Abstract
Second-generation biofuel production has emerged as a prominent sustainable and alternative energy. The biochemical properties of cellulolytic enzymes are imperative for cellulosic biomass conversion into fermentable sugars. In the present study, thermostable CMCase and β-glucosidase were purified and characterized from Aspergillus fumigatus JCM 10253. The enzymes were purified through 80% ammonium sulfate precipitation, followed by dialysis and DEAE-cellulose ion-exchange chromatography. The molecular masses of the purified CMCase and β-glucosidase were estimated to be 125 kDa and 90 kDa, respectively. The CMCase and β-glucosidase demonstrated optimum activities at pH 6.0 and 5.0, respectively. Their respective maximum temperatures were 50 and 60 °C. The cellulase activities were stimulated by 10 mM concentration of Ca2+, Ni2+, Fe2+, Mg2+, Cu2+, Mn2+, Zn2+, and Pb2+ ions. The CMCase activity was enhanced by surfactant Triton X-100 but marginally influenced by most inhibitors. The β-glucosidase retained its activity in the presence of organic solvents (30%) isoamyl alcohol, heptane, toluene, and ethyl acetate, while CMCase was retained with acetone during a prolonged incubation of 168 h. The Km and Vmax values of the two cellulases were studied. The properties of high thermostability and good tolerance against organic solvents could signify its potential use in biofuel production and other value-added products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose is a major polysaccharide component of plant cell walls [1]. Cellulosic waste materials are a renewable carbon source used by various microorganisms and promising feedstock to lower the production cost of cellulosic ethanol and other chemical sources [2, 3]. Microbial cellulases are involved in hydrolyzing β-1, 4-glycosidic linkages of cellulose molecules, one of the most stable and prevalent bonds in nature [4, 5]. The cellulases are categorized into three types: endoglucanase or carboxymethyl cellulase (E.C. 3.2.1.4), cellobiohydrolase or exoglucanase (E.C.3.2.1.91), and cellobiase or β-glucosidase (E.C.3.2.1.21) [2, 6, 7]. Endoglucanases randomly cleaves β-1,4-glycosidic bonds in the polymeric structure of cellulose, demonstrating reducing and non-reducing ends. Cellobiohydrolases generate cellobiose and other small oligosaccharides units by acting on reducing and non-reducing ends. Finally, β-glucosidases is a rate-limiting enzyme that cleaves cellobiose to release monomeric sugar molecules [6, 8,9,10,11]. Thus, β-glucosidase plays a key role in the final step of cellulose hydrolysis to produce glucose for bioethanol production.
Fungi and bacteria are well-known for their good potential to be used in cellulase production. However, fungal cellulases are preferred over bacterial sources because of easy recovery, high activity, wide thermal and pH stability, and versatile substrate utilization [6, 10, 12]. The low cost and readily available solid substrates like forest wastes, different grasses, cotton stalks, bagasse, rice husk, wheat husk, and other crops residues are reported to provide a nourishing environment for filamentous fungi [6, 13, 14].
Cellulase has become the world’s third largest group of enzymes used in the industrial sector [15, 16]. Microbial cellulases have demonstrated their broad applications in biotechnological and other manufacturing industries [17, 18]. The rising concerns of producing cellulase are due to their huge demand in different sectors such as cotton processing, cellulosic-based bioethanol production, detergent formulation, textile, laundry, food, feed, leather, juice extraction, paper, and pulp making industries [2, 19,20,21,22]. However, several bacteria and actinomycetes have been reported cellulase yield [22,23,24]. Species of the genera Trichoderma, Penicillium, and Aspergillus are renowned producers of commercial cellulase [4]. The cellulase accounts for about 20% of the world market as a commercial enzyme [6, 14, 16, 25, 26].
Thermostable cellulases play a significant role in the enzymatic hydrolysis of cellulose. Thermostable enzymes can be used directly after the heating step without pre-cooling, which decreases the processing time and improves fermentation yields [27]. The selective production of more efficient thermostable enzymes has become the objective of much research worldwide. The thermostability of cellulases secreted by fungi for industrial applications has been extensively studied in several model organisms, like Trichoderma reesei [28], Aspergillus fumigatus [29], and Phanerochaete chrysosporium [30]. The cellulases from Aspergillus species are broadly studied, such as β-glucosidase by Aspergillus terreus [31], the β-glucosidase from Aspergillus niger [32], a highly glucose-tolerant β-glucosidase from Aspergillus oryzae [33], thermostable β-glucosidase from A. fumigatus Z5 [34], and thermostable CMCase from Aspergillus fumigatus N2 [29]. However, the high titer of active enzyme production that cleaves β-1,4-glycosidic bonds is still challenging and a bottleneck for biomass conversion.
The present investigation was focused on the extraction and purification of extracellular cellulolytic enzymes by ammonium sulfate precipitation followed by dialysis and DEAE-cellulose ion-exchange chromatography. To the best of our knowledge, this is the first study on the biochemical characterization of purified carboxymethyl cellulase and β-glucosidase from Aspergillus fumigatus JCM 10253 with the supplement of agro-industrial waste ragi (Eleusine coracana) husk in the fermentation under statistically optimized parameters. In this context, the major goal of this work was to purify cellulases and determine their biocatalytic efficiency under different conditions to assess their potential applications in bioprocessing industries.
Materials and Methods
Fungal Strain and Culture Conditions
A cellulolytic Aspergillus fumigatus JCM 10253 was isolated from a soil sample from Warangal district, Telangana, India. The fungus was identified by 18S rRNA sequencing [35]. The fungal strain JCM 10253 was selected based on the zone of clearance after Congo red staining demonstrated by the plate assay method [35]. For the shake flask fermentation, the Mandels and Weber [36] production medium was added with 1% (w/v) carboxymethyl cellulose (CMC) as a carbon source. The composed medium was sterilized at 121 °C, 15 psi pressure. A loopful of Aspergillus fumigatus JCM 10253 spores was inoculated into a sterilized medium. The above-prepared medium was supplemented with 1.6% (w/v) ragi husk as a substrate. The medium was adjusted to pH 2.0 and incubated at 48.6 °C, 160 rpm for 8.5 days for the production of CMCase. Simultaneously, the second production medium consisted of 1.17% (w/v) ragi husk. The medium was maintained at pH 3.8, 48.8 °C, and agitation at 160 rpm for 7.6 days for the production of β-glucosidase under previously statistically optimized conditions [37].
Purification of Cellulase
The crude culture from Aspergillus fumigatus JCM 10253 was subjected to the purification process at 4 °C. The cells and residual medium were separated from the crude culture by centrifugation at 10,000 rpm for 30 min. The supernatant was exposed to ammonium sulfate precipitation (60–90% saturation) for overnight. After precipitation, a pellet was recovered from the supernatant by centrifugation at 10,000 rpm for 20 min. The pellet was resuspended in 10 mL of 10 mM sodium phosphate buffer (pH 7.0) and dialysis tubing with a molecular weight cut off 10 kDa (HiMedia, Mumbai, India) against the same buffer. The dialyzed sample was equilibrated with freshly prepared sodium phosphate buffer and loaded onto the DEAE-cellulose column (2.5 × 20 cm, Bio-Rad Laboratories, USA). The enzyme fraction was allowed to bind with the matrix, and the unbound fraction was collected to estimate the enzyme activity and protein content. The elution of the bound fraction was done by a linear gradient of NaCl (0.1–1.0 M) at a flow rate of 1.0 mL/min. The active fractions were collected separately and dialyzed against the same buffer. The standard assays were used to measure the enzyme activity and protein content in the dialyzed samples.
Enzyme Assays and Protein Quantification
Carboxymethyl cellulase (CMCase) was assayed according to the method described by Ghose [38]. The CMCase activity was determined by reaction mixture containing 0.25 mL of the purified enzyme and 0.25 mL of 2% substrate (CMC dissolved in 50 mM sodium citrate buffer (pH 4.8)) incubated at 50 °C for 30 min. After incubation, 1.5 mL of 3,5-dinitrosalicylic acid (DNS) reagent was added to the reaction mixture and kept in a boiling water bath for 5 min [39]. The reducing sugar was quantified at 540 nm by UV–visible spectrophotometer (Jasco-V-630). β-Glucosidase was assayed according to the method demonstrated by Grover et al. [40] using the p-nitrophenyl-β-D-glucopyranoside (pNPG) as a substrate [41]. The reaction mixture contains 0.5 mL of sodium acetate buffer (0.1 M; pH 5.0), 0.25 mL of the purified enzyme, and 0.25 mL of 0.02 M pNPG incubated at 37 °C for 15 min. After incubation, 1.0 mL sodium carbonate (0.2 M) was added to the reaction mixture and measured at 400 nm. One unit (U) of enzyme activity is defined as the amount of enzyme releasing 1 µmol of glucose or p-nitrophenol per min from their respective substrates under the standard assay conditions. The protein content of each fraction was monitored at 280 nm using UV spectrophotometer after every step of chromatography. The protein was quantified by the Bradford method [42] using the standard as bovine serum albumin (BSA).
