Abstract
Myocardial infarction (MI) causes cardiomyocyte death, provokes innate immune response, and initiates tissue remodeling. The intrinsic healing process is insufficient to replace the lost cells, or regenerate and restore the functional features of the native myocardium. Autologous bone marrow-derived mesenchymal stem cell (BM-MSC) transplantation is being explored to offer therapeutic potential after MI. Here, we cultured human BM-MSC spheroids in three-dimensional collagenous gels for 28 days under exposure to tumor necrosis factor-alpha (+ TNFα), and coculture with adult human cardiomyocytes, or with conditioned media (CM) pooled from TNFα-stimulated adult cardiomyocytes. MSC differentiation marker (CD90, GATA4, cTnI, cTnT, Cx43, MHC, α-actin) expression, matrix protein (elastin, hyaluonic acid, sulfated glycosaminoglycans, laminin, fibrillin, nitric oxide synthase) synthesis, and secretome (cytokines, chemokines, growth factors) release at days 12 and 28 were assessed. MSC density decreased with duration in all culture conditions, except in controls. GATA4 expression was higher in cocultures but lower in + TNFα cultures. Synthesis and deposition of various extracellular matrix proteins and lysyl oxidase within MSC cultures, as well as secretome composition, were strongly dependent on the culture condition and duration. Results suggest that TNFα-induced inflammation suppresses BM-MSC survival and differentiation into mature cardiomyocytes by day 28, while promoting matrix protein synthesis and cytokine release conducive to MI remodeling. These findings could have implications in developing tissue engienering and cell transplantation strategies targeting MI, as well as to develop therapuetics to target inflammation-induced matrix remodeling post-MI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocardial infarction (MI) occurs when occuluded coronary arteries block the supply of oxygen and nutrients to the heart [1, 2], resulting in cardiomyocyte death. Post-MI, various signaling molecules are released, immune response is provoked via upregulation of complement cascade, cytokine/chemokine expression is enhanced [1, 3], and new extracellular matrix deposition and scar formation occur [3]. Several intrinsic cellular and biomolecular events are sequentially and spatiotemporally orchestrated to induce the repair process, though the outcomes are usually determined by the extent of inflammation [4]. Chemokines are significantly upregulated following MI, which are crucial for immune cell infiltration to the infarct tissue [4]. The infiltrating macrophages release anti-inflammatory and pro-fibrotic mediators [4] which suppress T cell and B cell proliferation, inhibit matrix metalloproteinases (MMPs) formation and upregulate tissue inhibitors of matrix metalloproteinases (TIMPs) release, and promote myofibroblast differentiation, thereby contributing to matrix remodeling [4].
Myofibroblasts synthesize matrix proteins such as collagens, although higher fibrotic deposition leads to impaired cell–cell coupling and reduced mechanical function of ventricles, which eventually cause adverse cardiac function [5]. Myocardial resident and infiltrating immune cells secrete higher levels of growth factors upon ischemic insult via activation of hypoxia-inducible factor 1-alpha (HIF-1α) and nitric oxide (NO) generation, which in turn promote invasion of endothelial progenitor cells, marrow stromal/stem cells, and native stem/progenitor cells into the infract tissue to promote angiogenesis, vasculogenesis, and neo-matrix formation [3]. In addition, several chemokines, growth factors, proteases, and inhibitors were reported to play specific roles during progression and repair of heart disease.
However, the intrinsic cardiac healing is insufficient to regenerate the native structural, functional, and biological integrity of the myocardium [6, 7]. Cell therapy has been pursued as a potential approach to provide regenerative cues to the diseased myocardium following MI [8]. Among the various cell types suitable for such therapy, bone marrow-derived mesenchymal stem cells (BM-MSCs) are a promising autologous cell source [9,10,11] as they prevent apoptosis, inhibit fibrosis, promote angiogenesis, proffer immunomodulation, and can be coaxed to differentiate into cardiomyocytes [12,13,14]. We and others have shown that MSCs secrete a cocktail of angiogenic, anti-apoptotic, and mitogenic factors when cultured in vitro [15, 16].
Previously, we reported on the ability of human BM-MSCs to differentiate into cardiomyocyte-like cells and secrete several growth factors, chemokines, and proteases pertinent for cardiac tissue regeneration [15, 17, 18]. Our study utilized three-dimensional (3D) spheroid model of human BM-MSCs as a mechanism to precondition MSCs and enhance their differentiation and paracrine signaling ability. Since the fate of human MSCs is largely dependent on their local microenvironment [19, 20], it is important to understand how their differentiation, paracrine secretion, and matrix regeneration ability would be modulated when implanted in vivo within a dynamic and inflammatory infarct cardiac tissue. More recently, we elucidated the cardiac differentiation potential of human MSC spheroids cultured in 3D collagen hydrogel in the presence of 5-azacytidine (aza) [18]. Such gel-laden MSCs showed potential in cardiac regeneration as they might replace cardiac cells and help regenerate the cardiac matrix via synthesis of several cardiac matrix components and secretion of essential paracrine signaling molecules [15].
Here, we investigated the roles of inflammatory conditions and coculture with adult human cardiomyocytes, on the ability of human MSC spheroids within 3D collagen gels to survive and differentiate into cardiomyocyte-like cells, synthesize cardiac matrix components, and secrete paracrine signaling molecules relevant for cardiac tissue regeneration. We hypothesize that (i) such inflammatory and coculture conditions partly mimic the in vivo microenvironment of the infarct tissue and modulate the differentiation and secretome ability of human MSCs and (ii) paracrine secretion by MSC spheroids within inflammatory conditions would be enhanced to provide higher cell survival and regenerative milieu.
