Abstract
To recover a nitrogen resource from high-ammonia-nitrogen wastewater, two amphitrophic hydrogen-oxidizing bacteria (HOB), Paracoccus denitrificans Y5 and P. versutus D6, capable of nitrogen assimilation for single-cell protein (SCP) production were isolated. These two HOB strains could grow autotrophically with H2 as an electron donor, O2 as an electron acceptor, CO2 as a carbon source, and ammonia nitrogen (NH4+-N) as a nitrogen source. The cell molecular formulas of strains Y5 and D6 determined by autotrophic cultivation were C3.33H6.83O2.58N0.77 and C2.87H5.34O3.17N0.57, respectively. The isolated strains could synchronously remove NH4+-N and organic carbon and produce SCP via heterotrophic cultivation. The rates of removal of NH4+-N and soluble chemical oxygen demand reached 35.47 and 49.04%, respectively, for Y5 under mixotrophic cultivation conditions with biogas slurry as a substrate. SCP content of strains Y5 and D6 was 67.34–73.73% based on cell dry weight. Compared with soybean meal, the SCP of Y5 contained a variety of amino acids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen pollution has become an important environmental problem [1]. High-ammonia-nitrogen wastewater (HAW) is mainly derived from breeding, chemical fertilizers, meat-processing, the petrochemical industry, coking wastewater, and municipal sewage. It is difficult to treat such wastewater owing to the wide range of ammonia nitrogen (NH4+-N) concentrations and high chemical oxygen demand (COD). The major treatments of HAW are based on physicochemical and biological technologies. Biological removal of nitrogen has been widely adopted due to its effectiveness and low cost [2]. Biological processes of nitrogen removal include traditional nitrification–denitrification and the emerging technologies of simultaneous nitrification–denitrification [3], short nitrification–gentrification [4], and anaerobic ammonium oxidation [5]. Nonetheless, all these biological processes convert ammonia into nitrogen gas. Nitrogen is a macronutrient used by living organisms for growth and survival [3]. HAW contains organic carbon and micronutrient resources. Theoretically, these nitrogen and carbon resources can be utilized by microorganisms to produce protein.
Single-cell protein (SCP), i.e., dried cells of microorganisms, is a high-value-added product that can serve as a protein supplement in animal feed [6]. For cell growth, hydrogen-oxidizing bacteria (HOB) can heterotrophically utilize NH4+-N and organic carbon and/or autotrophically capture NH4+-N and CO2 using H2 as an electron donor and O2 as an electron acceptor [7]. There are some advantages of HOB use. First, compared with fungi, algae, and yeast, HOB have high protein content per cell dry weight (CDW) [8, 9]. Second, HOB have a flexible lifestyle: they can alternatively or concomitantly grow in heterotrophic and autotrophic modes [10]. Third, although algae can grow heterotrophically and autotrophically, the growth of HOB is often not limited by light. Fourth, the other cell material of HOB, polyhydroxybutyrate, can serve as a prebiotic [11]. SCP with a prebiotic has greater nutritional value than other microbial proteins. Advantages of the production of SCP from HAW by HOB include the offset running cost of wastewater treatment; lower dependence of SCP production on land, with relieved pressure on agriculture [12]; and capacity for CO2 fixation, with a reduction in the greenhouse effect.
So far, only a few HOB genera have been reported to produce SCP. These include Hydrogenomonas [13], Alcaligenes [14], Aquaspirillum [15], and Pseudomonas [11]. In these bacteria, SCP production is carried out in an autotrophic environment with CO2 as the carbon source. No report has described both autotrophic and heterotrophic nitrogen assimilation for SCP production and wastewater treatment by HOB. The aims of this study were to (1) isolate HOB capable of utilizing NH4+-N, (2) investigate the ability of the isolates to produce SCP under autotrophic and heterotrophic conditions, and (3) to evaluate the feasibility of SCP production from biogas slurry via mixotrophic cultivation of HOB.
