate was visualized using TLC visualizer 2 under RT white, and UV—254 nm [18, 19].
Fourier transform infrared spectroscopy (FTIR) for EEAF was analyzed using Cary 630 FTIR (Agilent Technologies, US) in ATR mode in the spectral range of 4000 to 400 cm−1 with a resolution of 4 cm−1 and a cycle of 32 scans. The spectrum was obtained using OriginPro 8 software [20].
The molecular mass of the EEAF was measured using Fourier transform mass spectrometry (FT-MS/MS) in positive–electron spray ionization (ESI) mode as the source. Mass range was set to 50 to 2000 Da with a scan speed of 0.2 s per scan. The operation of the instrument, data acquisition, and processing was performed using Xcalibur 2.0 software (Thermo Fisher Scientific) [21, 22].
Proton–Nuclear Magnetic resonance spectroscopy (1H NMR) spectrum for the EEAF in deuterated DMSO was measured in Bruker AM 500 NMR spectrometer (400 MHz) using tetramethysilane (TMS) (δ, 0.0) as standard. The spectrum was analyzed using Bruker TOPSPIN 4.0.7 acquisition software and ACD/Spectrus Processor package (Advanced Chemistry Development, Inc., Toronto, ON, Canada) [23, 24].
In Vitro Scratch Wound Assay
L929 fibroblast cells were seeded onto three sets of dishes: control (no coating), 0.5 µg/ml of collagen-coated, and 0.5 µg/ml of collagen with 100 µL of EEAF coated dishes. The scratch wound closure assay was performed as per the protocol [25].
Collagen Film Preparation and Characterization
Collagen film was prepared by mixing 5 ml of (3 mg/ml) purified collagen with 100 to 200 µl of EEAF stock according to the procedure of Varkey et al., (2015) [26]. The films were subjected to physical tests to determine the swelling property and tensile strength [27].
In Vivo Wound Healing Property of Collagen Films
Male Wistar rats were split into five groups and maintained in a central animal house facility, VIT, Vellore. A 2 × 2 cm2 full-thickness open excision wound was created on the back of the rats, dressed up with respective collagen films topically and observed for a period of 16 days. Rats were sacrificed and the wound tissues were removed on the 4, 8, 12, and 16th day post-wound infliction which were used for histological analyses (hematoxylin and eosin stain). The contraction of the wound site was measured to assess the percentage and quality of wound healing according to the formula of Hill et al., (2004) [28]. The tissue samples from the wound site were subjected to hydroxyproline (HP) quantification [29].
Statistical Analysis
All data were presented as mean and standard deviation using GraphPad Prism. One-way analysis of variance was used for the analysis of differences between groups. P-value < 0.05 was considered statistically significant.
Results and Discussion
Preliminary Phytochemical Screening and Purification of the CA Ethanolic Extract
The preliminary studies revealed the presence of tannins, anthocyanins, proanthocyanidins, and other polyphenols in ethanolic extracts of CA. The crude ethanol extract was chosen for further analysis as it contained many of the phytochemicals (Table S1). The key molecular interactions exerted by polyphenols on proteins are colloidal turbidity, denaturation of enzymes, astringency, and tanning [12]. Proanthocyanidins, commonly occurring polyphenols, are further grouped based on substitution and degree of oxidation. Procyanidins, the most commonly occurring proanthocyanidins in plants, have varied biological activity depending on the degree of polymerization [30]. Procyanidins are also known as condensed tannins are known to interact and cross-link with proline-rich protein collagen to form a compact protein network [31]. Proanthocyanidin extracted from plants were natural cross-linkers of gelatin [32]. Hence, the procyanidin oligomers have been gaining attention in the tissue engineering field for their cross-linking ability and therapeutic properties [13, 31, 32].
Gelatin precipitation assay was used to screen the chromatographic fractions obtained by gradient elution using solvents of varying polarity. The fraction that precipitated gelatin was examined by visual assessment and turbidity measurement at 600 nm (Fig. 1a and b). The screened EEAF were pooled and evaporated to dryness. The stock was prepared with 25 mg of the dried extract in 1 ml of ethanol and used for further experiments.