Materials and Methods
Human Primary Cardiomyocyte Culture
Cryopreserved human primary cardiomyocytes (Catalog # T4037; lots: RZ8149 and 0017825754001) were obtained from Applied Biological Materials Inc. (Richmond, BC, Canada). The cells were isolated from normal human heart ventricles (59-year-old Caucasian male for RZ8149; 48-year-old Caucasian male for 0017825754001), characterized using flow cytometry for cardiac-specific markers such as sarcomeric α-actinin and myosin, and cryopreserved. For all experiments, the cryopreserved cell ampules were thawed in Prigrow I medium reconstituted with 10% fetal bovine serum and 1% penicillin–streptomycin, centrifuged at 300 × g for 3 min, and cultured on tissue culture plastics coated with Applied Cell Extracellular Matrix (ECM; G422). Cells were treated with 10 ng/mL TNFα throughout the culture duration (Fig. 1A) to induce inflammation [12]. Cells from lot RZ8149 were used for all experiments related to biochemical analysis, while those from 0017825754001 were used for differentiation and survival assays to account for any potential donor to donor variability. All media and associated ingredients were purchased from Applied Biological Materials Inc., or Thermo Fisher Scientific (Waltham, MA, USA).
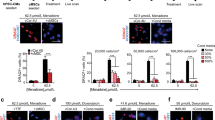
(A) Representative phase-contrast and immunofluorescence (Live/Dead assay) images of human primary cardiomyocytes seeded on ECM-coated tissue culture plastic, in control and TNFα-stimulated cultures, at D28. Scale bar: 100 µm. (B) Schematic of coculture setup between human primary cardiomyocytes and human MSCs. (C) Representative immunofluorescence images of MSCs in collagen 3D gels, from Live/Dead® assay, under respective culture conditions at D28. Scale bar: 100 µm. Average cell densities of MSCs (D) and cardiomyocytes (E) in various culture conditions, at D12 and D28. * indicates p < 0.05
Human BM-MSC and Cardiomyocyte Cultures
Human BM-MSCs (cryopreserved Poietics™ normal HBM‐MSCs; PT‐2501; Lonza, Walkersville, MD, USA) were expanded in Gibco™ DMEM with media changes every 24 h. They were then formed into spheres and cultured within rat tail-derived type I collagen as we reported earlier [15, 18]. Direct cocultures of human MSC spheroids and human cardiomyocytes were established by seeding one cell type in the 24-well plates and the other in the Transwell® cell culture inserts (Falcon®, PET Membrane, 1-µm membrane pore size), with the culture media for each cell type mixed at 1:1 ratio (Fig. 1B). Separately, indirect cocultures were established by supplementing MSC spheroids with spent conditioned media retrieved from cardiomyocyte cultures treated with 10 ng/mL TNFα. The culture medium specific to each cell type in the indirect cocultures was also maintained at 1:1 ratio. In all cases, MSC spheroids were sandwiched within 2 mg/mL rat tail-derived collagen hydrogels, whereas cardiomyocytes were cultured on 2D tissue culture plastic or Transwell® inserts coated with the proprietary ECM (G422) to enhance cell adhesion. Cardiomyocyte differentiation, matrix synthesis, and paracrine release by MSC spheroids were investigated under four different culture conditions: MSC spheroids alone (MSC alone), MSC spheroids treated with 10 ng/mL TNFα (+ TNFα), MSC spheroids cocultured with cardiomyocytes (coculture), and MSC spheroids treated with conditioned medium from TNFα-exposed cardiomyocytes (+ CM). Results from coculture conditions were included in Supplementary information. In parallel, the matrix synthesis and paracrine release by human adult cardiomyocytes in the presence (+ TNFα) or absence (Control) of 10 ng/mL TNFα were quantified.
Immunofluorescence Labeling and Imaging
All antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA) or Abcam (Cambridge, MA, USA). Primary antibodies for CD90, CD73, GATA4, cardiac Troponin T (cTnT), cardiac Troponin I (cTnI), connexin-43 (Cx-43), cardiac myosin heavy chain (MHC), and α-sarcomeric actin were used for immunofluorescence staining to evaluate the stemness and progression to early, mature, and contractile lineages. Similarly, primary antibodies for type III collagen, laminin, fibrillin-1, and nitric oxide synthases (NOS2, NOS3) were used to assess the cardiac matrix proteins and enzymes released by the cells in selective culture conditions. Staining procedures and imaging were described earlier [18]. Live/Dead® Viability/Cytotoxicity Kit (Thermo Fisher Scientific) was used to determine the cell viability and toxicity at various culture conditions as per the manufacturer’s recommendations. The immunofluorescence images of cells stained with various markers were quantified, where appropriate, to compare fluorescence intensities across the conditions stained with the same marker. Briefly, multiple cells were selected per image, one at a time, using freeform tool in NIH ImageJ software, and the area integrated intensity as well as the mean gray values was measured. Similar measurements were obtained from regions (on the same images) that have no fluorescence to capture the background levels. The corrected total cell fluorescence (CTCF) was derived as the difference between integrated density and the product of area of selected cell and mean fluorescence of background readings. CTCF has arbitrary units [21, 22].
DNA and ECM Quantification
The ECM types and amounts, i.e., total proteins, sulfated glycosaminoglycans (sGAG), hyaluronic acid (HA), elastin, and lysyl oxidase enzyme (LOX), released by cells into the pooled spent culture media or deposited as matrix within the cell layers in respective scaffolds, were quantified using protocols we detailed earlier [15]. A fluorescence-based DNA quantification kit (Quant-iT™ PicoGreen® dsDNA kit, Thermo Fisher Scientific) was used to quantify the total double-stranded deoxyribonucleic acid (dsDNA) in all cases. The numbers of cardiomyocytes and MSCs in cultures were obtained by assuming 20 pg DNA/cell and 8.5 pg DNA/cell, respectively, and cell proliferation compared under various culture conditions [23, 24]. Total sGAG deposition within the cell layers and that released in the pooled media were quantified using Sulfated Glycosaminoglycan Quantification Kit (Amsbio LLC, Cambridge, MA, USA).