Polyacrylamide Gel Electrophoresis
SDS-PAGE was performed according to the method described by Laemmli [43]. The molecular weight was determined using 3 mm slab gel with 12% polyacrylamide separating gels. The gel was prepared by mixing 1.68 mL of MilliQ water, 2.0 mL of acrylamide mixture (30%), 1.25 mL of Tris–HCl (1.5 M, pH 8.8), 50 µL of sodium dodecyl sulfate (SDS) (10%), 25 µL of ammonium persulfate (10%), and 2.5 µL of tetramethylethylenediamine (TEMED). The stacking gel (4%) was prepared of 3.05 mL of MilliQ water, 665 µL of acrylamide (30%), 1.25 µL of Tris–HCl (0.5 M, pH 6.8), 50 µL of SDS (10%), 25 µL of ammonium persulfate (10%), and 5 µL of TEMED. The staining solution was used (0.1% Coomassie Brilliant Blue R-250, 50% methanol, and 10% glacial acetic acid) for 4 h, and destaining (40% methanol and 10% glacial acetic acid) was performed overnight on a gel rocker. The unstained protein markers (10–250 kDa, Thermo Fisher Scientific, USA) were used to determine the molecular weight of the purified enzyme.
Screening of Purified CMCase and β-Glucosidase Activities
CMCase Activity
The activity staining was performed by adding 1% (w/v) of CMC and agarose in 25 mL of 10 mM sodium phosphate buffer (pH 7.0) and vigorously mixed while heating and poured onto the sterile Petri plates. Wells were punched into the gel, and 0.05 mL of the purified enzyme was pipetted into wells. Then, plates were incubated at 30 °C for 24 h. After the incubation period, the staining of plates with 1% Congo red solution was performed, a clear zone marked as cellulose degradation.
β-Glucosidase Activity
Esculin gel diffusion assay was performed to screen the β-glucosidase activity [44]. Approximately 4% of agar mixed in 0.2 M sodium acetate buffer (pH 5) was autoclaved and incubated at 50 °C to avoid agar solidification. Esculin of 0.2% was mixed in 6 mL of 1% FeCl3 and incubated at 50 °C in a boiling water bath. These two solutions were mixed gently and poured into sterile Petri dishes. Esculin gel was allowed to solidify, and wells were punched. A total of 18 μL of the purified enzyme was pipetted into wells and incubated at 37 °C for 24 h. Plates were perceived for the appearance of a brown-black zone, demonstrating esculin hydrolysis.
Biochemical Characterization of Purified Cellulase
Effect of Temperature on Enzyme Activity and Stability
The impact of temperature on cellulase activity was studied by incubating purified enzyme at different temperatures (40, 45, 50, 55, 60, 65, and 70 °C) for 30 min. The thermal stability was measured by incubating purified enzyme at different temperatures (40, 45, 50, 55, 60, 65, and 70 °C) for 1 h. The CMCase activity was determined by conducting a reaction at pH 6.0 and 50 °C. Similarly, the β-glucosidase activity was measured at pH 5.0 and 60 °C. The relative activity without incubation (unheated) was defined as 100% under the standard assay conditions.
Effect of pH on Enzyme Activity and Stability
The impact of pH on the cellulase activity was determined from 3.0 to 10.0 pH range using suitable buffers at 10 mM concentration (3–5.5 sodium citrate buffer, 4.5–6 sodium acetate buffer, 6–8 sodium phosphate buffer, 8.5–9 Tris–HCl buffer, 9.5–10 glycine–NaOH buffer) under the standard assay conditions. The pH stabilities of CMCase and β-glucosidase were measured by incubating 100 μL of the purified enzyme mixed with 100 μL of the buffer solutions of different pH (3.0–10.0) for 1 h at 50 and 60 °C, respectively. The aliquots of the reaction mixture were used to evaluate the relative activities under the standard assay conditions.
Effect of Metal Ions on Enzyme Activity
The impact of metal ions (Ca2+, Ni2+, Fe2+, Mg2+, Cu2+, Hg2+, Mn2+, K+, Na+, Zn2+, Ba2+, Co2+, Cd2+, and Pb2+) were determined (at concentrations of 2, 5, and 10 mM of metal chlorides) on the cellulase activity. The incubation of purified CMCase and β-glucosidase was carried out using different metal ions at 50 and 60 °C for 1 h, respectively, and assayed under standard conditions. The relative activity in the absence of metal ions was defined as 100% under standard assay conditions.
Effect of Inhibitors on Enzyme Activity
The effect of inhibitors (urea, dithiothreitol (DTT), β-mercaptoethanol (v/v), sodium dodecyl sulfate (SDS), sodium sulfite, ethylenediaminetetraacetic acid (EDTA), phenylmethylsulfonyl fluoride (PMSF), and sodium azide) on the cellulase activity was studied at 5 and 10 mM concentrations. The incubation of purified CMCase and β-glucosidase was carried out using the above reagents at 50 and 60 °C for 1 h, respectively. The relative activity without inhibitors was defined as 100% under standard assay conditions.
Effect of Organic Solvents on Enzyme Stability
The purified cellulase was mixed with 30% (v/v) concentration of different organic solvents, viz., 2-propanol, propan-1-ol, isoamyl alcohol, 1-butanol, n-pentane, heptane, ethanol, methanol, acetone, xylene, benzene, toluene, ethylene glycol, chloroform, and ethyl acetate, and incubated for 7 days at 120 rpm in the tight screw cap tubes. The incubation of purified CMCase and β-glucosidase was carried out using 30% organic solvents at 50 and 60 °C, respectively, and assayed under standard conditions. The relative activity without the solvents was defined as 100% under standard assay conditions.
Effect of Surfactants on Enzyme Activity
The impact of surfactants on cellulase activity was determined by incubating a reaction mixture of a purified enzyme with surfactants Triton-X-100, Tween-20, Tween-80, and dimethyl sulfoxide (DMSO) at concentrations of 1% and 5% (v/v). The incubation of purified CMCase and β-glucosidase was carried out using different surfactants at 50 and 60 °C for 1 h, respectively, and assayed under standard conditions. The relative activity without surfactants was defined as 100% under the standard assay conditions.
Determination of Substrate Specificity
The purified cellulase activity was measured against different substrates. The substrates were carboxymethyl cellulose (CMC), p-Nitrophenyl-β-D-glucopyranoside (pNPG), cellobiose, Avicel, filter paper (strip), and beechwood xylan. The reaction mixtures of 1% (w/v) substrates for CMCase and β-glucosidase were prepared in 10 mM sodium phosphate buffer (pH 6.0) and sodium acetate buffer (pH 5.0) and assayed at 50 and 60 °C under standard assay conditions, respectively.
Kinetic Parameters
To determine the kinetic parameters (Km and Vmax), the purified CMCase and β-glucosidase were assayed at 50 and 60 °C using substrates CMC and pNPG concentrations ranging from 1.0 to 40 mg/mL in 10 mM sodium phosphate buffer of pH 6.0 and sodium acetate buffer of pH 5.0 under standard assay conditions, respectively. Kinetic parameters were determined by the Michaelis–Menten constant (Km, mg/mL) and maximum reaction velocity (Vmax, μmol/mg/min) through the non-linear regression of the Michaelis–Menten equation.
Results and Discussion
Screening Plates of CMCase and β-Glucosidase Activities
Cellulases are well known for breaking down cellulose polymer into glucose subunits. The plate assays were employed to screen the activities of purified enzymes from Aspergillus fumigatus JCM 10253. The purified enzymes were transferred to screening plates to assess CMCase and β-glucosidase activities. After 24 h of incubation, the plates were stained with Congo red dye that produced a clear zone surrounding the inoculated purified CMCase, as depicted in Fig. 1B. The area covered by a clear zone demonstrates the ability of the enzyme to hydrolyze cellulose [45]. The enzyme breaks down the polysaccharide into smaller monosaccharides. Thus, the Congo red dye cannot bind strongly with monosaccharides resulting in a clear zone [46]. In the gel diffusion assay, esculin (6,7-dihydroxycoumarin-6-β-D-glucopyranoside) was used as a substrate for β-glucosidase activity, which is expressed by the production of a brown-black zone on esculin gel (Fig. 1C).
Screening of purified CMCase and β-glucosidase activities from Aspergillus fumigatus JCM 10253 by plate assays. Plate assay show the zone of clearance after 24 h of Congo red staining by purified CMCase (B), and plate without enzyme considered as control (A); C Esculin gel diffusion assay of purified β-glucosidase activity showing brown-black zone after 24 h of incubation, and wells without enzyme considered as control (no dark regions)
Polyacrylamide Gel Electrophoresis for Molecular Weight Determination
The cellulase from Aspergillus fumigatus JCM 10253 was purified from crude enzyme extract by fractional ammonium sulfate precipitation, followed by dialysis and DEAE-cellulose ion-exchange chromatography. The results of purification steps are summarized in Tables 1 and 2. The optimum activity was obtained in the fraction by the extension of 80% ammonium sulfate. The active fraction collected from DEAE-cellulose column showed CMCase activity of 19.5 U/mL with a protein content of 0.34 mg/mL. The purified CMCase had a specific activity of 57.3 U/mg of protein, demonstrating a 1.45 fold increase in activity with a yield of 20.5% (Table 1). Besides, the active fraction released from DEAE-cellulose column showed a β-glucosidase activity of 0.083 U/mL with a protein content of 0.16 mg/mL. The purified β-glucosidase exhibited a 2.73 fold increase in activity with a recovery of 46.2%. The specific activity was 0.51 U/mg of protein, as described in Table 2.