Materials and Methods
Isolation and Identification of HOB
Bacteria and the Culture Medium
Soybean rhizosphere soil, pond water, and biogas slurry of chicken manure (BSCM) were used for the enrichment of HOB. The modified mineral medium (MM) was prepared for HOB enrichment and isolation. MM was composed of the following (in g/L): 4.0 NH4Cl, 1 KH2PO4, 2 K2HPO4, 2 NaCl, 0.01 CaCl2, 0.2 MgSO4·7H2O, 0.01 FeSO4·7H2O, 10 mL of a trace element solution, and 10 mL of a vitamin solution. The trace element solution consisted of (in mg/L) 200 ZnSO4·7H2O, 20.0 Na2MoO4·2H2O, 1000 MnSO4·H2O, 20.0 CuCl2·2H2O, 200 CoCl2·6H2O, 20.0 NiCl·6H2O, 20.0 Na2SeO4, and 20.0 Na2WO4. The vitamin solution was composed of (in mg/L) 2.0 biotin, 5.0 riboflavin, 10.0 pyridoxine, 2.0 folic acid, 5.0 thiamin, 5.0 nicotinic acid, 5.0 calcium pantothenate, 0.1 cyanocobalamine, 5.0 aminobenzoic acid, and 5.0 thioctic acid. MM was sterilized at 121 °C for 15 min. Solutions of NH4HCO3, NH4OH, and vitamins were sterilized by membrane filtration (0.22-μm pore size) and were added to the medium. Initial pH of the medium was adjusted to 7.0 ± 0.2 with 2-mol/L NaOH and 2-mol/L HCl solutions. Three ammonium salts (NH4Cl 4.0 g/L, NH4HCO3 5.9 g/L, and NH4OH 2.6 g/L) with the same NH4+-N concentration (1046 mg/L) were employed to evaluate the effect of an anion on CDW and SCP production. The characteristics of BSCM are listed in Table 1.
Alcaligenes latus DSM 1122, Herbaspirillum autotrophicum DSM 732, Pseudomonas carboxydohydrogena DSM 1083, and Cupriavidus necator DSM 531, which are all known HOB [14, 16], served as control strains to evaluate the capacity for SCP production. These four strains were purchased from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ).
Enrichment, Isolation, and Identification of HOB
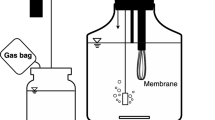
Serum bottles and tubes were used for enrichment and isolation. Approximately 5 g of soybean rhizosphere soil, pond water, or BSCM was added separately to 500-mL serum bottles with 200 mL of MM. A gas mixture (H2:O2:CO2 at 7:2:1, v/v) was injected into each serum bottle [17]. The replacement-and-exhaust method was adopted for the gaseous H2/O2/CO2 atmosphere [18]. The gas replacement-and-injection equipment is illustrated in Fig. 1. Enrichment was conducted as described by Bae et al. [19]. Isolation of HOB was carried out by means of serum tubes supplied with the same gas mixture. Determination of the optimal growth temperature and pH of the isolated strains involved the single-variable methodology (30 °C and 7.0).
The genomic DNA of each bacterial isolate was extracted using the Sangon Biotech Bacteria DNA Kit (Shanghai, China). The DNA fragment containing the 16S ribosomal RNA (rRNA) gene was amplified by polymerase chain reaction with universal primers as previously described [20]. The amplicons were sequenced and analyzed by Sangon Biotech. Sequence similarity searches were performed in the GenBank data library via the BLAST program.
SCP Production by HOB
For SCP production, the autotrophic cultivation of the isolated and purchased strains was performed in 250-mL serum bottles using MM and the gas mixture. The 2% (v/v) inocula were aseptically injected into MM with a sterile syringe. The pressure was maintained at ~ 0.06 MPa. The bottles were shaken at 30 °C and 180 rpm, and the gas consumption was monitored by a pressure gauge every day. When the gauge pressure decreased to zero, the gas mixture was injected again. The CDW and SCP content were measured after gas was no longer consumed. All the experiments were conducted in triplicate.
The heterotrophic cultivation of HOB was performed in a 250-mL triangular flask containing MM and glucose with a breathable plug. Glucose was added to a final concentration of 38.9 g/L to adjust the C/N ratio to a reasonable level (i.e., 15) for microbial growth. The medium was autoclaved at 115 °C for 30 min and inoculated with a 2% HOB seed solution. Cultivation was carried out at 30 °C and 180 rpm. Glucose concentration was measured every day. When the glucose was consumed, CDW and SCP contents were determined. All the experiments were carried out in triplicate.
Mixotrophic cultivation was conducted in 500-mL serum bottles using BSCM and the gas mixture. The BSCM was diluted fivefold, and pH was adjusted to 7.0 ± 0.2. The bottles were shaken at 30 °C and 180 rpm. The procedure of pressure monitoring and gas charging was the same as that used for autotrophic cultivation. Soluble chemical oxygen demand (SCOD) and NH4-N concentration were measured every 4 days. The CDW and SCP contents were determined after there was no gas consumption. All the experiments were conducted in triplicate.
