Abstract
L-aspartate-α-decarboxylase was extracellularly expressed to enhance its production for β-alanine biosynthesis. L-aspartate-α-decarboxylase and cutinase were coexpressed in Escherichia coli; more than 40% of the L-aspartate-α-decarboxylase was secreted into the medium. Selection of best conditions among tested variables enhanced L-aspartate-α-decarboxylase production by the recombinant strain. The total L-aspartate-α-decarboxylase activity reached 20.3 U/mL. Analysis of the enzymatic properties showed that the optimum temperature and pH for L-aspartate-α-decarboxylase were 60 °C and 7.5, respectively. Enzyme activity was stable at pH 4.0–8.5 and displayed sufficient thermal stability at temperatures < 50 °C. In addition, enzymatic synthesis of β-alanine was performed using extracellularly expressed L-aspartate-α-decarboxylase, and a mole conversion rate of > 99% was reached with a substrate concentration of 1.5 M. Extracellular expression of L-aspartate-α-decarboxylase resulted in increased enzyme production, indicating its possible application in the enzymatic synthesis of β-alanine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
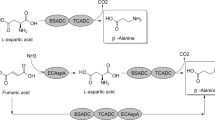
L-aspartate-α-decarboxylase (ADC) catalyzes the decarboxylation of L-aspartate at the α position to form β-alanine and CO2 [1]. β-Alanine, a naturally occurring β-amino acid, is involved in the pantothenic acid metabolic pathway and plays important roles throughout the development of organisms. β-Alanine is widely used for the production of industrially important nitrogen-containing chemicals, such as pantothenic acid and nylon-3 [2]. β-Alanine can be prepared by chemical synthesis and enzymatic synthesis. Among these approaches, enzymatic synthesis using L-aspartate decarboxylation catalyzed by ADC is favorable because this method is environmentally friendly, has few byproducts, and has low costs [3, 4].
Currently, a number of studies investigating ADCs from a variety of microorganisms, including Escherichia coli, Mycobacterium tuberculosis, Corynebacterium glutamicum, and Bacillus subtilis, have been described, but the productivity of ADCs from these wild strains is relatively low, partly resulting in high production costs for β-alanine [5,6,7,8]. Since protein expression in E. coli represents the most facile approach [9], several attempts at the recombinant expression of ADCs in E. coli were performed to increase the productivity of ADC for β-alanine production [2, 4, 8, 10], but satisfactory results were still not achieved. Compared to conventional expression, extracellular expression of proteins has the advantages of no space limitations for the accumulation of protein, minimization of the potentially harmful action of recombinant proteins against host cells, reduced inclusion body formation, and improved protein expression [11, 12]. Several extracellular expression approaches have been adopted to promote the yield of recombinant proteins by E. coli. Target proteins with signal peptides can undergo transmembrane transport to the periplasmic space and can then be further secreted into the medium via nonspecific seepage or upon the addition of chemical reagents [13,14,15]. Extracellular secretion can also be achieved by coexpression with cutinase, which catalyzes the hydrolysis of membrane phospholipids, resulting in increased membrane permeability [12, 16]. These studies showed that extracellular expression significantly increased the yield of the target protein. Thus, extracellular expression may be a more favorable approach for large-scale ADC preparation.
In this study, the ADC-encoding gene from Bacillus tequilensis, which encodes a very effective enzyme for the preparation of β-alanine [17], was extracellularly produced by coexpression with cutinase (cut) and signal peptides in E. coli. The best recombinant strain was chosen to further optimize culture conditions for ADC production. In addition, the conditions for β-alanine production with extracellularly expressed ADC were identified, and an efficient bioconversion system was developed.
Materials and Methods
Plasmids, Strains, and Mediums
The wild-type strain, Bacillus tequilensis, was isolated in our previous study and deposited in the China General Microbiological Culture Collection Center (Beijing, China) with an accession number of CGMCC 10506 [17]. The expression plasmid pET-28a(+), the plasmid pET-20b(+), and the host strain BL21(DE3) were obtained from Novagen (Shanghai, China).