Characterization and Identification of the EEAF
The TLC spots of purified EEAF were visualized under RT white and UV 254 nm with an Rf value around 0.63 (Fig. 2I) [33]. The distinct purified spot encircled in red in the image of the HP-TLC plate (Fig. 2I) was further subjected to structural characterization. The analysis of the FTIR spectrum indicated the characteristic bands of polyphenols at 3286.7, 2920.23, 2850.75, 1560.41, 1409.96, 1361.74, 1267.23, 1201.65, 1114.86, and 1064.71 cm−1 corresponding to the O–H bond stretching in alcohol group (polyphenols); C-H stretching in alkane and alkene group; C = C bond stretching in aromatic compounds, O–H bending of phenols; C-O stretching in aromatic rings, O–H in the deformation of polyphenols, and sp3 C-O–H bond stretching of primary alcohol respectively (Fig. 2II) [20, 34, 35]. Mass spectrum revealed the relative abundance of molecular ions at m/z 413.14, 291.05, 211.06, and 103.07 which relates to 8—(2,4-dihydroxybenzyl) – 2—(3,4-dihydroxyphenyl) chromane—3,5,7—triol; 2—(3,4-dihydroxyphenyl)—3,4-dihydro—2H – 1 – benzopyran—3,5,7-triol; 2,8-dimethyloctahydro-2H—1 – benzopyran—3,5,7-triol; and oxan-3-ol respectively. The structures presented in Fig. 2III were the probable molecular ion fragments from the backbone structure of procyanidins [18]. The 1H–NMR spectra (Fig. 2IV) contain δ 10.2 (a – H, alcohol, H-7), δ 9.48 (b – H, alcohol, H-5), δ 9.32 (c – 2H, alcohol, H-3′ and H-4′), δ 6.61 and δ 6.58 (d – 3H, benzene, H-2′, H-5′ and H-6′), δ 5.76 (e – 2H, benzene, H-6, and H-8), δ 5.32 (f – H, alcohol, H-3), δ 4.65, δ 4.62 and δ 4.6 (g – 2H, methine, H-2, and H-4), and δ 1.88 and δ 1.82 (h – 2H, methine, H-4) (Fig. S1). The structure was identified to be procyanidin B which contains two monomers of epicatechin molecules. The results are well corroborating with the previously reported result of procyanidin B structure [23, 36, 37].
Structural characterization of EEAF. I Images of HP-TLC plate visualized under visible, and UV-254; lane 1—crude extract of CA and lane 2—EEAF. The spot of active compound is marked in EEAF lane (red circle). II FTIR spectrum of EEAF. The wavenumber (cm−1) of the transmittance peaks is given. III FT–MS/MS spectrum of EEAF. The probable fragmentation pathway of the compound with m/z and chemical structures of the fragment ions. IV 1H–NMR spectrum of EEAF (δ in ppm). The protons present in the identified compound are marked in the chemical structure (right) corresponding to the signal in the NMR spectrum (left)
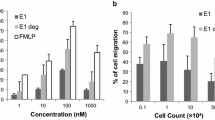
Cell Migration and In Vitro Wound Closure
The stages of wound healing involve the hemostasis, inflammatory, proliferation, and remodeling phase. The migration of fibroblasts to the wound area is important to secrete more ECM proteins to provide a scaffold for further cell attachment, proliferation, interaction, and migration. In the in vitro scratch assay of the present study, the cells started to migrate into the wound area at 24 h and the wound closure was almost complete in 48 h in EEAF-treated collagen-coated dishes (Fig. 3). The major ECM protein involved in the wound healing process is type I collagen which has a prime role in enhancing cell proliferation, migration, and differentiation [38]. The ability of the EEAF-treated collagen to promote cell fibroblast migration was confirmed by in vitro wound closure assay [25]. The fibroblast migration with fish collagen treated with EEAF was comparatively faster than bovine collagen. Proanthocyanidin nanodispersion was found to induce fibroblast migration and inhibit the expression of inflammatory cells in the wound area to hasten the wound healing process [39] Similarly, collagen has also been reported to influence the migration of cells and promote wound healing[40].
