Abstract
The pretreatment of plant biomass negatively impacts the economics of many bioenergy and bioproduct processes due to the thermochemical requirements for deconstruction of lignocelluluose. An effective strategy to reduce these severity requirements is to pretreat the biomass with white-rot fungi, such as Trametes versicolor, which have the innate ability to deconstruct lignocellulose with a suite of specialized enzymes. In the present study, the effects of 12 weeks of pretreatment with a wild-type strain (52J) and a cellobiose dehydrogenase-deficient strain (m4D) of T. versicolor on hardwood and Miscanthus were explored. Both strains of T. versicolor led to significant decreases of insoluble lignin and significant increases of soluble lignin after acid hydrolysis, which suggests improved lignin extractability. The glucose yields after saccharification using an enzyme cocktail containing chitinase were similar or significantly higher with 52J-treated biomass compared to untreated hardwood and Miscanthus, respectively. The fungal treated biomass, regardless of the strain used, also showed significant increases in energy content and compressive strength of pellets. Overall, the use of T. versicolor as a pretreatment agent for hardwood and Miscanthus could be an environmentally friendly strategy for conversion technologies that require delignification and saccharification, and/or processes that require densification and transport.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the widespread availability of lignocellulose, efficient and economical conversion of lignocellulosic biomass to biofuels is impeded by the chemical recalcitrance of the material. Within lignocellulose, the hemicellulose and lignin fractions act as a substantial chemical barrier to the glucose-rich cellulose polymer. Thus, enzyme penetration to the cellulose that would convert the carbohydrate polymer into fermentable glucose monomers is challenging. This inability to efficiently hydrolyze lignocellulose has been the impetus to investigate pretreatment strategies that deconstruct lignocellulose into its various constituents, thereby allowing access to the energy-rich cellulose for biofuel production.
The leading pretreatment systems involve various thermochemical or thermo-mechanical processes, such as steam explosion and ammonia fiber expansion [1, 2]. Currently, many of these pretreatment processes involve expensive corrosion-resistant reactors, produce large amounts of hazardous waste, and require extensive washing of treated solids in order to remove compounds that are inhibitory to ethanol-fermenting microorganisms [3]. Consequently, pretreatment typically adds significant costs to lignocellulosic biofuel production strategies, which can have negative economic consequences at commercialized scales [2, 3]. Therefore, inexpensive and environmentally friendly pretreatment alternatives are desirable in order to lower the cost of biofuel production and minimize negative environmental impacts.
A potential alternative to these traditional pretreatment strategies is biological pretreatment, which can mitigate the cost associated with and severity of the current thermochemical and thermo-mechanical processes due to the ability of various microbial species to deconstruct the recalcitrant components of lignocellulosic biomass [4,5,6]. Microorganisms, including white-, brown-, and soft-rot fungi, and some ruminant bacteria, have the innate ability to deconstruct lignocellulosic biomass by using a suite of specialized enzymes as a means to access and utilize cellulose as a carbon source [7,8,9,10]. As a result, much research has been dedicated to identifying these organisms, understanding their deconstruction processes, and demonstrating their ability to deconstruct lignocellulose from a variety of substrates [11, 12].
An additional limitation to the use of low-density lignocellulosic feedstocks (e.g., Miscanthus) is the efficient transportation of the material. To minimize transportation costs, the material may need to be compacted into briquettes or pellets [13, 14]. However, these densification strategies also have the potential to negatively impact the economics of bioenergy and bioproduct strategies because of the additional energy (e.g., heat) and chemical (e.g., binding agent) requirements [14]. As a result, alternative methods for compaction have been investigated in the context of direct combustion processes like gasification or pyrolysis [15], and for lignocellulosic ethanol production [7].
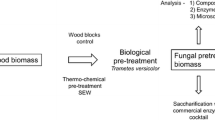
The present study explored the efficacy of a white-rot fungus, Trametes versicolor, as a biological pretreatment agent for hardwood (oak/ash blend) and Miscanthus, which are two biomass feedstocks that may have contributed significantly to the biomass portfolio in temperate climates. The effects of wild-type (52J) and mutant (m4D) strains of T. versicolor after 12 weeks of pretreatment with these feedstocks were explored to assess the feasibility of using T. versicolor as a biological pretreatment in the context of conversion and densification strategies. Specifically, the lignocellulosic chemistry of both biomass types was assessed, along with enzymatic saccharification efficiency, pellet strength, and overall energy content.