E. coli cells used for gene cloning, plasmid propagation, and inoculum preparation were cultured in lysogeny broth (LB). E. coli cells used for producing target protein were cultured in modified terrific broth (TB) containing 5 g/L glycerol, 24 g/L yeast extract, 12 g/L peptone, 2.31 g/L KH2PO4, and 16.43 g/L K2HPO4. Fed-batch fermentation was carried out in a 5-L fermenter, and 3 L of TB was initially loaded. When the initial glycerol was exhausted and dissolved oxygen rose sharply, feeding medium containing 500 g/L glycerol and 5 g/L MgSO4 was added to the fermenter at a rate of 25 mL/h. Additionally, 50 mg/L kanamycin and 100 mg/L ampicillin were added to the medium as needed.
Construction of Recombinant E. coli for Extracellular Expression of ADC
The ADC-encoding gene panD (GenBank KY123117) was amplified from the genomic DNA of B. tequilensis by PCR by utilizing primers with recognition sites for the restriction enzymes NcoI and XhoI (ADC-F: 5′-AACCATGGGCATGTATCGAACAATGATGAG-3′ and ADC-R: 5′-AACTCGAGCAAAATTGTACGGGCGGGT-3′). Then, the amplified gene was digested with NcoI and XhoI and ligated into the commercially available vector pET20b(+) or pET28a(+), resulting in the plasmid pET20b-panD or pET28a-panD. The plasmid pET20b-panD was digested with Nde I and Xho I. The fragment containing pelB-panD was ligated with pET28a digested with the same enzymes, leading to the formation of pET28a-pelB-panD, a plasmid harboring panD with the signal pelB. The sequence of the ompA signal (GenBank CP028714) was synthesized commercially with the recognition sites for Nde I and Nco I on both ends. After digestion with the restriction enzymes Nde I and Nco I, the ompA signal fragment was ligated into pET28a-panD, leading to the formation of pET28a-ompA-panD. The published sequence of Thermobifida fusca cutinase (GenBank: YP_288944) was synthesized commercially with the recognition sites for Nde I and Nco I. At the 3′ end of the cutinase sequence, a ribosome-binding sequence (RBS) was inserted. After digestion with the restriction enzymes Nde I and Nco I, the fragment of the cutinase gene was ligated into pET28a-panD, leading to the formation of pET28 -cut-panD. All plasmids obtained were transformed into E. coli BL21(DE3) for protein expression.
Recombinant E. coli Strain Cultivation
The seed culture was incubated for 6 h on a rotary shaker (200 rpm) at 37 °C in a 500-mL flask containing 50 mL of LB medium supplemented with 50 mg/L kanamycin sulfate. Fermentation was conducted in a 500-mL flask containing 50 mL of TB medium at 37 °C and 220 rpm in a rotary shaker. When the dry cell weight (DCW) reached 2.1 g/L, 100 g/L lactose solution was added to the culture to obtain a final concentration of 2 g/L, and expression was continued for 30 h. For 5-L fermenter cultivation, the seed culture was prepared as described for seed cultivation in shake flasks and then inoculated into TB medium at a temperature of 37 °C and at pH 7.0. When the DCW of the culture reached 8.6 g/L, the culture temperature was reduced to 26 °C by rapid water circulation, and 100 g/L lactose solution was added to the fermenter to obtain a final concentration of 2 g/L to induce enzyme expression. The dissolved oxygen level was maintained at around 30% of air saturation by regulating the agitation speed from 300 to 900 rpm and by regulating the ventilation level.
Enzymatic Characterization of Recombinant Extracellular ADC
The fermentation broth was centrifuged at 13,000×g for 10 min, and the supernatant was used for enzymatic characterization of the extracellular ADC. The best pH of the recombinant extracellular ADC was determined by using 50 mM acetate buffer (pH 4.0, 5.0), 50 mM phosphate buffer (pH 6.0, 7.0, 7.5), or 50 mM Tris–HCl buffer (pH 8.0, 8.5). To test pH stability, enzyme activity was measured after maintaining the enzyme at the indicated pH for 24 h at 4 °C. The best temperature was determined by measuring the enzyme activity between 30 and 80 °C as previously described. To test thermal stability, enzyme activity was measured after incubating the enzyme at the indicated temperature for 6 h. Relative activities are expressed as percentages of maximum enzyme activity.