Collagen Film Preparation and Characterization
Collagen is well known to promote wound healing, but extracted collagen is degradable. The films with weak physical strength would fail to stay on the wound area for long and tend to degrade faster. The collagen should be made robust to combat degradation and inhibit the entry of invading microflora in the skin. Earlier work on the incorporation of catechin, a plant polyphenol into rat tail collagen enhanced the thermal stability of the protein for use as biomaterials. The stabilized collagen showed an increase in shrinkage temperature and maintained the secondary structure of the protein even upon chemical denaturation treatment [11]. The influence of tannic acid concentration in the degree of cross-linking of dermal sheep collagen and in vivo burn wound healing activity of the cross-linked films was assessed in a study [12]. Hence, an attempt has been made to study the effect of the extract on improving the stability and wound healing property of collagen films [27]. The EEAF-treated films were compared to the control films based on physical properties. The control films appeared to be translucent and resilient (Fig. 4a). The EEAF-treated bovine collagen films were found to be less resilient among the prepared collagen film groups. The EEAF-treated collagen films were slightly brown in color due to the cross-linking. The tensile strength of the fish collagen films increased from 8 to 13 MPa, whereas the bovine collagen films showed a significant increase from 15 to 25 MPa upon EEAF incorporation (Fig. 4b). This could be due to the higher percentage of proline and hydroxyproline in tendon collagen, which interacts with procyanidins. The swelling tests were done to assess the strength and moisture absorption capacity of the films. The swelling rates of fish and bovine collagen films decreased upon EEAF incorporation. The EEAF-treated bovine collagen film showed the least swelling activity than the collagen film groups (Fig. 4c). This could be attributed to the decreased polarity of the film upon treatment with the EEAF probably due to stacking interactions between proline rings of collagen and phenol rings (C-H–-π hydrogen bonding) of procyanidins by displacing water. The other main interaction between the polyphenols and collagen protein is mainly due to hydrogen bonding involving the hydroxyl groups of polyphenols with the functional carbonyl group of Pro residues present in the collagen (O–H–-O) (Fig. 4d) [13]. Thus, the collagen polypeptide chains are interlocked with each other through procyanidin compounds without disrupting the triple helix structure of collagen [32]. Unlike the chemical cross-linkers glutaraldehyde and formaldehyde, procyanidins are cytocompatible and are known to be non-toxic [41].
Comparison of characterization of untreated and treated collagen films. A—collagen film without EEAF treatment; B—collagen film with 100 µL EEAF treatment; C—collagen film with 200 µL EEAF treatment. a Images of prepared collagen films. b Tensile strength measurement of collagen films. c Swelling test of the collagen films. A single dollar sign ($) indicates significant change between control untreated fish collagen and EEAF-treated fish collagen film (values-value < 0.05); a single number sign (#) indicates significant change between untreated bovine tendon collagen film and EEAF-treated bovine collagen film (P-value < 0.05), and d probable interaction of procyanidin B with the Pro residues of collagen protein through hydrogen bonding
In Vivo Wound Healing
In the present study, the wounds were completely closed by the 16th day in the EEAF-treated collagen film groups and there was no sign of infection (Fig. 5a). However, the EEAF-treated bovine collagen film group showed a noticeable wound closure compared to other groups at the end of 12 days. On day 4, the wound created on the rat skin contracted to 19.6 ± 1.6, 32.75 ± 4.13, 45 ± 1.39, 36.73 ± 0.64, 59.23 ± 1.41% in groups I to V respectively (Fig. 5b). The better healing in EEAF-treated films could be due to the longer retention of stabilized collagen films or maybe a synergistic therapeutic effect on healing. Groups III and V (EEAF-treated fish and bovine films) showed the maximum amount of collagen deposition in the wound sites that would have helped in accelerating the wound healing process which was well correlated with the wound contraction measurements (Fig. 5c).
