Abstract
Feeding cadmium (II) and selenium (IV) simultaneously to anaerobic granular sludge with the aim of synthesizing cadmium selenide (CdSe) nanoparticles induces compositional changes in the extracellular polymeric substances (EPS) matrix of this sludge. A methanogenic anaerobic granular sludge was repeatedly exposed to Cd(II) (10–50 mg L−1) and selenite (79 mg L−1) for 300 days at pH 7.3 and 30 °C in a fed-batch feeding regime for enrichment of Se-reducing bacteria and synthesis of CdSe nanoparticles. EPS fingerprints of the granular sludge, obtained by size exclusion chromatography coupled to a fluorescence detector, showed a significant increase in the intensity of protein-like substances with > 100 kDa apparent molecular weight (aMW) upon repeated exposure to Cd(II) and Se(VI). This was accompanied by a prominent decrease in protein-like substances of aMW < 10 kDa. The fingerprint of the humic-like substances showed emergence of a new peak with aMW of 13 to 300 kDa in the EPS extracted from the Cd/Se fed granular sludge. Experiments on metal(loid)–EPS interactions showed that the CdSe nanoparticles interact mainly with loosely bound EPS (LB-EPS). This study showed that the formation of Se(0) and CdSe nanoparticles occurs in the LB-EPS fraction of the granular sludge and repeated exposure to Cd and Se induces compositional changes in the EPS matrix.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Extracellular polymeric substances (EPS) are a complex mixture of high molecular weight macromolecules comprising mainly of proteins, carbohydrates, and humic-like substances [1,2,3]. EPS play a significant role in microorganism-metal interactions, i.e., sorption, bioreduction, and biomineralization, thus determining the biogeochemical cycling of metal ions as well as the transformation and immobilization of metals in natural environments [4,5,6]. EPS make direct contact and interact with the metals and nanoparticles before the metals enter the cells and thus play a vital role in protecting the microbial cells [7,8,9,10,11].
An intimate association of EPS with biogenic nanoparticles (NPs), e.g., those of selenium, tellurium, silver, and palladium NPs, has also been reported [12,13,14,15]. The EPS may mediate reduction of metal(loid)s or provide nucleation sites for biogenic NPs formation [5, 16, 17]. Jain et al. [13] showed that EPS of granular sludge play a pivotal role in governing the surface characteristics and fate of biogenic Se(0) NPs. Raj et al. [18] reported that EPS extracted from Pseudomonas aeruginosa JP-11 isolated from marine environments facilitated stabilization of cadmium sulfide (CdS) NPs formed via chemical reduction. In our previous study, the EPS matrix of anaerobic granular sludge was shown to play a prominent role in the binding of Cd(II) and Se(IV) ions and to act as nucleation site for the formation of CdSe NPs during the biological synthesis of CdSe/CdS core/shell structures along with CdSe NPs using anaerobic granular sludge [19]. Formation of CdSe/CdS core/shell structures was possible due to the interaction of the Cd(II) ions available on the surface of the CdSe NPs with the sulfhydryl (–SH) groups of the EPS components [19].
Further study of this mechanism requires a better characterization of the EPS of the anaerobic granular sludge and a better understanding of its role in the synthesis and immobilization of Se(0) and CdSe NPs. The complexity of the EPS of anaerobic granular sludge and their interaction with Se(IV) and Cd(II) hinder the understanding of the parameters that govern the size and charge of NPs and their retention in the EPS. To the best of our knowledge, there is no detailed study on the compositional changes in the EPS matrix of biofilms upon synthesis of Se and CdSe NPs following Se-oxyanion reduction and Cd exposure.
The main objective of this study was, therefore, to investigate the differences in the EPS composition extracted from anaerobic granular sludge enriched in the presence of Cd(II) and Se(IV) for microbial synthesis of CdSe NPs. The three-dimensional excitation-emission matrix (3D-EEM) of the EPS was used to understand the changes in chemical properties of the fluorescence compounds in the EPS. Size exclusion chromatography (SEC) coupled to fluorescence detectors was used to differentiate fingerprints of protein and humic-like substances in the EPS extracted from anaerobic granules before and after the enrichment to determine the compositional changes in the EPS matrix due to enrichment of granular sludge in the presence of Cd(II) and Se(IV). Experiments on metal(loid)–EPS interactions to decipher the role of EPS in CdSe biosynthesis were also performed.
Materials and Methods
Source of Biomass
Anaerobic granular sludge was collected from a full-scale upflow anaerobic sludge blanket (UASB) reactor treating paper mill wastewater (Industriewater Eerbeek B.V., Eerbeek, The Netherlands). A detailed description of the anaerobic granular sludge is given in detail by Roest et al. [20].
The anaerobic granular sludge, further referred to as EGS, was fed for 300 days with cadmium chloride (CdCl2) and sodium selenite at pH 7.3 and 30 °C in a fed-batch experiment aimed at CdSe NPs production [19]. Sodium selenite (1 mM = 79 mg L−1) was used as selenium source, while a stock solution of CdCl2 (1 g L−1) was used as Cd(II) source. The initial Cd(II) concentration was increased in a stepwise manner from 10 to 50 mg L−1 and then later decreased to 30 mg L−1 as Se(IV) reduction was negatively affected and incomplete in the presence of 50 mg L−1 Cd(II) [19]. An appropriate control with untreated granular sludge (UGS) was set up by incubating serum bottles containing anaerobic granular sludge for 300 days using the same fed-batch feeding regime under similar conditions, but without Se(IV) and Cd(II) in the mineral medium (Fig. 1). The composition of the mineral medium used for enrichment of both EGS and UGS was (mg L−1) NH4Cl (300), CaCl2.2H2O [15], KH2PO4 (250), Na2HPO4 (250), MgCl2 (120), and KCl (250). Sodium lactate (5 mM) was added as carbon source and electron donor. The granular sludge, i.e., EGS and UGS, was transferred to the freshly prepared medium after every 2 weeks of incubation and the process was repeated over 300 days for a total of 20 transfers for the enrichment of the microbial community and further experiments. Details of the synthesis of Se(0) and CdSe NPs as well as separation and characterization of Se(0) and CdSe NPs are reported in our previous article [19].