Cytokine/Chemokine Analysis
Essential MSC cytokines and chemokines were quantified using Discovery Assays® (Eve Technologies, Alberta, Canada) according to the manufacturer’s protocol. Briefly, the pooled spent media from each culture condition at day 12 (D12) and day 28 (D28) time points were processed using Human MMP & TIMP Panel assay (sensitivity: 0.5–14 pg/mL); Human Cytokine/Chemokine Array assay (sensitivity: 0.5–10 pg/mL); and TGF-β array (sensitivity: 2–6 pg/mL). Analytes were quantified using multiplex LASER bead technology and bead analyzer (Bio-Plex 200), where antibody-conjugated fluorophore beads simultaneously detect multiple analytes from a single assay.
Statistical Analysis
All data are represented as mean ± standard error, with at least 3 independent replicates, unless otherwise stated. Statistical analyses were performed using GraphPad Prism 9, and one-way analysis of variance (ANOVA) followed by Tukey’s test, or two-way ANOVA followed by Bonferroni post hoc test, as required, to determine the significance in differences between groups (p < 0.05).
Results
Cell Viability and Proliferation
Adult human primary cardiomyocytes in controls and inflammatory conditions (TNFα) had good viability at D28 (Fig. 1A). Representative immunofluorescence images of MSCs from Live/Dead® assay in respective culture conditions at D28 (Fig. 1C) showed significant cell survival in all cases. MSC density decreased with duration in all culture conditions, except in controls (Fig. 1D). The results from cocultures condition also showed similar trends (Fig. S1). MSCs in control cultures by D28 showed significantly higher cell density compared to D12 (2.25-fold; p < 0.001), while MSC density in MSC + CM condition was significantly lower at D28 compared to D12 (p = 0.0064). Similarly, from D12 to D28, cardiomyocyte density significantly (p < 0.05) increased in controls and TNFα-stimulated cultures, while it decreased in cocultures (Fig. 1E). Taken together, TNFα stimulation significantly suppressed MSC density at both D12 and D28, although such effects of TNFα were not evident in adult cardiomyocyte cultures.
Cardiac Marker Expression in Adult Human Cardiomyocytes
The expression of cardiac markers in adult primary human cardiomyocytes within controls, cocultures, and TNFα-treated cultures was characterized using immuno- fluorescence imaging. Cardiomyocytes expressed Cx43, MHC, and α-actin in all culture conditions (Fig. 2). Most cardiomyocytes in all the three culture conditions expressed Cx43 (≈ 90%), while cocultures had a lower expression of MHC and α-actin (Fig. S2). The strong staining for Cx43 (cardiac gap junction protein), surface marker α-MHC, and sarcomeric α-actin in these cultures at D28 attest to the continued retention of functionality of adult primary cardiomyocytes even in the presence of TNFα (and within MSC cocultures to a certain extent).
MSC Differentiation in Various Culture Conditions
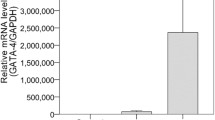
The exclusive expression of CD90 within spheroids differed among culture conditions by D28, with the highest expression in controls and the least in TNFα-exposed cultures (Fig. 3). MSCs co-expressing CD90 and GATA4 were significantly higher in controls compared to all other culture conditions. Interestingly, GATA4 exclusive expression was significantly higher in cocultures (Fig. S3) than in TNFα-exposed cultures (Fig. 3). However, GATA4 + cTNT and cTnT + CD90 co-expressions were much lower in all culture conditions except in controls. Similar trends could be evinced in the weak expression of cardiac conduction (Cx43) and contractile markers (MHC, α-actin) in all culture conditions except in controls (Fig. S4). Representative fluorescence images of these markers for all MSC culture conditions, as well as CTCF quantification of such images, are shown in Figure S4. Together, these results suggest that TNFα presence might be suppressing BM-MSC differentiation into more mature cardiomyocyte-like cells within the cardiomyocyte lineage spectrum by D28 as evident from myocyte marker expression.
(A) Quantification of MSC differentiation into cardiomyocyte lineage under healthy and inflammatory conditions. The percentages of cells expressing stemness, early, and late cardiac markers at D28 of culture were shown. (B) Representative immunofluorescence images of MSCs treated with TNFα or conditioned media. Scale bar: 100 µm
ECM Protein Synthesis in Various Culture Conditions
In general, (i) the release and deposition of normalized levels of total protein content, sGAGs, HA, and elastin within MSC alone cultures decreased with culture duration whereas those within cocultures increased over time (Fig. 4A, Table S1), and (ii) protein deposition as matrix was at least 1–2 orders of magnitude lower than their release into pooled media over the culture duration. Significantly higher amounts of these proteins were noted within cocultures compared to respective MSC alone cultures on D28 (p < 0.05; Table S1). The deposition and release of normalized levels of total protein, HA, and elastin by MSCs stimulated with TNFα or supplemented with conditioned media increased steadily from D12 to D28 (Fig. 4A). Immunofluorescence labeling also showed higher expression of other cardiac ECM proteins, mainly collagen III, laminin, fibrillin, and NOS2 at D28 within cocultures compared to monocultures (Fig. S5).