Methods
Biomass Sources
The hardwood mixture was an approximately equal mixture of ash and oak, sourced from Foster Brothers Wood Products (Auxvasse, MO). The particle size of the wood chips ranged from 1.5 to 4 cm in length and width. The Miscanthus (Miscanthus × giganteus cv. Illinois) was grown at a farm near Pesotum, IL, and chopped into 2-cm segments during harvest at the end of the third growing season. All biomass was stored at ambient temperature and humidity prior to inoculation.
Fungal Growth and Biomass Inoculation
Trametes versicolor wild-type strain (52J; ATCC 20869) and the cellobiose dehydrogenase-deficient strain (m4D) [16] were maintained with weekly subculturing at 25 °C in Petri plates (100 × 15 mm) containing potato dextrose agar (PDA) supplemented with an additional 7.5 g L−1. After 1 week of growth, the fungi were removed from the PDA plates by scraping with a spatula. Liquid cultures were prepared by adding the collected fungi to malt extract broth (MEB), at a ratio of 10 Petri plates of fungi per 1 L of broth. The mixtures were then blended with a DeLonghi DHB716 hand mixer using several short bursts of 2–3 s each. The blended mixture was then added to an additional 3 L of MEB to create a final 4-L volume. The mixture was incubated at 25 °C for 4 days at 225 rpm in an autoclaved 14-L bucket with a lid loosely attached. After incubation, the fungi were filtered (75 μm mesh), rinsed with deionized water to remove residual MEB, and resuspended in 4 L of deionized water.
For each replicate, 1 L (dry volume) of wood chips or Miscanthus straw was heated at 121 °C for 35 min using an autoclave. The biomass was placed into ethanol-sterilized 3-L polypropylene containers, which had predrilled 0.5-cm holes on opposite sides that were covered with micropore tape for gas exchange. Each container with biomass was inoculated with 100 mL of the T. versicolor suspension. The biomass and inoculum were mixed by shaking with a sealed lid, and incubated at 25 °C for 12 weeks.
Acid Hydrolysis
The fungal treated and untreated biomass was milled to pass a 20-mesh sieve (0.85 mm) using a Thomas Wiley® Mini-Mill (Thomas Scientific, Swedesboro, NJ) and dried at 105 °C for 16 h. The dried material was hydrolyzed using acid hydrolysis according to Sluiter et al. [17]. The carbohydrate content of the hydrolysates was quantified using a System Gold 166 HPLC (Beckman Coulter, Brea, CA) and an Aminex HPX-87P (300 mm × 7.8 mm) column (Bio-Rad, Hercules, CA) at 85 °C. The mobile phase was 100% water at a flow rate of 0.6 mL min−1. Sugars were detected using an RI-1530 refractive index detector (JASCO, Easton, MD). Insoluble lignin and soluble lignin were determined according to Sluiter et al. [17], with an absorbance wavelength of 240 nm and absorptivity constant of 25 L g−1 cm−1 used for the determination of soluble lignin.
Thioacidolysis
Lignin moieties were extracted from dried and milled biomass (described in the “Acid Hydrolysis” section) using a microscale thioacidolysis procedure [18]. After extraction, 100 μL of the lignin moieties (in methylene chloride) was added to 100 μL of pyridine and 500 μL of N,O-bis(trimethylsilyl)trifluoroacetamide and trimethylchlorosilane (99:1 v/v) for derivitization. The methyl silylated lignin moieties were quantified using a Trace 1310 GC (Thermo Scientific, Waltham, MA) equipped with a flame ionization detector and a TG-5MS (30 m × 0.25 mm × 0.25 μm) column (Thermo Scientific). The GC oven settings were 130 °C for 3 min, 3 °C min−1 to 250 °C, and 5 min hold. The inlet and detector were set at 250 °C with a carrier gas (nitrogen) flow of 1.3 mL min−1. Samples were injected at 2 μL under splitless conditions. Lignin peaks were identified as per Robinson and Mansfield [18].
