Abstract
Green macroalgae are an abundant and undervalued biomass with a specific cell wall structure. In this context, different pretreatments, namely ethanol organosolv (Org), alkaline, liquid hot water (LHW), and ionic liquid (IL) pretreatments, were applied to the green macroalgae Ulva lactuca biomass and then evaluated. Their effects on chemical composition, biomass crystallinity, enzymatic digestibility, and theoretical ethanol potential were studied. The chemical composition analysis showed that the Org and LHW pretreatments allowed the highest glucan recovery (80.8 ± 3.6 and 62.9 ± 4.4 g/100 g DM, respectively) with ulvan (80.0 and 99.1%) and hemicellulose (55.0 and 42.3%) removal. These findings were in agreement with both thermogravimetric analysis and scanning electron microscopy results that confirm significant structural changes of the pretreated biomasses. It was found that the employed pretreatments did not significantly affect the cellulose crystallinity; however, they both increased the whole crystallinity and the enzymatic digestibility. This later reached 97.5% in the case of LHW pretreatment. Our results showed high efficiency saccharification of Ulva lactuca biomass that will constitute the key step of the implementation of a biorefinery process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The exhaustion of fossil fuels has brought growing concerns since various areas are dependent on these fuels, but since they also created many environmental problems such as global warming, air quality deterioration, and oil spills. Consequently, research is now focused on decreasing CO2 formation and on developing alternative energy sources to substitute the limited fossil fuels [1].
Among the most attractive alternative energy sources is biomass. As renewable material, it is environment-friendly and cost-effective. At the same time, recent advances in biotechnology and green chemistry developed new concepts for converting renewable biomass into valuable products such as biofuels [2]. With the use of alimentary polysaccharides for first-generation ethanol production, numerous doubts rose about its possible impact on food supply and security, generating an urgent demand for alternative, sustainable fuels and feedstock to replace food-based feedstock [3].
In this context, algae can provide a high-yield source of third-generation biofuels without compromising food supplies or rainforests [4, 5]. The global market of algal biofuels is expected to witness a huge expansion during the next decade. Since, petroleum and aviation industries started investing in algae biofuels with the aim of replacing fossil fuels [6]. Beside lipid-rich microalgae for the production of sustainable biodiesel, a huge interest was given to macroalgae as source of third-generation bioethanol seen their specific chemical composition and their availability. Macroalgal biomass does not require agricultural additives or fertilizers and does not compete with cultivation space as they can be cultivated in open aquatic media. Some green macroalgae species, namely Ulvacae and Cladophoracae, raised more attention because of their availability at coasts and their easy harvesting [7, 8].
The stability of bonds between the major components of green macroalgae cell walls, cellulose, hemicellulose, and ulvan makes its pretreatment essential for an efficient saccharification of their polysaccharides to fermentable sugars. In fact, the goals of all pretreatment technologies are to improve access of hydrolytic enzymes to cell wall polysaccharides, while minimizing degradation of sugars and formation of fermentation inhibitors [9].
Pretreatment technologies can be categorized as chemical, physical, or biological, or as a combination of these categories. The chemical-based pretreatment technologies (e.g., alkaline, organosolv) are commonly used with or without fiber explosion. Alkaline pretreatments, such as the sodium hydroxide pretreatment, typically solubilize lignin and a portion of the hemicellulose, generating a more accessible structure [10]. Solvent-based pretreatments include organosolv processes using alcohols (e.g., methanol, ethanol, and glycerol) or organic acids (e.g., formic, maleic, and acetic). In such processes, lignin and/or hemicellulose is removed, thereby increasing pore volume and hydrolytic enzyme accessibility [11]. Liquid hot water (LHW) pretreatment using pressure to maintain water in a liquid state at elevated temperatures (160–240 °C) is also an attractive approach because it does not require the addition of chemicals such as sulfuric acid, lime, or ammonia [12].
In addition to these solvents, ionic liquids (ILs) have emerged as promising non-derivating solvents for the dissolution of lignocellulose. Especially, it has been reported that 1-ethyl-3-methylimidazolium acetate ([Emim][Ac]) has a good dissolving capability for lignocelluloses [13].
The green macroalgae Ulva lactuca is very abundant in the coasts of Tunisia and causes many environmental problems. Therefore, in order to implement an eco-friendly solution aimed at the biodegradation of this green macroalgae, we studied four pretreatments (alkaline, organosolv, LHW, and IL) as a key step for the whole bioenergetic conversion of algal biomass. The evaluation of these pretreatments was mainly based on the chemical, thermal, and crystalline properties, but also the global enzymatic saccharification and ethanol formation of the pretreated biomasses.
Material and Methods
Materials
The green macroalgae Ulva lactuca was collected from the lagoon of Tunis (GPS 36.813095, 10.192673; salinity 33.8 psu). Samples were transported to the laboratory and were immediately washed with distilled water, air-dried, hand milled, and stored in plastic bags until use.
Algae Pretreatment
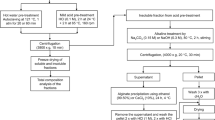
All pretreatment reactions of Ulva lactuca were realized in a 300-ml Parr steel reactor (type 4566) except for the ionic liquid (IL) one which was realized in centrifugal tubes.