Recombinant ADC Production
Frequently used TB medium was employed to determine ADC activity, and conditions for the expression of recombinant ADC were examined. The default culture conditions were those described for cultivation in 5-L fermenters. The induction temperatures examined were 22 °C, 26 °C, 30 °C, 33 °C, and 37 °C; the lactose concentrations investigated were 0.5 g/L, 1 g/L, 2 g/L, 5 g/L, and 7 g/L; and fumarate was fed and maintained at concentrations of 5 g/L and 10 g/L.
Production of β-Alanine by Transformation of L-Aspartate Via ADC
The transformation reaction was optimized under the following conditions: 100 mL of 0.1 M phosphate buffer solution (pH 7.5) in a 250-mL flask on a rotary shaker (200 rpm) at 50 °C. Different substrate feeding strategies were employed to optimize β-alanine production. For batch mode, 0.5 M substrate was first suspended in phosphate buffer solution and dissolved with the addition of NaOH. For intermittent fed mode, substrate was fed in 5 batches, which were added at 0.1 M per hour for up to 4 h. For continuous fed mode, the initial concentration of the substrate was 50 mM, and the rest of the substrate was fed continuously at a rate of 1.8 mM/min. The optimized transformation reaction was subsequently amplified at a 1-L scale.
Analytical Methods
The cell density was measured using a spectrophotometer against distilled water at 600 nm after appropriate dilution. The OD600 was calculated as follows: OD600 = absorbance×dilution factor. One OD600 was equivalent to 0.358 g/L DCW. The fumarate concentration was measured as previously described using high-performance liquid chromatography (HPLC) [18]. L-aspartate and β-alanine levels were analyzed by the HPLC-based o-phthalaldehyde (OPA) derivatization method with detection at 338 nm [19].
ADC Activity Assays
One milliliter of culture broth was centrifuged at 13,000×g for 10 min, and the supernatant was labeled as the extracellular fraction. The pelleted cells were resuspended in an approximately equal volume of 50 mM sodium phosphate buffer (pH 7.0) to an OD600 of approximately 5 and disrupted by sonication on ice. Cellular debris was separated by centrifugation (13,000×g, 4 °C for 10 min), and the supernatant was collected as the intracellular fraction. The enzymatic activities in the cell fractions were measured.
Under standard conditions, the reaction mixture contained 0.1 M L-aspartate (substrate), 100 μL of appropriately diluted ADC solution, and 50 mM sodium phosphate buffer (pH 7.0) in a final volume of 1 mL. The mixture was incubated at 37 °C for 30 min, and the reaction was stopped by the addition of 1 mL of 10% trichloroacetic acid. ADC activity was calculated from the β-alanine concentration, with 1 U of activity defined as the amount of ADC that catalyzes the conversion of 1 μmol of L-aspartate to β-alanine per min at 37 °C.
Statistical Analysis
The mean values and standard deviations were calculated from the data obtained from three independent experiments. Analysis of variance was performed by one-way ANOVA followed by Tukey’s HSD post hoc multiple comparison analysis using the IBM SPSS statistics package, version 22. Statistical differences at p < 0.05 were considered significant.
Results
Cloning and Expression of ADC from B. tequilensis
To achieve high ADC productivity by E. coli, the recombinant plasmids pET28a-panD (control), pET28a-cut-panD, pET28a-pelB-panD, and pET28a-ompA-panD were constructed and individually transformed into E. coli BL21(DE3). These strains were cultivated in shake flasks with TB medium for ADC production, as shown in Fig. 1. With the exception of control E. coli BL21(DE3)/pET28a-panD, all strains secreted ADC into the supernatant. The highest total ADC activity of 2.8 U/mL was achieved in E. coli BL21(DE3) harboring pET28a-cut-panD and was significantly higher than that of the control strain (p < 0.01); 42.8% of the ADC was released outside the cell. However, no significant improvement in total ADC activity was found in E. coli BL21(DE3)/pET28a-pelB-panD and E. coli BL21(DE3)/pET28a-ompA-panD compared with the control strain, and the total ADC activity of E. coli BL21(DE3)/pET28a-ompA-panD was even slightly lower than that of the control strain (p < 0.05). Subsequent SDS-PAGE experiments also confirmed these results (Fig. 2). The coexpressed signal peptides pelB and ompA did not effectively increase ADC activity. Target protein attached to a signal peptide is only exported to the periplasmic space of E. coli, and it is difficult to achieve further, sufficient secretion into the supernatant without adding chemical reagents [15]. However, coexpressed cutinase catalyzes E. coli membrane phospholipid hydrolysis, resulting in the free, nonspecific release of cytosolic proteins [16]. Under these conditions, ADC was continuously secreted outside the cell, the negative feedback effect caused by exogenous protein accumulation in cells was relieved, and more ADC was produced by E. coli. Therefore, E. coli BL21(DE3)/pET28a-cut-panD was used for further investigation.
