Abstract
Fluorescent and incandescent lighting systems were applied for batch photofermentative hydrogen production by four purple non-sulfur photosynthetic bacteria (PNSB). The hydrogen production efficiency of Rhodopseudomonas palustris, Rhodobacter sphaeroides, Rhodobacter capsulatus, and Rhodospirillum rubrum was evaluated using different carbon sources (acetate, butyrate, lactate, and malate). Incandescent light was found to be more effective for bacteria cell growth and hydrogen production. It was observed that PNSB followed substrate selection criteria for hydrogen production. Only R. palustris was able to produce hydrogen using most carbon sources. Cell density was almost constant, but cell growth rate and hydrogen production were significantly varied under the different lighting systems. The kinetics study suggested that initial substrate concentration had a positive correlation with lag phase duration. Among the PNSB, R. palustris grew faster and had higher hydrogen yields of 1.58, 4.92, and 2.57 mol H2/mol using acetate, butyrate, and lactate, respectively. In the integrative approach with dark fermentation effluents rich in organic acids, R. palustris should be enriched in the phototrophic microbial consortium of the continuous hydrogen production system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In view of the global energy crisis related to fossil fuels, hydrogen is considered as a promising alternative fuel and “clean energy carrier” due to its renewability and clean burning nature [1, 2]. Hydrogen has a higher energy yield than hydrocarbon fuels [3, 4]. It can also be used as feedstock for the synthesis of ammonia, alcohol, and aldehydes and for the hydrogenation of various petroleum products [5]. Biological hydrogen production has several advantages compared with the traditional electrolysis or thermochemical processes (e.g., low energy requirement). Purple non-sulfur photosynthetic bacteria (PNSB) have been reported to convert a wide variety of organic compounds to hydrogen and carbon dioxide under anaerobic conditions in the presence of light [6]. These bacteria species mainly include Rhodopseudomonas sp., Rhodobacter sp., and Rhodospirillum sp. [7,8,9]. They grow in mesophilic freshwater and neutral pH, while Rhodobacter sp. is also found in the sewage ponds or eutrophic lakes [10]. Ren et al. isolated Rhodopseudomonas faecalis RLD-53 from indigenous pond sludge to produce hydrogen [11]. Sasikala et al. reported hydrogen production by isolated Rhodobacter sphaeroides O.U. 001 from distillery wastewater [12].
PNSB convert light energy into chemical bond energy (stored in ATP) for cell growth. Organic compounds are catalyzed by nitrogenase to produce hydrogen under nitrogen-deficient environment. Broad spectra of light wavelengths can be used by PNSB [13]. Light sources such as tungsten, fluorescent, infrared, and halogen lamps have been reported for hydrogen production, but the performance varies among different conditions [14, 15]. The light energy strongly affects hydrogen production rate and yield in the photofermentation process, and it should not be neglected during hydrogen evolution [16,17,18].
Photosynthetic bacteria utilize a wide variety of substrates for hydrogen production including carbohydrates such as glucose, and organic acids like short chain volatile fatty acids (VFAs) [6]. Thus, industrial or agricultural effluents rich in carbohydrates and VFAs could be used as feedstock for photofermentative hydrogen production. For instance, acetic, butyric, and lactic acids (dark fermentation effluent from food waste) and malic acid (distillery waste) are common substrates for PNSB utilization [19, 20]. However, the reported substrate conversion efficiencies vary among different carbon sources and bacteria strains [21]. These discrepancies related to carbon sources and concentrations should be outreached before scale-up implementation.
In this study, two lighting systems (fluorescent and incandescent) were investigated for biohydrogen production using four PNSB at different carbon sources and concentrations. The substrates were selected with reference to dark fermentation effluents. Kinetics study on cell growth and hydrogen production was further performed to reveal hydrogen evolution.
Materials and Methods
Bacteria Strains and Medium
R. palustris DSM 127, R. sphaeroides DSM 158, and R. capsulatus DSM 1710 were purchased from Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ) in lyophilized form. R. rubrum WT was obtained from Karolinska University (Sweden).