Saccharification
A laboratory-scale organosolv technique was used to delignify the fungal treated and untreated biomass according to previously established methods [16, 19]. Briefly, 250 mg of milled material (0.85 mm) and 5 mL of 60% ethanol acidified with sulfuric acid (pH 3.4) were added to a stainless steel 23-mL acid digestion bomb (Parr Instruments, Moline, IL) and heated for 30 min in Ucon HTF 14 oil (Sigma-Aldrich, St. Louis, MO). The liquid fraction was separated from the insoluble, carbohydrate-rich material under a vacuum using a coarse (40–60 μm) glass frit filter. The solid fraction was rinsed with 100 mL of water and then resuspended in a total of 20 mL of citrate buffer (25 mM; pH 4.8) containing tetracycline at 10 μg mL−1. Celluclast 1.5 L (C2730, Sigma-Aldrich) and Novozyme 188 (C6105, Sigma-Aldrich) were added to the citrate solution at final concentrations of 67 and 1 FPU g−1 of initial biomass, respectively, with or without Glucanex (L1412, Sigma-Aldrich) at 1 mg mL−1. The suspensions were incubated at 50 °C for 3 days at 80 rpm. The glucose content of the supernatant from the enzymatic digest and the liquid fraction from organosolv were assessed for soluble carbohydrates by HPLC analysis as described in the “Acid Hydrolysis” section.
Compressive Strength
For the determination of compressive strength, a disc was created from 6 g of milled (0.85 mm) biomass under 1600 N of force (5 s hold) using a SimpliMet 2 mounting press with a 30-mm die (Buehler, Lake Bluff, IL) as previously described [20]. The resulting discs were approximately 30 mm in diameter and 10 mm thick. Each disc was placed on the short edge on a 60-mm platen that was mounted to a vertical wheel test stand (HV-110; Imada, Inc., Northbrook, IL), and compressed with a DS2 digital force gauge (Imada, Inc.) until the disc failed. The peak force required to break the disc (compressive strength) was recorded from the digital force gauge.
Energy Content
The energy content of biomass was assessed using a 6200 Isoperibol Calorimeter (Parr Instrument Company, Moline, IL) according to the manufacturer’s instructions. The higher heating value (HHV) was determined from 1 g of milled (0.85 mm) biomass.
Statistical Analyses
All data were subjected to one-way ANOVA followed by Tukey’s pairwise comparisons at the 95% level using Origin 8.1 (OriginLab, Northampton, MA). Means are reported with standard error of the mean.
Results and Discussion
Fungal Growth
The wild-type (52J) and cellobiose dehydrogenase mutant (m4D) [21] strains of T. versicolor were able to establish growth on both the hardwood chips and Miscanthus during the 12 weeks of pretreatment. After 12 weeks, the fungal mycelia had effectively covered the exposed surfaces of both types of biomass. As observed with previous research [16], the m4D strain, which is unable to effectively catabolize cellulose as a carbon source [21], had visibly less growth on each substrate than the wild-type strain (52J). Presumably, this reduced fungal growth of the m4D strain is a result of the reduced amount of carbohydrate available to this strain as compared to the 52J strain [16]. The m4D strain was explored in the present study because of the potential for this fungus to lead to improved delignification of biomass without appreciable loss of glucose through cellulolytic activity.
Carbohydrate Content
In the context of liquid biofuels, particularly ethanol, the soluble carbohydrate content of biomass is a critical parameter. Accordingly, the five primary carbohydrates of lignocellulose were examined using acid hydrolysis (Fig. 1). The glucose content of the untreated hardwood mixture and Miscanthus was 57.4 ± 1.6 and 35.6 ± 2.0%, respectively. With both 52J and m4D pretreatment, the level of glucose resulting from acid hydrolysis generally increased in both the hardwood and Miscanthus samples, with m4D leading to statistically higher glucose release than untreated biomass (Fig. 1). This is in contrast to the results from an earlier study with canola straw, where treatment with 52J for 12 weeks led to a significant reduction in glucose released after acid hydrolysis [16]. In that same study, biomass treated with m4D led to a similar glucose release compared to untreated material. The increase in glucose released by acid hydrolysis from the present study may be due to an increase in accessibility of the sugar as a result of changes to lignin structure (described in the “Lignin Analysis” section) and/or a contribution of glucose from the fungus itself during acid hydrolysis of the material.