Experimental protocol for EPS extraction and characterization of the different phases of EGS and UGS reported in this study. Note that the characterization of the nanoparticles (Raman, XPS, etc.) has been reported in detail in previous study [19]. In pellet selenium was precipitated as Se(0)-Cd complexes, while in sludge different Se compounds such as Se(0), CdSe, and CdSxSe1 − x NPs were formed [19]
In continuation to the previous study [19], Se/Cd exposed anaerobic granular sludge (EGS) was subjected to EPS extraction and characterization after the 300-day enrichment (Fig. 1). Se(0) NPs were collected as pellet (Fig. 1) from the liquid phase by centrifugation at 37,000 × g for 20 min at 4 °C, as detailed in our previous article [19]. The pellet was re-dispersed in Milli-Q water for the characterization of the EPS associated with Se(0) NPs as a capping agent.
EPS Extraction
EPS was extracted from Se/Cd exposed (EGS) and control (UGS) anaerobic granular sludge after the 300-day enrichment according to a previous protocol with modification [21]. Prior to extraction, granular sludge was washed twice with Milli-Q water. EGS and UGS were centrifuged at 10,000 × g for 20 min at 4 °C and the supernatants were collected as loosely bound EPS (LB-EPS) extracts. The pellet was re-suspended in Milli-Q water and used for extracting tightly bound EPS (TB-EPS). Subsequent heating at 60 °C for 10 min followed by centrifugation at 10,000 × g for 20 min at 4 °C was performed for extracting TB-EPS from the same granular sludge samples.
Excitation Emission Matrix Fluorescence Spectroscopy
EPS extracts were first diluted with 50 mM phosphate buffer (25 mM Na2HPO4 + 25 mM NaH2PO4) at pH 7.0 (± 0.1) by following Bhatia et al. [1]. Excitation emission matrix (EEM) spectra of the EPS extracts were obtained at 22 (± 1) °C using a spectrofluorophotometer (RF-5301pc, Shimadzu). Emission was scanned from 220 to 500 nm after excitation ranging from 220 to 400 nm using 10 nm increments (Fig. 2). The fluorescence data were processed using the Panorama Fluorescence 3.1 software (Lab Cognition, Japan). The 3-D spectra were divided into five different regions [1, 22]. Each region is associated with different compounds: derived from PN—tyrosine and tryptophan (region I or II) corresponding to aromatic proteins, fulvic-like acids (region III), soluble microbial by-product-like (SMP) substances (region IV), and HS-like substances (region V) [1, 22].
Size Exclusion Chromatography
Fingerprints of EPS extracts from EGS and UGS were obtained as described previously using a Merck Hitachi LaChrom chromatography equipped with a L7200 autosampler, a L7100 quaternary pump, a D7000 interface, and a L7485 fluorescence detector [1]. To improve the resolution of the EPS fingerprints, two columns were used in series [23]: Bio SEC 300 Å and 100 Å (Agilent) with theoretical selective permeation ranges of 5–1250 kDa and 0.1–100 kDa, respectively. A 50 mM phosphate buffer (25 mM Na2HPO4 and 25 mM NaH2PO4, Prolabo) with 150 mM NaCl at pH 7.0 (± 0.1) was used as mobile phase at a constant flow of 0.7 mL/min. Mass calibration was performed for both protein and humic-like substances. For proteins, the following molecules were used: Ferritin (Sigma), 440 kDa; immunoglobulin G from human serum (Sigma), 155 kDa; bovine serum albumin (Sigma), 69 kDa; ribonuclease A (Sigma), 13.7 kDa; and thyrotropin releasing hormone (Sigma), 362.38 Da [23]. Humic-like substances calibration curves were made with synthetic polymers of polystyrene sulfonate (Sigma): 350 kDa, 150 kDa, 77 kDa, 32 kDa, 13 kDa, 4.3 kDa, and 510 Da [1].
For calibration, the logarithm of the MW (log (MW)) was plotted as a function of the elution volume (Ve):
Calibration with protein standards:
Calibration with polystyrene sulfonate standards:
where, MW is the molecular weight in Da and Ve represents the elution volume in mL.
The fingerprints of the EPS were monitored by fluorescence detection and excitation/emission wavelengths for the fluorescence detector were selected as 221/350 nm for protein-like substances and 345/443 nm for humic-like substances. Signals were standardized as a function of the TOC content of the EPS samples to allow comparison of the chromatograms.