(A) Normalized protein amounts deposited as matrix or released into pooled media in MSC alone, MSC + TNFα, and MSCs receiving conditioned medium from cardiomyocytes (+ CM), at D12 and D28 time points. * indicates p < 0.05. LOX levels in pooled media and deposited matrix layers within (B) MSC cultures and (C) cardiomyocyte cultures, at D12 and D28, under various TNFα-stimulatory culture conditions. * indicates p < 0.05
Except in MSC alone cultures, LOX deposition as matrix improved with culture duration (Fig. 4B). However, LOX release decreased with culture duration except in + TNFα cultures. Finally, one-way ANOVA followed by Tukey’s test or two-way ANOVA followed by Bonferroni’s post hoc test suggested that the release (into media) and deposition (as matrix) of normalized total protein, sGAG, HA, elastin, and LOX in MSC cultures were dependent on the culture condition, culture duration, and the interaction of these two factors (p < 0.01, in all cases). These results collectively suggest that TNFα stimulate significant increases in the synthesis and deposition of various matrix proteins (elastin, sGAGs, HA, LOX) in MSC cultures. On the other hand, cocultures with cardiomyocytes contribute to the deposition of basement membrane proteins (e.g., collagen type III, laminin, fibrillin), shown by the representative fluorescence images and CTCF quantification in Fig. S5. The synthesis and deposition of these protein types are relevant for cardiac tissue remodeling post-MI, where both TNFα and adult cardiomyocytes co-inhabit the inflammatory niche with implanted MSCs.
ECM Protein Synthesis by Adult Cardiomyocytes Within Different Cultures
Within controls, significant reduction in total protein deposition (p < 0.0001) but not in total protein release was observed by D28 compared to D12 (Fig. 5A). Within TNFα-receiving cardiomyocytes, no significant change was observed in total protein deposition but normalized total protein release significantly increased from D12 to D28 (p = 0.0005). sGAG deposition increased at least by fourfold by D28 in control cultures compared to D12 (p < 0.0001). Addition of TNFα stimulated higher sGAG deposition at D28 compared to D12, but not versus controls. HA deposition as matrix was dependent on culture condition (p = 0.0002) and on the interaction of culture condition and durations, with the highest amounts in D28 cocultures and the lowest in D28 controls. HA release in pooled media was also dependent on culture duration (p < 0.0001). Elastin deposition and release were independent of culture conditions. On the other hand, immunofluorescence images and quantification of their normalized intensities showed higher expression of cardiac ECM proteins such as collagen III, laminin, fibrillin, NOS2, and NOS3 within TNFα-stimulated cardiomyocyte cultures compared to controls (Fig. 5B).
(A) Normalized protein amounts deposited as matrix or released into pooled media in adult human cardiomyocyte cultures, receiving no TNFα (Control) or 10 ng/mL of TNFα (+ TNFα), at D12 and D28 time points. * indicates p < 0.05. (B) Representative immunofluorescence images of cardiac ECM proteins deposited by cardiomyocytes within control and TNFα-stimulated conditions. Scale bar: 100 µm. Corrected total cell fluorescence intensities of the primary markers expression were quantified from these images and analyzed. * indicates p < 0.05
In general, protein deposition as matrix in cardiomyocyte cultures is at least 1–2 orders of magnitude lower than that released into pooled media, but within the similar ranges noted in MSC cultures (Fig. 4A). Normalized LOX deposition (Fig. 4C) was dependent on culture condition (p < 0.0001), while LOX release was dependent on culture duration and interaction of culture condition and duration (p < 0.0001 for both). LOX release and deposition were lower in D28 cultures compared to D12 cultures in both the culture conditions, and LOX deposition as matrix was at least an order of magnitude lower than that released into media in all cases. In conclusion, results suggest that by D28 in adult cardiomyocyte cultures, TNFα suppressed synthesis and deposition of various extracellular matrix proteins (elastin, HA, sGAG) although basement membrane protein synthesis was significantly stimulated.
Cytokine Expression Within MSC and Adult Cardiomyocyte Cultures
By D12, GM-CSF, MCP-3, IP-10, and RANTES were higher in MSC cultures receiving TNFα and in cocultures, but very low in MSC + CM cultures and cardiomyocyte cultures (Fig. 6). The cytokine levels measured within cocultures are shown in Table S2. At least half of the cytokines tested were undetectable in MSC alone cultures, at both D12 and D28. Eotaxin and G-CSF levels were almost threefold higher in MSC cultures exposed to TNFα compared to that in cocultures. MDC was highly expressed only in cocultures, but mostly very low in all other cultures. IL-6 was low in MSC controls but high in cocultures and cultures with TNFα. IL-8 was more than tenfold higher in cocultures and cultures exposed to TNFα, compared to MSC alone or conditioned media receiving cultures. MCP-1 was almost fourfold higher in cocultures and MSCs exposed to TNFα and conditioned media, compared to MSC alone controls. Interestingly, TNFα was not expressed at all in MSC controls but was high in cocultures. The levels of growth factors (VEGF, TGF-β1, TGF-β2, TGF-β3) did not vary considerably among MSC and cardiomyocyte culture conditions. MMP-1 was relatively higher in cocultures and MSC cultures receiving the conditioned media, while MMP-2 was higher in MSC cultures receiving conditioned media. However, these metalloproteinases were relatively low in MSC alone cultures and TNFα-receiving cultures. TIMPs-1 and 2 were highly expressed in all culture conditions, while TIMP-3 was not expressed in any cultures except in MSC alone controls.
