Abstract
Corn stover was treated using low-moisture anhydrous ammonia (LMAA) at controlled ammoniation temperature. Moisturized corn stover (50 % moisture) was contacted with anhydrous ammonia (0.1 g NH3/g-biomass) in a batch reactor at various temperatures (ambient to 150 °C). After ammoniation at elevated and controlled temperature, ammoniated corn stover was pretreated at various temperatures (60–150 °C) for 72–144 h. Change in composition was marginal at low pretreatment temperature but was relatively severe with pretreatment at high temperature (130–150 °C). The latter resulted in low enzymatic digestibility. It was also observed that extreme levels (either high or low) of residual ammonia affected enzymatic digestibility, while residual ammonia improved by 1.0–1.5 %. The LMAA method enhanced enzymatic digestibility compared to untreated corn stover (29.8 %). The highest glucan and xylan digestibility (84.1 and 73.6 %, respectively) was obtained under the optimal LMAA conditions (i.e., ammoniation at 70 °C for 20 min, followed by pretreatment at 90 °C for 48 h).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The demand to find alternative fuels has risen due to environmental issues that are being experienced globally. As a clean alternative transportation fuel, ethanol has been getting the most attention. Up to the present, most of the fuel ethanol produced globally has come from sugar and starch (sugarcane and corn). Although possible, these approaches have encountered serious problems when upscaling production, including a sharp increase in global food insecurity. If fuel ethanol is produced from food crops, then more production of ethanol directly results in reduction of potential food supplies [1, 2]. On the other hand, cellulosic ethanol can be produced from lignocellulosic biomass, which is an abundant and renewable source. It was estimated that there is an annual supply of 200 billion metric tons worldwide [3]. Lignocellulosic biomass typically includes agricultural by-products and forest residue. However, lignocellulosic biomass is much more difficult to saccharify than crop biomass (starch and sugar) because its cellulosic part is recalcitrant to bacterial breakdown in nature [4, 5]. Moreover, it is a heterogeneous polymer of cellulose, hemicellulose, and lignin, making it difficult for enzymes to access the cellulosic fibers [6, 7]. The reported digestibility of lignocellulosic biomass is low (<20 %) without proper pretreatment.
To date, various chemical, mechanical, and biological pretreatment methods have been investigated and developed to enhance the enzymatic saccharification yield (digestibility) of lignocellulosic biomass. In our laboratory, several different alkaline pretreatment methods using ammonia have been developed. This is because ammonia has been found effective as a pretreatment catalyst of herbaceous biomass. Lignin is a polyphenolic compound and major bonds between lignins are carbon-to-carbon (C–C) and ether (C–O–C) bonds. Ammonia is known to be effective for cleavage of the bonds in lignin [8]. Therefore, it removes lignin effectively, can easily be recovered and re-used, and is non-corrosive to most construction materials.
These pretreatment methods include (1) ammonia recycle percolation (ARP) [9], (2) soaking in aqueous ammonia (SAA) at room temperature and SAA at moderate temperature [10, 11], and (3) low-moisture anhydrous ammonia (LMAA) [7]. Among them, ARP produces a high level of delignification (70–90 %) but dissolves about 50 % of the xylan during the process, which makes the downstream process for xylan recovery complicated. In addition, ARP runs at relatively high temperature (170–210 °C) with high liquid throughput, which requires high capital and operating costs. SAA was developed to resolve the aforementioned problems with the ARP method. Both SAA methods are typically conducted at mild temperature (room temperature–90 °C), which gives high hemicellulose retention (80–90 %), while it removes a considerable amount of lignin (60–70 %). High retention of hemicellulose (pentose) with the solid residue is a desirable feature because it can eliminate pentose recovery and detoxification of hydrolysate downstream. However, the SAA methods also require high chemical and water inputs (6–10 times the biomass weight: oven dry weight (ODW)), which then subsequently requires more water for washing than the water used in the pretreatment reaction. In order to solve the problem of high water input in SAA and ARP, the LMAA method was developed.
For the LMAA, biomass adjusted to 50 % moisture (S/L = 1) is contacted with anhydrous gaseous ammonia (called the ammoniation step) under ambient conditions without temperature control and then heat treated at 90 °C. In the LMAA, a washing step is not necessary; therefore, it was anticipated that the LMAA method would require low water and ammonia inputs, compared to the other methods discussed. The LMAA has advantages such as low water usage and high retention of both cellulose and hemicellulose (almost 100 %); however, it requires extended reaction time (96 h). Although it does not remove lignin, it was reported that LMAA was effective in significantly improving enzymatic digestibility. The previous study on LMAA method was conducted at ambient ammoniation temperature (25 °C) for 10 min, but a gradual increase of temperature (to 60–70 °C) occurs due to the exothermic reaction between ammonia and water.