Analytical Methods
Characteristics of original BSCM were analyzed. Total solids (TS), volatile solids (VS), suspended solids (SS), and volatile suspended solids were determined by standard techniques [21]. Total N, total P, total K, NH4+-N, NO3−-N, COD, and SCOD were analyzed on a DR-1900 spectrophotometer (HACH, USA). Electrical conductivity was determined using a CHA-C258 conductivity meter (Shenzhen Ruihao, China). pH was determined with a pHS-3C pH meter (Shanghai Precision & Scientific Instrument Co., Ltd., China). Humus acid was quantified based on the Chinese Standard (HGT3276-1999). Total coliform counts and total colony counts and determination of metallic elements and amino acids followed the National Institute of Measurement and Testing Technology protocols.
The broth of HOB cultures was centrifuged at 10,000 rpm for 3 min for the determination of CDW and SCP content. CDW was measured after water was evaporated at 105 °C for 12 h. Kjeldahl nitrogen quantification was conducted on an Automatic Kjeldahl Apparatus (FOSS 2000). SCP was determined by multiplying the Kjeldahl nitrogen value by a conversion factor of 6.25. Analyses of C, H, N, and S involved a Vario EL element analyzer (Elementar Analysensysteme GmbH, Germany). Glucose consumption was measured by means of an SBA-40E biosensor. The gas mixture (H2/O2/CO2) was analyzed on an Agilent 6890N Gas Chromatograph with a thermal conductivity detector and a 2-m stainless-steel column packed with Porapak Q (50/80 mesh) (Agilent Technologies, USA). The amino acid composition was analyzed by Sci-Tech Innovation (China).
Calculations
The average fixation rates of NH4+-N by strains Y5 and D6 under autotrophic, heterotrophic, and mixotrophic conditions were calculated as
where NH4+-Ni indicates the initial NH4+-N concentration in a medium, NH4+-Nr indicates the residual NH4+-N in the culture supernatant, and t is the cultivation time.
The NH4+-N conversion and utilization efficiency by Y5 and D6 were calculated as
In Eq. (3), CDW-N was determined according to the Kjeldahl method.
The CDW yield from the different gases and NH4+-N in the autotrophic condition was calculated as
The carbon and nitrogen balance in the autotrophic condition was calculated as
In Eqs. (6) and (7), Ccell and Ncell indicate the cell carbon and nitrogen content determined by elemental analysis.
Results and Discussion
Isolation, Identification, and Optimization
Two strains capable of growth on CO2 and NH4Cl as the sole inorganic carbon and nitrogen source, respectively, were isolated. According to their 16S rRNA gene sequences, these strains were identified as Paracoccus denitrificans Y5 and P. versutus D6, respectively. The effects of temperature and pH on cell growth are depicted in Fig. 2. The optimal growth temperature and pH of Y5 and D6 were 30 °C and 7.0, respectively. Figure 3 illustrates the CDW yield and SCP content of strain Y5 obtained in the three-ammonium-salt medium. NH4HCO3 was the most suitable nitrogen source for SCP production because of its neutrality. It is known that HCO3− is alkalescent, which enabled the adjustment and maintenance of the soda acid balance in the culture. The average fixation rate of NH4+-N and volumetric productivity of Y5 and D6 with NH4HCO3 as a nitrogen source were slightly higher than the values obtained on NH4Cl (Table 2). Thus, NH4HCO3, which simulated the NH4+-N of BSCM, was favorable for producing SCP from biogas slurry containing a high NH4+-N concentration and carbon dioxide derived from biogas utilization as substrates.