Heat maps showing levels of cytokines under various MSC and cardiomyocyte culture conditions, at D12 and D28, quantitated using laser bead technology. The gray intensity corresponds to blank values, while values outside the defined range were shown in dark brown. Abbreviations: CXC and CC, chemokine; FGF, fibroblast growth factor; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte/macrophage colony-stimulating factor; GRO, growth-regulated oncogene; HGF, hepatocyte growth factor; HIF-1α, hypoxia-inducible factor-1α; IFN-γ, interferon-gamma; IGF, insulin-like growth factor; IL, interleukins; IP-10, interferon-γ-inducible protein 10; MCP, monocyte chemoattractant protein; MDC, macrophage-derived chemokine; MHC, cardiac myosin heavy chain; PDGF, platelet-derived growth factor; RANTES, regulated on activation, normal T cell expressed and secreted; SDF-1, stromal cell-derived factor 1; TGF, transforming growth factor; VEGF, vascular endothelial growth factor
By D28, RANTES, GRO-α, IL-6, and IL-8 were drastically lower in MSC alone and MSC + CM cultures than in cocultures and MSC + TNFα cultures (Fig. 6). TNFα was significantly lower in MSC + CM cultures compared to MSC + TNFα cultures. MIP-1α was the highest within cocultures and mostly not expressed in the rest of the MSC cultures. VEGF levels were lowered in MSCs exposed to TNFα, but it was at least threefold higher in MSC alone controls and cocultures. Growth factors (VEGF, TGF-β1, TGF-β2, TGF-β3) did not vary considerably among culture conditions. MMP-1 was selectively expressed in lower amounts in MSC alone and MSC + TNFα cultures, but twofold higher in cocultures and fourfold higher in MSC + CM cultures. MMP-2 levels were at least twofold higher in MSC alone controls compared to MSCs exposed to TNFα and conditioned media. TIMP-1 was highly expressed in all culture conditions, with the highest in MSC alone controls and the lowest in MSC + TNFα cultures. TIMP-2 was high in all cultures except in MSC + TNFα cultures. TIMP-3 was high in all cases except MSC + cocultures and MSC + TNFα cultures, and TIMP-4 was least expressed in all culture conditions.
Overall, it could be concluded that (i) the types and levels of the secretome depend on the culture condition and duration; (ii) TNFα stimulated release of significantly more number and higher amounts of cytokines in MSC cultures but not in cardiomyocyte cultures, at both early (D12) and late time points (D28); and (iii) TNFα led to higher levels of MMPs with concomitant suppression of TIMPs in MSC cultures while such trends were not evident in cardiomyocyte cultures. These findings have implications in our understanding of matrix remodeling (e.g., MMP-induced degradation) and migration of cardiomyocytes and cardiac fibroblasts into the inflammatory niche in response to the growth factors and their gradients (e.g., EGF, FGF, VEGF, PDGF, TGF), as well as infiltration of circulating white blood cells owing to release of interleukins and cytokines.
Discussion
Towards investigating the suitability of human BM-MSCs for treating MI, we have recently shown that MSCs release a variety of ECM proteins and secretome over a 28-day period, when cultured in 3D type I collagen niche in vitro [15, 18, 25]. Others have shown that mechanical stimuli (e.g., shear flow, uniaxial strain, substrate rigidity) regulate MSC differentiation towards cardiac lineages, secretome release, marker expression, gene and protein expression, survival, and proliferation [26,27,28,29,30]. For cell transplantation approaches to be successful, the potential roles of inflammatory microenvironment, disorganized ECM, and presence of other innate cell types such as mature cardiomyocytes should be considered and investigated. While the role of cocultures on matrix synthesis by MSCs and their progenies has been reported [31,32,33,34,35], the influence of adult mature cardiomyocytes on MSCs has received less attention [36, 37].
TNFα was selected in our study to stimulate the cells and mimic plasma levels of TNFα in chronic heart failure patients [38]. In alignment with literature on the roles of TNFα and IL-1 on the upregulation of chemokine release by MSCs [12], we note that TNFα-stimulated MSC spheroids elicited a wide spectrum and higher amounts of inflammatory cytokines than MSC alone controls. Specifically, inflammatory cytokines such as GM-CSF, MCP-3, IP-10, Fractalkine, MDC, RANTES, and TNFα were observed within cocultures and MSCs exposed to TNFα. Cocultures additionally expressed potent anti-proliferative cytokine IFNα2 and anti-inflammatory cytokines such as IL-1RA and IL-4. While most of these cytokines in cocultures were expressed until D12, those by MSCs exposed to TNFα were predominant by D28. Potent inflammatory mediator TNFα, which induces cellular apoptosis by activating cell death pathways, was high in all MSC and cardiomyocyte cultures at both time points [39,40,41]. To conclude, the absence of most of these inflammatory chemokines within MSC alone controls (D12 and D28) but their upregulation in rest of the MSC cultures (+ TNFα, cocultures, + CM) might be contributing for higher cell density in controls and reduction in other MSC cultures (Fig. 1D).
On the other hand, potent growth factors mediating MSC survival and proliferation (e.g., EGF, FGF-2, PDGF-BB) [42, 43] were only expressed within cocultures on D12 (Table S2), but mostly not expressed in all other cultures. FGF-2 induces cardiac differentiation of stem cells and cardiac precursors, promotes reprogramming of cardiac fibroblasts [44, 45], and plays a crucial role in early cardiomyogenesis [46]. IFN-γ that induces MSC secretion [42] was only expressed within D12 cocultures. We conjecture that direct paracrine signaling between MSCs and cardiomyocytes promoted induction of mitogenic and differentiation cues in cocultures, leading to enhanced standalone expressions of GATA4 and cTnI in MSC layers, compared to MSCs supplemented with CM or TNFα. Inflammatory cytokines induce rapid reorganization of cytoskeletal and cell–cell gap junctions and reduce Young’s modulus of treated cells compared to untreated controls [47, 48]. This suggests that higher levels of inflammatory cytokines (e.g., TNFα, IL-6) in all MSC cultures in our study, except in controls, could have inhibited the expression of mature cardiac conduction and contractile markers. Thus, while the cell–cell and cell–matrix interactions of MSC spheroids within 3D collagen promoted cardiac conduction and contractile markers of MSCs [15, 18], the induced inflammatory cytokines might have abrogated the expression of such cardiac markers in MSC spheroids within such microenvironment.