A 100 μL of filtered (0.2 μm, Whatman™, GE Health) EPS extracts (LB-EPS and TB-EPS) was injected for each SEC analysis. In order to remove potential metal ions retained in the columns, a cleaning step was applied after each chromatographic run [24]. Se(0) NPs samples were also analyzed by SEC after filtering with a 0.2-μm membrane to characterize the EPS associated with Se(0) NPs. Fractions were collected during SEC analysis for each EPS sample at different elution times to study metal-EPS interactions: the five collected fractions are detailed in Fig. 3A. Se and Cd concentrations in each fraction were measured by ICP-MS (Agilent 7700×). These concentrations were used to determine the repartition (% of Se or Cd) in a given fraction compared to the sum of the element detected in each fraction.
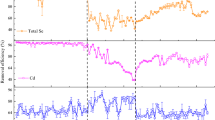
SEC chromatograms of LB (A, C) and TB-EPS (B, D) from UGS and Cd/Se-enriched EGS and EPS associated with Se(0) NPs. Fluorescence detection at Ex/Em: 221/350 nm for protein-like substances (A, B) and at 345/443 nm for humic-like substances (C, D). Vertical dotted lines in (A) correspond to the five fractions collected for determining Cd and Se concentrations in LB-EPS extracted from EGS. Legend of symbols are inside panel (A)
Analytical Methods
For quality control and method validation, different control experiments were performed to have better understanding on metal(loid)–EPS interaction and fluorescence quenching [25]. First, 0.1 mM each of Cd(II) and Se(IV) was added to the LB-EPS extract obtained from UGS followed by the collection of EEM spectra after 20 min of incubation [25]. In order to study a potential impact of the presence of Cd and Se on SEC-fluorescence analysis, the same LB-EPS extracts incubated with both Cd(II) and Se(IV) (0.1 mM each) were also analyzed by SEC and compared with the fingerprints of LB-EPS extracted from EGS.
The biochemical composition of the EPS extracts, i.e., humic-like (HS-like) substances, protein (PN) (using Lowry modified by Frølund et al. 1995), and polysaccharide (PS) (using the phenol-sulfuric acid method by Dubois et al. 1956), was determined as described earlier [21]. The total organic carbon (TOC), total Se, and Cd content in the EPS extracts were quantified using a Phoenix 8000 TOC-analyzer (Dohrmann) and a ICP-MS (Agilent 7700x), respectively. The compressive strength of EGS and UGS was determined with 13 replicates using a LLOYD FR K plus Ametek instrument to detect “breaks/compressions” with a LC 5 Newton probe. Rupture 2 software was used for data treatment.
Results
Chemical Composition of the EPS of the Granular Sludge
There was no significant difference in total LB-EPS of EGS and UGS as measured by TOC after the enrichment of the sludge, but the total amount of extracted TB-EPS was ~ 6 times lower in EGS (Table 1). The PN content of the LB- and TB-EPS was, respectively, three and six times higher for EGS (Table 1). The HS-like and PS content were standardized to the PN content to highlight a strong decrease of HS-like components after the Cd/Se feeding, especially in the TB-EPS (tenfold reduction). The PS/PN ratios also decreased but to a lower extent (twofold reduction). In contrast, the standardized HS-like and PS contents were similar in LB- and TB-EPS for UGS (Table 1). Selenium was not detected in the EPS of UGS before enrichment, while only a small amount of Cd (0.11 μg mg C−1 of EPS) was found in the TB-EPS (Table 1). The total Se and Cd concentration amounted to 70.6 and 45.8 μg mg C−1 of EPS, respectively, in the LB-EPS extracted from the EGS, while it was only 27.6 and 10.1 μg mg C−1 of EPS, respectively, in the TB-EPS extracted.
The Se(0) NPs separated from the aqueous phase by centrifugation contained ~ 8 mg Se L−1 and 0.5 mg Cd L−1 (Table 1). Se(0) NPs also contained a significant amount of organic matter (Table 1). The PN content of the EPS on the Se(0) NPs was lower than in EPS extracted from EGS: 10 and 6 times lower compared to LB- and TB-EPS, respectively. The standardized PS content was the same as in EGS EPS, but the standardized amount of HS-like components was 4 times higher than in EGS EPS.
The strength compression study reveals that the compressive strength of the EGS (11.5 ± 0.8 N mm−1) was higher compared to that of UGS (8 ± 1 N mm−1).
Fluorescence Properties of the EPS Extracts
Figure 2 shows the EEM spectra corresponding to the different EPS extracts. All EPS extracts exhibited fluorescence in the areas associated with the PN—tyrosine and tryptophan (region I or II) at excitation/emission wavelengths (Ex/Em) of 220–250/275–375 nm. The relative intensity of fluorescence of PN was higher in both LB and TB-EPS extracts after the enrichment (Fig. 2B and D). The fluorescence of humic-like substances at Ex/Em wavelengths of 310–350/275–375 nm was only observed in LB-EPS in UGS (Fig. 2C). These EEM spectra were further used to determine the optimal settings for fluorescence detection by SEC as mentioned in the “Materials and Methods” section. The following excitation/emission wavelengths were thus used: 221/350 nm for generating protein-like fingerprints and 345/443 nm for humic-like fingerprints.
Fingerprints of EPS Extracts by SEC
The SEC chromatograms show that the fingerprints of PN-like molecules of both LB- (Fig. 3A) and TB-EPS (Fig. 3B) extracted from untreated and enriched sludge are quite similar: they only differ by the intensity of the chromatographic peaks. Both LB- and TB-EPS fingerprints show an increase in intensity in the range of higher aMW (HMW) of > 100 kDa after the enrichment in the presence of Cd(II) and Se(IV). In contrast, the presence of PN-like substances with lower aMW (LMW) < 10 kDa became negligible, particularly in the LB-EPS fraction of EGS.