The histopathological analysis was done to assess the wound healing in epidermal and dermal regions of the rat skin (Fig. 6). The healed skin in histopathology analysis would indicate well-defined epidermis with regenerated epithelial tissues and dermis regions; densely packed connective tissue; the appearance of hair follicles, sebaceous glands, and blood vessels; cellular infiltration of keratinocytes and fibroblasts; and absence of inflammatory cells such as neutrophils and lymphocytes [2, 3, 42]. Histological findings and scoring are given in Table 1. The control group which did not receive any treatment showed very high infiltration of inflammatory cells (IC) near the wound site. On day 8, the epidermis remained damaged and the granulation tissue layer was observed to be loosened. Acanthosis, thickening of the skin layers stratum basale and stratum spinosum, and scaring were observed until the 16th day. In groups II and IV, scab formation was observed in the wound site and epidermis was still damaged on day 4 and the IC infiltration was seen near the wound site. On days 8 and 12, the infiltration of IC continued with damaged epidermis and acanthosis but hair follicles (HF) and blood vessels (BV) started to form indicating the onset of angiogenesis in the wound. On day 16, the re-epithelialization was seen distinctively with BV and HF formation. In group III, the onset of re-epithelialization was observed from day 4 and the infiltration of IC was lesser compared to control and group II treated tissues. In groups III and V, the IC infiltration was considerably reduced and the dermis started to appear compact on the 8th day. At the end of the 12th day, the re-epithelialization was completed with BV formation and fibroblast migration. The firm and distinct epidermis and dermis with HF and BV were seen which almost appeared like normal skin on day 16. In group V, the re-epithelialization rate was very high compared to other treatment groups.
It was obvious that the EEAF treatment had an impact on wound healing and improved the applicability of collagen films. Procyanidin B2 has been proven to accelerate wound healing by enhancing the function of epithelial progenitor cells (EPC) for promoting angiogenesis in the wound sites of diabetic mice. It also attenuated the levels of reactive oxygen species level in the EPCs by activating the Nrf2 signaling pathway. Thereby, procyanidin B2 facilitated the survival, function, and migration of EPCs in angiogenesis impaired wound [43]. Earlier studies also established that dietary procyanidins possess anti-inflammatory and anti-tumor properties because of their high antioxidant potential [44, 45]. Therefore, the incorporation of procyanidin B in the collagen films not only offers mechanical strength but also aids in wound healing. Evidently, the in vivo study showed that the EEAF-treated bovine collagen film tends to accelerate wound healing better in comparison to fish collagen films. The improvement in physical properties of EEAF-treated bovine collagen film could have supported wound healing greatly. However, fish collagen has a certain advantage as it would be devoid of the potential risk of bovine spongiform encephalopathy got from the bovine source [46].
A study was performed on the reinforcement of bovine skin collagen scaffold with furfural, a plant-based inorganic compound to improve its mechanical and thermal stability. The scaffolds re-established the tissue integrity in the wounds and were found to be hemocompatible [47]. Another study involved the impregnation of rat tail tendon collagen scaffolds with juglone, a quinone functionalized silver nanoparticles for improving thermal stability and wound healing potential [48]. The calf skin collagen films were stabilized with magnesium ascorbyl phosphate and have proven to enhance the thermal properties and mechanical strength of the films. The stabilized collagen films fastened the wound healing process and completely closed the wound on 16th day of the wound healing process and increased the rate of collagen fibrillation [49]. The studies conducted on collagen films in combination with plant extracts or chemical stabilizing agents enhanced the positive effect of stabilized exogenous collagen on wound healing.
Conclusion
Though several wound care management strategies have been investigated, the present study pitches the role of plant-based compounds incorporated in collagen films for external wound treatments. In summary, collagen films treated with procyanidin B from CA leaf extract showed promising results in wound healing. The materials used in preparing the wound dressing are of natural origin that are compatible and promote the wound repair process. The plant extract improved the physical properties of collagen films that improved ease of application and controlled degradation. Collagen films cross-linked with phytochemicals might provide a newer approach in finding wound treatment strategies and can be taken to the next level of experimentation.
Data Availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Broughton, G., Janis, J. E., & Attinger, C. E. (2006). The basic science of wound healing. Plastic and Reconstructive Surgery, 117(7), 12–34.
Rezvanian, M., Ng, S. F., Alavi, T., & Ahmad, W. (2021). In-vivo evaluation of Alginate-Pectin hydrogel film loaded with Simvastatin for diabetic wound healing in Streptozotocin-induced diabetic rats. International Journal of Biological Macromolecules, 171, 308–319.
Thao, N. T. T., Wijerathna, H. M. S. M., Kumar, R. S., Choi, D., Dananjaya, S. H. S., & Attanayake, A. P. (2021). Preparation and characterization of succinyl chitosan and succinyl chitosan nanoparticle film: In vitro and in vivo evaluation of wound healing activity. International Journal of Biological Macromolecules, 193, 1823–1834.