Growth medium (per L) consisted of 0.3 g yeast extract, 1 g sodium succinate, 0.5 mL ethanol, 0.4 g NH4Cl, 0.5 g ammonium acetate, 5 mL ferric citrate (0.1%), 0.5 g KH2PO4, 0.4 g MgSO4∙7H2O, 0.4 g NaCl, 0.05 g CaCl2∙2H2O, 0.4 mL vitamin B12 solution (0.1 g/L), and 1 mL trace element solution SL-6. The trace element solution SL-6 (per L) contained 0.1 g ZnSO4∙7H2O, 0.03 g MnCl2∙4H2O, 0.3 g H3BO3, 0.2 g CoCl2∙6H2O, 0.01 g CuCl2∙2H2O, 0.02 g NiCl2∙6H2O, and 0.03 g Na2MoO4∙2H2O. The initial pH value was adjusted to 6.8 ± 0.2 with 1 M HCl or NaOH solution. The substrate was also adjusted according to growth medium. The carbon source was replaced by acetate, butyrate, lactate, or malate, while 10 mM glutamate was used as the nitrogen source. Both growth medium and substrate were transferred into 120- or 60-mL serum bottles, purged with argon gas to create anaerobic conditions, and then sealed with butyl rubber stoppers and aluminum crimp caps. Afterwards, the sealed serum bottles were autoclaved at 121 °C for 15 min.
Batch Photofermentation Experiments
For cell growth, the bacteria strains were initially cultivated at 30 °C in 120-mL serum bottles containing 60 mL growth medium. When the cells reached the mid-exponential growth phase, they were injected into 60-mL serum bottles containing 30 mL hydrogen production substrate (acetate, butyrate, lactate, or malate) for the hydrogen production study. The incubator was operated at 180 rpm and 30 °C, and illuminated by fluorescent or incandescent light. The fluorescent lighting system consisted of six fluorescent lamps (Phillips Essential TL5 14 W/830) with an average intensity of 2400 lx. The incandescent lighting system comprised six incandescent bulbs (Phillips A55 25 W) with an average intensity of 2000 lx.
A summary of the batch photofermentation experimental conditions is shown in Table 1. In Run 1, the four PNSB were cultivated in growth medium illuminated by fluorescent or incandescent light. Cell density and growth kinetics were investigated. In Run 2, the four carbon sources (acetate, butyrate, lactate, and malate) were used to evaluate the photofermentative hydrogen production by R. palustris under fluorescent or incandescent lighting systems. The concentrations were ranged based on dark fermentation effluent [19]. Run 2 lasted 32 days (50 days for butyrate study). In Run 3, the optimized concentration of each carbon source was selected and tested by the four photofermentative bacteria. The hydrogen production yields were evaluated.
Analytical Methods
Gas production was measured periodically by using a manometer (SAMCO, UK). Gas contents (H2 and CO2) were analyzed by gas chromatography equipped with thermal conductivity detector (GC-TCD-TCD, Agilent, USA). Argon and helium were used as carrier gases. Columns used in the analysis were molecular sieve 13X and 5A. Oven and detector temperatures were set at 115 and 150 °C, respectively. The cumulative hydrogen volume was calculated from the H2 content in gas samples and total volume of the gas produced. All gas production data were normalized at standard temperature (0 °C) and pressure (760 mm of Hg).
Acetate and butyrate concentrations were analyzed using gas chromatography with flame ionization detector (GC-FID, Agilent, USA), equipped with a selective capillary GC column (DB-FFAP) for acid separation using nitrogen as carrier gas. Lactate and malate concentrations were analyzed at 210 nm by high-performance liquid chromatography (HPLC, Thermo Scientific Dionex, USA), with diode-array detector and a 300 × 7.8 mm Aminex HPX-87H column (Bio-Rad, USA). The mobile phase was an aqueous solution of 0.005 M H2SO4 with a flow rate of 0.7 mL/min. The liquor samples were filtered through a 0.45-μm nylon membrane before free acid analysis.
Light intensity was measured by using a light meter (LT Lutron LX-1108, Taiwan). Optical density (OD) was quantitated as cell concentration and measured by a spectrophotometer at 660 nm (Agilent, USA).