The alkali pretreatment consisted of using NaOH 6% (w/v) for 2 h at 180 °C [14]. The pretreated biomass was then washed and dried for further studies. The ratio of the used biomass was 10% (m/v).
For the ethanol organosolv pretreatment, 15 g of biomass was mixed with 150 ml (10% ratio) of a 50% (v/v) ethanol solution and then introduced to the reactor and then kept for 2 h at 200 °C. The remaining biomass was then washed and air-dried.
For the liquid hot water pretreatment (LHW), 15 g of algal biomass was mixed with 150 ml of distilled water (10% ratio) and the pretreatment reaction was carried out for 2 h at 170 °C [15].
The IL pretreatment of Ulva lactuca was realized based on Viell et al. (2013) [16] and further analytical investigations in Viell et al. (2016) [17]. The IL used was 1-ethyl-3-methylimidazolium acetate ([EMIM][Ac], > 95% (w/w) purity). Five grams of algae was added to the IL in a centrifugal tube which was placed in an aluminum heating block on a magnetic stirrer (500 rpm). The ionic liquid was added until to mixture weight reached 10 g. The pretreatment was carried out for 1 h at 115 °C. After the pretreatment, the algae was thoroughly washed with water to remove the residual IL and then air-dried for 2 days before compositional analysis and enzymatic hydrolysis.
Enzymatic Hydrolysis
The enzymatic saccharification experiment was realized using a proportion of 10% (w/v) of native or pretreated algae prepared in 0.1 M sodium acetate buffer pH 4.8. The hydrolysis is started after the addition of the re-buffered enzyme solution Celluclast 1.5L appropriately diluted to achieve a cellulase activity of 10 U per gram of biomass. The mix was shaken in a thermomixer (MKR 10, HLC BioTech, Bovenden, Germany) at 900 rpm and 50 °C [17]. Samples were withdrawn periodically to evaluate the progress of the enzymatic saccharification. The enzymes were inactivated at 100 °C and then centrifuged for 5 min at 3000 rpm. The supernatant was kept for quantification of sugars by high-performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) [18].
The evaluation of enzymatic conversion was assessed by determining two yields:
-
The global conversion yield, which corresponds to the amount (mg) of total soluble sugars, determined from the HPAEC-PAD analysis, released per gram of dried pretreated biomass.
-
The glucan or xylan conversion yields, which are specific yields related only to the enzymatic conversion of either glucan or xylan. They refer to glucose or xylose amounts (mg) reported to initial glucan or xylan (mg) content in either native or pretreated biomasses. Amounts of glucose or xylose were determined by HPAEC-PAD analysis.
Cellulose Extraction
Cellulose was extracted from the native and pretreated biomasses using the method previously described by Jmel et al. (2016) [4]. The extraction process consisted briefly in lipids elimination as a first step, followed by ulvan extraction. The residual biomass was then bleached and subjected to an acid and alkali bath. The extracted cellulose was finally dried at 105 °C.
Analytical Methods
Compositional Analysis of the Green Macroalgae
Two-step acid hydrolysis was performed according to NREL Laboratory Analytical Procedure to determine the chemical composition of the native and pretreated algae. Biomass (25 mg) was hydrolyzed with 250 μl of 72% sulfuric acid for 1 h at 30 °C. Hydrolyzates were then diluted to 4% of sulfuric acid with distilled water and then heated for 1 h at 120 °C [19]. After hydrolysis, 2 ml of the supernatant was centrifuged and then filtered before sugar monomers analysis.
HPAEC-PAD Analysis of Monomeric Sugars
The monosaccharides released after hydrolysis of the native and pretreated [19] were determined based on HPAEC-PAD method [20]. As enzymes for biomass degradation only cleave saccharide linkages, the HPAEC-PAD method [20] was shortened to an 11-min run starting with 99% 100 mM NaOH (A) and 1% 100 mM NaOH and 500 mM NaAc (B) on an ICS-5000+ device (Thermo Scientific, USA). After 6 min, the eluent composition reaches 75% A and 25% B. An increase of eluent B to 70% and a decrease of eluent A to 30% start by minute six. Finally, after 8.5 min, the eluent composition changes from 30% A and 70% B back to the starting conditions and the subsequent equilibration step of 5 min will start before the next injection.
The recovery yields correspond to the percentages of monosugars contents after HPAEC-PAD analysis (glucose, xylose, rhamnose, or mannose) in each pretreated biomass reported to their initial content on the untreated biomass.
Scanning Electron Microscopy
The surface morphology characteristics of the native and pretreated biomass were observed using a scanning electron microscope model S-3000 from Hitachi. Before the observation, samples were spread on a conductive adhesive and then coated with gold.
Thermogravimetric Analysis
Thermogravimetric analysis (TGA) studies were carried out using a TG 209 C thermogravimetric analyzer instrument. About 10 mg of the sample was placed in a crucible, and the TGA spectra were recorded in an ambient nitrogen atmosphere from 25 to 900 °C at a heating rate of 10 °C/min.