Autotrophic SCP Production by HOB
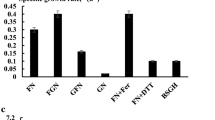
Autotrophic SCP production was carried out in a shake serum bottle with CO2 as the sole carbon source. This test was completed after 5 days, when no further gas consumption was evident. The profiles of CDW and SCP content of different HOB are presented in Fig. 4. All the strains were able to grow autotrophically. The maximum CDW production of 2.12 g/L and SCP content of 73.73% were obtained with strain Y5. C. necator DSM 531 has been widely studied concerning autotrophic production of SCP [22, 23]. SCP content of Y5 was much higher than that of DSM 531 (67%), algae (40–60%), yeast (45–55%), fungi (30–45%), and soybean (45%) [9]. The elemental composition of Y5 was as follows (major components only): 40.00% C, 10.84% N, 6.83% H, and 1.00% S, with a cell molecular formula of C3.33H6.83O2.58N0.77. The stoichiometry of strain Y5 is roughly indicated by Formula (8). The elemental composition of D6 (major components only) was 34.42% C, 7.99% N, 5.34% H, and 1.58% S, with a cell molecular formula of C2.87H5.34O3.17N0.57. The stoichiometry of D6 is indicated by Formula (9):
The low conversion efficiency resulted in a high concentration of residual NH4+-N (Table 2). The cause of the low conversion efficiency may be that the HOB growth was limited by the partial vacuum caused by gas consumption and discontinuous gas feeding. This situation was confirmed on Hydrogenomonas eutropha [24]. Cohen [25] has also found that HOB grow better under steady gas pressure near atmospheric pressure with continuous gas feeding. The continuous bioreactor culture system will be used in future research. As demonstrated in Table 3, the CDW yield per carbon dioxide in strain Y5 was higher than the maximum value reported in a sequencing batch reactor and continuous reactor [26]. The rates of utilization of carbon and nitrogen by Y5 were greater than the rates manifested by D6. On the other hand, the utilization rates of carbon and nitrogen were not 100%. This state of affairs may be associated with produced secondary metabolites including amino acids and volatile fatty acids.
Heterotrophic SCP Production by HOB
The main soluble organic carbon was reduced sugar instead of volatile fatty acids and amino acids (Table 1). Hence, glucose, which simulated the carbon source of BSCM, was added into MM for heterotrophic SCP production. After 5 days, the glucose was completely consumed. The CDW and SCP contents are shown in Fig. 4b. The maximal CDW (5.17 g/L) and SCP content (72.2%) were also obtained with strain Y5. The results also revealed that the CDW and SCP content of the six strains under heterotrophic conditions were substantially higher than those under autotrophic conditions, except for P. carboxydohydrogena DSM 1083. The volumetric productivity of heterotrophic cultivation in terms of CDW was higher than that for autotrophic cultivation (Table 2). This result is similar to that on the HOB growth on organic substrates in the air atmosphere; this number was twofold higher than that under autotrophic conditions [15]. The reason was the restricted supply of a carbon source owing to the slower gas-liquid mass transfer rate during autotrophic cultivation [17].
For heterotrophic cultivation, although the conversion efficiency of NH4+-N was 92.38–95.55%, only 43.83–49.78% of NH4+-N was converted to CDW. This situation resulted from the volatilization of ammonia during shaking cultivation. Therefore, the values of average fixation rate, conversion efficiency, and utilization efficiency of NH4+-N were meaningless.
Mixotrophic SCP Production by HOB
The COD/TN ratio of BSCM was 3.76, with the corresponding C/N ratio of 1.41, which is much lower than the required value for SCP production (Table 1). Therefore, mixotrophic SCP production was carried out with the gas mixture and BSCM as substrates. The mixotrophic cultivation was terminated when there was no further ammonia consumption. Unfortunately, none of the six strains could grow with the sterilized original BSCM without dilution. This result may be explained as follows: the HOB could not adapt to a new environment containing high concentrations of metal ions and other complex salts. Hence, BSCM was diluted fivefold with water. All six strains grew in the diluted and sterilized BSCM. As demonstrated in Fig. 5a, the NH4+-N concentration and SCOD of the strains both declined. Taking this one step further, the drop of SCOD was greater in the first 8 days than in the last 8 days. There was no gas consumption during the first 3 days, indicating that heterotrophic metabolism occurred preferentially during the mixotrophic cultivation. The results supported the hypothesis that key enzymes for autotrophic metabolism form during heterotrophic growth [23]. The remaining difficult-to-degrade organic matter could not be used by HOB. During the latter 8 days of cultivation, the NH4+-N concentrations of strains Y5 and D6 decreased slowly, and the amount of SCOD and gas consumption hardly decreased. The results showed that the cultivation period of 8 days was suitable for mixotrophic SCP production from BSCM. The rates of removal of NH4+-N and SCOD reached 35.47 and 49.04% for strain Y5. The CDW and SCP content of Y5 was 3.19 g/L and 69.6%, respectively, and was 3.3 g/L and 67.3%, respectively, for strain D6 (Fig. 5b). This result showed that it is possible that strain Y5 synchronously removed NH4+-N and SCOD and produced SCP. The order of volumetric productivity for strains Y5 and D6 was heterotrophic > mixotrophic > autotrophic cultivation.
On the basis of the data above, a possible process scheme for SCP production from HAW by HOB is proposed in Fig. 6.