The sugar content after acid hydrolysis of milled oak/ash hardwood mix (HW) and Miscanthus (Mxg) after no fungal treatment (light gray) or treatment with Trametes versicolor wild-type strain (52J; gray) or CDH-deficient strain (m4D; dark gray) for 12 weeks. Letter differences within biomass and sugar type indicate significant differences at the 95% level
The second most abundant sugar released from most lignocellulose substrates is xylose, which has been shown to be an effective substrate for fermentation [22, 23]. In the current study, the xylose content generally decreased in biomass after treatment with 52J and m4D; however, there were no statistical differences with xylose content between fungal pretreated and untreated biomass. The more prominent decrease in xylose with 52J-treated material (particularly with Miscanthus) may be due to the more aggressive growth observed with this wild-type strain, which would require more carbohydrates than the m4D strain.
Arabinose is typically the third most abundant structural carbohydrate in plant biomass. In the present study, untreated hardwood had 2.6 ± 0.1% arabinose, while untreated Miscanthus had 10.0 ± 0.5%. The relatively high proportion of arabinose in Miscanthus was expected due to the prominence of this sugar in the cell wall of most grasses. With both biomass types, 52J and m4D treatments led to decreased arabinose, with significantly less from Miscanthus. It is probable that the fungi were metabolizing this sugar in both cases, with a bigger decrease with 52J attributed to the more vigorous growth on the substrate with this strain as compared to the m4D strain.
Mannose and galactose are two additional, commonly measured sugars in the structural cell wall of plant biomass, although at relatively minor percentages (<2% of dry weight in the present study). In hardwoods, the percentage of mannose and galactose had similar trends as arabinose, with a general reduction in these sugars as a result of fungal pretreatment, which is likely the result of catabolism by the fungal strains. With treated Miscanthus, mannose levels were significantly reduced in fungal treated biomass compared to untreated material, which may suggest that these sugars were readily accessible to the fungus. Nevertheless, the changes to these minor carbohydrates are unlikely to impact saccharification-based processes due to the relatively low levels of these sugars and the preference of fermentation strategies for glucose and xylose.
Lignin Analysis
White-rot fungi are equipped with a variety of enzymes (e.g., oxidases) that allow these organisms to deconstruct lignocellulose as means to reach the carbohydrates necessary for survival [11, 24]. A common technique for assessing changes to the covalent linkages of lignin is thioacidolysis, which utilizes a thiol reagent to liberate individual lignin moieties that make the lignin polymer, such as syringyl (S) and guaiacyl (G). The ratio of S to G determined using this technique is often used as a descriptor of the chemical structure of lignin [25, 26]. In the present study, the S to G ratio of the untreated hardwood mix was approximately 2:1, which agrees with previous studies using this same technique on hardwoods [18]. Treatment with 52J led to a significant decrease in the S to G ratio, while m4D led to a significant increase in the S to G ratio compared to untreated hardwood (Table 1). This overall pattern was observed previously with fungal treatment of canola straw using these same fungal strains [16]. With Miscanthus, the untreated S to G ratio was 0.8:1, which is in agreement with previous studies that found higher levels of G lignin moieties than S in Miscanthus [27]. As with the hardwood, the 52J strain on Miscanthus led to a significant reduction in the S to G ratio; however, m4D led a ratio that was not statistically different than the untreated biomass.
Lignin content was also examined with an acid hydrolysis method [17]. Using this technique, the insoluble lignin was significantly reduced with fungal treatment of the hardwood mix (Table 1). This may indicate that the fungal pretreatment allowed more lignin to be solubilized by sulfuric acid treatment. The significant increase in soluble lignin from these same samples supports this hypothesis (Table 1). With Miscanthus, 52J led to the same pattern as observed with the hardwood; however, m4D on Miscanthus led to insoluble and soluble lignin values that were not significantly different than untreated biomass. This is in agreement with unchanged S to G ratios in m4D-treated Miscanthus (Table 1).