X-ray Diffraction
The crystallinity analysis of the different samples was realized using an Empyrean X-ray diffractometer (PANalytical) with CuKα radiation at 40 kV and 40 mA. The recorded range was from 1° to 50° with a step size of 0.013. The crystallinity index (CrI) was calculated using the formula \( CrI=\frac{I_{0.02}-{I}_{\mathrm{am}}}{I_{0.02}}\times 100 \) where I 0.02 represents the intensity of the diffraction plane 0.02 at 2θ = 22.5° and I am is the intensity at about 2θ = 18°.
Results and Discussion
Pretreatment Effect on the Chemical Composition and Thermal Properties of Ulva lactuca
Previous reports indicated the presence of four types of polysaccharides in the cell wall of the Ulva genus. Cellulose and ulvan represented the major fractions. However, a lower presence of the alkali soluble xyloglucan and glucuronan was also reported [21]. In this work, the sugar composition of the green macroalgae Ulva lactuca was studied before and after different pretreatments (alkaline, organosolv, LHW, and IL). In addition, the acid soluble residue including lignin-like molecules, ash, and sulfate derived from sulfated polysaccharides was also determined.
The composition of native and pretreated Ulva lactuca in comparison with the initial dry mass is summarized in Table 1.
As can be deduced from Table 1, the chemical composition of Ulva lactuca indicates a total sugar content of about 30% (w/w) of the dry weight. A sugar content of approximately 20% (w/w) was previously reported by Bobin Dubigeon et al. (1997) [22] and Van der Wal et al. (2013) [14].
The glucan, xylan, rhamnan, and mannan composition of the native Ulva lactuca were respectively 11.2, 3.4, 12, and 3.7 g/100 g DM and were in agreement with Lahaye and Robic (2007) findings about the presence of glucan and ulvan as main polysaccharides [21].
Van der Wal et al. (2013) reported lower amounts of glucan and rhamnan (8.2 and 7.0 g/100 g DM, respectively) but a higher amount of xylan (4.5 g/100 g DM) [14]. These variations in total sugar content and in different polysaccharides can be explained by the seasonal periods [23] or climate variation [24] since the origin of the green macroalgae differed.
The results show that the sugar composition of Ulva lactuca varied depending on the pretreatment used. Recovery yields (Table 1) for the different pretreatments were calculated for each kind of carbohydrate. They gave an estimation of the effect of pretreatment on the residual content of each carbohydrate. Table S2 (supplementary materials) shows the residual biomass after each pretreatment, allowing the calculation of recovery yields mentioned in Table 1.
The highest glucan content was obtained for the ethanol organosolv pretreatment (80.8 ± 3.6 g/100 g DM) followed by the LHW pretreatment (62.9 ± 4.4 g/100 g DM). To our knowledge, no previous studies reported the glucan content after pretreatment of Ulva lactuca biomass. However, several pretreatments of the genus Chaetomorpha were reported by Schultz-Jensen et al. (2013) [25], where a similarly high content of glucan was obtained from green macroalgae after LHW pretreatment (64 ± 2 g/100 g DM). However, the glucan content of Chaetomorpha linum (38 g/100 g DM) is higher than the content for Ulva lactuca used in this work (11.2 g/100 g DM) [25].
The xylan content was decreased by ethanol organosolv (1.2 ± 0.3 g/100 g DM), alkaline (1.1 ± 0.1 g/100 g DM), and LHW (1.8 ± 0.1 g/100 g DM) pretreatments. However, it was increased by the IL pretreatment (5.1 ± 0.0 g/100 g DM). The presence of xylose and mannose indicates the incomplete removal of hemicelluloses in the remaining solid after the pretreatments. The most effective pretreatment in hemicellulose elimination was the alkaline pretreatment which is considered as the most effective in breaking the ester bonds between cell wall polysaccharides. It also removes acetyl groups and various uronic acid substitutions in hemicelluloses [26].
Except for the IL pretreatment, the rhamnan amount was decreased by all the pretreatments especially by LHW (0.1 ± 0.0 g/100 g DM) and alkaline pretreatment (0.2 ± 0.0 g/100 g DM) indicating the elimination of ulvan, one of the main constituents of Ulva lactuca cell wall. The elimination of ulvan represents a crucial step for algae biorefinery processes as it has been proven that this polymer inhibits the sugars fermentation [27].
The IL pretreatment increased the amount of glucan but showed no effect on hemicellulose and ulvan elimination. In contrast, sugar composition results showed an increase in the amount of rhamnose, xylose, and mannose (22.4 ± 0.1, 5.1 ± 0.2, and 3.0 ± 0.1 g/100 g DM, respectively). ILs are not frequently used for algae pretreatment in a biorefinery purpose mainly because of the high costs of these solvents. In this work, the aim of using ILs for algal biomass pretreatment is to study a possible improvement of the enzymatic saccharification of the green macroalgae since they are known for their ability to de-crystallize cellulose in forestry biomass such as wood and disrupt the lignin and hemicellulose matrix [16]. However, we observed a slight increase in the enzymatic conversion and no elimination of hemicellulose and ulvan. This can be explained by the high viscosity of [EMIM][Ac] in comparison to other solvents used in the other pretreatments. This high viscosity does not allow the diffusion of the catalyst through the carbohydrate matrix of Ulva lactuca [28]. This work represents the first assay of a green macroalgae pretreatment with [EMIM][Ac] followed by an enzymatic hydrolysis. Previous works assayed the deconstruction of Ulva rigida without any biorefinery perspective [29]. Also, Ravanal et al. (2016) [28] pretreated brown algae with ILs and had the same difficulties concerning the IL viscosity and accessibility to the internal carbohydrate matrix [28].