Kinetics Study and Data Analysis
The modified Gompertz equation was used to simulate cell growth and hydrogen production. For cell growth, Eq. (1) was used [22]:
Where, y is the OD at time t, A represents the maximum OD reached, μ max is the maximum OD increase rate (as ΔOD/h), λ is the lag time (h) for OD increase, and e is the natural constant equal to 2.718.
For hydrogen production, Eq. (2) was used [23, 24]:
Where, H represents the cumulative hydrogen production (mL-H2/L-medium) at time t (h), H max denotes the maximum cumulative H2 production (mL-H2/L-medium), R max is the maximum hydrogen production rate (mL-H2/L-medium/h), and λ is the lag-phase duration (h) for hydrogen production. Statistical significance was evaluated by one-way ANOVA.
Results and Discussion
Effect of Light Source on the Kinetics of PNSB Cell Growth
The fluorescent and incandescent lighting systems were applied to investigate the effect of different light source on PNSB cell growth (Run 1). As the bacteria strains grow in different colors, the color is used as an indicator of PNSB growth status [10]. For instance, anaerobic phototrophic cultures of Rhodobacter species are yellow-green to yellow-brown, while aerobic cultures are pink to red [25]. The cell growth experiments showed that the four PNSB could grow under both lights: R. palustris in red, R. rubrum in purple-red, and R. capsulatus and R. sphaeroides in yellow-brown. However, it took longer for PNSB to start growing under the fluorescent light compared to the incandescent light. The lag phase of R. capsulatus and R. sphaeroides was around 4 days under the incandescent light, but more than 10 days under the fluorescent light. Among the four strains, R. palustris grew faster than the other three strains, and it was the only culture producing hydrogen within 21 days of fermentation under the fluorescent light. Therefore, R. palustris was selected to further investigate the effect of light source on cell growth and hydrogen production (Run 2).
Cell growth kinetics of R. palustris were evaluated under the two lighting systems (Fig. 1). Under incandescent light, R. palustris had a very short lag phase and reached its maximum cell density (OD660) of 1.78 in 78 h. The cells grew considerably slower under fluorescent light, and the maximum cell density (OD660) of 1.69 was obtained in 162 h. In order to further investigate the effect of light on cell growth, the simulated cell growth kinetics of R. palustris were determined according to Eq. (1). The model fit the experimental results well with a correlation coefficient (R 2) of 0.965 and 0.977 for fluorescent and incandescent lights, respectively. The estimated maximum cell growth rates (μ max) for the fluorescent and incandescent lighting systems were 0.01344/h and 0.03675/h, respectively. The cell growth rate under the incandescent light was three times higher than that under the fluorescent light. The lag phase time (λ) under fluorescent light was around 9.2 h, while λ was 0.1 h under incandescent light. The simulated ODmax were 1.75 and 1.77 under fluorescent and incandescent lights, respectively. The cell growth rate and the lag phase were significantly different (p < 0.05) under the two lighting systems, but the maximum cell density had no significant difference (p = 0.14). The kinetics study suggests that although the light did not affect biomass concentration, it was a limiting factor for cell growth rate that affects hydrogen production rate.
Effect of Lighting System and Carbon Sources on Hydrogen Evolution by R. palustris
In Run 2, four carbon sources (acetate, butyrate, lactate, and malate) were tested for hydrogen production by R. palustris under the two lighting systems (fluorescent and incandescent). Substrate conversion efficiency (SCE) is used to evaluate the hydrogen production performance of each specific carbon source [26]. It is the ratio of the actual hydrogen produced to the theoretical hydrogen production.
The theoretical hydrogen production is calculated according to the following hypothetical reaction, which denotes that all of the substrate is utilized for hydrogen and carbon dioxide production.
Table 2 lists the photofermentation characteristics under fluorescent and incandescent lights. R. palustris was able to utilize acetate for hydrogen production, but it was ineffective to convert butyrate, malate, and lactate to hydrogen under the fluorescent light. Hydrogen yield at different initial acetate concentrations was ranged between 1.52 to 1.69 mol H2/mol substrate. R. palustris did not grow well using butyrate and lactate as carbon sources. Only small amounts of butyrate and lactate were consumed (less than 17%), which resulted in limited cell growth and nugatory hydrogen evolution. R. palustris was able to utilize malate for cell growth, but the cell density decreased as the initial malate concentration increased. Furthermore, the final pH values of malate fermentation effluents were above 9, which set unfavorable conditions for hydrogen evolution.