Amino Acid Profiles
The amino acid composition of SCP from strains Y5 and D6 was measured and compared with soybean meal as reported by Lo and Moreau [27]. The profiling (Table 4) indicated that strains Y5 and D6 contained a variety of amino acids, whose concentrations exceeded those of soybean meal. Proline, aspartic acid, glutamic acid, and serine, which were not present in soybean meal, were evident in the SCP of strains Y5 and D6. The arginine content of Y5 was relatively higher than that of soybean meal. Arginine is one of the important amino acid additives in fish feed. The total content of methionine and cystine in strain Y5 was similar to that reported for microbial protein [26]. Methionine and cystine are two amino acids that are essential for poultry and pigs [27]. Concentrations of threonine, valine, alanine, and glycine were higher than those in soybean meal.
Conclusions
Isolated strains P. denitrificans Y5 and P. versutus D6 produce SCP under autotrophic, heterotrophic, and mixotrophic conditions. The optimal growth temperature and pH of Y5 and D6 are 30 °C and 7.0, respectively. The cell molecular formulae of strain Y5 (C3.33H6.83O2.58N0.77) and D6 (C2.87H5.34O3.17N0.57) were determined during autotrophic cultivation. The order of volumetric productivity for Y5 and D6 was heterotrophic > mixotrophic > autotrophic cultivation. SCP contents of strain Y5 and D6 were 67.34–73.73% based on CDW with NH4HCO3 as a nitrogen source. The SCP contains proline, aspartic acid, glutamic acid, and serine, which are not present in soybean meal.
References
Xing, W., Zhang, W. Q., Li, D. S., Li, J. L., Jia, F. F., Cui, Y. W., & Ren, F. M. (2017). An integrated O/A two-stage packed-bed reactor (INT-PBR) for total nitrogen removal from low organic carbon wastewater. Chemical Engineering Journal, 328, 894–903.
Khardenavis, A. A., Kapley, A., & Purohit, H. J. (2007). Simultaneous nitrification and denitrification by diverse Diaphorobacter sp. Applied Microbial and Cell Physiology, 77, 403–409.
Medhi, K., Singhal, A., Chauhan, D. K., & Thakur, I. S. (2017). Investigating the nitrification and denitrification kinetics under aerobic and anaerobic conditions by Paracoccus denitrificans ISTOD1. Bioresource Technology, 242, 334–343.
Hou, J., Xia, L., Ma, T., Zhang, Y. Q., Zhou, Y. Y., & He, X. G. (2017). Achieving short-cut nitrification and denitrification in modified intermittently aerated constructed wetland. Bioresource Technology, 232, 10–17.
Wei, H. W., Wang, J., Hassan, M., Han, L., & Xie, B. (2017). Anaerobic ammonium oxidation-denitrification synergistic interaction of mature landfill leachate in aged refuse bioreactor: Variations and effects of microbial community structures. Bioresource Technology, 243, 1149–1158.
Jalasutram, V., Kataram, S., Gandu, B., & Anupoju, G. (2013). Single cell protein production from digested and undigested poultry litter by Candida utilis: optimization of process parameters using response surface methodology. Clean Technologies and Environmental Policy, 15(2), 265–273.
Matassa, S., Boon, N., Arends, J. B. A., & Verstraete, W. (2015). H2-oxidizing bacteria for single cell protein production and sustainable nitrogen cycling, Resource Recovery, 1st IWA Conference, Abstracts.
Nangul, A., & Bhatia, R. (2013). Microorganisms: a marvelous source of single cell proteins. The Journal of Microbiology, Biotechnology and Food Sciences, 31, 15–18.
Nasseri, A. T., Rasoul-Amini, S., Morowvat, M. H., & Ghasemi, Y. (2011). Single cell protein: production and process. American Journal of Food Technology, 6, 103–116.
Pohlmann, A., Fricke, W. F., Reinecke, F., Kusian, B., Liesegang, H., Cramm, R., Eitinger, T., Ewering, C., Potter, M., Schwartz, E., Strittmatter, A., Vosz, I., Gottschalk, G., Steinbuchel, A., Friedrich, B., & Bowien, B. (2006). Genome sequence of the bioplastic-producing “Knallgas” bacterium Ralstonia eutropha H16. Nature Biotechnology, 24(10), 1257–1262.
Matassa, S., Boon, N., & Verstraete, W. (2015). Resource recovery from used water: the manufacturing abilities of hydrogen-oxidizing bacteria. Water Research, 68, 467–478.