Barrientos, S., Stojadinovic, O., Golinko, M. S., Brem, H., & Tomic-Canic, M. (2008). Growth factors and cytokines in wound healing. Wound Repair Regen., 16(5), 585–601.
Moholkar, D. N., Sadalage, P. S., Peixoto, D., Paiva-Santos, A. C., & Pawar, K. D. (2021). Recent advances in biopolymer-based formulations for wound healing applications. European Polymer Journal, 160, 110784.
Chattopadhyay, S., & Raines, R. T. (2014). Collagen based biomaterials for wound healing. Biopolymers, 101, 821–833.
Albini, A., & Adelmann-Grill, B. C. (1985). Collagenolytic cleavage products of collagen type I as chemoattractants for human dermal fibroblasts. European Journal of Cell Biology, 36(1), 104–107.
Gould, L. J. (2016). Topical collagen-based biomaterials for chronic wounds: Rationale and clinical application. Advances in Wound Care, 5(1), 19–31.
Sun, L., Li, B., Jiang, D., & Hou, H. (2017). Nile tilapia skin collagen sponge modified with chemical cross-linkers as a biomedical hemostatic material. Colloids and Surfaces. B, Biointerfaces, 159, 89–96.
Elango, J., Bu, Y., Bin, B., Geevaretnam, J., Robinson, J. S., & Wu, W. (2017). Effect of chemical and biological cross-linkers on mechanical and functional properties of shark catfish skin collagen films. Food Bioscience, 17, 42–51.
Madhan, B., Subramanian, V., Rao, J. R., Unni, B., & Ramasami, T. (2005). Stabilization of collagen using plant polyphenol: Role of catechin. International Journal of Biological Macromolecules, 37, 47–53.
Heijmen, F. H., Du Pont, J. S., Middelkoop, E., Kreisn, R. W., & Hoekstra, M. J. (1997). Cross-linking of dermal sheep collagen with tannic acid. Biomaterials, 18(10), 749–754.
Petelski, A. N., Pamies, S. C., & Sosa, G. L. (2021). How procyanidin C1 sticks to collagen: The role of proline rings. Biophysical Chemistry, 276, 106627.
Juan-badaturuge, M., Habtemariam, S., & Thomas, M. J. K. (2011) Antioxidant compounds from a South Asian beverage and medicinal plant, Cassia auriculata. Food Chem, 125(1), 221–225.
Monisha, M., Sowmiya, M., Ragunathan, R., & Johney, J. (2017). Extraction of bio active compounds from Cassia auriculata pods and leaves and its medicinal uses. Int J Curr Microbiol Appl Sci, 6(8), 425–434.
Esakkirajan, M., Prabhu, N. M., Arulvasu, C., Beulaja, M., Manikandan, R., & Thiagarajan, R. (2014). Anti-proliferative effect of a compound isolated from Cassia auriculata against human colon cancer cell line HCT 15. Spectrochim. Acta A Mol Biomol. Spectrosc., 120, 462–466.
Gómez-Estaca, J., Montero, P., Fernández-Martín, F., Alemán, A., & Gómez-Guillén, M. C. (2009). Physical and chemical properties of tuna-skin and bovine-hide gelatin films with added aqueous oregano and rosemary extracts. Food Hydrocoll., 23(5), 1334–1341.
Pedan, V., Weber, C., Do, T., Fischer, N., Reich, E., & Rohn, S. (2018). HPTLC fingerprint profile analysis of cocoa proanthocyanidins depending on origin and genotype. Food Chemistry, 267, 277–287.
Avula, B., Bae, J. Y., Zhao, J., Wang, Y. H., Wang, M., Zhang, Z., Khan, I. A. (2021) Quantitative determination and characterization of polyphenols from Cissus quadrangularis L. and dietary supplements using UHPLC-PDA-MS, LC-QToF and HPTLC. J. Pharm. Biomed. Anal., 199, 114036.
Scano, P. (2021). Characterization of the medium infrared spectra of polyphenols of red and white wines by integrating FT IR and UV–Vis spectral data. LWT, 147, 111604.