For the UGS, the HS-like fingerprints are quite similar for LB and TB-EPS (Fig. 3C and D): three main fractions with aMW ≤ 13 kDa are observed, but the fluorescence intensities are about tenfold higher for LB-EPS. After the enrichment, both LB and TB-EPS fingerprints are modified and differ from one another. For LB-EPS, compared to the UGS, intensities are strongly reduced (~ 20-fold lower) and the first peak is eluted significantly later, corresponding to a decrease of the aMW of the EPS extracted from EGS. TB-EPS fingerprints are quite similar to LB-EPS after enrichment at the exception of the apparition of a broad peak corresponding to aMW in the range of 13 to 300 kDa.
Figure 3 also compares the SEC chromatograms of Se(0) NPs with the LB-EPS extracted from EGS and UGS. The fingerprints of PN-like substances (Fig. 3A) are quite similar, but the peak intensities for EPS associated with Se(0) NPs were about two- and fivefold lower compared to LB-EPS from UGS and EGS, respectively. Interestingly, for HS-like substances, the Se(0) NPs fingerprint is quite similar to LB-EPS from UGS, but not EGS with about 20-fold lower peak intensity (Fig. 3C).
Fractionation of Metal(loid)s Associated with the Extracted EPS
To investigate the interaction of the EPS with Cd and Se, five different fractions were collected during the SEC analysis of EPS extracted from EGS (fractions are indicated in Fig. 3A). The Cd and Se repartition in each fraction is given in Table 2. Se was detected only in the 4th fraction (19–21 mL), which is close to the total permeation volume, and thus corresponds to the elution of very small compounds. In LB-EPS, Cd was also mainly found in the 4th fraction (89% of total Cd), whereas in TB-EPS the majority of Cd (63%) was present in the 1st fraction (9–12 mL). For Se(0) NPs (size < 200 nm), Se was eluted in the 4th fraction, while the majority of Cd was mainly present in the 1st (9–12 mL) and 5th (21–33 mL) fraction (Table 2).
Effect of Metal(loid)–EPS Interactions on EPS Composition
In the control experiment for method validation, significant fluorescence quenching was evident after 20 min of the addition of Cd(II) and Se(IV) to the LB-EPS extracted from UGS (Fig. 4). The addition of Se(IV) (alone or with Cd) to the LB-EPS of UGS decreased the fluorescence intensities for fulvic and humic-like substances (III and V), while it became negligible for aromatic protein-like (I and II) substances (Fig. 4). Se(IV) thus induces a specific fluorescence quenching in the regions attributed to PN and HS-like substances. LB-EPS of UGS samples were analyzed further by SEC before and after spiking with Cd(II) and Se(IV) to study the effect of Cd and Se ions on the EPS elution profile (Fig. 5). The addition of Cd(II) or Se(IV) to the LB-EPS did not cause any significant changes in the fingerprint of PN and HS-like molecules for LB-EPS (Fig. 5).
Discussion
Changes in Chemical Composition of EPS Extracts by Cd/Se Exposure
This study showed that both LB- and TB-EPS extracted from the EGS had a higher PN/PS ratio during exposure to Cd(II) and Se(IV) for 300 days (Table 1). This could be due to the stress exerted by the metal(loid)s Cd(II) and Se(IV) or the NPs, i.e., Se(0) and/or CdSe NPs on the microorganisms. PN, PS, and HS-like substances are the major components of the EPS matrix which influence the physicochemical properties of the sludge in biological wastewater treatment systems [1]. Some studies have, however, reported that extracellular proteins are more important than polysaccharides in mediating electrostatic bonds in the biofilm matrix due to the presence of negatively charged amino acids in the proteins [26], which have a high metal binding affinity [27]. Moreover, proteins play a crucial role in microbial synthesis of Se(0) NPs including transport of Se oxyanions (in)to the cell, the redox reactions, export of Se(0) nuclei out of the cell, and assembly of Se(0) into NPs [28]. Thus, an increase of the protein content and/or synthesis of new specific proteins in the EPS matrix of the granular sludge might offer sites for binding of Cd(II) and Se(IV), redox reactions, and capping of Se(0) and CdSe NPs during the microbial synthesis of Se(0) and CdSe NPs.
The HS-like substances are the second major component of Se(0) NPs, unlike EPS (Table 1). This suggests that the HS-like substances may also contribute to nucleation for Se(0) and CdSe NPs formation and provide redox-active sites. Humic acids influenced the bioreduction of Pu(IV) polymers by Shewanella putrefaciens: bioreduction rates increased at humic acid concentrations below 15 mg L−1 but decreased at more elevated humic acid concentrations [29]. Humic acid assisted synthesis and stabilization of silver nanoparticles does indeed occur [30]. This is consistent with the present study, as Fig. 4 shows the quenching of fluorescence of HS-like substances when the EPS are incubated with Se(IV), implying a close interaction between HS-like substances and selenite. The HS-like substances are not present in the synthetic growth media and thus come from the granular sludge as a by-product of the bacterial metabolism. In addition to the effects of protein, this study shows that it is also necessary to focus on the role of HS-like substances in the reduction, nucleation, and formation (size and shape) of Se(0) and CdSe NPs.