The intracellular and extracellular fractions from different cells were analyzed by using SDS-PAGE. For SDS-PAGE analysis, 15 μL of protein samples was mixed with 5 μL of 5 × loading buffer, the mixture was boiled for 10 min, and then10 μL samples were loaded in each lane of an SDS-PAGE gel. M, molecular weight markers. 1, 2, Intracellular and extracellular ADC of E. coli BL21(DE3)/pET28a-panD. 3, 4, Intracellular and extracellular ADC of E. coli BL21(DE3)/pET28a- ompA-panD. 5, 6, Intracellular and extracellular ADC of E. coli BL21(DE3)/pET28a-pelB-panD. 7, 8, Intracellular and extracellular ADC of E. coli BL21(DE3)/pET28a-cut-panD
Effect of Induction Conditions on ADC Production
ADC production increased with increasing temperature when the induction temperature was below 30 °C (Fig. 3a). The highest total ADC activity of 14.3 U/mL was achieved at 30 °C, and 45.4% of ADC was secreted extracellularly. However, ADC activity decreased significantly as the temperature further increased (p < 0.05). This result was likely due to the rapid production of ADC at high temperatures, leading to some proteins losing the ability to fold correctly, which possibly resulted in the formation of inclusion bodies. The optimal lactose concentration for ADC production was determined to be 2 g/L (Fig. 3b), and an increase or decrease in the dose resulted in significantly lower expression levels. In addition, induced temperature and lactose concentration had no obvious effect on cell growth.
Effect of Fumarate Concentration on ADC Production
Recombinant protein expression was significantly inhibited by fast-acting carbon sources such as glucose and glycerol. In the induced expression period, the concentration of these fast-acting carbon sources should be kept as low as possible [20, 21]. However, a carbon source concentration that is too low will not meet the needs of bacterial growth and protein synthesis. Slow-acting carbon sources such as fumarate are good supplements to prevent the negative effects of a lack of carbon sources during expression. The addition of slow-acting carbon sources such as fumarate and succinate during the induction period was reported to be extremely beneficial for recombinant tryptophan synthase expression [22]. Therefore, the fumarate feeding strategy was selected to improve the yield of ADC. Fumarate was not conducive to cell growth, and the biomasses of both experimental groups with added fumarate were lower than that of the control group with no fumarate added (Fig. 4a). However, fumarate was beneficial to ADC production, as shown in Fig. 4b; the highest total ADC activity of 20.3 U/mL was achieved at 36 h when the concentration of fumarate was maintained at 5 g/L. This activity was 28.4% higher than that of the control group (p < 0.05). However, a further increase in fumarate concentration was not favorable for improving ADC yield. Thus, the optimal fumaric acid concentration for ADC production was determined to be 5 g/L. At this concentration, extracellular ADC activity could be detected starting at 20 h and gradually improved, along with the release of intracellular enzymes (Fig. 4c), reaching a maximum value of 10.8 U/mL at 40 h, accounting for 53% of total ADC activity.
pH- and Temperature-Based Characterization of Recombinant Extracellular ADC
The characteristics of extracellular ADC were investigated to provide the basic parameters for β-alanine production. The best pH for ADC was determined to be 7.5 (Fig. 5a). There was little loss of activity when the enzyme was held at pH 4.0–8.5 over 24 h. The best temperature for ADC was 60 °C (Fig. 5b), with activity decreasing sharply at temperatures higher or lower than 60 °C. Thermostability experiments showed that approximately 97% of the initial enzyme activity was retained after incubation at 50 °C for 6 h.