Anupama, & Ravindra, P. (2000). Value-added food: single cell protein. Biotechnology Advances, 18(6), 459–479.
Repaske, R. (1966). Characteristics of hydrogen bacteria. Biotechnology and Bioengineering, 8(2), 217–235.
Volova, T. G., & Barashkov, V. A. (2010). Characteristics of proteins synthesized by hydrogen-oxidizing microorganisms. Applied Biochemistry and Microbiology, 46(6), 574–579.
Aragno, M., & Schlegel, H. G. (1978). Physiological characterization of the hydrogen bacterium Aquaspirillum autotrophicum. Archives of Microbiology, 116(3), 221–229.
Vandamme, P., & Coenye, T. (2004). Taxonomy of the genus Cupriavidus: a tale of lost and found. International Journal of Systematic and Evolutionary Microbiology, 54(6), 2285–2289.
Yu, J., Dow, A., & Pingali, S. (2013). The energy efficiency of carbon dioxide fixation by a hydrogen-oxidizing bacterium. International Journal of Hydrogen Energy, 38(21), 8683–8690.
Hu, J., Wang, L., Li, Y., Fu, X., Le, Y., Xu, D., Lu, B., & Yu, J. (2009). Breeding, optimization and community structure analysis of non-photosynthetic CO2 assimilation microbial flora. Environmental Science, 30, 2438–2444.
Bae, S., Kwak, K., Kim, S., Chung, S. Y., & Igarashi, Y. (2001). Isolation and characterization of CO2-fixing hydrogen-oxidizing marine bacteria. Journal of Bioscience and Bioengineering, 91(5), 442–448.
Nguyen, S., Ala, F., Cardwell, C., Cai, D., McKindles, K. M., Lotvola, A., Hodges, S., Deng, Y., & Tiquia-Arashiro, S. M. (2013). Isolation and screening of carboxydotrophs isolated from composts and their potential for butanol synthesis. Environmental Technology, 34(13-14), 1995–2007.
APHA. (1998). Standard methods for the examination of water and wastewater. 21st Edition. American Public Health Association.
Tanaka, K., Miyawaki, K., Yamaguchi, A., Khosravi-Darani, K., & Matsusaki, H. (2011). Cell growth and P(3HB) accumulation from CO2 of a carbon monoxide-tolerant hydrogen-oxidizing bacterium, Ideonella sp. O-1. Applied Microbiology and Biotechnology, 92(6), 1161–1169.
Garcia-Gonzalez, L., Mozumder, M. S. I., Dubreuil, M., Volcke, E. I. P., & De Wever, H. (2015). Sustainable autotrophic production of polyhydroxybutyrate (PHB) from CO2 using a two-stage cultivation system. Catalysis Today, 257, 237–245.
Repaske, R. (1962). Nutritional requirements for Hydrogenomonas eutropha. Journal of Bacteriology, 83, 418–422.
Cohen, J. S., & Burris, R. H. (1955). A method for the culture of hydrogen bacteria. Journal of Bacteriology, 69(3), 316–319.
Matassa, S., Verstraete, W., Pikaar, I., & Boon, N. (2016). Autotrophic nitrogen assimilation and carbon capture for microbial protein production by a novel enrichment of hydrogen-oxidizing bacteria. Water Research, 101, 137–146.
Lo, S. N., & Moreau, J. R. (1986). Mixed-culture microbial protein from waste sulfite pulping liquor II: its production on pilot-plant scale and use in animal feed. Canadian Journal of Chemical Engineering, 64(4), 639–646.
Acknowledgements
This study was supported jointly by the National Key R & D Program of China (2018YFD0501405), by the Youth Innovation Promotion Association CAS (2017423), the Key Project for Foreign Cooperation of the International Cooperation Bureau of the Chinese Academy of Sciences (182344KYSB20170009), the Science and Technology Service Network Initiative (STS) of the Chinese Academy of Sciences, the Key Laboratory of Environmental and Applied Microbiology of Chengdu Institute of Biology CAS (KLCAS-2016-10, KLCAS-2017-9), and the Chengdu Science and Technology Huimin Project (2016-HM02-00092-SF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Dou, J., Huang, Y., Ren, H. et al. Autotrophic, Heterotrophic, and Mixotrophic Nitrogen Assimilation for Single-Cell Protein Production by Two Hydrogen-Oxidizing Bacterial Strains. Appl Biochem Biotechnol 187, 338–351 (2019). https://doi.org/10.1007/s12010-018-2824-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-2824-1