The IL pretreatment was not optimized in this work; a potential increase in the temperature of pretreatment might have a beneficial effect on IL accessibility to the internal matrix especially under consideration of [EMIM][Ac] being stable up to 180 °C [30].
The thermal properties of the native and pretreated Ulva lactuca biomass were also analyzed by the mean of thermogravimetric analysis (TGA) which allows the quantitative measurement of changes in materials mass related to its dehydration and decomposition versus time and temperature [31]. Figure 1 shows the thermogravimetric (TGA, A) and the differential thermogravimetric (DTG, B) curves of the native and pretreated samples.
The TGA-DTG curves showed a similar shape for all pretreated biomass with a slight difference for the native algae regarding its complex composition in comparison to the pretreated ones. The thermal decomposition process of Ulva lactuca can be divided into three phases. The first phase goes from the starting temperature to about 180 °C. This phase corresponds to the dehydration of the biomass [32]. The second phase of mass loss goes from 240 to 400 °C, and it can be attributed to the depolymerization or the decomposition of Ulva lactuca organic substances, namely proteins and carbohydrates [33]. The major weight loss for the native and pretreated samples was observed during this second phase. The three highest peaks for DTG curves in this second phase were for IL, LHW, and organosolv which correlate perfectly with the results of the chemical analysis. Here, it was shown that the IL pretreated samples were composed mainly of glucan and ulvan which both decompose thermally in this phase [31] and that the LHW and organosolv samples were composed mainly of glucan. The third phase of mass loss occurs between 600 and 800 °C. This phase is attributed to the thermal degradation of the carbonaceous components of the native and pretreated biomass [34].
Although the shapes of the curves were similar, slight differences existed between the native algae decomposition curve and the pretreated ones; this difference is mostly due to the difference in the composition of every sample [33]. In fact, the native biomass contained all the existing polysaccharides, namely glucan, xylan, rhamnan, and mannan, that were selectively eliminated depending on each other pretreatment. This explains the shift in the degradation pattern of the native algae. On the other hand, the pretreated samples showed a similar shape including all the degradation temperatures of algal biomass. The different biopolymers constituting the biomass cell wall are thermally degradable in different ranges of temperatures. For examples, hemicellulose or other non-cellulosic components reveal generally a lower thermal stability in comparison with cellulose [33].
The TGA-DTG results including the three thermal decomposition phases were in agreement with the findings of Chen et al. (2014) who used physical pretreatments in order to improve the thermal decomposition characteristics of algae [33].
Pretreatment Effect on Structural Features of Ulva lactuca
The sugar composition of the pretreated algae was complemented by the structural features which generally have a huge effect on the enzymatic hydrolysis efficiency (Fig. 2).
The native algae showed a smooth, compact, continuous, and well-ordered structure in comparison with the pretreated samples which all showed morphological changes.
The alkaline pretreated Ulva lactuca revealed a loss of the ordered structure (Fig. 2c). In addition, a swelling phenomenon was caused by the alkaline catalyst generating wrinkles on the surface of the green algae. Actually, the alkaline pretreatment is classified as one of the most effective treatments for the swelling of biomass allowing for an enhanced enzymatic hydrolysis [11]. For the organosolv pretreatment, it can be observed that inner fibers of treated samples were fully exposed which may facilitate the cellulase action during enzymatic hydrolysis (Fig. 2d). The IL pretreated algae exhibited a less-defined and a hairy-like organization compared to the native algae but unlike the organosolv samples, fibers were not fully exposed. This less important exposure may be caused by the presence of ulvan indicated by the high amount of rhamnose after the pretreatment.
Finally, the scanning electron microscopy (SEM) observation of the LHW pretreated samples showed cracks and holes on the algae surface (Fig. 2b). The regular surface observed for the native Ulva lactuca was replaced by folds and irregular holes. It can also be observed that the structure is more exposed to the external surface which may be beneficial for cellulase activity during enzymatic hydrolysis.
To support the biomass structural changes observed by SEM images, the crystallinity index (CrI) was measured for the native, LHW, organosolv, alkaline, and IL pretreated samples as well as cellulose extracted from these materials by the means of X-ray diffraction. The obtained values are summarized in Table 2.