While it is difficult to ascertain the underlying mechanisms responsible for the difference between S to G ratios resulting from treatment with 52J and m4D, it is clear from this study and Canam et al. [16] that the two strains of T. versicolor are deconstructing and/or altering the lignin structure differently. The primary difference between these two strains at the genetic and biochemical level is the lack of cellobiose dehydrogenase (CDH) expression in m4D compared to 52J [21]. This enzyme has been shown to be involved in cellulose catabolism [28], but it has also been proposed to be involved with lignin deconstruction, for example, by mediating Fenton’s chemistry leading to secondary reactions resulting in lignin degradation [29]. Previous work with m4D has shown that the mutant shows a greatly decreased ability to penetrate hardwood, but its ability to degrade modified Kraft lignin is intact [21]. The current study may provide evidence for CDH’s role in delignification, although the differences in lignin ratios observed may be partly or entirely a secondary effect as a result of differences to carbohydrate deconstruction and catabolism.
Delignification and Enzymatic Saccharification
To assess the effects of altered carbohydrate and lignin chemistry in the treated material on bioenergy strategies, enzymatic saccharification was undertaken. Separation of the cellulosic material from lignin was conducted using an organosolv (acidified ethanol) technique followed by enzymatic digestion of the resulting biomass using Celluclast and Novozyme 188 with or without Glucanex. With the hardwood mix, the glucose released after saccharification without Glucanex was significantly reduced with fungal treated material compared to untreated (Fig. 2). However, digests that also contained Glucanex showed no significant difference compared to the untreated material. Miscanthus that was treated with the wild-type strain of T. versicolor (52J) led to glucose release in the absence of Glucanex that was not significantly different than untreated material, yet the presence of Glucanex in the enzyme cocktail led to a significant increase when compared to untreated material under the same conditions. By comparison, Miscanthus that was treated with m4D led to significantly less glucose released after saccharification either with or without Glucanex, although the addition of Glucanex to the enzyme cocktail did increase the overall yield of glucose when compared to untreated material (Fig. 2).
Glucose released during enzymatic saccharification without Glucanex (−GLX) or with Glucanex (+GLX) of milled oak/ash hardwood mix (HW) and Miscanthus (Mxg) after no fungal treatment (light gray) or treatment with Trametes versicolor wild-type strain (52J; gray) or CDH-deficient strain (m4D; dark gray) for 12 weeks. Letter differences within biomass type and enzyme cocktail type indicate significant differences at the 95% level
It was assumed that the liquid fraction from organosolv treatment, which typically has high levels of hemicellulose sugars [30], was enriched with glucose in the fungal treated samples, which would explain the reduced glucose from enzymatic saccharification in the absence of Glucanex. However, HPLC analysis of the organosolv liquid fraction confirmed that high levels of glucose were not present (data not shown). Another possible explanation for the reduced glucose levels after saccharification without Glucanex is that the combination of fungal treatment and organosolv leads to inhibitors of one or both of the enzymes used for saccharification.
Compressive Strength and Energy Content
A key component to bioenergy and biomaterial supply chains is transport, which often requires densification of biomass as a means to reduce shipping volume and minimize transportation costs. However, densification of some forms of biomass (e.g., grasses) may require heat and/or binding agents [14], which leads to additional energy input and expense. To this end, the pelletability (without added heat or binding agents) of the treated biomass was assessed and compared to untreated material. In all cases, the fungal treated biomass led to significantly stronger pellets (as measured by compressive strength) than untreated biomass (Fig. 3). The wild-type T. versicolor treatment (52J) led to hardwood pellets and Miscanthus pellets that were 5.4× and 6.5× stronger, respectively, than pellets formed from untreated material. Increases in strength were also observed with m4D-treated material, but the increases were less substantial at 1.5× and 2.4× for hardwood and Miscanthus, respectively. The difference in pellet strength between m4D and 52J was significant, and it is not clear if more vigorous growth (i.e., more fungal mass per sample) by the 52J strain is entirely responsible, or if differences in lignin structure, as a result of the fungus, leads to altered chemical adhesion under compression.