Optimization of Reaction Conditions for β-Alanine Production
Substrates at high concentrations have an inhibitory effect on ADC activity [4]. The potential application of B. tequilensis ADC for the synthesis of β-alanine was investigated by optimizing substrate feeding strategies. Two different substrate feeding strategies were used to achieve L-aspartate decarboxylation for the sake of increasing β-alanine productivity (Fig. 6). Compared with the batch reaction, both substrate fed modes accelerated the reaction rate, leading to shortened reaction times for complete conversion. The substrate concentration was kept at a low level throughout the course of the reaction when employing substrate feeding strategies. Therefore, the significant inhibitory effect was consequently relieved, resulting in a faster reaction rate and higher reaction efficiency. The highest space-time yield (213 g/L/day) was achieved when substrate was fed continuously, which indicated that this strategy effectively improved the reaction rate and thus overcame the inhibition effect caused by a high L-aspartate concentration.
Effect of the substrate feeding strategy on the decarboxylation of L-aspartate. Reaction conditions: L-aspartate (0.5 M) and fermentation broth of E. coli (200 mL/L) in phosphate buffer (0.1 M, pH 7.5), 50 °C, 200 rpm. The reaction volume was 100 mL. Conv., the mole conversion. The space-time yield is defined as the production rate of β-alanine per day (g/L/day)
Subsequently, experiments were designed to further increase the substrate concentration for the decarboxylation reaction using continuous fed-batch mode. As shown in Fig. 7, when the substrate concentration was increased to 1.00 M, a satisfactory result could still be obtained with mole conversion of > 99%. A mole conversion of 72.6% was reached after 20 h when the substrate concentration was increased to 1.50 M. This may be due to the irreversible mechanism-based inactivation of the enzyme caused by pyruvoyl group protonation [8]. This unsatisfactory result was improved with an increased amount of enzyme. A mole conversion rate of > 99% was achieved at 17 h when the enzyme amount was increased by 150%. For economic reasons, experiments with higher substrate concentrations were not continued because of the longer reaction times and requirement for additional enzyme. In conclusion, the substrate concentration was increased threefold by selecting the best conditions among tested conditions for β-alanine production, and the β-alanine yield reached 123.3 g/L, higher than that obtained in previous described procedures for the synthesis of β-alanine [2, 4, 7, 8, 10]. When the reaction system was scaled up to 1 L, as expected, the results were still desirable, indicating the developed process showed great potential for large-scale availability.
Conclusion
In summary, we successfully developed an extracellular expression system for ADC from B. tequilensis by coexpressing T. fusca cutinase for the first time. Compared with normal recombinant expression, the production of ADC was increased by 40%. Under optimal conditions, the yield of expressed ADC reached 20.3 U/mL, which is the highest ADC production yield ever reported. Furthermore, recombinant ADC expressed in E. coli showed higher thermostability, which makes it more suitable for use in industrial applications. Because approximately 50% of enzyme was released outside the cell, ADC fermentation broth could be used directly as a catalyst for L-aspartate decarboxylation. Finally, the substrate inhibition effect on ADC was alleviated by using a substrate continuous feeding strategy. Even at high concentrations, L-aspartate was completely decarboxylated to β-alanine. This study will pave the way for increased production of β-alanine by ADC.
References
Könst, P. M., Franssen, M. C. R., Scott, E. L., & Sanders, J. P. M. (2009). A study on the applicability of L-aspartate alpha-decarboxylase in the biobased production of nitrogen containing chemicals. Green Chemistry, 11(10), 1646–1652.
Song, C. W., Lee, J., Ko, Y. S., & Lee, S. Y. (2015). Metabolic engineering of Escherichia coli for the production of 3-aminopropionic acid. Metabolic Engineering, 30(3), 121–129.
Ford, J. H. (2002). The alkaline hydrolysis of β-aminopropionitrile. Journal of the American Chemical Society, 67(5), 876–877.
Shen, Y., Zhao, L., Li, Y., Zhang, L., & Shi, G. (2014). Synthesis of β-alanine from L-aspartate using L-aspartate-α-decarboxylase from Corynebacterium glutamicum. Biotechnology Letters, 36(8), 1681–1686.
Williamson, J. M., & Brown, G. M. (1979). Purification and properties of L-aspartate-alpha-decarboxylase, an enzyme that catalyzes the formation of beta-alanine in Escherichia coli. Journal of Biological Chemistry, 254(16), 8074–8082.
Gopalan, G., Chopra, S., Ranganathan, A., & Swaminathan, K. (2010). Crystal structure of uncleaved L-aspartate-alpha-decarboxylase from Mycobacterium tuberculosis. Proteins-Structure Function and Genetics, 65(4), 796–802.