Hernandes, V. V., Franco, M. F., Santos, J. M., Melendez-Perez, J. J., de Morais, D. R., de Rocha, W. F., & C., & Correa, D. N. (2015). Characterization of ANFO explosive by high accuracy ESI (±)-FTMS with forensic identification on real samples by EASI (-)-MS. Forensic Science International, 249, 156–164.
Domínguez-Rodríguez, G., Plaza, M., & Marina, M. L. (2021). High-performance thin-layer chromatography and direct analysis in realtime-high resolution mass spectrometry of non-extractable polyphenols from tropical fruit peels. Food Research International, 147, 110455.
González-Manzano, S., Pérez-Alonso, J. J., Salinas-Moreno, Y., Mateus, N., Silva, A. M. S., de Freitas, V., & Santos-Buelga, C. (2008). Flavanol-anthocyanin pigments in corn: NMR characterisation and presence in different purple corn varieties. Journal of Food Composition and Analysis, 21(7), 521–526.
Ha, T. J., Park, J. E., Lee, K. S., Seo, W. D., Song, S. B., Lee, M. H., & Lee, J. H. (2021). Identification of anthocyanin compositions in black seed coated Korean adzuki bean (Vigna angularis) by NMR and UPLC-Q-Orbitrap-MS/MS and screening for their antioxidant properties using different solvent systems. Food Chemistry, 346, 128882.
Liang, C. C., Park, A. Y., & Guan, J. L. (2007). In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nature Protocols, 2(2), 329–333.
Varkey, M., Ding, J., & Tredget, E. (2015). Advances in skin substitutes—Potential of tissue engineered skin for facilitating anti-fibrotic healing. J. Funct. Biomater., 6(3), 547–563.
Ghodbane, S. A., & Dunn, M. G. (2016). Physical and mechanical properties of cross-linked type I collagen scaffolds derived from bovine, porcine, and ovine tendons. Journal of Biomedical Materials Research. Part A, 104(11), 2685–2692.
Hill, D. P., Poore, S., Wilson, J., Robson, M. C., & Cherry, G. W. (2004). Initial healing rates of venous ulcers: Are they useful as predictors of healing? American Journal of Surgery, 188(1), 22–25.
Woessner, J. F. (1961). The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Archives of Biochemistry and Biophysics, 93(2), 440–447.
Yang, H., Tuo, X., Wang, L., Tundis, R., Portillo, M. P., Simal-Gandara, J., & Deng, J. (2021). Bioactive procyanidins from dietary sources: The relationship between bioactivity and polymerization degree. Trends in Food Science & Technology, 111, 114–127.
Li, X., Wang, G., Chen, D., & Lu, Y. (2014). Interaction of procyanidin B3 with bovine serum albumin. RSC Advances, 4(14), 7301–7312.
He, L., Mu, C., Shi, J., Zhang, Q., Shi, B., & Lin, W. (2011). Modification of collagen with a natural cross-linker, procyanidin. International Journal of Biological Macromolecules, 48(2), 354–359.
Saigo, T., Wang, T., Watanabe, M., & Tohge, T. (2020). Diversity of anthocyanin and proanthocyanin biosynthesis in land plants. Current Opinion in Plant Biology, 55, 93–99.
Hayat, J., Akodad, M., Moumen, A., Baghour, M., Skalli, A., Ezrari, S., & Belmalha, S. (2020) Phytochemical screening, polyphenols, flavonoids and tannin content, antioxidant activities and FTIR characterization of Marrubium vulgare L. from 2 different localities of Northeast of Morocco. Heliyon, 6(11) e05609.
Wu, G., Fan, G., Zhou, J., Liu, X., Wu, C., & Wang, Y. (2021). Structure and main polyphenols in the haze of blackberry wine. LWT, 149, 111821.
Abe, Y., Shoji, T., Kawahara, N., Kamakura, H., Kanda, T., Goda, Y., & Ozeki, Y. (2008). Structural characterization of a procyanidin tetramer and pentamer from the apple by low-temperature NMR analysis. Tetrahedron Letters, 49(45), 6413–6418.
Nakashima, S., Oda, C., Masuda, S., Tagashira, M., & Kanda, T. (2012). Isolation and structure elucidation of tetrameric procyanidins from unripe apples (Malus pumila cv. Fuji) by NMR spectroscopy. Phytochemistry, 83, 144–152.