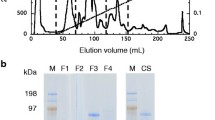
The dialyzed sample obtained after the ammonium sulfate precipitation method was employed in the next step of the purification process by applying ion-exchange chromatography. The dialyzed sample (2 mL) was equilibrated with sodium phosphate buffer (10 mM, pH 7.0) before being loaded into the DEAE-cellulose column. The unbound fraction was collected, passing through the column, and simultaneously determined its total protein content and enzyme activity. The no activity in the unbound fraction indicates that total cellulase is bound to a matrix. The bound enzyme with matrix was eluted using sodium phosphate buffer of pH 7 having NaCl with increasing concentration at gradient of 0.1 M. The maximum activities of CMCase and β-glucosidase were detected in the fractions released from 0.2 M and 0.3 M NaCl using DEAE-cellulose column, respectively. The active fractions were pooled together, followed by dialysis to remove excess salt. Thereafter, the molecular masses of purified enzymes were determined. The SDS-PAGE showed that the molecular masses of CMCase and β-glucosidase were approximately 125 and 90 kDa, respectively (Fig. 2). Hence, this demonstrated the purity of elution fractions by a single band on SDS-PAGE. Two β-glucosidases purified from strains T1 and T2 of Aspergillus tubingensis showed molecular masses of 111 kDa and 102 kDa, respectively [47]. The molecular weight of purified cellulase from Aspergillus ochraceus MTCC 1810 was ∼78 kDa described by Asha et al. [48]. Oh et al. [49] reported two β-glucosidases, BGL1 and BGL2, from cellulolytic fungi Aspergillus sp. YDJ216 with molecular masses of 97 kDa and 45 kDa, respectively. Narasimha et al. [50] described the molecular mass of β-glucosidase from Aspergillus niger as estimated to be 95 kDa. The β-glucosidase from Penicillium piceum showed a molecular weight of 92 kDa [51]. The molecular mass of the purified cellulase from Rhizopus oryzae SN5 was determined as ~ 80 kDa [52]. In another study, Penicillium citrinum and Aspergillus clavatus were demonstrated three isoforms of cellulase with a molecular weight of 130 kDa [53].
Biochemical Characterization of Purified Cellulase
Effect of Temperature on Enzyme Activity and Stability
Temperature is one of the significant factors affecting the microorganism’s growth and metabolic activities [54, 55]. As the incubation temperature increases, the collision rate increases along with the reaction rate, as seen in the several chemical reactions. The enzyme stability decreases due to thermal degradation, and holding the enzyme at a high temperature may denature the enzyme. In the present study, the temperature effect on purified enzyme was measured across a wide range from 40 to 70 °C. The optimum CMCase and β-glucosidase activities were obtained at 50 and 60 °C, respectively. Thus, further increasing temperature showed a decline in enzyme activities (Fig. 3a and b). In fungi, cellulase activity reached maximum temperatures, usually between 50 to 60 °C and stable until 50 to 55 °C [56, 57]. The purified cellulase from Aspergillus terreus showed optimum activity in the range of 46–62 °C [58]. The purified CMCase from halophilic fungi Aspergillus flavus TISTR 3637 exhibited maximal activity at 60 °C [59]. The report on purified thermostable CMCase and β-glucosidase from Penicillium sp. LMI01 showed optimum activities at 60 °C [60]. In addition, β-glucosidases usually demonstrate maximum temperatures in the range of 40 to 60 °C [61, 62]. The report revealed that the purified β-glucosidases (BGL1 and BGL2) from Aspergillus sp. YDJ216 were optimally active at 60 °C [49]. Similarly, purified endo-β-1,4-glucanase and β-glucosidase from Penicillium chrysogenum were exhibited optimum activities at 50 and 60 °C, respectively [63].
Effect of temperature on cellulase activity and stability. The optimum temperature was determined by incubating the enzyme at different temperatures (40–70 °C). The stability of the enzyme was measured by pre-incubating at respective temperatures for 1 h and assayed under standard conditions. Results are the mean of triplicate experiments with standard deviation represented by error bars
The thermophilic filamentous fungi are an excellent source of cellulases due to their high thermostability, which is one of the most desirable characteristics for industrial applications [64]. The purified CMCase and β-glucosidase from Aspergillus fumigatus JCM 10253 showed stabilities in the temperature range of 40–65 °C. At temperatures 50 and 60 °C, CMCase and β-glucosidase activities retained 100% after 1 h of incubation, respectively. At 65 °C, the CMCase and β-glucosidase were retained 85% and 96% after 1 h, respectively, demonstrating a significant decrease in activities (Fig. 3 a and b). The CMCase from Penicillium sp. LMI01 showed stability between 50 and 60 °C, about 95.7% of activity was retained after 1 h [60]. Martins et al. [65] studied β-glucosidase thermostability revealed 100% and 48.6% when exposed at 60 and 65 °C, respectively. The cellulase from Penicillium pinophilum MS 20 exhibited stability up to 2 h at 50 °C and retained 100% of the activity [66]. The report described that β-glucosidase from Fusarium chlamydosporum HML278 was stable below 70 °C and had 75% of the enzyme activity after 1 h [67]. The enzymes that are stable at 60 °C and above are known to be heat-resistant enzymes. These enzymes play a significant role in the enzymatic saccharification and fermentation of lignocellulosic biomass [68].
Effect of pH on Enzyme Activity and Stability
The pH is a critical factor that affects enzyme activity. The cellulase activity was active over a pH range from 3.0 to 9.0. The optimum activities were obtained at pH 6.0 and 5.0 for CMCase and β-glucosidase, respectively (Fig. 4a and b). The β-glucosidases of different fungal species reported the highest activity in the acidic medium between pH 4.0 and 6.0 [61, 69, 70]. The high levels of these enzymes are an important factor for biotechnological processes.
Effect of pH on cellulase activity and stability. The optimum pH was measured by incubating at different pH (pH 3–10) using various buffers for 30 min at 50 and 60 °C for CMCase (a) and β-glucosidase (b), respectively. The stability of the enzyme was measured by pre-incubation at respective temperatures for 1 h and assayed under standard conditions. Results are the mean of triplicate experiments with standard deviation represented by error bars
Microorganisms are susceptible to hydrogen ion concentration present in the fermentation medium. The pH factor is important in inducing microbial morphology and enzyme production [54, 71]. Gupta et al. [72] described that pH is crucial machinery that affects the production of enzymes, and changes in pH influence the medium’s stability. The reports on purified β-glucosidases from Penicillium chrysogenum and Penicillium citrinum UFV1 showed maximal activities at pH 5.0 [63, 64]. Narasimha et al. [50] determined the optimum activity of β-glucosidase from Aspergillus niger at pH 5.0. The purified cellulase from Aspergillus terreus demonstrated maximal activity from a pH range of 5.0 to 6.0 [58]. In another studies, cellulases from Penicillium pinophilum MS 20 and Aspergillus awamori showed optimum activities at pH 5.0 [66, 73].
In the present study, the purified CMCase and β-glucosidase of A. fumigatus JCM 10253 were stable over pH 4.0 to 9.0 (Fig. 4a and b). The pH stabilities of CMCase and β-glucosidase were retained 100% after 1 h of incubation at pH 6.5 and 5.0, respectively. The purified CMCase and β-glucosidase show more than 90% of the activities within a pH range from 4.0 to 7.0. The report on cellulase from Trichoderma longibrachiatum showed stability of pH range from 3 to 6 [46]. The cellulase from P. pinophilum MS 20 showed wide pH (4–7) stability and retained 90% of the activity [66]. The β-glucosidase from the thermophilic fungus Malbranchea pulchella (MpBg3) showed stability between the pH range of 4.0 to 6.0 [61]. Qin et al. [67] described the β-glucosidase stability of F. chlamydosporum HML278 from pH 4.0 to 10.0 and optimum activity at pH 5.0. Da Costa et al. [64] described the β-glucosidase of Penicillium citrinum UFV1 showed stability in a pH range from 5 to 8. The β-glucosidase of Penicillium verruculosum determined maximum activity at pH 4.5 and maintained 90% of the activity at pH 4.0–5.0 [74]. Similarly, β-glucosidase (bgl T2) of Aspergillus fresenii revealed optimum stability in the range of pH 3.0–8.0 [75].
Effect of Metal Ions on Enzyme Activity
Metal ions are known to influence enzyme activity. However, the interference mechanisms are not well understood due to a lack of supporting data to confirm that activation or inhibition occurs via allosteric or non-allosteric mechanisms [76]. In this study, the effects of common metal ions on CMCase and β-glucosidase activities were measured. The metal ions positively modulated the CMCase activity at 10 mM concentration of Ca2+ (131.5%), Ni2+ (129.3%), Fe2+ (130.7%), Cu2+ (141.9%), Mn2+ (145%), Zn2+ (128.6%), and Pb2+ (115.9%), as shown in Fig. 5A. However, metal ions such as Mg2+, Hg2+, K+, Na+, Ba2+, Co2+, and Cd2+ inhibited the CMCase activity (negative modulation). On the other hand, metal ions positively modulated the β-glucosidase activity by Ca2+ (134%), Ni2+ (139.8%), Fe2+ (127.8%), Mg2+ (124.9%), Cu2+ (126.5%), Mn2+ (146.5%), and Pb2+ (128%) at concentration of 10 mM (Fig. 5B), whereas Hg2+, K+, Na+, Zn2+, Ba2+,Co2+, and Cd2+ showed negative modulation on the β-glucosidase activity. In addition, metal ions like Hg2+, K+, Na+, Zn2+, Ba2+, Co2+, and Cd2+ at concentrations of 2 and 5 mM moderately declined the cellulase activities. The CMCase and β-glucosidase activities were enhanced with Mn2+ by 145% and 146.5%, respectively. The result suggests that Mn2+ might be acting as a co-factor to enhance cellulase activity.