Changes in Fingerprints and Macromolecule Size Distribution of EPS Extracts
The intensity of the HMW (> 100 k Da) PN-like substances increased in the EPS after the enrichment, while the LMW (< 10 k Da) PN-like substances disappeared (Fig. 3). The proteins with HMW offer more binding sites and interaction points with the metal cations and polymers, which offer a higher structural stability to the granules [31, 32]. On the other hand, LMW substances are considered low-molecular-weight acids (< 10 kDa) and generally comprised of low molar mass organic acids (including amino acids and peptides), while the low amphiphilic (slightly hydrophobic) compounds represent sugars, alcohols, aldehydes, and ketones [33]. A possible conversion of these LMW substances (amino acids and peptides) to HMW biopolymers during repetitive exposure of granular sludge to Cd(II) and Se(IV) for 300 days can thus not be ruled out [33].
LMW substances can also interact with Cd(II) and Se(IV) and form larger molecules due to aggregation that are eluted as HMW substances. However, addition of Cd(II) or Se(IV) to the LB-EPS did not cause any significant changes in the elution profile of the LB-EPS (Fig. 5). This suggests that the changes in fingerprint of PN- or HS-like substances revealed previously (Fig. 3) can only be attributed to modifications in the EPS composition due to the long exposure of the granular sludge to Cd(II) and Se(IV), and not to a simple alteration of the fluorescence detector response due to metal(loid) binding to the EPS samples. These changes in EPS composition in EGS could thus be due to a modification of (i) the metabolism, (ii) the microbial communities after enrichment, and/or (iii) the release of different EPS components, i.e., PN, PS, and HS-like substances induced by the enrichment. Further studies, e.g., microbial communities analysis, proteomics, as well as Cd and Se interactions with HS-like substances, should be carried out to improve the understanding of the EPS composition in detail (e.g., different functional groups of EPS and changes in binding properties, identification of proteins associated with the Se(0), or CdSe NPs produced by the anaerobic granular sludge) and their role in microbial synthesis of nanoparticles.
It is suggested that the abundant proteins in the outer layer of the selenite-reducing granular sludge during the microbial synthesis of Se(0) from Se(IV) can be totally altered compared to the protein profile extracted from the granular sludge unexposed to Se(IV) [34]. Interestingly, a new 95-kDa protein, SefA (selenium factor A), was isolated from the extracellular Se(0) NPs secreted by Thauera selenatis [35]. Therefore, an increase of certain periplasmic and cytoplasmic proteins that are present within the HMW region due to the exposure to Cd(II) and Se(IV) cannot be ruled out (Fig. 3). Surprisingly, PN-like substances which represent the major part of Se(0) NPs’ organic matter have a similar aMW repartition to LB-EPS (Fig. 3A). This similarity can lead to the assumption that the nucleation of Se(0) NPs partly involves PN-like substances present in LB-EPS and association of HMW PN-like substances with Se(0) NPs. The living cells in a granular sludge may be induced to proliferate and produce more HMW PN-like substances in order to either govern the formation and influence the shape and size of Se and CdSe NPs [28, 36] or to prevent the toxicity of Cd(II) and/or Se(IV) ions or both [5, 9]. The potential mechanisms inducing the increase of HMW PN-like substances need to be further explored. For HS-like substances, the fingerprint obtained for Se(0) NPs and LB-EPS of EGS is rather unique (Fig. 3C), with some common peaks with LB-EPS from UGS, which suggests that part of the HS-like substances of Se(0) NPs comes from metabolized LB-EPS.
Interaction of Se and Cd with EPS
This study shows no significant differences in the LB-EPS content of EGS and UGS (Table 1), which could be due to either a lower content in EPS or a less efficient EPS extraction [37]. The decrease in EPS content of the enriched granules is not consistent with the literature which claims that metal stress (at sublethal concentration) generally induces a higher EPS content [3]. However, Gopal et al. [38] reported that CdS quantum dots can degrade the EPS of E. coli cells. Raman spectroscopy and X-ray photoelectron spectroscopy (XPS) of the same EGS after 300 days of enrichment confirmed the deposition of selenium either as biogenic Se(0) or CdSxSe1-x NPs on the EGS [19]. Thus, partial degradation of EPS due to the deposition of biogenic Se(0) and/or CdSxSe1-x NPs cannot be ruled out.
The lower extraction efficiency could also be a result of the higher granular strength as highlighted by the compression tests that both density and compressive strength of the EGS (11.5 ± 0.8 N mm−1) were increased compared to UGS (8 ± 1 N mm−1). Moreover, the ion bridging interactions between metal(loid) cations and the EPS components can also play a beneficial role in bacterial aggregation and compressibility of the sludge [3]. Significant fluorescence quenching was observed when Cd and Se(IV) were added to the LB-EPS extracted from UGS (Fig. 4), which clearly indicates that LB-EPS has a strong binding capacity for Cd(II) and Se(IV) [25]. The presence of high concentrations of Cd and Se in the EPS extracts of EGS (Table 1) further supports the strong metal(loid)–EPS interactions.
Interestingly, the total Se and Cd concentrations were 2.6 and 4.5 times higher, respectively, in the LB-EPS than in the TB-EPS extracted from EGS (Table 1). This further suggests that the majority of Cd and Se are retained in the LB-EPS, but not in the TB-EPS. Moreover, Se and Cd eluted separately in the TB-EPS possibly as Se(0) and Cd-EPS complex, but not as CdSe NPs (Table 2). In our previous study, it was also shown that Cd strongly interacts with the –SH (thiol) of EPS and forms [Cd–S–EPS] complexes [19]. This might result in the formation of large aggregates of HS-like-Cd complexes or association of Cd with macromolecules in TB-EPS [39]. It can also explain the appearance of a big peak of HS-like substances of TB-EPS with aMW > 100 kDa, but not in the LB-EPS (Fig. 3). The majority of Cd eluted in the same 1st fraction (9–12 mL) also corroborates with this finding (Table 2).