Under the incandescent light, butyrate, lactate, and malate were well utilized by R. palustris for hydrogen production and cell growth. Hydrogen yield from butyrate was higher than that from lactate and malate. Concentration increase of butyrate and malate had little effect on hydrogen yield, while concentration increase of lactate decreased hydrogen yield. Limited hydrogen was produced from acetate, although R. palustris grew well. Effluent analysis showed that final pH was above 8.7, which was unfavorable for hydrogen production by PNSB [26]. It seems that pH change was detrimental for hydrogen production, but not for cell growth. A neutral pH range (6.64 to 8.02) was found at other carbon source effluents. Overall, the photofermentation process under incandescent light was more efficient for hydrogen production compared to that under fluorescent light.
Figure 2 presents the photofermentative hydrogen evolution using different carbon sources. R. palustris easily produced hydrogen from acetate. As the initial concentration increased, more hydrogen was produced (Fig. 2a). The maximum cumulative hydrogen production of 1735 mL/L was produced at 3 g/L acetate. When butyrate was used as the carbon source (Fig. 2b), R. palustris started hydrogen production after 220 h at 1 g/L. It took even longer for hydrogen evolution using higher concentrations of butyrate. Maximum cumulative hydrogen was obtained at 2 g/L butyrate. Regarding lactate and malate, R. palustris could easily convert them into hydrogen. Figure 2c shows steady hydrogen production with increased initial lactate concentration. Furthermore, the cumulative hydrogen production increased as the initial malate concentration increased (Fig. 2d). The optimal initial carbon concentrations for cumulative hydrogen production were 2.5 g/L lactate and 6 g/L malate.
The hydrogen production kinetics are summarized in Table 3. With increase of carbon concentration, the estimated maximum hydrogen production rate (R max) decreased for acetate, lactate, and malate. The R max of butyrate did not present any trend and varied between 13.9 to 19.9 mL/L/h. The highest R max (27.6 mL/L/h) was achieved using 2 g/L malate. The lag phase duration (λ) for hydrogen production increased as the initial carbon concentration increased. λ was much longer in butyrate than that in other carbon sources, which implies that more time was required for R. palustris to acclimatize butyrate for hydrogen production. The maximum cumulative hydrogen production (H max) among the carbon sources was 2170.9 mL/L/h at 6 g/L malate, 2153.7 mL/L/h at 2 g/L butyrate, 1866.1 mL/L/h at 3 g/L acetate, and 1670.7 mL/L/h at 2.5 g/L lactate. The maximum SCE was consistent with the H max for each carbon source except malate. The highest SCE (49.2%) was obtained at the initial butyrate concentration of 2 g/L.
Based on the experimental findings, the lighting system had significant effect on hydrogen production. Although under fluorescent light only acetate was converted to hydrogen, most carbon sources (butyrate, malate, and lactate) could be converted under incandescent light. Few researches have indicated the effect of light source on substrate selection for hydrogen production. Herein, the effects of carbon sources were focused on lag phase, hydrogen production rate, and substrate conversion efficiency. Butyrate had the highest SCE for hydrogen evolution, in agreement with that of the previous photofermentation studies [27, 28]. The high initial carbon concentration resulted in a longer lag phase suggesting that lower concentrations at the beginning of the photofermentation process could be beneficial for bacterial acclimatization and hydrogen production. Interestingly, acetate and malate could be utilized by R. palustris for cell growth, but no hydrogen was observed due to high pH. Tao et al. have reported that the photosynthetic bacterial strain R. sphaeroides ZX-5 could perform self pH-adjustment for maximum hydrogen production [29]. In this study, a potential pH control could sustain R. palustris to evolve hydrogen, since there was sufficient cell growth.