A Effect of metal ions on CMCase activity. The CMCase activity was determined in the presence of different metal ions by pre-incubation with a purified enzyme in the reaction mixture at 50 °C for 1 h and assayed under standard conditions. The enzyme activity without incubation with metal ions was defined as 100%. Results are the mean of triplicate experiments with standard deviation represented by error bars. B Effect of metal ions on β-glucosidase activity. The β-glucosidase activity was determined in the presence of different metal ions by pre-incubation with a purified enzyme in the reaction mixture at 60 °C for 1 h and assayed under standard conditions. The enzyme activity without incubation with metal ions was defined as 100%. Results are the mean of triplicate experiments with standard deviation represented by error bars
In several studies, Ca2+ has been reported as cellulase activators [63, 77]. Feng et al. [78] showed that the Ca2+ enhanced β-glucosidase CfGlu1C activity by 39.4%. Similarly, the Ca2+ activated the cellulase from Aspergillus awamori by 53.4% [73]. The activation and inhibition of cellulases occur through multiple interactions among ions and enzymes. Metal ions such as Na+, K+, Co2+, and Hg2+ are involved in reducing cellulase activity. In contrast, divalent cations like Ca2+, Fe2+, Zn2+, and Pb2+ had enhanced the cellulase activity [63, 66, 73]. The reports on bivalent ions Hg2+ and Co2+ inhibited enzyme activities. The authors revealed that Hg2+ could interact with cysteine residues in sulfhydryl groups, particularly -SH group, which can modify the tertiary structure of the protein [67, 79,80,81]. Bai et al. [82] described that the β-glucosidase activity from Penicillium simplicissimum H-11 was enhanced by Mn2+ while inhibited by Co2+. Similarly, the β-glucosidase activity of Aspergillus niger was enhanced by Fe2+ and Mn2+ [83]. Ba2+ inhibited two β-glucosidases (BGL1 and BGL2) activities from cellulolytic fungus Aspergillus sp.YDJ216 [49]. The report described that the β-glucosidase activity of Aspergillus niger was stimulated by Mn2+ up to 20% and inhibited by Hg2+ [50]. The study demonstrated that the Co2+, Zn2+, and Hg2+ inhibited the β-glucosidase activity of F. chlamydosporum HML278 and was significantly activated by Mn2+, Ca2+, Mg2+, and Fe3+ [67]. Pachauri et al. [46] studied that cellulase from Trichoderma longibrachiatum was activated by Ca2+, Mg2+, Zn2+, and Fe2+. Bansal et al. [84] described that the purified cellulase activity of A. niger NS-2 was enhanced by Ca2+ and Mn2+ while inhibited by Hg4+. Similarly, Fe2+ and Mn2+ ions had enhanced cellulase activity of Cochliobolus sativus up to fivefold [85]. Prajapati et al. [86] described that cellulase activity from Aspergillus tubingensis NKBP-55 had significantly stimulated through Cu2+ and Mn2+, whereas Hg2+ inhibited. The activating effect in cellulase by Mn2+ ions is due to changes in electrostatic bonding, which stabilize the enzymes’ tertiary structure [86]. Subsequently, the authors suggest that this activation is due to ions’ effect on the enzyme structural conformation or may be due to the reaction between cations and amino acid residues associated with the catalytic site. The enzymes act differently with these metal ions shows differences in their catalytic domains. Therefore, this kind of interaction may be either positive or negative modulation [76, 80, 87, 88].
Effect of Inhibitors on Enzyme Activity
The impact on cellulase activity by urea, DTT, β-mercaptoethanol, SDS, sodium sulfite, EDTA, PMSF, and sodium azide was taken into account at 5 and 10 mM concentrations. The effect of inhibitors on cellulase activities is summarized in Table 3. The results found no significant inhibitory effect on cellulase activities. Approximately10–20% of the reduction was determined in the CMCase activity. The minimal reduction of CMCase activity was determined by urea, β-mercaptoethanol, and SDS. The report described that the chemical reagents EDTA, SDS, PMSF, β-mercaptoethanol, DTT, and sodium azide had no significant inhibitory effect on cellulase activity from T. longibrachiatum [46]. The cellulase activity of Aspergillus awamori exhibited a minor inhibitory effect by PMSF, DTT, and sodium azide, while SDS had a major effect [73]. The report described that the cellulase activity of Aspergillus niger was inhibited by EDTA [89]. The inhibitory effect by metal chelating agents makes an inactive complex with inorganic groups of the enzyme [73, 89].
The β-glucosidase showed approximately 35–45% loss of activity. The SDS showed a maximal reduction of β-glucosidase activity to 53.2% at 10 mM and 59.7% at 5 mM. Approximately 13–19% loss of activity was determined by PMSF, a minimal reduction among inhibitors. The β-glucosidase activity was inhibited up to 40% by urea, DTT, EDTA, and sodium azide at 10 mM concentration. The β-glucosidase activity from Lycoperdon pyriforme was not affected by EDTA, DTT, and PMSF [90]. In contrast, SDS completely inhibited the activity, which revealed that the integrity of its three-dimensional conformation is crucial for catalytic activity [90]. SDS and DMSO had no significant effect on the β-glucosidase activity from Aspergillus niger, while EDTA declined the activity to 27% [83]. The glucose-tolerant β-glucosidase of Malbranchea pulchella (MpBg3) showed a lack of effect by β-mercaptoethanol and EDTA [61]. The β-glucosidase from the thermophilic fungus Myceliophthora thermophila M.7.7 had no appreciable impact by SDS, EDTA, and DTT when pNPG was used as substrate [80].
Effect of Organic Solvents on Enzyme Stability
The product inhibition of cellulases by various factors is a basic concern that influences the efficiency of the enzyme. The motive of this study was to measure the stability of cellulase in the presence of a wide range of hydrophilic and hydrophobic organic solvents. These studies are important in the enological applications like ethanol-tolerant enzymes for alcohol fermentation. In addition, tolerance to solvents can also be significant for its potential application in solvent-based industries for high value-added products [91, 92]. In the present study, the purified CMCase from Aspergillus fumigatus JCM 10253 was incubated with different organic solvents (30%, v/v) at 50 °C for a week, and obtained values are summarized in Table 4. The results showed that only acetone retained its activity with purified CMCase throughout 168 h of incubation among various organic solvents. The prolonged incubation of 30% acetone with purified CMCase exhibited relative activity to 106 (1 h), 110.4 (24 h), 115.7 (48 h), 119.3 (72 h), 114.2 (96 h), 107.5 (120 h), 104.3 (144 h), and 101.8% (168 h). However, CMCase in the presence of other organic solvents did not retain its activity. Most of the organic solvents with purified CMCase showed relative activity over 50% after 24 h of incubation.
In the same approach, the purified β-glucosidase was incubated with 30% (v/v) organic solvents at 60 °C for a week, and obtained values are summarized in Table 5. In this study, the purified β-glucosidase in the presence of isoamyl alcohol, heptane, toluene, and ethyl acetate retained the activity throughout 168 h of the incubation period. The purified β-glucosidase with isoamyl alcohol, heptane, toluene, and ethyl acetate demonstrated optimum relative activities increased to 187.9, 158.3, 164.2, and 182% of the activity at 72 h, respectively. While in the presence of other organic solvents, the β-glucosidase was unable to retain its activity. The β-glucosidase relative activities were decreased to 35–50% approximately by 2-propanol, propan-1-ol, 1-butanol, n-pentane, ethanol, methanol, acetone, xylene, ethylene glycol, and chloroform after 24 h of incubation. The greater value of log P, the partition coefficient, demonstrates that the higher hydrophobic nature of solvents caused higher enzyme activity than that in the presence of hydrophilic solvents [93,94,95]. The enzyme activity is influenced by the addition of solvent, which interacts with the water molecules around the enzyme. The enzymes loss the catalytic activities in the presence of highly polar solvents (like ethanol, methanol, and 1-propanol) due to various factors such as their high degree of partitioning, hydrophobic interactions, disruption of native H-bonds, and stripping off eminent water layer [96,97,98,99]. Besides, the enzymes from thermophiles are more tolerant to organic solvents than mesophilic enzymes [100, 101].