The compressive strength of pellets made from milled oak/ash hardwood mix (HW) and Miscanthus (Mxg) after no fungal treatment (untreated) or treatment with Trametes versicolor wild-type strain (52J) or CDH-deficient strain (m4D) for 12 weeks. Letter differences within biomass type indicate significant differences at the 95% level
The energy content of biomass is a valuable parameter for a variety of bioenergy strategies, such as direct combustion and gasification. Accordingly, the energy content of untreated and treated biomass in the present study was assessed using bomb calorimetry. The untreated hardwood mixture in this study had an energy content (HHV) of 18.9 ± 0.2 MJ kg−1, which is in agreement with previous studies of hardwoods [31, 32]. Similarly, the energy content determined for untreated Miscanthus (17.3 ± 0.1 MJ kg−1) was comparable to previous studies with this feedstock [33, 34]. With both biomass types, treatment with T. versicolor led to significant increases in energy content regardless of the strain examined (Fig. 4). Although the overall increases to energy content with the treated biomass are minor relative to untreated biomass (∼2–4%), it is noteworthy that treatment of the biomass with T. versicolor did not negatively affect the energy content. This is particularly important for bioenergy strategies that involve direct combustion of densified biomass. Therefore, the use of T. versicolor as a pretreatment agent may be a viable option for direct combustion simply due to the increased compressive strength of the material.
The energy content of milled oak/ash hardwood mix (HW) and Miscanthus (Mxg) after no fungal treatment (untreated) or treatment with Trametes versicolor wild-type strain (52J) or CDH-deficient strain (m4D) for 12 weeks. Letter differences within biomass type indicate significant differences at the 95% level
Conclusions
The use of the CDH-deficient strain (m4D) did not appear to offer a significant advantage over the wild-type strain (52J) of T. versicolor with respect to the parameters examined in the current study with either biomass type, despite previous findings with canola straw [16]. However, the data suggest that hardwood and Miscanthus treated with the wild-type strain (52J) leads to biomass that has improved lignin extractability, compressive strength, and energy content, along with similar or enhanced saccharification yield (with the addition of Glucanex) compared to untreated biomass. Conditioning hardwood and Miscanthus with T. versicolor should therefore be considered as an inexpensive and environmentally friendly strategy for enhancing the physicochemical properties of the biomass for bioenergy applications.
References
Elgharbawy, A. A., Alam, M. Z., Moniruzzaman, M., & Goto, M. (2016). Ionic liquid pretreatment as emerging approaches for enhanced enzymatic hydrolysis of lignocellulosic biomass. Biochemical Engineering Journal, 109, 252–267.
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y. Y., Holtzapple, M., & Ladisch, M. (2005). Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresource Technology, 96, 673–686.
Wan, C., & Li, Y. (2012). Fungal pretreatment of lignocellulosic biomass. Biotechnology Advances, 30, 1447–1457.
Anderson, W. F., & Akin, D. E. (2008). Structural and chemical properties of grass lignocelluloses related to conversion for biofuels. Journal of Industrial Microbiology & Biotechnology, 35, 355–366.
Ray, M. J., Leak, D. J., Spanu, P. D., & Murphy, R. J. (2010). Brown rot fungal early stage decay mechanism as a biological pretreatment for softwood biomass in biofuel production. Biomass and Bioenergy, 34, 1257–1262.
Sindhu, R., Binod, P., & Pandey, A. (2016). Biological pretreatment of lignocellulosic biomass—an overview. Bioresource Technology, 199, 76–82.
Canam, T., Town, J., Iroba, K., Tabil, L., Dumonceaux, T.J. (2013) In: A.K. Chandel, S.S. da Silva (Eds.), Pretreatment of lignocellulosic biomass using microorganisms: approaches, advantages, and limitations, sustainable degradation of lignocellulosic biomass—techniques, applications and commercialization, InTech.
Floudas, D., Binder, M., Riley, R., Barry, K., Blanchette, R. A., Henrissat, B., Martinez, A. T., Otillar, R., Spatafora, J. W., Yadav, J. S., Aerts, A., Benoit, I., Boyd, A., Carlson, A., Copeland, A., Coutinho, P. M., de Vries, R. P., Ferreira, P., Findley, K., Foster, B., Gaskell, J., Glotzer, D., Gorecki, P., Heitman, J., Hesse, C., Hori, C., Igarashi, K., Jurgens, J. A., Kallen, N., Kersten, P., Kohler, A., Kues, U., Kumar, T. K. A., Kuo, A., LaButti, K., Larrondo, L. F., Lindquist, E., Ling, A., Lombard, V., Lucas, S., Lundell, T., Martin, R., McLaughlin, D. J., Morgenstern, I., Morin, E., Murat, C., Nagy, L. G., Nolan, M., Ohm, R. A., Patyshakuliyeva, A., Rokas, A., Ruiz-Duenas, F. J., Sabat, G., Salamov, A., Samejima, M., Schmutz, J., Slot, J. C., St John, F., Stenlid, J., Sun, H., Sun, S., Syed, K., Tsang, A., Wiebenga, A., Young, D., Pisabarro, A., Eastwood, D. C., Martin, F., Cullen, D., Grigoriev, I. V., & Hibbett, D. S. (2012). The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science, 336, 1715–1719.