In contrast, the majority of Se and Cd elute together in the 4th fraction (19–21 mL) (Table 2) in the LB-EPS, corresponding to the total permeation volume, indicating that both Se and Cd were either present in ionic form or were present as smaller NPs (i.e., CdSe). However, Raman spectroscopy and XPS confirmed that no selenite was present in sludge repeatedly exposed to selenite and that selenium was only present in the sludge either as Se(0), CdSe, or CdSxSe1-x with a size ranging between 10 and 190 nm [19]. It is, thus, possible that due to the formation of CdSe and CdSxSe1-x NPs with a very small size, Cd and Se elute together at the total permeation volume, i.e., 4th fraction. Interestingly, when Se(0) NPs with a size of < 200 nm were run through SEC, the majority of the Se eluted similarly in the 4th fraction (Table 2). All these results suggest that formation of Se(0) and CdSe NPs occurs mainly in the LB-EPS, highlighting the pivotal role of LB-EPS in the microbial synthesis of Se(0) and CdSe NPs by providing the microbial reduction and nucleation sites for NPs synthesis. Thus, the influence of LB-EPS on the microbial synthesis of CdSe should not be ignored. Moreover, this study also suggests that a simple, non-destructive EPS extraction method combined with SEC of LB-EPS can be an exciting alternative method for separation and purification of the CdSe NPs, which are difficult to separate and harvest when using granular sludge for their biosynthesis [19].
Effect on Granular Sludge Rheology
Many waste streams, especially acid mine drainage and flue gas desulfurization scrubbing liquors, contain both selenium oxyanions (concentration can be up to 1–53 and 162 mg L−1, respectively) and cadmium (concentration can be up to 2–44 and 82 mg L−1, respectively) which needs to be treated before their discharge into the environment [40, 41]. Designing a UASB bioreactor is, thus, essential in order to develop a novel microbial synthesis process for the simultaneous treatment of Cd(II) and Se(IV) and recover them as Se and CdSe NPs. Mechanical strength is one of the most important physical properties of granular sludge along with its settleability (mainly related to the size and density of the sludge) in a UASB reactor. However, toxicity of the wastewater containing Cd(II) and Se(IV) and newly formed Se and CdSe NPs can affect the strength of the granules. Although the effect of decreasing the granule strength in batch experiments is not much detrimental, the turbulence, shear forces, and other internal forces are much more powerful in large-scale UASB reactors and the decrease in granule strength can be much more serious. Interestingly, addition of Cd(II) and Se(IV) and/or the formation of Se and CdSe NPs results in improved mechanical strength of the granular sludge. Recently, Liang et al. [42] demonstrated that introducing magnetic nanoparticles (MNPs) into an activated sludge system can improve the sludge surface hydrophobicity and mechanical strength. Thus, the toxicity associated with Cd(II) and Se(IV) and newly formed Se and CdSe NPs are not a limitation in the case of designing UASB reactors for large-scale operation and might even contribute to the strength of the anaerobic granular sludge.
Conclusion
Size-exclusion chromatographic fingerprints were used to investigate the compositional changes in EPS after the enrichment of anaerobic granular sludge in the presence of Cd(II) and Se(IV) for microbial synthesis of CdSe NPs. The PN and PS-like content increased, while the concentration of HS-like substances decreased and variations in mass distribution for both PN and HS-like substances were observed. HMW protein-like substances of > 100 kDa increased significantly, whereas LMW protein-like substances of < 10 kDa disappeared. The differences observed in the fingerprints of HS-like substances were not only in the peak intensities but also in peak positions, meaning that the composition of the EPS changes in terms of concentration and chemical composition. Total metal(loid) analysis and fractionation by SEC of Se/Cd-enriched sludge revealed that the majority of Se and Cd was present in the LB-EPS and eluted together, possibly as CdSxSe1-x or CdSe NPs.
References
Bhatia, D., Bourven, I., Simon, S., Bordas, F., van Hullebusch, E. D., Rossano, S., Lens, P. N. L., & Guibaud, G. (2013). Fluorescence detection to determine proteins and humic-like substances fingerprints of exopolymeric substances (EPS) from biological sludges performed by size exclusion chromatography (SEC). Bioresource Technology, 131, 159–165.
Geyik, A. G., Kılıç, B., & Çeçen, F. (2016). Extracellular polymeric substances (EPS) and surface properties of activated sludges: Effect of organic carbon sources. Environmental Science and Pollution Research International, 23(2), 1653–1663.
Sheng, G. P., Yu, H. Q., & Li, X. Y. (2010). Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems, a review. Biotechnology Advances, 28(6), 882–894.
Stylo, M., Alessi, D. S., Shao, P. P., Lezama-Pacheco, J. S., Bargar, J. R., & Bernier-Latmani, R. (2013). Biogeochemical controls on the product of microbial U(VI) reduction. Environmental Science & Technology, 47(21), 12351–12358.
Tourney, J., & Ngwenya, B. T. (2014). The role of bacterial extracellular polymeric substances in geomicrobiology. Chemical Geology, 386, 115–132.