The biomass CrI reflects the total sample crystallinity and is affected by hemicellulose, ulvan cellulose domains, and other components which contribute together to produce the amorphous signal of the studied sample [35]. In contrast, the extracted cellulose CrI indicates the crystallinity character of the isolated cellulose from the native and the pretreated samples. When comparing cellulose CrI, it can be deduced that the applied pretreatments did not significantly affect the cellulose crystallinity of Ulva lactuca. However, comparison between biomass CrI and cellulose CrI could give information about pretreatment efficiency and hemicellulose/ulvan removal. Indeed, compared to the native biomass CrI, all the pretreatments increased the biomass crystallinity. This increase is caused by the elimination of amorphous carbohydrates and the components around the cellulose [36]. As shown by Table 1, this elimination was variable and not complete for all the studied pretreatments, which explain the lower values of biomass CrI.
The structural study using crystallinity indexes supports the results of the chemical composition as the pretreated biomasses richest in glucan, namely organosolv (80.8 ± 3.6 g/100 g DM) and LHW (62.9 ± 4.4 g/100 g DM), showed the highest increase in biomass CrI (52 and 50%, respectively). In addition, the IL pretreatment that showed no elimination of hemicellulose and ulvan showed a slight increase of the biomass CrI (39%) probably due to the elimination of some amorphous carbohydrates from the external surface, assuming that the IL does not reach the inner carbohydrates of the matrix due to its high viscosity [28].
On the other hand, when we only focus on the cellulose CrI, and even if we consider that any extraction protocol could theoretically affect cellulose CrI, our obtained results for different algal biomasses showed significant differences between cellulose CrI values (data not shown). These differences seem due to the origin and intrinsic organization of celluloses rather than to the effect of extraction protocol. If it was the case, we expect that CrI values will be close with a tendency to an amorphous character due essentially to NaOH action. Thus, the extraction process does not affect the crystallinity index of the extracted celluloses [4].
The cellulose purity was confirmed by infrared spectroscopy (FTIR) which showed the presence of only cellulose functional groups [4] (Fig. S1) and by acid hydrolysis followed by HPAEC-PAD analysis of sugar monomers (Table S1) that proved the release of glucose as a major component, indicating an insignificant contamination.
Enzymatic Hydrolysis of Native and Pretreated Algae
The recovered biomass after pretreatment was thoroughly washed and subjected to enzymatic hydrolysis using Trichoderma reesei cellulases in order to assess the effectiveness of each pretreatment in increasing the digestibility of Ulva lactuca polysaccharides.
The enzymatic hydrolysis experiments were realized at 50 °C and with an enzyme activity equal to 10 U/g of biomass.
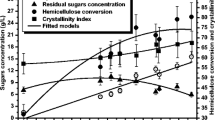
The effect of solid loading was estimated by determining the global conversion yield as showed in Fig. 3. Substrate loading was optimized for all pretreatments using 5, 10, 20, and 50% concentrations. The obtained global conversion yields showed that the LWH and organosolv pretreatments gave the best digest biomass that perfectly correlate with results of the above physicochemical study. Furthermore, it was found that the enzymatic hydrolysis with a substrate loading of 10% showed the highest yields after 72 and 120 h (Fig. 3).
To interpret more deeply, the conversion kinetics of the major carbohydrates, namely glucan and xylan, were assessed using a substrate loading of 10% for each pretreatment (Fig. 4). The evaluation of the more suitable pretreatment was also based on determining the best glucan and xylan conversion yields. These specific yields inform about digestibility of both cellulose and hemicellulose considered as a key step for the successful valorization of the whole Ulva lactuca biomass.
Figure 4 shows that all pretreatments allowed an increase of the glucan conversion compared to hydrolysis of the native Ulva lactuca. The most effective pretreatments were the LHW and the organosolv pretreatments with a yield of 97.5 and 91.3%, respectively. The highest xylan conversion yield was obtained for LHW (96.45%) and for the alkaline pretreatment (96.41%). Choi et al. (2013) reported conversion yields of 60.7% for glucan and 22.2% for xylan after high-pressure steam pretreatment of Ulva pertusa Kjellmann [37].
The LHW pretreatment presented the best conversion yields compared to the other pretreatments. A correlation with the compositional analysis indicates that the almost complete removal of ulvan (indicated by the absence of rhamnose in the remaining solid after pretreatment) allowed a better accessibility of the enzymes to the sugar polymers. These results seem quite interesting, efficient, and cost-effective for the implementation of a green algae biorefinery process seen the low costs of this pretreatment method [38].
The organosolv pretreatment showed also interesting results for the polysaccharide yield (91.2% for glucan and 82.6% for xylan). Although these yields were lower than the LHW pretreatment, the organosolv pretreatment remains a good candidate for a cost-effective process since the glucan content of the remaining solid after pretreatment was quite important (80.8%) and as the used ethanol for the pretreatment can be recycled.
The alkaline and the IL pretreatments showed a yield of 74.8 and 62.5%, respectively, after enzymatic hydrolysis. The alkaline pretreatment allowed for a respectable glucan yield in addition to a high xylan conversion (96.4%). However, the glucan yield obtained after this pretreatment is the lowest among all pretreatments. On the other hand, the IL pretreatment improved the glucan yield from 27.9 to 62.5% but it was not efficient on xylan conversion as here, the yield was lower than for the native biomass (38.7%). As discussed above, the IL eliminated the exposed hemicellulose and had no effect on the internal matrix due to its high viscosity; the internal xylan then remained intact and inaccessible for hydrolytic enzymes [28].