Hess, M., Sczyrba, A., Egan, R., Kim, T. W., Chokhawala, H., Schroth, G., Luo, S., Clark, D. S., Chen, F., Zhang, T., Mackie, R. I., Pennacchio, L. A., Tringe, S. G., Visel, A., Woyke, T., Wang, Z., & Rubin, E. M. (2011). Metagenomic discovery of biomass-degrading genes and genomes from cow rumen. Science, 331, 463–467.
Hittinger, C. T. (2012). Evolution. Endless rots most beautiful. Science, 336, 1649–1650.
MacDonald, J., Doering, M., Canam, T., Gong, Y., Guttman, D. S., Campbell, M. M., & Master, E. R. (2011). Transcriptomic responses of the softwood-degrading white-rot fungus Phanerochaete carnosa during growth on coniferous and deciduous wood. Applied and Environmental Microbiology, 77, 3211–3218.
Martinez, D., Berka, R. M., Henrissat, B., Saloheimo, M., Arvas, M., Baker, S. E., Chapman, J., Chertkov, O., Coutinho, P. M., Cullen, D., Danchin, E. G. J., Grigoriev, I. V., Harris, P., Jackson, M., Kubicek, C. P., Han, C. S., Ho, I., Larrondo, L. F., de Leon, A. L., Magnuson, J. K., Merino, S., Misra, M., Nelson, B., Putnam, N., Robbertse, B., Salamov, A. A., Schmoll, M., Terry, A., Thayer, N., Westerholm-Parvinen, A., Schoch, C. L., Yao, J., Barabote, R., Nelson, M. A., Detter, C., Bruce, D., Kuske, C. R., Xie, G., Richardson, P., Rokhsar, D. S., Lucas, S. M., Rubin, E. M., Dunn-Coleman, N., Ward, M., & Brettin, T. S. (2008). Genome sequencing and analysis of the biomass-degrading fungus Trichoderma reesei (syn. Hypocrea jecorina). Nature Biotechnology, 26, 553–560.
Gonzales, D., Searcy, E., & Eksioglu, S. (2013). Cost analysis for high-volume and long-haul transportation of densified biomass feedstock. Transportation Research Part A: Policy and Practice, 49, 48–61.
Kaliyan, N., & Morey, V. (2009). Factors affecting strength and durability of densified biomass products. Biomass and Bioenergy, 33, 337–359.
Biswas, A. K., Rudolfsson, M., Brostrom, M., & Umeki, K. (2014). Effect of pelletizing conditions on combustion behaviour of single wood pellet. Applied Energy, 119, 79–84.
Canam, T., Town, J. R., Tsang, A., McAllister, T. A., & Dumonceaux, T. J. (2011). Biological pretreatment with a cellobiose dehydrogenase-deficient strain of Trametes versicolor enhances the biofuel potential of canola straw. Bioresource Technology, 102, 10020–10027.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., Crocker, D. (2008) Determination of structural carbohydrates and lignin in biomass, NREL/TP-510-42618.
Robinson, A. R., & Mansfield, S. D. (2009). Rapid analysis of poplar lignin monomer composition by a streamlined thioacidolysis procedure and near-infrared reflectance-based prediction modeling. The Plant Journal, 58, 706–714.
Pan, X., Xie, D., Kang, K. Y., Yoon, S. L., & Saddler, J. N. (2007). Effect of organosolv ethanol pretreatment variables on physical characteristics of hybrid poplar substrates. Applied Biochemistry and Biotechnology, 137–140, 367–377.
Thapa, S., Johnson, D. B., Liu, P. P., & Canam, T. (2014). Algal biomass as a binding agent for the densification of Miscanthus. Waste and Biomass Valorization, 6, 91–95.