Dusch, N., Pühler, A., & Kalinowski, J. (1999). Expression of the Corynebacterium glutamicum panD gene encoding L-aspartate-alpha-decarboxylase leads to pantothenate overproduction in Escherichia coli. Applied and Environmental Microbiology, 65(4), 1530–1539.
Pei, W., Zhang, J., Deng, S., Tigu, F., Li, Y., & Li, Q. (2017). Molecular engineering of L-aspartate-α-decarboxylase for improved activity and catalytic stability. Applied Microbiology and Biotechnology, 101(15), 6015–6021.
Mahalik, S., Sharma, A. K., & Mukherjee, K. J. (2014). Genome engineering for improved recombinant protein expression in Escherichia coli. Microbial Cell Factories, 13(1), 177.
Li, H., Lu, X., Chen, K., Yang, J., Zhang, A., Wang, X., & Ouyang, P. (2018). β-Alanine production using whole-cell biocatalysts in recombinant Escherichia coli. Molecular Catalysis, 449, 93–98.
Khushoo, A., Pal, Y., Singh, B. N., & Mukherjee, K. J. (2004). Extracellular expression and single step purification of recombinant escherichia coli L-asparaginase II. Protein Expression & Purification, 38(1), 29–36.
Su, L., Yue, M., & Jing, W. (2015). Extracellular expression of natural cytosolic arginine deiminase from Pseudomonas putida and its application in the production of l -citrulline. Bioresource Technology, 196, 176–183.
Cheng, J., Wu, D., Chen, S., Chen, J., & Wu, J. (2011). High-level extracellular production of α-cyclodextrin glycosyltransferase with recombinant Escherichia coli BL21 (DE3). Journal of Agricultural & Food Chemistry, 59(8), 3797–3802.
Choi, J. H., & Lee, S. Y. (2004). Secretory and extracellular production of recombinant proteins using Escherichia coli. Applied Microbiology & Biotechnology, 64(5), 625–635.
Mergulhão, F. J., Summers, D. K., & Monteiro, G. A. (2005). Recombinant protein secretion in Escherichia coli. Biotechnology Advances, 23(3), 177–202.
Su, L., Woodard, R. W., Chen, J., & Wu, J. (2013). Extracellular location of Thermobifida fusca cutinase expressed in Escherichia coli BL21(DE3) without mediation of a signal peptide. Applied and Environmental Microbiology, 79(14), 4192–4198.
Feng, Z., Zhang, J., Chen, G., Cha, Y., Liu, J., Ge, Y., Cheng, S., & Yu, B. (2016). Isolation, identification and fermentation optimization of Bacillus tequilensis PanD37 producing L-aspartate α- decarboxylase. Acta Microbiologica Sinica, 56(1), 44–55.
Song, C. W., Kim, D. I., Choi, S., Jang, J. W., & Lee, S. Y. (2013). Metabolic engineering of Escherichia coli for the production of fumaric acid. Biotechnology and Bioengineering, 110(7), 2025–2034.
Bartolomeo, M. P., & Maisano, F. (2006). Validation of a reversed-phase HPLC method for quantitative amino acid analysis. Journal of Biomolecular Techniques Jbt, 17(17), 131–137.
Zhu, H. Y., & Li, Q. (2006). Strategies for expression of soluble heterologous proteins in Escherichia coli. Chinese Journal of Process Engineering, 6(1), 150–155.
Makino, T., Georgiou, G., & Skretas, G. (2011). Strain engineering for improved expression of recombinant proteins in bacteria. Microbial Cell Factories, 10(1), 32–32.
Terasawa, M., Inui, M., Uchida, Y., Kobayashi, M., Kurusu, Y., & Yukawa, H. (1991). Application of the tryptophanase promoter to high expression of the tryptophan synthase gene in Escherichia coli. Applied Microbiology & Biotechnology, 34(5), 623–627.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Feng, Z., Zhang, J., Chen, G. et al. Extracellular Expression of L-Aspartate-α-Decarboxylase from Bacillus tequilensis and Its Application in the Biosynthesis of β-Alanine. Appl Biochem Biotechnol 189, 273–283 (2019). https://doi.org/10.1007/s12010-019-03013-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03013-1