Comparison of Pretreatment Methods
Several criteria characterize an effective pretreatment such as minimizing energy demand, low pretreatment cost, or its inexpensive recycling [39]. In addition, the macroalgal biomass used in this work presents several advantages compared to lignocellulosic biomass. First, it represents an abundant biomass with high yields; nevertheless, it can be also cultivated without the use of arable lands. Secondly, macroalgae present a specific composition in comparison with other biomasses. This specific composition consists mainly in the absence of lignin and the more flexible structure [4].
In this study, four different pretreatment methods were compared in order to understand their impact on the chemical, thermal, structural and particularly on the enzymatic digestibility of Ulva lactuca polysaccharides. As a part of a biorefinery process, the most efficient pretreatment should remove hemicellulose and ulvan and increase the digestibility of cellulose to release fermentable sugars and should not be expensive [40].
Considering these factors, the IL pretreatment should not be considered as it is the most expensive one. In addition, it showed the lowest enzymatic conversion yield and did not eliminate ulvan and hemicellulose. The alkaline pretreatment showed a better conversion yield than the IL one and a better elimination of ulvan and hemicellulose. However, this pretreatment generates large amounts of waste water after the washing step which is considered as a limiting factor for the process efficiency.
The highest glucan yields after enzymatic hydrolysis were obtained with the LHW and the organosolv pretreatment (96.4 and 82.6%, respectively). In addition, these pretreatments showed the highest glucan content in comparison to the other pretreatments. They both represent a good perspective for the implementation of an efficient process. Although the ethanol used for the organosolv process can be recycled [41], the LHW has the advantage of using water without any chemical addition and does not require a washing step after the pretreatment which makes this pretreatment a more suitable candidate for the implementation of an efficient biorefinery process based on Ulva lactuca biomass or for the production of sugar syrup from a biomass that causes an environmental problem in the beginning.
However, the calculations of the theoretical ethanol yields per gram of raw material of Ulva lactuca considering 100% fermentation efficiency were 0.2, 1.7, 34, 22, and 7.9 g ethanol/100 g DM, respectively, for the native, alkaline, organosolv, LHW, and IL. These results showed that the organosolv pretreatment was more efficient for bioethanol production (34 g ethanol/100 g DM) than the LHW (22 g ethanol/100 g DM). The organosolv pretreatment is one of the most promising biomass pretreatments as it presents several advantages such as its minor effects on cellulose, the efficient fractionation of hemicellulose, and the organic solvent recycling [41].
A previous work by Schultz-Jensen et al. (2013) reported an ethanol yield of 11 g ethanol/100 g DM from the native Chaetomorpha linum and compared five physical pretreatments, and the highest ethanol yield was obtained for ball milling (18 h) [25]. Although that Chatomorpha linum contains four times more glucan than Ulva lactuca, this work showed that the organosolv and LHW pretreatments can provide higher ethanol yields for 100 g of biomass.
The fermentation of the monomeric sugars obtained after enzymatic hydrolysis of macroalgae is one of the most promising ways for the production of ethanol or chemicals. However, several other processes can be applied for the production of different metabolites such as sorbitol by glucose hydrogenation [42], alkyl-glucosides by transglucosylation [4], or glucose-rich syrup by concentration.
The remaining solid after the hydrolysis of sugar polymers is enriched in proteins which were not extracted or eliminated [18]. Thus, this remaining solid should be the focus of future studies for applications in fields such as food or additives [14].
Conclusion
The green macroalgae Ulva lactuca represents an abundant renewable and undervalued biomass that could be an excellent candidate for providing bioethanol. Therefore, we evaluated four pretreatments preceding enzymatic saccharification. The LHW pretreatment gave the best results in terms of glucan yield, and an efficient elimination of ulvan and hemicelluloses. Although, the organosolv pretreatment was more efficient for the ethanol production. Significant changes in both thermal and crystalline properties were observed for both pretreatments. These results indicate that the LHW and organosolv pretreatments could constitute a promising and central step in a biorefinery process based on Ulva lactuca.
Abbreviations
- [EMIM][Ac]:
-
1-ethyl-3- methylimidazolium- acetate
- CrI:
-
Crystallinity index
- DM:
-
Dry matter
- HPAEC-PAD:
-
High-performance anion exchange chromatography coupled to pulsed amperometric detection
- IL:
-
Ionic liquid
- LHW:
-
Liquid hot water
- Org:
-
Ethanol organosolv
- SEM:
-
Scanning electron microscopy
- TGA:
-
Thermogravimetric analysis
References
Suganya, T., Varman, M., Masjuki, H., & Renganathan, S. (2016). Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: a biorefinery approach. Renewable and Sustainable Energy Reviews, 55, 909–941.
Borines, M. G., de Leon, R. L., & Cuello, J. L. (2013). Bioethanol production from the macroalgae Sargassum spp. Bioresource Technology, 138, 22–29.
Binod, P., Sindhu, R., Singhania, R. R., Vikram, S., Devi, L., Nagalakshmi, S., Kurien, N., Sukumaran, R. K., & Pandey, A. (2010). Bioethanol production from rice straw: an overview. Bioresource Technology, 101, 4767–4774.