Dumonceaux, T., Bartholomew, K., Valeanu, L., Charles, T., & Archibald, F. (2001). Cellobiose dehydrogenase is essential for wood invasion and nonessential for kraft pulp delignification by Trametes versicolor. Enzyme and Microbial Technology, 29, 478–489.
Casey, E., Mosier, N. S., Adamec, J., Stockdale, Z., Ho, N., & Sedlak, M. (2013). Effect of salts on the co-fermentation of glucose and xylose by a genetically engineered strain of Saccharomyces cerevisiae. Biotechnology for Biofuels, 6, 83.
Ha, S. J., Galazka, J. M., Kim, S. R., Choi, J. H., Yang, X., Seo, J. H., Glass, N. L., Cate, J. H. D., & Jin, Y. S. (2011). Engineered Saccharomyces cerevisiae capable of simultaneous cellobiose and xylose fermentation. Proceedings of the National Academy of Sciences of the United States of America, 108, 504–509.
Wymelenberg, A. V., Gaskell, J., Mozuch, M., Kersten, P., Sabat, G., Martinez, D., & Cullen, D. (2009). Transcriptome and secretome analyses of Phanerochaete chrysosporium reveal complex patterns of gene expression. Applied and Environmental Microbiology, 75, 4058–4068.
Huntley, S. K., Ellis, D., Gilbert, M., Chapple, C., & Mansfield, S. D. (2003). Significant increases in pulping efficiency in C4H-F5H-transformed poplars: Improved chemical savings and reduced environmental toxins. Journal of Agricultural and Food Chemistry, 51, 6178–6183.
Nunes, C. A., Lima, C. F., Barbosa, L. C. A., Colodette, J. L., Gouveia, A. F. G., & Silverio, F. O. (2010). Determination of eucalyptus spp lignin S/G ratio: a comparison between methods. Bioresource Technology, 101, 4056–4061.
Liu, Z., Padmanabhan, S., Cheng, K., Schwyter, P., Pauly, M., Bell, A. T., & Prausnitz, J. M. (2013). Aqueous-ammonia delignification of miscanthus followed by enzymatic hydrolysis to sugars. Bioresource Technology, 135, 23–29.
Phillips, C. M., Beeson, W. T., Cate, J. H., & Marletta, M. A. (2011). Cellobiose dehydrogenase and a copper-dependent polysaccharide monooxygenase potentiate cellulose degradation by Neurospora crassa. ACS Chemical Biology, 6, 1399–1406.
Hammel, K. E., Kapich, A. N., Jensen Jr., K. A., & Ryan, Z. C. (2002). Reactive oxygen species as agents of wood decay by fungi. Enzyme and Microbial Technology, 30, 445–453.
Zhao, X., & Liu, D. (2009). Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Applied Microbiology and Biotechnology, 82, 815–827.
Jenkins, B. M., Baxter, L. L., Miles Jr., T. R., & Miles, T. R. (1998). Combustion properties of biomass. Fuel Processing Technology, 54, 17–46.
Fang, S., Liu, Z., Cao, Y., Liu, D., Yu, M., & Tang, L. (2011). Sprout development, biomass accumulation and fuelwood characteristics from coppiced plantations of Quercus acutissima. Biomass and Bioenergy, 35, 3104–3114.
Burner, D. M., Tew, T. L., Harvey, J. J., & Belesky, D. P. (2009). Dry matter partitioning and quality of Miscanthus, Panicum, and Saccharum genotypes in Arkansas, USA. Biomass and Bioenergy, 33, 610–619.
Jeguirim, M., Dorge, S., & Trouve, G. (2010). Thermogravimetric analysis and emission characteristics of two energy crops in air atmosphere: Arundo donax and Miscanthus giganthus. Bioresource Technology, 101, 788–793.
Acknowledgements
This research was funded by a grant awarded to T.C. (SU835708) from the People, Prosperity and the Planet (P3) program of the US Environmental Protection Agency, along with internal research grants from Eastern Illinois University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalinoski, R.M., Flores, H.D., Thapa, S. et al. Pretreatment of Hardwood and Miscanthus with Trametes versicolor for Bioenergy Conversion and Densification Strategies. Appl Biochem Biotechnol 183, 1401–1413 (2017). https://doi.org/10.1007/s12010-017-2507-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2507-3