Compared to MSC alone cultures, higher levels of ECM proteins (total protein, HA, and elastin, on per cell basis) were released in other cultures suggesting that MSCs synthesize higher cardiac matrix proteins under inflammatory and coculture conditions. Moreover, concomitant higher LOX and protein deposition implies higher cross-linking and maturation of the matrix within treated MSC cultures compared to controls. However, significant decrease in sGAG release observed in MSC + TNFα cultures at D12 and D28 suggests specific inhibitory role of TNFα on sGAG synthesis. Matrix release and deposition in cardiomyocytes cultures were lower in TNFα-added cultures compared to controls, indicating their matrix regeneration inability under inflammatory conditions.
MSCs treated with TNFα generated inflammatory chemokines in higher concentrations than MSCs treated with CM, possibly due to the differences in the mode of signaling among the cells. Inflammatory cytokines released from TNFα-stimulated cardiomyocytes could have acted on MSCs in a paracrine manner in + CM cultures. Conversely, MSCs treated with TNFα released a cocktail of inflammatory cytokines which might have influenced MSCs in an autocrine manner. MSCs released higher levels of anti-inflammatory cytokines and growth factors in cocultures, implying that paracrine signaling from cardiomyocytes, both during normal and inflammatory states, activates MSCs differentially to tune their inflammatory and anti-inflammatory properties.
As opposed to MSCs within cocultures, MSCs receiving CM released minimal amounts of inflammatory cytokines and measurable amounts of cardiac matrix proteins, although spheroids under those conditions did not show enhanced differentiation into cardiomyocytes. However, MSCs receiving CM had reduced levels of TNFα compared to + TNFα or + coculture conditions. These findings hold potential from the perspective of translational medicine since MSCs treated with CM showed reduced release of inflammatory cytokines, but higher or comparable amounts of cardiac matrix components compared to MSCs within cocultures. Reduced inflammation is crucial for tissue repair since inflammatory cytokines also stimulate chemokine secretion by MSCs, which not only elicit migration of inflammatory cells but also chemoattract native stem/progenitor cells to the infarct region, including native MSCs which predominantly express receptors for such chemokines [11, 12, 19, 49]. In addition, chemokine secretion by MSCs also provides immunosuppressive effect as they will drive immune cells to close proximity of MSCs, which will release immunosuppressive factors such as IDO, PG, TGF-β, and NO that will act via paracrine or direct cell–cell contact to suppress the immune cells [12, 49]. MMPs and TIMPs release by MSC spheroids hold advantage as they would enable matrix turnover needed for cell invasion and angiogenesis initiation in the infarct tissue [12]. Thus, the inflammatory cytokines, chemokines, and proteolytic enzymes expressed by MSC spheroids hold promise in cardiac tissue regeneration. Inflammatory myocardial tissue hosts multiple chemokines which not only help recruit immune cells, hematopoietic cells, and endothelial progenitors but also provide chemotactic gradient for the homing and engraftment of native stem cells including MSCs which express receptors for various chemokines, growth factors, and ECM molecules. Expressions of chemokine receptors, chemokines, and MMPs are enhanced by MSCs during inflammation, which improves their homing and invasion to the injured tissues [12].
Conclusions
We here investigated how the inflammatory and coculture condition alters the cytokine/chemokine expression by MSCs. Our results collectively suggest that (i) TNFα suppressed MSC survival but not cardiomyocyte survival over the 28-day culture period; (ii) expression of Cx43, MHC, and α-actin in cardiomyocyte cultures remained relatively unaffected, but their expressions in MSC cultures were severely inhibited in TNFα presence; (iii) TNFα suppressed MSC differentiation and maturation into cardiomyocyte lineage beyond D12; (iv) synthesis and deposition of matrix proteins increased in general in the presence of TNFα and over time in MSC cultures but not in cardiomyocyte cultures; (v) MSCs receiving conditioned media showed higher or comparable amounts of cardiac matrix components versus cocultures; and (vi) TNFα stimulated release of numerous types and higher levels of cytokines and MMPS while simultaneously suppressing TIMPs release in BM-MSC cultures but not in adult human cardiomyocyte cultures. Future studies could investigate how the chemokine receptors and adhesion molecules of MSC spheroids are altered under such conditions, as well as the behavior of BM-MSCs when implanted in vivo under inflammatory conditions. Those findings would enable to expand our knowledge on the in vivo behavior of MSC spheroids when transplanted within infarct tissue environment.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Jourdan-LeSaux, C., Zhang, J., & Lindsey, M. L. (2010). Extracellular matrix roles during cardiac repair. Life Sciences, 87, 391–400.
Yabluchanskiy, A., Li, Y., Chilton, R. J., & Lindsey, M. L. (2013). Matrix metalloproteinases: Drug targets for myocardial infarction. Current Drug Targets, 14, 276–286.
Altara, R., Manca, M., Sabra, R., Eid, A. A., Booz, G. W., & Zouein, F. A. (2016). Temporal cardiac remodeling post-myocardial infarction: Dynamics and prognostic implications in personalized medicine. Heart Failure Reviews, 21, 25–47.
Frangogiannis, N. G. (2012). Regulation of the inflammatory response in cardiac repair. Circulation Research, 110, 159–173.
Fan, D., Takawale, A., Lee, J., & Kassiri, Z. (2012). Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair, 5, 15.
Bergmann, O., Bhardwaj, R. D., Bernard, S., Zdunek, S., Barnabé-Heider, F., Walsh, S., Zupicich, J., Alkass, K., Buchholz, B. A., & Druid, H. (2009). Evidence for cardiomyocyte renewal in humans. Science, 324, 98–102.
Silvestri, A., Boffito, M., Sartori, S., & Ciardelli, G. (2013). Biomimetic materials and scaffolds for myocardial tissue regeneration. Macromolecular Bioscience, 13, 984–1019.
Joshi, J., & Kothapalli, C. R. (2015). Nanofibers based tissue engineering and drug delivery approaches for myocardial regeneration. Current Pharmaceutical Design, 21, 2006–2020.