The study of purified cellulase from Aspergillus tubingensis NKBP-55 showed inhibition by 5% of solvents [86]. The cellulase activity from Ganoderma lucidum was partially or severely inhibited by 1% of acetone, butanol, ethanol, methanol, isopropanol, and toluene [2]. Similarly, the cellulase from Peniophora sp. NDVN01 demonstrated low activity at 10 to 20% of methanol, ethanol, and 1-butanol, in which loss of activity is up to 36% [102]. The β-glucosidase (BglH) from Aspergillus oryzae was inactivated by 25% ethanol [103]. The activity of β-glucosidase (BG2) from Trichosporon asahii had slightly declined by 10% of methanol, ethanol, and isopropanol [104]. The study described that the β-glucosidase activity was reduced when exposed to (30%) methanol and (20%) 2-propanol [105]. Thus, large doses of organic solvents reduced the enzyme activity. This decrease may be due to the denaturing effect of organic solvents at high concentrations [69].
Effect of Surfactants on Enzyme Activity
Surfactants are an essential constituent of detergents industries and can alter the properties of the enzyme. The CMCase activity was measured using surfactants at concentrations of 1% and 5% (v/v). In this study, Tween-20, Tween-80, and DMSO were retained 102%, 97.8%, and 105.2% of the activity at 1%, respectively (Fig. 6A). The CMCase activity was enhanced by Tween-20 and DMSO, probably due to increasing substrate solubility or stabilizing enzyme structure [69, 106]. However, a slightly decline in the CMCase activity by Tween 80 at concentrations of 1% and 5% approximately 2–4% loss of activity was determined. The CMCase activity was enhanced with Triton X-100 by 180.6% and 206.5% at concentrations of 1% and 5%, respectively. The permeability of the cell membrane could increase by Triton X-100, leading to improve membrane transport and, thus, higher enzyme activity [46]. The stimulating effect of surfactants may result from their action on the cell membrane, causing enhanced permeability by promoting the release of cell-bound enzymes [107, 108]. The previous reports demonstrated that the cellulase activities from Trichoderma reesei Rut C30 and T. longibrachiatum were enhanced by Triton X-100 [46, 109]. The report also described that the CMCase activity was improved by Triton-X-100 [108].
Effect of surfactants on cellulase activity. The purified CMCase (A) and β-glucosidase (B) were pre-incubated with different surfactants at 50 and 60 °C for 1 h, respectively, and assayed under standard conditions. The enzyme activity without incubation with surfactants was defined as 100%. Results are the mean of triplicate experiments with standard deviation represented by error bars
On the other hand, Tween-20 (90.4%), Tween-80 (95.5%), and DMSO (84.2%) slightly declined the β-glucosidase activity, whereas Triton X-100 retained 103.5% at a concentration of 1%. The β-glucosidase activity was inhibited by Tween-20 (83.5%), Tween-80 (82.5%), and DMSO (73.8%), while Triton X-100 retained 109% of the activity at a concentration of 5% (Fig. 6B). As a result, surfactants have no remarkable impact on the β-glucosidase activity [110]. The β-glucosidase of Myceliophthora thermophila M.7.7 showed a loss of activity by 7% with DMSO [80]. The previous report described that the β-glucosidase from Chalara paradoxa CH32 was inhibited by Tween 80 and Triton X-100 [111]. Wang et al. [104] described that two purified β-glucosidases from Trichosporon asahii, named BG1 and BG2, exhibited less susceptibility by Triton X-100. The β-glucosidase BGL2 from Aspergillus sp.YDJ216 was inhibited by 1% Triton X-100, while β-glucosidase BGL1 had no significant effect [49]. Similarly, β-glucosidase from Aspergillus oryzae was inactivated by DMSO [103]. The previous studies revealed that Tween 20, Tween 80, and Triton-X 100 had no significant effects on cellulases from Ganoderma lucidum and Aspergillus tubingensis NKBP-55 [2, 86]. In the hydrolysis process, the non-ionic surfactants have the ability to alter the enzyme’s surface property and assist in reducing the irreversible inactivation of cellulase or compensating the negative effects during larger conversions which are greater advantages to the paper industry [112, 113].
Substrate Specificity
The substrate specificity of the purified cellulases showed broad substrate cleaving activities in Table 6. The purified CMCase exhibited high activity towards CMC (100%), followed by Avicel. The hydrolyzing activity on pNPG, cellobiose, and filter paper demonstrated a low correlation with purified CMCase. In addition, CMCase exhibited the least hydrolyzing activity with the beechwood xylan substrate. The β-glucosidase showed utmost activity against pNPG (100%) and hydrolyzed dimeric compound cellobiose up to 92.5% despite the main substrate. However, β-glucosidase was not reactive towards substrates CMC, filter paper, Avicel, and beechwood xylan [54, 114]. This finding agreed with previously reported purified endoglucanase (AS-HT-Celuz A) and β-glucosidase (AS-HT-Celuz B) from Aspergillus ochraceus MTCC 1810 [48].
Kinetic Studies
Kinetic parameters of purified CMCase and β-glucosidase from A. fumigatus JCM 10253 revealed Km values of 10.79 and 32.59 mg/mL, respectively. The Vmax values for CMCase and β-glucosidase were 128.7 and 9.36 μmol/mg/min, respectively. The previous reports demonstrated the Km values of cellulases from Aspergillus niger (0.23 mg/mL towards CMC) [54] and Thermoascus aurantiacus RBB-1(37 mg/mL towards CMC) [115]. The β-glucosidase from A. fumigatus JCM 10253 showed lower affinity towards pNPG in comparison with Km values of β-glucosidases from Aspergillus flavus (0.124 mg/mL towards pNPG) [116] and Fusarium chlamydosporum HML278 (2.76 mg/mL towards pNPG) [67]. When the Km value is lesser, it has been suggested that the enzyme has a stronger affinity with the substrate [73]. Therefore, the substrate is a significant factor that affects enzyme activity. Enzymes are complex machines that work with a wide range of chemical mechanisms [117]. In an enzyme-catalyzed reaction, enzyme activity increases as the substrate expand [117]. Although at the same time a continuous increase in substrate concentration will have minor or no effect on the enzyme activity, at that time, the enzyme is said to be saturated with its substrate [54, 117]. Moreover, the lower Km of the substrate is a significant factor for the industrial saccharification process because it can reduce the end-product inhibition in the enzymatic system [118, 119].
Conclusion
The present study was carried out for the biochemical characterization of purified CMCase and β-glucosidase from Aspergillus fumigatus JCM 10253. The production rate and cost reduction are essential factors in converting cellulose into glucose. The synergistic action of β-glucosidases with multiple cellulase enzymes could efficiently enhance the biomass transformation process. The CMCase and β-glucosidase exhibited optimum activities at 50 and 60 °C, respectively, and stabilities under elevated temperatures indicated the enzymes’ thermo-tolerant nature. Furthermore, the purified cellulases showed significant tolerance against various metal ions, denaturing agents, and organic solvents. The present findings demonstrated by the cellulolytic enzymes make ideal candidates for cellulose hydrolysis, which is a crucial step for bioethanol production and other value-added products. Future studies may be designed to assess their potential in the saccharification of lignocellulosic biomass.
Availability of Data and Materials
Not applicable.
References
Gupta, R., Mehta, G., Deswal, D., Sharma, S., et al. (2013). Cellulases and their biotechnological applications. In R. C. Kuhad & A. Singh (Eds.), Biotechnology for Environmental Management and Resource Recovery (pp. 89–106). Springer.
Manavalan, T., Manavalan, A., Thangavelu, K. P., & Heese, K. (2015). Characterization of a novel endoglucanase from Ganoderma lucidum. Journal of basic Microbiology, 55(6), 761–771.
Tiwari, R., Nain, L., Labrou, N. E., & Shukla, P. (2018). Bioprospecting of functional cellulases from metagenome for second generation biofuel production: A review. Critical Reviews in Microbiology, 44(2), 244–257.
Zhao, C. H., Liu, X., Zhan, T., & He, J. (2018). Production of cellulase by Trichoderma reesei from pretreated straw and furfural residues. RSC advances, 8(63), 36233–36238.
Behera, B. C., Sethi, B. K., Mishra, R. R., Dutta, S. K., & Thatoi, H. N. (2017). Microbial cellulases–Diversity & biotechnology with reference to mangrove environment: A review. Journal of Genetic Engineering and Biotechnology, 15(1), 197–210.
Srivastava, N., Srivastava, M., Mishra, P. K., Gupta, V. K., Molina, G., Rodriguez-Couto, S., Manikanta, A., & Ramteke, P. W. (2018). Applications of fungal cellulases in biofuel production: Advances and limitations. Renewable and Sustainable Energy Reviews, 82, 2379–2386.
Rawat, R., Srivastava, N., Chadha, B. S., & Oberoi, H. S. (2014). Generating fermentable sugars from rice straw using functionally active cellulolytic enzymes from Aspergillus niger HO. Energy & Fuels, 28(8), 5067–5075.
Singhania, R. R., Patel, A. K., Sukumaran, R. K., Larroche, C., & Pandey, A. (2013). Role and significance of beta-glucosidases in the hydrolysis of cellulose for bioethanol production. Bioresource technology, 127, 500–507.
Singh, G., Verma, A.K. and Kumar, V., 2016. Catalytic properties, functional attributes and industrial applications of β-glucosidases. 3 Biotech, 6(1), p.3.
Ramani, G., Meera, B., Vanitha, C., Rao, M., & Gunasekaran, P. (2012). Production, purification, and characterization of a β-glucosidase of Penicillium funiculosum NCL1. Applied biochemistry and biotechnology, 167(5), 959–972.