Sivaperumal, P., Kamala, K., & Rajaram, R. (2018). Adsorption of cesium ion by marine actinobacterium Nocardiopsis sp. 13H and their extracellular polymeric substances (EPS) role in bioremediation. Environmental Science and Pollution Research International, 25(5), 4254–4267.
Ozturk, S., & Aslim, B. (2008). Relationship between chromium(VI) resistance and extracellular polymeric substances (EPS) concentration by some cyanobacterial isolates. Environmental Science and Pollution Research International, 15(6), 478–480.
Li, W. W., & Yu, H. Q. (2014). Insight into the roles of microbial extracellular polymer substances in metal biosorption. Bioresource Technology, 160, 15–23.
Thuptimdang, P., Limpiyakorn, T., McEvoy, J., Prüß, B. M., & Khan, E. (2015). Effect of silver nanoparticles on Pseudomonas putida biofilms at different stages of maturity. Journal of Hazardous Materials, 290, 127–133.
Hou, J., Yang, Y., Wang, P., Wang, C., Miao, L., Wang, X., Lv, B., You, G., & Liu, Z. (2017). Effects of CeO2, CuO, and ZnO nanoparticles on physiological features of Microcystis aeruginosa and the production and composition of extracellular polymeric substances. Environmental Science and Pollution Research International, 24(1), 226–235.
Wei, L., Li, Y., Noguera, D. R., Zhao, N., Song, Y., Ding, J., Zhao, Q., & Cui, F. (2017). Adsorption of Cu2+ and Zn2+ by extracellular polymeric substances (EPS) in different sludges: Effect of EPS fractional polarity on binding mechanism. Journal of Hazardous Materials, 321, 473–483.
Yates, M. D., Cusick, R. D., & Logan, B. L. (2013). Extracellular palladium nanoparticle production using Geobacter sulfurreducens. ACS Sustainable Chemistry & Engineering, 1(9), 1165–1171.
Jain, R., Jordan, N., Weiss, S., Foerstendorf, H., Heim, K., Kacker, R., Hübner, R., Kramer, H., van Hullebusch, E. D., & Lens, P. N. L. (2015). Extracellular polymeric substances govern the surface charge of biogenic elemental selenium nanoparticles. Environmental Science & Technology, 49(3), 1713–1720.
Mal, J., Nancharaiah, Y. V., Maheswari, N., van Hullebusch, E. D., & Lens, P. N. L. (2017). Continuous removal and recovery of tellurium in an upflow anaerobic granular sludge bed reactor. Journal of Hazardous Materials, 327, 79–88.
Huangfu, X., Xu, Y., Liu, C., He, Q., Ma, J., Ma, C., & Huang, R. (2019). A review on the interactions between engineered nanoparticles with extracellular and intracellular polymeric substances from wastewater treatment aggregates. Chemosphere, 219, 766–783.
Kang, F., Alvarez, P. J., & Zhu, D. (2014). Microbial extracellular polymeric substances reduce Ag+ to silver nanoparticles and antagonize bactericidal activity. Environmental Science & Technology, 48(1), 316–322.
Li, S. W., Zhang, X., & Sheng, G. P. (2016). Silver nanoparticles formation by extracellular polymeric substances (EPS) from electroactive bacteria. Environmental Science and Pollution Research International, 23(9), 8627–8633.
Raj, R., Dalei, K., Chakraborty, J., & Das, S. (2016). Extracellular polymeric substances of a marine bacterium mediated synthesis of CdS nanoparticles for removal of cadmium from aqueous solution. Journal of Colloid and Interface Science, 462, 166–175.
Mal, J., Nancharaiah, Y. V., Bera, S., Maheshwari, N., van Hullebusch, E., & Lens, P. N. L. (2017). Biosynthesis of CdSe nanoparticles by anaerobic granular sludge. Environmental Science. Nano, 4(4), 824–833.
Roest, K., Heilig, H. G., Smidt, H., Stams, A. J. M., & Akkermans, A. D. L. (2005). Community analysis of a full-scale anaerobic bioreactor treating paper mill wastewater. Systematic and Applied Microbiology, 28(2), 175–185.
D'Abzac, P., Bordas, F., van Hullebusch, E. D., Lens, P. N. L., & Guibaud, G. (2010). Extraction of extracellular polymeric substances (EPS) from anaerobic granular sludges: Comparison of chemical and physical extraction protocols. Applied Microbiology and Biotechnology, 85(5), 1589–1599.
Chen, W., Westerhoff, P., Leenheer, J. A., & Booksh, K. (2003). Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environmental Science & Technology, 37(24), 5701–5510.
Bourven, I., Simon, S., Bhatia, D., van Hullebusch, E. D., & Guibaud, G. (2014). Effect of various size exclusion chromatography (SEC) columns on the fingerprints of extracellular polymeric substances (EPS) extracted from biological sludge. Journal of the Taiwan Institute of Chemical Engineers, 149, 148–155.
Polec-Pawlak, K., Ruzik, R., Abramski, K., Ciurzynska, M., & H, G. (2005). Cadmium speciation in Arabidopsis thaliana as a strategy to study metal accumulation system in plants. Analytica Chimica Acta, 540(1), 61–70.
Zhang, D., Pan, X., Mostofa, K. M., Chen, X., Mu, G., Wu, F., Liu, J., Song, W., Yang, J., Liu, Y., & Fu, Q. (2010). Complexation between Hg(II) and biofilm extracellular polymeric substances: An application of fluorescence spectroscopy. Journal of Hazardous Materials, 175(1-3), 359–365.