Rangaraj, A., Harding, K., & Leaper, D. (2011). Role of collagen in wound management. Wounds UK, 7(2), 54–63.
Rajakumari, R., Volova, T., Oluwafemi, O. S., Rajeshkumar, S., Thomas, S., & Kalarikkal, N. (2020) Nano formulated proanthocyanidins as an effective wound healing component. Mater. Sci. Eng., C, 106, 111821.
Wang, C. H., Hsieh, D. J., Periasamy, S., Chuang, C. T., Tseng, F. W., Kuo, J. C., & Tarng, Y. W. (2020). Regenerative porcine dermal collagen matrix developed by supercritical carbon dioxide extraction technology: Role in accelerated wound healing. Materialia, 9, 100576.
Yang, Y., Ritchie, A. C., & Everitt, N. M. (2017) Comparison of glutaraldehyde and procyanidin cross-linked scaffolds for soft tissue engineering. Mater. Sci. Eng., C, 80, 263–273.
Liu, T., Dan, W., Dan, N., Liu, X., Liu, X., & Peng, X. (2017) A novel graphene oxide-modified collagen-chitosan bio-film for controlled growth factor release in wound healing applications. Mater. Sci. Eng., C, 77, 202–211.
Fan, J., Liu, H., Wang, J., Zeng, J., Tan, Y., Wang, Y., & Dai, X. (2021). Procyanidin B2 improves endothelial progenitor cell function and promotes wound healing in diabetic mice via activating Nrf2. Journal of Cellular and Molecular Medicine, 25(2), 652–665.
Jiang, Y., Wang, X., Yang, W., & Gui, S. (2020). Procyanidin B2 suppresses lipopolysaccharides-induced inflammation and apoptosis in human type II alveolar epithelial cells and lung fibroblasts. Journal of Interferon and Cytokine Research, 40(1), 54–63.
Gopalakrishnan, S., Ediga, H. H., Reddy, S. S., Reddy, G. B., & Ismail, A. (2018). Procyanidin-B2 enriched fraction of cinnamon acts as a proteasome inhibitor and anti-proliferative agent in human prostate cancer cells. IUBMB Life, 70(5), 445–457.
Song, E., Yeon Kim, S., Chun, T., Byun, H. J., & Lee, Y. M. (2006). Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials, 27(15), 2951–2961.
Lakra, R., Kiran, M. S., & Sai Korrapati, P. (2022). Collagen scaffold reinforced with furfural for wound healing application. Materials Letters, 315, 131956.
Natarajan, D., & Kiran, M. S. (2019). Fabrication of juglone functionalized silver nanoparticle stabilized collagen scaffolds for pro-wound healing activities. International Journal of Biological Macromolecules, 124, 1002–1015.
Lakra, R., Kiran, M. S., & Korrapati, P. S. (2021). Effect of magnesium ascorbyl phosphate on collagen stabilization for wound healing application. International Journal of Biological Macromolecules, 166, 333–341.
Acknowledgements
The authors thank the management of Vellore Institute of Technology (VIT), Vellore, India for the encouragement and financial support to carry out this work. The authors also thank Dr. Pundlik R. Bhagat and Mr. Subodh U. Raut, Department of chemistry, School of Advanced Sciences, VIT, Vellore for their help in NMR studies.
Funding
This study was funded by the VIT Seed grant (VIT/SG/2018–19/62). Author C Shanthi received the grant from VIT, Vellore.
Author information
Authors and Affiliations
Contributions
Experiments, data curation, data analysis, software, validation, and writing—original draft were done by K Sivaraman and P Sujitha. Material preparation, data curation, and data analysis were done by A Arunkumar. Conceptualization, methodology, funding acquisition, project administration, resources, supervision, and writing—review, and editing were done by C Shanthi.
Corresponding author
Ethics declarations
Ethical Approval
The experimental procedures were approved by the institutional animal ethical committee and the guidelines were strictly followed (VIT/IAEC/14/Nov5/44).
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sivaraman, K., Sujitha, P., Arunkumar, A. et al. Biocompatible Films of Collagen-Procyanidin for Wound Healing Applications. Appl Biochem Biotechnol 194, 4002–4017 (2022). https://doi.org/10.1007/s12010-022-03956-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03956-y