Evaluation of Hydrogen Production by PNSB
R. palustris, R. sphaeroides, R. capsulatus, and R. rubrum could grow under both lighting systems, but the cell growth rate was lower under fluorescent light. Therefore, evaluation of hydrogen production was conducted under incandescent light. Figure 3 presents the findings using the optimized concentration of carbon sources: 2 g/L acetate, 2 g/L butyrate, 2.5 g/L lactate, and 6 g/L malate (Run 3). Similar to previous data, limited hydrogen was produced from acetate by all bacteria strains. The consumed acetate was only used for cell growth but not for hydrogen production. R. palustris effectively utilized butyrate for hydrogen production, while the other three strains produced little hydrogen using butyrate as the carbon source. R. capsulatus only produced hydrogen well from malate, while R. sphaeroides preferred to utilize lactate for hydrogen production. Both lactate and malate could be easily converted to hydrogen by R. rubrum, while the hydrogen yields were 2.02 and 2.13 mol H2/mol substrate, respectively. R. palustris had a wider range of carbon source utilization and a higher hydrogen yield than the other strains. Hydrogen production rate of lactate and malate fermentation was higher than that of butyrate fermentation. The results showed that PNSB had a significant preference on substrate selection for photofermentative hydrogen production, which would be beneficial for the treatment of complex waste effluent. Additionally, Table 4 presents the SCE and final pH of the carbon source effluents by PNSB. R. palustris had the highest SCE among the bacteria strains. Unfavorable conditions (high pH) for hydrogen production were observed in the effluents from acetate, lactate by R. capsulatus, and malate by R. sphaeroides. As previous discussed, carbon types and pH will affect hydrogen production. It was also reported that different PNSB species have distinct types of nitrogenase: Mo-nitrogenase, V-nitrogenase, and Fe-nitrogenase, which is the main enzyme for hydrogen production [30]. Different lighting conditions might have distinct effects on different types of nitrogenase and on alternative metabolic pathways available, promoting or inhibiting the accumulation of final products, such as hydrogen and biomass synthesis [17].
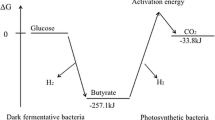
Theoretical Hydrogen Production from Integrated Dark-Photofermentation System
A two-stage sequential dark and photofermentation system using food waste as substrate is assumed for estimation of theoretical hydrogen production (Fig. 4). Based on previous [19] and current findings, the dark fermentation stage is carried out using Clostridium butiricum and the photofermentation stage using R. palustris. Food waste (27 g VS/L) is fed in the dark fermentation stage. Based on process efficiency, the maximum hydrogen yield by C. butiricum is 38.9 mL-H2/g-VSadded, which is equal to 1050 mL-H2 from 100 g/L FW. The main fatty acids in the dark fermentation effluent are 28.9 mM acetate, 38.9 mM butyrate, and 51.2 mM lactate. The dark fermentation effluent is subsequently used as substrate in the photofermentation stage. According to the hydrogen yield by R. palustris based on the current study (1.58 mol H2/mol acetate, 4.92 mol H2/mol butyrate, and 2.57 mol H2/mol lactate), 369 mM H2 equal to 8257 mL H2/L could be produced in the photofermentation stage (under optimized conditions). Theoretically, 9.3 L-H2 could be produced from 100 g/L FW in the integrated dark and photofermentation system.
Several challenges should be considered in the sequential dark and photofermentation system using mixed substrates. (1) The diverse components could cause instability in the dark fermentation process. Some certain pre-treatment (pH, ultrasound, thermal, etc.) is required to minimize such impact. (2) Specific and efficient inoculums should be used for each fermentation stage. (3) The dark fermentation effluent should be monitored before feeding in the photofermentation stage for metabolites (e.g., sulfides, long chain fatty acids, etc.) that could compromise process efficiency. (4) Limited light penetration in the photofermentation stage that might require dilution of substrate or varying light intensity. (5) Optimization of operating parameters for each stage (substrate to inoculum ratio, pH control, pre-treatment, inoculum sources, temperature, reactor design, hydraulic retention time, etc.). (6) Scalability and industrialization of the sequential dark and photofermentation system in relation to substrate type.