Yeoman, C. J., Han, Y., Dodd, D., Schroeder, C. M., Mackie, R. I., & Cann, I. K. (2010). Thermostable enzymes as biocatalysts in the biofuel industry. Advances in applied microbiology, 70, 1–55.
Budihal, S. R., Agsar, D., & Patil, S. R. (2016). Enhanced production and application of acidothermophilic Streptomyces cellulase. Bioresource technology, 200, 706–712.
Bisen, P.S. and Sharma, A., 2012. Introduction to instrumentation in life sciences. Crc Press
Kalsoom, R., Ahmed, S., Nadeem, M., Chohan, S., & Abid, M. (2018). Biosynthesis and extraction of cellulase produced by Trichoderma on agro-wastes. International Journal of Environmental Science and Technology, 16(2), 921–928.
Acharya, S., & Chaudhary, A. (2012). Bioprospecting thermophiles for cellulase production: A review. Brazilian Journal of Microbiology, 43(3), 844–856.
Srivastava, M., Srivastava, N., Ramteke, P.W. and Mishra, P.K. eds., 2019. Approaches to enhance industrial production of fungal cellulases. Springer.
Bhat, M. (2000). Cellulases and related enzymes in biotechnology. Biotechnology advances, 18(5), 355–383.
Singh, R.S., Singh, T. and Pandey, A., 2019. Microbial enzymes—An overview. Advances in Enzyme Technology, pp.1–40.
Phitsuwan, P., Laohakunjit, N., Kerdchoechuen, O., Kyu, K. L., & Ratanakhanokchai, K. (2013). Present and potential applications of cellulases in agriculture, biotechnology, and bioenergy. Folia microbiologica, 58(2), 163–176.
Kumar, V., Singh, D., Sangwan, P., & Gill, P. K. (2014). Global market scenario of industrial enzymes (pp. 173–196). Trends, scope and relevance. Nova Science Publishers, New York.
Singh, R., Kumar, M., Mittal, A. and Mehta, P.K., 2016. Microbial enzymes: Industrial progress in 21st century. 3 Biotech, 6(2), pp.1–15.
Islam, F., & Roy, N. (2018). Screening, purification and characterization of cellulase from cellulase producing bacteria in molasses. BMC research notes, 11(1), 1–6.
Prasad, P., Singh, T. and Bedi, S., 2013. Characterization of the cellulolytic enzyme produced by Streptomyces griseorubens (Accession No. AB184139) isolated from Indian soil. Journal of King Saud University-Science, 25(3), pp.245–250.
Nguyen, K.A., Kumla, J., Suwannarach, N., Penkhrue, W. and Lumyong, S., 2019. Optimization of high endoglucanase yields production from polypore fungus, Microporus xanthopus strain KA038 under solid-state fermentation using green tea waste. Biology open, 8(11).
Ilyas, U., Gohar, F., Saeed, S., Bukhari, Z. and Ilyas, H., 2013. Screening of locally isolated Aspergillus species for their cellulolytic potential and their optimization on Vigna mungo in solid state fermentation. Biotechnology Journal International, pp.350–358.
Soliman, S.A., El-Zawahry, Y.A. and El-Mougith, A.A., 2013. Fungal biodegradation of agro-industrial waste. Cellulose-Biomass Conversion, pp.75–100.
Liu, D., Zhang, R., Yang, X., Xu, Y., Tang, Z., Tian, W., & Shen, Q. (2011). Expression, purification and characterization of two thermostable endoglucanases cloned from a lignocellulosic decomposing fungi Aspergillus fumigatus Z5 isolated from compost. Protein expression and purification, 79(2), 176–186.
Jiang, X., Du, J., He, R., Zhang, Z., Qi, F., Huang, J., & Qin, L. (2020). Improved production of majority cellulases in Trichoderma reesei by integration of cbh1 gene from Chaetomium thermophilum. Frontiers in microbiology, 11, 1633.
Lin, C., Shen, Z., & Qin, W. (2017). Characterization of xylanase and cellulase produced by a newly isolated Aspergillus fumigatus N2 and its efficient saccharification of barley straw. Applied biochemistry and Biotechnology, 182(2), 559–569.
Machado, A. S., Valadares, F., Silva, T. F., Milagres, A. M., Segato, F., & Ferraz, A. (2020). The secretome of Phanerochaete chrysosporium and Trametes versicolor grown in microcrystalline cellulose and use of the enzymes for hydrolysis of lignocellulosic materials. Frontiers in bioengineering and biotechnology, 8, 826.
Workman, W. E., & Day, D. F. (1982). Purification and properties of β-glucosidase from Aspergillus terreus. Applied and Environmental Microbiology, 44(6), 1289–1295.
Watanabe, T., & SATO, T., YOSHIOKA, S., KOSHIJIMA, T. and KUWAHARA, M. (1992). Purificication and properties of Aspergillus nigerβ-glucosidase. European Journal of Biochemistry, 209(2), 651–659.
Riou, C., Salmon, J. M., Vallier, M. J., Günata, Z., & Barre, P. (1998). Purification, characterization, and substrate specificity of a novel highly glucose-tolerant β-glucosidase from Aspergillus oryzae. Applied and Environmental Microbiology, 64(10), 3607–3614.
Liu, D., Zhang, R., Yang, X., Zhang, Z., Song, S., Miao, Y., & Shen, Q. (2012). Characterization of a thermostable β-glucosidase from Aspergillus fumigatus Z5, and its functional expression in Pichia pastoris X33. Microbial Cell Factories, 11(1), 1–15.
Saroj, P., Manasa, P., & Narasimhulu, K. (2018). Characterization of thermophilic fungi producing extracellular lignocellulolytic enzymes for lignocellulosic hydrolysis under solid-state fermentation. Bioresources and Bioprocessing, 5(1), 1–14.
Mandels, M. and Weber, J., 1969. The production of cellulases.
Saroj, P., Manasa, P., & Narasimhulu, K. (2021). Assessment and evaluation of cellulase production using ragi (Eleusine coracana) husk as a substrate from thermo-acidophilic Aspergillus fumigatus JCM 10253. Bioprocess and Biosystems Engineering, 44(1), 113–126.
Ghose, T. K. (1987). Measurement of cellulase activities. Pure and Applied Chemistry, 59(2), 257–268.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical chemistry, 31(3), 426–428.
Grover, A.K., MacMurchie, D.D. and Cushley, R.J., 1977. Studies on almond emulsin βd-glucosidase I. Isolation and characterization of a bifunctional isozyme. Biochimica et Biophysica Acta (BBA)-Enzymology, 482(1), pp.98–108.
Ghose, T.K. and Bisaria, V.S., 1987. Measurement of hemicellulase activities. Part 1: xylanases. Pure Appl Chem, 59(12), pp.1739–1751.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry, 72(1–2), 248–254.
Laemmli, U.K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. nature, 227(5259), pp.680–685.
Saqib, A. A. N., & Whitney, P. J. (2006). Esculin gel diffusion assay (EGDA): A simple and sensitive method for screening β-glucosidases. Enyzme and Microbial Technology, 39, 182–184.
Gohel, H. R., Contractor, C. N., Ghosh, S. K., & Braganza, V. J. (2013). A comparative study of various staining techniques for determination of extra cellular cellulase activity on carboxy methyl cellulose (CMC) agar plates. International Journal of Current Microbiology and Applied Sciences, 3(5), 261–266.
Pachauri, P., More, S., Sullia, S. B., & Deshmukh, S. (2017). Purification and characterization of cellulase from a novel isolate of Trichoderma longibrachiatum. Biofuels, 11(1), 85–91.
Decker, C., Visser, J. and Schreier, P., 2001. Β-glucosidase multiplicity from Aspergillus tubingensis CBS 643.92: Purification and characterization of four β-glucosidases and their differentiation with respect to substrate specificity, glucose inhibition and acid tolerance. Applied microbiology and biotechnology, 55(2), pp.157–163.
Asha, P., Divya, J., & Singh, I. B. (2016). Purification and characterisation of processive-type endoglucanase and β-glucosidase from Aspergillus ochraceus MTCC 1810 through saccharification of delignified coir pith to glucose. Bioresource technology, 213, 245–248.
Oh, J. M., Lee, J. P., Baek, S. C., Kim, S. G., Do Jo, Y., Kim, J., & Kim, H. (2018). Characterization of two extracellular β-glucosidases produced from the cellulolytic fungus Aspergillus sp. YDJ216 and their potential applications for the hydrolysis of flavone glycosides. International journal of biological macromolecules, 111, 595–603.
Narasimha, G., Sridevi, A., Ramanjaneyulu, G., & Rajasekhar Reddy, B. (2016). Purification and characterization of β-glucosidase from Aspergillus niger. International Journal of Food Properties, 19(3), 652–661.
Gao, L., Gao, F., Zhang, D., Zhang, C., Wu, G., & Chen, S. (2013). Purification and characterization of a new β-glucosidase from Penicillium piceum and its application in enzymatic degradation of delignified corn stover. Bioresource technology, 147, 658–661.
Pandey, A.K. and Negi, S., 2020. Enhanced cellulase recovery in SSF from Rhizopus oryzae SN5 and immobilization for multi-batch saccharification of carboxymethylcellulose. Biocatalysis and Agricultural Biotechnology, 26, p.101656.