Karantalis, V., & Hare, J. M. (2015). Use of mesenchymal stem cells for therapy of cardiac disease. Circulation Research, 116, 1413–1430.
Singh, A., Singh, A., & Sen, D. (2016). Mesenchymal stem cells in cardiac regeneration: A detailed progress report of the last 6 years (2010–2015). Stem Cell Research & Therapy, 7, 82.
Williams, A. R., & Hare, J. M. (2011). Mesenchymal stem cells: Biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circulation Research, 109, 923–940.
Van Linthout, S., Stamm, C., Schultheiss, H.-P., & Tschöpe, C. (2011). Mesenchymal stem cells and inflammatory cardiomyopathy: Cardiac homing and beyond. Cardioliology Research and Practice, 2011, 757154.
Miao, C., Lei, M., Hu, W., Han, S., & Wang, Q. (2017). A brief review: The therapeutic potential of bone marrow mesenchymal stem cells in myocardial infarction. Stem Cell Research & Therapy, 8, 242.
Chen, Z., Chen, L., Zeng, C., & Wang, W. E. (2018). Functionally improved mesenchymal stem cells to better treat myocardial infarction. Stem Cells International, 2018, 7045245.
Joshi, J., Abnavi, M. D., & Kothapalli, C. R. (2019). Synthesis and secretome release by human bone marrow mesenchymal stem cell spheroids within three-dimensional collagen hydrogels: Integrating experiments and modelling. Journal of Tissue Engineering and Regenerative Medicine, 13, 1923–1937.
Nagaya, N., Kangawa, K., Itoh, T., Iwase, T., Murakami, S., Miyahara, Y., Fujii, T., Uematsu, M., Ohgushi, H., Yamagishi, M., Tokudome, T., Mori, H., Miyatake, K., & Kitamura, S. (2005). Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation, 112, 1128–1135.
Joshi, J., Brennan, D., Beachley, V., & Kothapalli, C. R. (2018). Cardiomyogenic differentiation of human bone marrow-derived mesenchymal stem cell spheroids within electrospun collagen nanofiber mats. Journal of Biomedical Materials Research. Part A, 106, 3303–3312.
Joshi, J., Mahajan, G., & Kothapalli, C. R. (2018). Three-dimensional collagenous niche and azacytidine selectively promote time-dependent cardiomyogenesis from human bone marrow-derived MSC spheroids. Biotechnology and Bioengineering, 115, 2013–2026.
Kyurkchiev, D., Bochev, I., Ivanova-Todorova, E., Mourdjeva, M., Oreshkova, T., Belemezova, K., & Kyurkchiev, S. (2014). Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells, 6, 552.
Murphy, M. B., Moncivais, K., & Caplan, A. I. (2013). Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Experimental & Molecular Medicine, 45, e54.
McCloy, R. A., Rogers, S., Caldon, C. E., Lorca, T., Castro, A., & Burgess, A. (2014). Partial inhibition of Cdk1 in G 2 phase overrides the SAC and decouples mitotic events. Cell Cycle, 13, 1400–1412.
Burgess, A., Vigneron, S., Brioudes, E., Labbé, J. C., Lorca, T., & Castro, A. (2010). Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proceedings of the National academy of Sciences of the United States of America, 107, 12564–12569.
Vliegen, H. W., Bruschke, A. V. G., & Van der Laarse, A. (1990). Different response of cellular DNA content to cardiac hypertrophy in human and rat heart myocytes. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 95, 109–114.
Wang, L., Tran, I., Seshareddy, K., Weiss, M. L., & Detamore, M. S. (2009). A comparison of human bone marrow–derived mesenchymal stem cells and human umbilical cord–derived mesenchymal stromal cells for cartilage tissue engineering. Tissue Engineering Part A, 15, 2259–2266.
Gishto, A., Farrell, K., & Kothapalli, C. R. (2015). Tuning composition and architecture of biomimetic scaffolds for enhanced matrix synthesis by murine cardiomyocytes. Journal of Biomedical Materials Research. Part A, 103, 693–708.
Huang, Y., Zheng, L., Gong, X., Jia, X., Song, W., Liu, M., & Fan, Y. (2012). Effect of cyclic strain on cardiomyogenic differentiation of rat bone marrow derived mesenchymal stem cells. PloS One, 7, e34960.
Li, Z., Guo, X., Palmer, A. F., Das, H., & Guan, J. (2012). igh-efficiency matrix modulus-induced cardiac differentiation of human mesenchymal stem cells inside a thermosensitive hydrogel. Acta Biomat, 8, 3586–3595.
Park, J. S., Huang, N. F., Kurpinski, K. T., Patel, S., Hsu, S., & Li, S. (2007). Mechanobiology of mesenchymal stem cells and their use in cardiovascular repair. Frontiers in Bioscience, 12, 5098–5116.
Tan, G., Shim, W., Gu, Y., Qian, L., Chung, Y. Y., Lim, S. Y., Yong, P., Sim, E., & Wong, P. (2010). Differential effect of myocardial matrix and integrins on cardiac differentiation of human mesenchymal stem cells. Differentiation, 79, 260–271.
Wen, Z., Zheng, S., Zhou, C., Wang, J., & Wang, T. (2010). Repair mechanisms of bone marrow mesenchymal stem cells in myocardial infarction. Journal of Cellular and Molecular Medicine, 15, 1032–1043.
Antonioli, E., Piccinato, C. A., Nader, H. B., Cohen, M., Goldberg, A. C., & Ferretti, M. (2015). Modulation of hyaluronan synthesis by the interaction between mesenchymal stem cells and osteoarthritic chondrocytes. Stem Cells International, 2015, 640218.