Conclusion
Two lighting systems were evaluated for photofermentative hydrogen production. Incandescent light was more efficient for PNSB growth and hydrogen production. Substrate selection criteria were observed by PNSB. Lactate was the preferable carbon source for R. capsulatus, while R. sphaeroides produced more hydrogen from malate. R. rubrum and R. palustris could convert several carbon sources to hydrogen, but with different efficiencies. Among the PNSB, R. palustris grew faster and showed high efficiency to a wide range of lighting and carbon sources, considering it as a potential microbial culture in the integrated dark and photofermentation system.
References
Logan, B. E. (2004). Extracting hydrogen and electricity from renewable resources. Environmental Science and Technology., 38(9), 160A–167A.
Hallenbeck, P. C., & Liu, Y. (2016). Recent advances in hydrogen production by photosynthetic bacteria. International Journal of Hydrogen Energy, 41(7), 4446–4454.
Yilmaz, F., Balta, M. T., & Selbaş, R. (2016). A review of solar based hydrogen production methods. Renewable and Sustainable Energy Reviews, 56, 171–178.
Sinha, P., & Pandey, A. (2011). An evaluative report and challenges for fermentative biohydrogen production. International Journal of Hydrogen Energy, 36(13), 7460–7478.
Lin, C. N., Wu, S. Y., Lee, K. S., Lin, P. J., Lin, C. Y., & Chang, J. S. (2007). Integration of fermentative hydrogen process and fuel cell for on-line electricity generation. International Journal of Hydrogen Energy, 32, 802–808.
Keskin, T., Abo-Hashesh, M., & Hallenbeck, P. C. (2011). Photofermentative hydrogen production from wastes. Bioresource Technology, 102(18), 8557–8568.
Ghosh, S., Dairkee, U. K., Chowdhury, R., & Bhattacharya, P. (2017). Hydrogen from food processing wastes via photofermentation using purple non-sulfur bacteria (PNSB)–a review. Energy Conversion and Management, 141, 299–314.
Kim, D. H., Son, H., & Kim, M. S. (2012). Effect of substrate concentration on continuous photo-fermentative hydrogen production from lactate using Rhodobacter sphaeroides. International Journal of Hydrogen Energy, 37(20), 15483–15488.
Dadak, A., Aghbashlo, M., Tabatabaei, M., Younesi, H., & Najafpour, G. (2016). Exergy-based sustainability assessment of continuous photobiological hydrogen production using anaerobic bacterium Rhodospirillum rubrum. Journal of Cleaner Production, 139, 157–166.
Brenner, D.J., Boone, D.R., Garrity, G.M., Goodfellow, M., Krieg, N.R., Rainey, F.A., Schleifer, K.H., Staley, J.T., and Vos, P. (2005) Bergey’s manual of systematic bacteriology. 2nd ed., USA.
Ren, N. Q., Liu, B. F., Ding, J., & Xie, G. J. (2009). Hydrogen production with R. faecalis RLD-53 isolated from freshwater pond sludge. Bioresource Technology, 100(1), 484–487.
Sasikala, K., Ramana, C. V., & Raghuveer Rao, P. (1992). Photoproduction of hydrogen from the waste water of a distillery by Rhodobacter sphaeroides O.U. 001. International Journal of Hydrogen Energy, 17(1), 23–27.
Zhang, D., Xiao, N., Mahbubani, K. T., del Rio-Chanona, E. A., Slater, N. K. H., & Vassiliadis, V. S. (2015). Bioprocess modelling of biohydrogen production by Rhodopseudomonas palustris: model development and effects of operating conditions on hydrogen yield and glycerol conversion efficiency. Chemical Engineering Science, 130, 68–78.
Ozmihci, S., & Kargi, F. (2010). Comparison of different mixed cultures for bio-hydrogen production from ground wheat starch by combined dark and light fermentation. Journal of Industrial Microbiology and Biotechnology, 37(4), 341–347.
Argun, H., & Kargi, F. (2010). Effects of light source, intensity and lighting regime on bio-hydrogen production from ground wheat starch by combined dark and photo-fermentations. International Journal of Hydrogen Energy, 35(4), 1604–1612.
Muzziotti, D., Adessi, A., Faraloni, C., Torzillo, G., & De Philippis, R. (2016). H2 production in Rhodopseudomonas palustris as a way to cope with high light intensities. Research in Microbiology, 167(5), 350–356.