Jmel, M. A., Ben, M. G., Marzouki, M. N., Mathlouthi, M., & Smaali, I. (2016). Physico-chemical characterization and enzymatic functionalization of Enteromorpha sp. cellulose. Carbohydrate Polymers, 135, 274–279.
Ben, Y. N., Jmel, M. A., Ben, A. M., Bouallagui, H., Marzouki, M. N., & Smaali, I. (2016). A biorefinery concept using the green macroalgae Chaetomorpha linum for the coproduction of bioethanol and biogas. Energy Conversion and Management, 119, 257–265.
Subhadra, B., & Edwards, M. (2010). An integrated renewable energy park approach for algal biofuel production in United States. Energy Policy, 38, 4897–4902.
Jung, K. A., Lim, S.-R., Kim, Y., & Park, J. M. (2013). Potentials of macroalgae as feedstocks for biorefinery. Bioresource Technology, 135, 182–190.
Bharathiraja, B., Chakravarthy, M., Kumar, R. R., Yogendran, D., Yuvaraj, D., Jayamuthunagai, J., Kumar, R. P., & Palani, S. (2015). Aquatic biomass (algae) as a future feed stock for bio-refineries: a review on cultivation, processing and products. Renewable and Sustainable Energy Reviews, 47, 634–653.
Hendriks, A., & Zeeman, G. (2009). Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresource Technology, 100, 10–18.
Fox, A., Nowycky, M., & Tsien, R. (1987). Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurons. The Journal of Physiology, 394, 149.
Zhao, X., Peng, F., Cheng, K., & Liu, D. (2009). Enhancement of the enzymatic digestibility of sugarcane bagasse by alkali–peracetic acid pretreatment. Enzyme and Microbial Technology, 44, 17–23.
Da Cruz, S. H., Dien, B. S., Nichols, N. N., Saha, B. C., & Cotta, M. A. (2012). Hydrothermal pretreatment of sugarcane bagasse using response surface methodology improves digestibility and ethanol production by SSF. Journal of Industrial Microbiology & Biotechnology, 39, 439–447.
Zavrel, M., Bross, D., Funke, M., Büchs, J., & Spiess, A. C. (2009). High-throughput screening for ionic liquids dissolving (ligno-) cellulose. Bioresource Technology, 100, 2580–2587.
van der Wal, H., Sperber, B. L., Houweling-Tan, B., Bakker, R. R., Brandenburg, W., & López-Contreras, A. M. (2013). Production of acetone, butanol, and ethanol from biomass of the green seaweed Ulva lactuca. Bioresource Technology, 128, 431–437.
Kim, D.-H., Lee, S.-B., & Jeong, G.-T. (2014). Production of reducing sugar from Enteromorpha intestinalis by hydrothermal and enzymatic hydrolysis. Bioresource Technology, 161, 348–353.
Viell, J., Wulfhorst, H., Schmidt, T., Commandeur, U., Fischer, R., Spiess, A., & Marquardt, W. (2013). An efficient process for the saccharification of wood chips by combined ionic liquid pretreatment and enzymatic hydrolysis. Bioresource Technology, 146, 144–151.
Viell, J., Inouye, H., Szekely, N. K., Frielinghaus, H., Marks, C., Wang, Y., Anders, N., Spiess, A. C., & Makowski, L. (2016). Multi-scale processes of beech wood disintegration and pretreatment with 1-ethyl-3-methylimidazolium acetate/water mixtures. Biotechnology for Biofuels, 9, 7.
Resch M. G., Baker J. O., & Decker S. R. (2015). Low solids enzymatic saccharification of lignocellulosic biomass. Laboratory analytical procedure, Denver, NREL.
Van Wychen, S., & Laurens, L. (2013). Determination of total carbohydrates in algal biomass. Laboratory analytical procedure, Denver, NREL.
Anders, N., Humann, H., Langhans, B., & Spieß, A. (2015). Simultaneous determination of acid-soluble biomass-derived compounds using high performance anion exchange chromatography coupled with pulsed amperometric detection. Analytical Methods, 7, 7866–7873.
Lahaye, M., & Robic, A. (2007). Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules, 8, 1765–1774.
Bobin-Dubigeon, C., Lahaye, M., & Barry, J. L. (1997). Human colonic bacterial degradability of dietary fibres from sea-lettuce (Ulva sp). Journal of the Science of Food and Agriculture, 73, 149–159.
Fleurence, J. (1999). Seaweed proteins: biochemical, nutritional aspects and potential uses. Trends in Food Science & Technology, 10, 25–28.
Ortiz, J., Romero, N., Robert, P., Araya, J., Lopez-Hernández, J., Bozzo, C., Navarrete, E., Osorio, A., & Rios, A. (2006). Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chemistry, 99, 98–104.
Schultz-Jensen, N., Thygesen, A., Leipold, F., Thomsen, S. T., Roslander, C., Lilholt, H., & Bjerre, A. B. (2013). Pretreatment of the macroalgae Chaetomorpha linum for the production of bioethanol—comparison of five pretreatment technologies. Bioresource Technology, 140, 36–42.