Chen, W., Liu, X., Chen, Q., Bao, C., Zhao, L., Zhu, Z., & Xu, H. H. K. (2018). Angiogenic and osteogenic regeneration in rats via calcium phosphate scaffold and endothelial cell co-culture with human bone marrow mesenchymal stem cells (MSCs), human umbilical cord MSCs, human induced pluripotent stem cell-derived MSCs and human embryonic stem cell-derived MSCs. Journal of Tissue Engineering and Regenerative Medicine, 12, 191–203.
McCorry, M. C., Puetzer, J. L., & Bonassar, L. J. (2016). Characterization of mesenchymal stem cells and fibrochondrocytes in three-dimensional co-culture: Analysis of cell shape, matrix production, and mechanical performance. Stem Cell Research & Therapy, 7, 39.
Shen, T., Shen, J., Zheng, Q. Q., Li, Q. S., Zhao, H. L., Cui, L., & Hong, C. Y. (2017). Cell viability and extracellular matrix synthesis in a co-culture system of corneal stromal cells and adipose-derived mesenchymal stem cells. International Journal of Ophthalmology, 10, 670–678.
Xu, Y., Zhang, X. J., Fang, L., & Zhao, T. B. (2015). Co-culture of annulus fibrosus cells and bone marrow mesenchymal stem cells. Genetics and Molecular Research, 14, 3932–3938.
Figeac, F., Lesault, P. F., Le Coz, O., Damy, T., Souktani, R., Trébeau, C., Schmitt, A., Ribot, J., Mounier, R., Guguin, A., Manier, C., Surenaud, M., Hittinger, L., Dubois-Randé, J. L., & Rodriguez, A. M. (2014). Nanotubular crosstalk with distressed cardiomyocytes stimulates the paracrine repair function of mesenchymal stem cells. Stem Cells, 32, 216–230.
Ma, Z., Yang, H., Liu, H., Xu, M., Runyan, R. B., Eisenberg, C. A., Markwald, R. R., Borg, T. K., & Gao, B. Z. (2013). Mesenchymal stem cell-cardiomyocyte interactions under defined contact modes on laser patterned biochips. PloS One, 8, e56554.
Zhao, S. P., & Zeng, L. H. (1997). Elevated plasma levels of tumor necrosis factor in chronic heart failure with cachexia. International Journal of Cardiology, 58, 257–261.
Haudek, S. B., Taffet, G. E., Schneider, M. D., & Mann, D. L. (2007). TNF provokes cardiomyocyte apoptosis and cardiac remodeling through activation of multiple cell death pathways. The Journal of Clinical Investigation, 117, 2692–2701.
Krown, K. A., Page, M. T., Nguyen, C., Zechner, D., Gutierrez, V., Comstock, K. L., Glembotski, C. C., Quintana, P. J., & Sabbadini, R. A. (1996). Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. The Journal of Clinical Investigation, 98, 2854–2865.
Song, W., Lu, X., & Feng, Q. (2000). Tumor necrosis factor-α induces apoptosis via inducible nitric oxide synthase in neonatal mouse cardiomyocytes. Cardiovascular Research, 45, 595–602.
Gharibi, B., & Hughes, F. J. (2012). Effects of medium supplements on proliferation, differentiation potential, and in vitro expansion of mesenchymal stem cells. Stem Cells Translational Medicine, 1, 771–782.
Rodrigues, M., Griffith, L. G., & Wells, A. (2010). Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Research & Therapy, 1, 32.
Itoh, N., Ohta, H., Nakayama, Y., & Konishi, M. (2016). Roles of FGF signals in heart development, health, and disease. Front Cell Dev Biol, 4, 110.
Rosenblatt-Velin, N., Lepore, M. G., Cartoni, C., Beermann, F., & Pedrazzini, T. (2005). FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. The Journal of Clinical Investigation, 115, 1724–1733.
Bartunek, J., Croissant, J. D., Wijns, W., Gofflot, S., de Lavareille, A., Vanderheyden, M., Kaluzhny, Y., Mazouz, N., Willemsen, P., Penicka, M., Mathieu, M., Homsy, C., De Bruyne, B., McEntee, K., Lee, I. W., & Heyndrickx, G. R. (2007). Pretreatment of adult bone marrow mesenchymal stem cells with cardiomyogenic growth factors and repair of the chronically infarcted myocardium. American Journal of Physiology. Heart and Circulatory Physiology, 292, H1095–H1104.
Liu, H., Wang, N., Zhang, Z., Wang, H., Du, J., & Tang, J. (2017). Effects of tumor necrosis factor-α on morphology and mechanical properties of HCT116 human colon cancer cells investigated by atomic force microscopy. Scanning, 2017, 2027079.
Wójciak-Stothard, B., Entwistle, A., Garg, R., & Ridley, A. J. (1998). Regulation of TNF-α-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. Journal of Cellular Physiology, 176, 150–165.
da Silva Meirelles, L., Fontes, A. M., Covas, D. T., & Caplan, A. I. (2009). Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine & Growth Factor Reviews, 20, 419–427.
Acknowledgements
CK expresses his gratitude to the CSU Office of Research funds, and JJ thanks the financial support from the Cellular and Molecular Medicine Specialization Fellowship and Dissertation Research Award from CSU.
Author information
Authors and Affiliations
Contributions
JJ: experiments, data analysis, methodology, writing original draft, review and editing of final draft. CK: conceptualization, data analysis, funding acquisition, methodology, project administration, resources, writing original draft, review and editing of final draft.
Corresponding author
Ethics declarations
Ethics Approval
The authors confirm that they have adhered to all ethical policies of the journal, as noted on the journal’s author guidelines page.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Joshi, J., Kothapalli, C.R. Role of Inflammatory Niche and Adult Cardiomyocyte Coculture on Differentiation, Matrix Synthesis, and Secretome Release by Human Bone Marrow Mesenchymal Stem Cells. Appl Biochem Biotechnol 194, 1938–1954 (2022). https://doi.org/10.1007/s12010-022-03803-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03803-0