Corona, V. M., Le Borgne, S., Revah, S., & Morales, M. (2017). Effect of light-dark cycles on hydrogen and poly-β-hydroxybutyrate production by a photoheterotrophic culture and Rhodobacter capsulatus using a dark fermentation effluent as substrate. Bioresource Technology, 226, 238–246.
Boran, E., Özgür, E., Gebicki, J., van der Burg, J., Yücel, M., Gündüz, U., Modigel, M., & Eroglu, I. (2009). Investigation of influencing factors for biological hydrogen production by R. capsulatus in tubular photo-bioreactors. Chemical Engineering Transactions, 18, 357–362.
Hu, C., Giannis, A., Chen, C. L., & Wang, J. Y. (2014). Evaluation of hydrogen producing cultures using pretreated food waste. International Journal of Hydrogen Energy, 39(33), 19337–19342.
Hay, J. X. W., Wu, T. Y., & Juan, J. C. (2013). Biohydrogen production through photo fermentation or dark fermentation using waste as a substrate: overview, economics, and future prospects of hydrogen usage. Biofuels, Byproducts and Biorefining, 7(3), 334–352.
Trchounian, A. (2015). Mechanisms for hydrogen production by different bacteria during mixed-acid and photo-fermentation and perspectives of hydrogen production biotechnology. Critical Reviews in Biotechnology, 35(1), 103–113.
Gardini, F., Martuscelli, M., Caruso, M. C., Galgano, F., Crudele, M. A., Favati, F., Guerzoni, M. E., & Suzzi, G. (2001). Effects of pH, temperature and NaCl concentration on the growth kinetics, proteolytic activity and biogenic amine production of Enterococcus faecalis. International Journal of Food Microbiology, 64(1), 105–117.
Xie, G. J., Liu, B. F., Xing, D. F., Ding, J., Nan, J., Ren, H. Y., Guo, W. Q., & Ren, N. Q. (2012). The kinetic characterization of photofermentative bacterium Rhodopseudomonas faecalis RLD-53 and its application for enhancing continuous hydrogen production. International Journal of Hydrogen Energy, 37(18), 13718–13724.
Lo, Y. C., Chen, W. M., Hung, C. H., Chen, S. D., & Chang, J. S. (2008). Dark H2 fermentation from sucrose and xylose using H2-producing indigenous bacteria: feasibility and kinetic studies. Water Research, 42(4), 827–842.
Uyar, B. (2013). A novel non-invasive digital imaging method for continuous biomass monitoring and cell distribution mapping in photobioreactors. Journal of Chemical Technology and Biotechnology, 88(6), 1144–1149.
Lazaro, C. Z., Varesche, M. B. A., & Silva, E. L. (2015). Effect of inoculum concentration, pH, light intensity and lighting regime on hydrogen production by phototrophic microbial consortium. Renewable Energy, 75, 1–7.
McKinlay, J. B., & Harwood, C. S. (2011). Calvin cycle flux, pathway constraints, and substrate oxidation state together determine the H2 biofuel yield in photoheterotrophic bacteria. MBio, 2(2), e00323–e00310.
Koku, H., Eroğlu, I., Gündüz, U., Yücel, M., & Türker, L. (2002). Aspects of the metabolism of hydrogen production by Rhodobacter sphaeroides. International Journal of Hydrogen Energy, 27(11), 1315–1329.
Tao, Y., He, Y., Wu, Y., Liu, F., Li, X., Zong, W., & Zhou, Z. (2008). Characteristics of a new photosynthetic bacterial strain for hydrogen production and its application in wastewater treatment. International Journal of Hydrogen Energy, 33(3), 963–973.
Kars, G., & Gündüz, U. (2010). Towards a super H2 producer: improvements in photofermentative biohydrogen production by genetic manipulations. International Journal of Hydrogen Energy, 35(13), 6646–6656.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hu, C., Choy, SY. & Giannis, A. Evaluation of Lighting Systems, Carbon Sources, and Bacteria Cultures on Photofermentative Hydrogen Production. Appl Biochem Biotechnol 185, 257–269 (2018). https://doi.org/10.1007/s12010-017-2655-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2655-5