Shruthi, B. R., Achur, R. N. H., & Nayaka Boramuthi, T. (2020). Optimized solid-state fermentation medium enhances the multienzymes production from Penicillium citrinum and Aspergillus clavatus. Current Microbiology, 77, 2192–2206.
Sulyman, A.O., Igunnu, A. and Malomo, S.O., 2020. Isolation, purification and characterization of cellulase produced by Aspergillus niger cultured on Arachis hypogaea shells. Heliyon, 6(12), p.e05668.
Bhanja, T., Rout, S., Banerjee, R., & Bhattacharyya, B. C. (2007). Comparative profiles of α-amylase production in conventional tray reactor and GROWTEK bioreactor. Bioprocess and biosystems engineering, 30(5), 369–376.
Tao, Y. M., Zhu, X. Z., Huang, J. Z., Ma, S. J., Wu, X. B., Long, M. N., & Chen, Q. X. (2010). Purification and properties of endoglucanase from a sugar cane bagasse hydrolyzing strain, Aspergillus glaucus XC9. Journal of Agricultural and Food Chemistry, 58(10), 6126–6130.
Liszka, M. J., Clark, M. E., Schneider, E., & Clark, D. S. (2012). Nature versus nurture: Developing enzymes that function under extreme conditions. Annual review of chemical and biomolecular engineering, 3, 77–102.
Shahriarinour, M., & Ramanan, R. N. (2015). Purification and characterisation of extracellular cellulase main components from Aspergillus terreus. BioResources, 10(3), 4886–4902.
Bano, A., Chen, X., Prasongsuk, S., Akbar, A., Lotrakul, P., Punnapayak, H., Anwar, M., Sajid, S., & Ali, I. (2019). Purification and characterization of cellulase from obligate halophilic Aspergillus flavus (TISTR 3637) and its prospects for bioethanol production. Applied biochemistry and biotechnology, 189(4), 1327–1337.
Santa-Rosa, P. S., Souza, A. L., Roque, R. A., Andrade, E. V., Astolfi-Filho, S., Mota, A. J., & Nunes-Silva, C. G. (2018). Production of thermostable β-glucosidase and CMCase by Penicillium sp. LMI01 isolated from the Amazon region. Electronic Journal of Biotechnology, 31, 84–92.
Monteiro, L. M. O., Vici, A. C., Pinheiro, M. P., Heinen, P. R., de Oliveira, A. H. C., Ward, R. J., Prade, R. A., Buckeridge, M. S., & de Moraes, M. D. L. T. (2020). A highly glucose tolerant ß-glucosidase from Malbranchea pulchella (mp bg3) enables cellulose saccharification. Scientific reports, 10(1), 1–12.
Langston, J., Sheehy, N. and Xu, F., 2006. Substrate specificity of Aspergillus oryzae family 3 β-glucosidase. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 1764(5), pp.972–978.
Silva, L. D. B., Gomes, T. C., Ullah, S. F., Ticona, A. R. P., Hamann, P. R. V., & Noronha, E. F. (2020). Biochemical properties of carbohydrate-active enzymes synthesized by Penicillium chrysogenum using corn straw as carbon source. Waste and Biomass Valorization, 11, 2455–2466.
Da Costa, S. G., Pereira, O. L., Teixeira-Ferreira, A., Valente, R. H., de Rezende, S. T., Guimarães, V. M., & Genta, F. A. (2018). Penicillium citrinum UFV1 β-glucosidases: Purification, characterization, and application for biomass saccharification. Biotechnology for biofuels, 11(1), 1–19.
Martins ME Da M., Martins E. Da S., Martins H.L., 2020. Production and characterization of a thermostable β-glucosidase from Myceliophthora heterothallica. Bioscience Journal, 36(1).
Pol, D., Laxman, R. S., & Rao, M. (2012). Purification and biochemical characterization of endoglucanase from Penicillium pinophilum MS 20. Indian Journal of Biochemistry & Biophysics, 49(3), 189–194.
Qin, Y., Li, Q., Luo, F., Fu, Y., & He, H. (2020). One-step purification of two novel thermotolerant β-1, 4-glucosidases from a newly isolated strain of Fusarium chlamydosporum HML278 and their characterization. AMB Express, 10(1), 1–11.
Alani, F., Anderson, W.A. and Moo-Young, M., 2008. New isolate of Streptomyces sp. with novel thermoalkalotolerant cellulases. Biotechnology letters, 30(1), pp.123–126.
Karami, F., Ghorbani, M., Mahoonak, A.S. and Khodarahmi, R., 2020. Fast, inexpensive purification of β-glucosidase from Aspergillus niger and improved catalytic/physicochemical properties upon the enzyme immobilization: Possible broad prospects for industrial applications. LWT, 118, p.108770.
Kudo, K., Watanabe, A., Ujiie, S., Shintani, T., & Gomi, K. (2015). Purification and enzymatic characterization of secretory glycoside hydrolase family 3 (GH3) aryl β-glucosidases screened from Aspergillus oryzae genome. Journal of bioscience and bioengineering, 120(6), 614–623.
Mrudula, S., & Murugammal, R. (2011). Production of cellulase by Aspergillus niger under submerged and solid state fermentation using coir waste as a substrate. Brazilian Journal of Microbiology, 42(3), 1119–1127.
Gupta, R., Gigras, P., Mohapatra, H., Goswami, V. K., & Chauhan, B. (2003). Microbial α-amylases: A biotechnological perspective. Process biochemistry, 38(11), 1599–1616.
Pachauri, P., More, S., Aranganathan, V., & Sullia, S. B. (2018). Kinetic study and characterization of cellulase enzyme from isolated Aspergillus niger subsp. awamori for cellulosic biofuels. Journal of Scientific and Industrial Research, 77, 55–60.
Volkov, P. V., Rozhkova, A. M., Zorov, I. N., & Sinitsyn, A. P. (2020). Cloning, purification and study of recombinant GH3 family β-glucosidase from Penicillium verruculosum. Biochimie, 168, 231–240.
Yang, Y., Wang, J., Guo, H. and Cao, Y., 2020. The enzymatic characters of heterologous expressed novel β-1, 4-glucosidase originated from Aspergillus fresenii. 3 Biotech, 10, pp.1–9.
Pereira, J., de, C., Giese, E.C., Moretti, M.M., de, S., Gomes, A.C., dos, S., Perrone, O.M., Boscolo, M., Da Silva, R., Gomes, E., Martins, D.A.B., 2017. Effect of metal ions, chemical agents and organic compounds on lignocellulolytic enzymes activities. In: Enzyme Inhibitors and Activators. InTech, pp. 139–164.
Okereke, O. E., Akanya, H. O., & Egwim, E. C. (2017). Purification and characterization of an acidophilic cellulase from Pleurotus ostreatus and its potential for agrowastes valorization. Biocatalysis and Agricultural Biotechnology, 12, 253–259.
Feng, T., Liu, H., Xu, Q., Sun, J., & Shi, H. (2015). Identification and characterization of two endogenous β-glucosidases from the termite Coptotermes formosanus. Applied biochemistry and biotechnology, 176(7), 2039–2052.
Stricks, W. and Kolthoff, I.M., 1953. Reactions between mercuric mercury and cysteine and glutathione. Apparent dissociation constants, heats and entropies of formation of various forms of mercuric mercapto-cysteine and-glutathione. Journal of the American Chemical Society, 75(22), pp.5673–5681.
Bonfá, E. C., de Souza Moretti, M. M., Gomes, E., & Bonilla-Rodriguez, G. O. (2018). Biochemical characterization of an isolated 50 kDa beta-glucosidase from the thermophilic fungus Myceliophthora thermophila M. 7.7. Biocatalysis and agricultural biotechnology, 13, 311–318.
Lee, H. J., Lee, Y. S., & Choi, Y. L. (2018). Cloning, purification, and characterization of an organic solvent-tolerant chitinase, MtCh509, from Microbulbifer thermotolerans DAU221. Biotechnology for biofuels, 11(1), 1–14.
Bai, H., Wang, H., Junde Sun, M. I., Han, M., Huang, Y., Han, X., & Yang, Q. (2013). Production, purification and characterization of novel beta glucosidase from newly isolated Penicillium simplicissimum H-11 in submerged fermentation. EXCLI journal, 12, 528.
Gong, G., Zheng, Z., Liu, H., Wang, L., Diao, J., Wang, P., & Zhao, G. (2014). Purification and characterization of a beta-glucosidase from Aspergillus niger and its application in the hydrolysis of geniposide to genipin. Journal of Microbiology and Biotechnology, 24, 788–794.
Bansal, N., Janveja, C., Tewari, R., Soni, R., & Soni, S. K. (2014). Highly thermostable and pH-stable cellulases from Aspergillus niger NS-2: Properties and application for cellulose hydrolysis. Applied biochemistry and biotechnology, 172(1), 141–156.
Aich, S., Singh, R. K., Kundu, P., Pandey, S. P., & Datta, S. (2017). Genome-wide characterization of cellulases from the hemi-biotrophic plant pathogen, Bipolaris sorokiniana, reveals the presence of a highly stable GH7 endoglucanase. Biotechnology for biofuels, 10(1), 1–14.