Cao, B., Shi, L., Brown, R. N., Xiong, Y., Fredrickson, J. K., Romine, M. F., Marshall, M. J., Lipton, M. S., & Beyenal, H. (2011). Extracellular polymeric substances from Shewanella sp. HRCR-1 biofilms: Characterization by infrared spectroscopy and proteomics. Environmental Microbiology, 13, 1018–1031.
Pan, X., Liu, J., Zhang, D., Chen, X., Song, W., & Wu, F. (2010). Binding of dicamba to soluble and bound extracellular polymeric substances (EPS) from aerobic activated sludge: A fluorescence quenching study. Journal of Colloid and Interface Science, 345(2), 442–447.
Tugarova, A. V., & Kamnev, A. A. (2017). Proteins in microbial synthesis of selenium nanoparticles. Talanta, 174, 539–547.
Xie, J., Han, X., Wang, W., Zhou, X., & Lin, J. (2017). Effects of humic acid concentration on the microbially-mediated reductive solubilization of Pu(IV) polymers. Journal of Hazardous Materials, 393, 347–353.
Akaighe, N., MacCuspie, R. I., Navarro, D. A., Aga, D. S., Banerjee, S., Sohn, M., & Sharma, V. (2011). Humic acid-induced silver nanoparticle formation under environmentally relevant conditions. Environmental Science & Technology, 45(9), 3895–3901.
Zhu, L., Zhou, J., Lv, M., Yu, H., Zhao, H., & Xu, X. (2015). Specific component comparison of extracellular polymeric substances (EPS) in flocs and granular sludge using EEM and SDS-PAGE. Chemosphere, 126, 26–32.
Dong, J., Zhang, Z., Yua, Z., Dai, X., Xu, X., Alvarez, P. J. J., & Zhu, L. (2017). Evolution and functional analysis of extracellular polymeric substances during the granulation of aerobic sludge used to treat p-chloroaniline wastewater. Chemical Engineering Journal, 330, 596–604.
Aryal, R., Lebegue, J., Vigneswaran, S., Kandasamy, J., & Grasmick, A. (2009). Identification and characterisation of biofilm formed on membrane bio-reactor. Separation and Purification Technology, 67(1), 86–94.
Gonzalez-Gil, G., Lens, P. N. L., & Saikaly, P. (2016). Selenite reduction by anaerobic microbial aggregates: Microbial community structure, and proteins associated to the produced selenium spheres. Frontiers in Microbiology, 7, 571–598.
Debieux, C. M., Dridge, E. J., Mueller, C. M., Splatt, P., Paszkiewicz, K., Knight, I., Florance, H., Love, J., Titball, R. W., Lewis, R. J., Richardson, D. J., & Butler, C. S. (2011). A bacterial process for selenium nanosphere assembly. Proceedings. National Academy of Sciences. United States of America, 108(33), 13480–13485.
Sathiyanarayanan, G., Dineshkumar, K., & Yang, Y. H. (2017). Microbial exopolysaccharide-mediated synthesis and stabilization of metal nanoparticles. Critical Reviews in Microbiology, 43(6), 731–752.
Zhang, X., Bishop, P. L., & Kinkle, B. K. (1999). Comparison of extraction methods for quantifying extracellular polymers in biofilms. Water Science and Technology, 39(7), 211–218.
Gopal, J., Wu, H.-F., & Gangaraju, G. (2011). Quantifying the degradation of extracellular polysaccharides of Escherichia coli by CdS quantum dots. Journal of Materials Chemistry, 21(35), 13445–13451.
Liu, C., Frenkel, A. I., Vairavamurthy, A., & Huang, P. M. (2001). Sorption of cadmium on humic acid: Mechanistic and kinetic studies with atomic force microscopy and X-ray absorption fine structure spectroscopy. Canadian Journal of Soil Science, 81(3), 337–348.
Moreau, J. W., Fournelle, J. H., & Banfield, J. F. (2013). Quantifying heavy metals sequestration by sulfate-reducing bacteria in an acid mine drainage-contaminated natural wetland. Frontiers in Microbiology, 4, 1–10.
Meawad, A. S., Bojinova, D. Y., & Pelovski, Y. G. (2010). An overview of metals recovery from thermal power plant solid wastes. Waste Management, 30(12), 2548–2559.
Liang, X., Gao, B.-Y., & Ni, S.-Q. (2017). Effects of magnetic nanoparticles on aerobic granulation process. Bioresource Technology, 227, 44–49.
Acknowledgments
The authors thank Marylène Viana (Université de Limoges, France) for her help with the compression tests. This research was supported through the Erasmus Mundus Joint Doctorate Environmental Technologies for Contaminated Solids, Soils, and Sediments (ETeCoS3) (FPA n00 2010-0009) and the European Commission Marie Curie International Incoming Fellowship (MC-IIF) Role of biofilm-matrix components in the extracellular reduction and recovery of chalcogens (BioMatch project, No. 103922). The authors acknowledge the Earth System Science and Environmental Management (ESSEM) COST Action ES1302 European Network on Ecological Functions of Trace Metals in Anaerobic Biotechnologies to support a short-term scientific mission (STSM) at the Université de Limoges (France).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mal, J., Nancharaiah, Y.V., Bourven, I. et al. Cadmium Selenide Formation Influences the Production and Characteristics of Extracellular Polymeric Substances of Anaerobic Granular Sludge. Appl Biochem Biotechnol 193, 965–980 (2021). https://doi.org/10.1007/s12010-020-03464-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03464-x