Gupta, R. (2008). Alkaline pretreatment of biomass for ethanol production and understanding the factors influencing the cellulose hydrolysis, ProQuest2008.
Robic, A., Gaillard, C., Sassi, J. F., Lerat, Y., & Lahaye, M. (2009). Ultrastructure of ulvan: a polysaccharide from green seaweeds. Biopolymers, 91, 652–664.
Ravanal, M. C., Pezoa-Conte, R., von Schoultz, S., Hemming, J., Salazar, O., Anugwom, I., Jogunola, O., Mäki-Arvela, P., Willför, S., & Mikkola, J.-P. (2016). Comparison of different types of pretreatment and enzymatic saccharification of Macrocystis pyrifera for the production of biofuel. Algal Research, 13, 141–147.
Pezoa-Conte, R., Leyton, A., Anugwom, I., von Schoultz, S., Paranko, J., Mäki-Arvela, P., Willför, S., Muszyński, M., Nowicki, J., & Lienqueo, M. (2015). Deconstruction of the green alga Ulva rigida in ionic liquids: closing the mass balance. Algal Research, 12, 262–273.
Wendler, F., Meister, F., Wawro, D., Wesolowska, E., Ciechanska, D., Saake, B., Puls, J., Le Moigne, N., & Navard, P. (2010). Polysaccharide blend fibres formed from NaOH, N-methylmorpholine-N-oxide and 1-ethyl-3-methylimidazolium acetate. Fibres and Textiles in Eastern Europe, 18, 21–30.
Alves, A., Caridade, S. G., Mano, J. F., Sousa, R. A., & Reis, R. L. (2010). Extraction and physico-chemical characterization of a versatile biodegradable polysaccharide obtained from green algae. Carbohydrate Research, 345, 2194–2200.
White, J. E., Catallo, W. J., & Legendre, B. L. (2011). Biomass pyrolysis kinetics: a comparative critical review with relevant agricultural residue case studies. Journal of Analytical and Applied Pyrolysis, 91, 1–33.
Chen, W.-T., Ma, J., Zhang, Y., Gai, C., & Qian, W. (2014). Physical pretreatments of wastewater algae to reduce ash content and improve thermal decomposition characteristics. Bioresource Technology, 169, 816–820.
Liu, H.-M., Li, M.-F., Yang, S., & Sun, R.-C. (2013). Understanding the mechanism of cypress liquefaction in hot-compressed water through characterization of solid residues. Energies, 6, 1590–1603.
Chen, L., Li, J., Lu, M., Guo, X., Zhang, H., & Han, L. (2016). Integrated chemical and multi-scale structural analyses for the processes of acid pretreatment and enzymatic hydrolysis of corn stover. Carbohydrate Polymers, 141, 1–9.
Li, M.-C., Wu, Q., Song, K., Lee, S., Qing, Y., & Wu, Y. (2015). Cellulose nanoparticles: structure–morphology–rheology relationships. ACS Sustainable Chemistry & Engineering, 3, 821–832.
Choi, W.-Y., Kang, D.-H., & Lee, H.-Y. (2013). Enhancement of the saccharification yields of Ulva pertusa kjellmann and rape stems by the high-pressure steam pretreatment process. Biotechnology and Bioprocess Engineering, 18, 728–735.
Wan, C., Zhou, Y., & Li, Y. (2011). Liquid hot water and alkaline pretreatment of soybean straw for improving cellulose digestibility. Bioresource Technology, 102, 6254–6259.
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y. Y., Holtzapple, M., & Ladisch, M. (2005). Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresource Technology, 96, 673–686.
Behera, S., Arora, R., Nandhagopal, N., & Kumar, S. (2014). Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renewable and Sustainable Energy Reviews, 36, 91–106.
Salapa, I., Katsimpouras, C., Topakas, E., & Sidiras, D. (2017). Organosolv pretreatment of wheat straw for efficient ethanol production using various solvents. Biomass and Bioenergy, 100, 10–16.
Romero, A., Alonso, E., Sastre, A., & Nieto-Marquez, A. (2016). Conversion of biomass into sorbitol: cellulose hydrolysis on MCM-48 and d-Glucose hydrogenation on Ru/MCM-48. Microporous and Mesoporous Materials, 224, 1–8.
Acknowledgements
This work was carried out as a part of the ALGAEVAL project (TUNGER – 72) jointly funded by the German Federal Ministry of Education and Research (BMBF) and the Tunisian Ministry for Higher Education and Scientific Research (MESRS).
Part of this work was performed as part of the Cluster of Excellence “Tailor-Made Fuels from Biomass,” which is funded by the Excellence Initiative of the German federal and state governments to promote science and research at German universities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
ESM 1
(DOCX 56 kb)
Rights and permissions
About this article
Cite this article
Jmel, M.A., Anders, N., Yahmed, N.B. et al. Variations in Physicochemical Properties and Bioconversion Efficiency of Ulva lactuca Polysaccharides After Different Biomass Pretreatment Techniques. Appl Biochem Biotechnol 184, 777–793 (2018). https://doi.org/10.1007/s12010-017-2588-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2588-z