Prajapati, B. P., Suryawanshi, R. K., Agrawal, S., Ghosh, M., & Kango, N. (2018). Characterization of cellulase from Aspergillus tubingensis NKBP-55 for generation of fermentable sugars from agricultural residues. Bioresource technology, 250, 733–740.
das Neves, C.A., de Menezes, L.H.S., Soares, G.A., dos Santos Reis, N., Tavares, I.M.C., Franco, M. and de Oliveira, J.R., 2020. Production and biochemical characterization of halotolerant β-glucosidase by Penicillium roqueforti ATCC 10110 grown in forage palm under solid-state fermentation. Biomass Conversion and Biorefinery, pp.1–12.
Souza, L. O., de Brito, A. R., Bonomo, R. C. F., Santana, N. B., Ferraz, J. L. A. A., Aguiar-Oliveira, E., & Franco, M. (2018). Comparison of the biochemical properties between the xylanases of Thermomyces lanuginosus (Sigma®) and excreted by Penicillium roqueforti ATCC 10110 during the solid state fermentation of sugarcane bagasse. Biocatalysis and Agricultural Biotechnology, 16, 277–284.
Ijaz, A., Anwar, Z., Irshad, M., Iqbal, Z., Arshad, M., Javed, M., & ZULFIQAR, M., AHMAD, A.R. and Ahmad, A. (2014). Purification and kinetic characterization of statistically optimized cellulase produced from Aspergillus niger. Romanian Biotechnological Letters, 19(6), 9836.
Akatin, M. Y. (2013). Characterization of a β-glucosidase from an edible mushroom, Lycoperdon pyriforme. International Journal of Food Properties, 16(7), 1565–1577.
Baffi, M. A., Martin, N., Tobal, T. M., Ferrarezi, A. L., Lago, J. H. G., Boscolo, M., Gomes, E., & Da-Silva, R. (2013). Purification and characterization of an ethanol-tolerant β-glucosidase from Sporidiobolus pararoseus and its potential for hydrolysis of wine aroma precursors. Applied biochemistry and biotechnology, 171(7), 1681–1691.
Arevalo-Villena, M., Ubeda Iranzo, J., & Briones Perez, A. (2007). Enhancement of aroma in white wines using a β-glucosidase preparation from Debaryomyces pseudopolymorphus (A-77). Food Biotechnology, 21(2), 181–194.
Liu, Y., Tan, H., Zhang, X., Yan, Y., & Hameed, B. H. (2010). Effect of monohydric alcohols on enzymatic transesterification for biodiesel production. Chemical Engineering Journal, 157(1), 223–229.
Wang, S., Meng, X., Zhou, H., Liu, Y., Secundo, F., & Liu, Y. (2016). Enzyme stability and activity in non-aqueous reaction systems: A mini review. Catalysts, 6(2), 32.
Su, E., & Wei, D. (2008). Improvement in lipase-catalyzed methanolysis of triacylglycerols for biodiesel production using a solvent engineering method. Journal of Molecular Catalysis B: Enzymatic, 55(3–4), 118–125.
Klibanov, A. M. (1990). Asymmetric transformations catalyzed by enzymes in organic solvents. Accounts of Chemical Research, 23(4), 114–120.
Mattos, C., & Ringe, D. (2001). Proteins in organic solvents. Current opinion in structural biology, 11(6), 761–764.
Sinha, R. and Khare, S.K., 2014. Effect of organic solvents on the structure and activity of moderately halophilic Bacillus sp. EMB9 protease. Extremophiles, 18(6), pp.1057–1066.
Kumar, A., Dhar, K., Kanwar, S. S., & Arora, P. K. (2016). Lipase catalysis in organic solvents: Advantages and applications. Biological Procedures Online, 18(1), 1–11.
Sonnleitner, B., & Fiechter, A. (1983). Advantages of using thermophiles in biotechnological processes: Expectations and reality. Trends in Biotechnology, 1(3), 74–80.
Cakmak, U., & Ertunga, N. S. (2016). Gene cloning, expression, immobilization and characterization of endo-xylanase from Geobacillus sp. TF16 and investigation of its industrial applications. Journal of Molecular Catalysis B: Enzymatic, 133, S288–S298.
Trinh, D.K., Quyen, D.T., Do, T.T. and Nghiem, N.M., 2013. Purification and characterization of a novel detergent-and organic solvent-resistant endo-beta-1, 4-glucanase from a newly isolated basidiomycete Peniophora sp. NDVN01. Turkish Journal of Biology, 37(4), pp.377–384.
Watanabe, A., Suzuki, M., Ujiie, S., & Gomi, K. (2016). Purification and enzymatic characterization of a novel β-1, 6-glucosidase from Aspergillus oryzae. Journal of bioscience and bioengineering, 121(3), 259–264.
Wang, Y., Li, J., & Xu, Y. (2011). Characterization of novel β-glucosidases with transglycosylation properties from Trichosporon asahii. Journal of agricultural and food chemistry, 59(20), 11219–11227.
Batra, J., & Mishra, S. (2013). Organic solvent tolerance and thermostability of a β-glucosidase co-engineered by random mutagenesis. Journal of Molecular Catalysis B: Enzymatic, 96, 61–66.
Piñuel, L., Breccia, J.D., Guisán, J.M. and López-Gallego, F., 2013. Production of hesperetin using a covalently multipoint immobilized diglycosidase from Acremonium sp. DSM24697. Journal of molecular microbiology and biotechnology, 23(6), pp.410–417.
Abdel-Fatah, O. M., Hassan, M. M., Elshafei, A. M., Haroun, B. M., Atta, H. M., & Othman, A. M. (2012). Physiological studies on carboxymethyl cellulase formation by Aspergillus terreus DSM 826. Brazilian journal of microbiology, 43(1), 01–11.
Prasanna, H.N., Ramanjaneyulu, G. and Reddy, B.R., 2016. Optimization of cellulase production by Penicillium sp. 3 Biotech, 6(2), pp.1–11.
Callow, N. V., & Ju, L. K. (2012). Promoting pellet growth of Trichoderma reesei Rut C30 by surfactants for easy separation and enhanced cellulase production. Enzyme and microbial technology, 50(6–7), 311–317.
Chan, C.S., Sin, L.L., Chan, K.G., Shamsir, M.S., Abd Manan, F., Sani, R.K. and Goh, K.M., 2016. Characterization of a glucose-tolerant β-glucosidase from Anoxybacillus sp. DT3–1. Biotechnology for biofuels, 9(1), pp.1–11.
Lucas, R., Robles, A., Alvarez de Cienfuegos, G., & Gálvez, A. (2000). β-Glucosidase from Chalara paradoxa CH32: Purification and properties. Journal of agricultural and food chemistry, 48(8), 3698–3703.
Wu, J., & Ju, L. K. (1998). Enhancing enzymatic saccharification of waste newsprint by surfactant addition. Biotechnology progress, 14(4), 649–652.
Asha, B. M., & Sakthivel, N. (2014). Production, purification and characterization of a new cellulase from Bacillus subtilis that exhibit halophilic, alkalophilic and solvent-tolerant properties. Annals of Microbiology, 64(4), 1839–1848.
Park, A. R., Hong, J. H., Kim, J. J., & Yoon, J. J. (2012). Biochemical characterization of an extracellular β-glucosidase from the fungus, Penicillium italicum, isolated from rotten citrus peel. Mycobiology, 40(3), 173–180.
Dave, B. R., Sudhir, A. P., & Subramanian, R. B. (2015). Purification and properties of an endoglucanase from Thermoascus aurantiacus. Biotechnology Reports, 6, 85–90.
Gudi, S. K., Gurramkonda, C., Chandra, S. M., Prasad, S. B. V., Yadav, S. P., Kumar, N. C. V. M., & Reddy, R. (2016). Thermostable β-D-glucosidase from Aspergillus flavus: Production, purification and characterization. International Journal of Clinical and Biological Sciences, 1, 1–15.
Scott, T. A. (1996). Concise encyclopedia biology. Walter de Gruyter.
Muensean, K., & Kim, S. M. (2015). Purification and characterization of β-glucosidase produced by Trichoderma citrinoviride cultivated on microalga Chlorella vulgaris. Applied biochemistry and microbiology, 51(1), 102–107.
Chen, Z., Liu, Y., Liu, L., Chen, Y., Li, S., & Jia, Y. (2019). Purification and characterization of a novel β-glucosidase from Aspergillus flavus and its application in saccharification of soybean meal. Preparative Biochemistry and Biotechnology, 49(7), 671–678.
Acknowledgements
The authors acknowledge the support provided by the Department of Biotechnology, National Institute of Technology Warangal, Telangana, India.
Author information
Authors and Affiliations
Contributions
Paramjeet Saroj performed the research experiments, data analysis, and writing of the manuscript. Manasa P contributed to the data collection and manuscript writing. Korrapati Narasimhulu supervised during research experiments and manuscript preparation. All authors contributed to the study conception and design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saroj, P., P, M. & Narasimhulu, K. Biochemical Characterization of Thermostable Carboxymethyl Cellulase and β-Glucosidase from Aspergillus fumigatus JCM 10253. Appl Biochem Biotechnol 194, 2503–2527 (2022). https://doi.org/10.1007/s12010-022-03839-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03839-2