In this study on the modified LMAA, the effects of various ammoniation parameters with temperature control at 70–150 °C for 10–30 min of reaction time were evaluated. In addition, pretreatment at higher temperature (up to 150 °C) was attempted to enhance digestibility and to reduce reaction time.
Materials and Methods
Materials
Feedstock
Corn stover was harvested from central Iowa in 2013 and air-dried at ambient temperature (to 5–10 % moisture content). The corn stover was then ground and sieved to achieve a graded series of sample particles that would pass through 10–35 mesh (US Standard, 0.5–2.0 mm of nominal sieve opening) sieves and then stored in sealed plastic containers at ambient temperature. The initial composition of the corn stover was 36.8 wt% glucan, 19.9 wt% xylan, 3.2 wt% arabinan, 1.7 wt% galactan, 20.1 wt% lignin (acid insoluble + acid soluble), 1.0 wt% ash, 5.2 wt% protein, and 11.8 wt% extractives.
Enzymes
Cellulase Cellic® CTech 2 (lot no. VCP10006) was provided by Novozymes, Inc. The average activities and protein content measured in our laboratory were 93.2 filter paper unit (FPU)/mL of enzyme and 76.1 mg-protein/mL, respectively. For enzymatic saccharification, enzyme loading of 15 FPU of cellulase/g-glucan was applied with no supplementation of other enzymes.
Methods
Experimental Setup and Operation
Moisturization and Ammoniation Steps
Corn stover was steeped in deionized water for 48 h to achieve the desired moisture level of 50 % (S/L = 1). The moisturized corn stover was placed in a SS-316 flow-through column reactor (2.3-cm internal diameter, 24.0-cm length, 99.7-mL internal volume) for ammoniation. Anhydrous ammonia in a high-pressure gas cylinder was introduced to the bottom of the reactor, held for the desired retention time (<30 min), and then released to the fume hood. Pressure and temperature gauges were installed to monitor the pressure and temperature changes during ammoniation. In this system, the pressure was maintained at 0.10 MPa (15 psig) for 10–30 min to achieve the desired ammonia loading of 0.1 g/g-ODW biomass. Ammoniation was conducted in a custom-made mechanical convection oven in order to increase the temperature. Temperature was controlled during ammoniation at the desired temperature. The reaction time started when the desired temperature was reached. All of the pretreatment experiments were performed in duplicate, at least.
After ammoniation, the treated solids were collected and divided into two portions. One-gram measure of biomass was used for residual ammonia analysis using ammonia ion selective electrode (ISE) meter (XL-250). The remainder was subjected to the next reaction (pretreatment). The procedure for ammonia analysis is described in the “Analytical Method” section.
Pretreatment and Evaporation Steps
After ammoniation, ammoniated corn stover was immediately transferred into several stainless tubular reactors (2.54-cm internal diameter, 30.0-cm length) for further pretreatment tests under various reaction conditions. Pretreatment was conducted in a convection oven with varying pretreatment temperature (60, 90, 120, or 150 °C) and pretreatment time (0–144 h). When pretreatment was completed, the reactors were removed from the oven and the pretreated solids were placed inside the fume hood to evaporate any excess ammonia. Evaporation was conducted at ambient temperature for 1 h. During the evaporation step, the moisture content of the pretreated samples was carefully monitored. Evaporation was stopped if the moisture level dropped below 30 % because over-drying could alter the pretreated sample’s structure and affect the enzymatic hydrolysis test [7].
Enzymatic Digestibility Test
The enzymatic digestibility test was conducted following the National Renewable Energy Laboratory’s Laboratory Analytical Procedure (NREL-LAP, Golden City, CO, USA) on enzyme saccharification of lignocellulosic biomass [12]. The enzymatic digestibility test was conducted in duplicate under the conditions of 50 °C, 150 r/min, and pH 4.8 (0.05 M sodium citrate buffer) in a shaking incubator. The initial glucan concentration was 1 % (w/v) based on 100 mL of total liquid. Antibiotics were used (40 mg/L tetracycline, 30 mg/L cyclohexamide). The Cellic® CTech 2 (lot no. VCP10006) was used and the enzyme loading was 15 FPU of cellulase/g-glucan, without any other supplementary enzyme.
Avicel® PH-101 (cat no. 11365, lot no. BCBJ0229V, Sigma-Aldrich Co., Ltd.) and untreated corn stover were put through the same procedure to provide a reference and control, respectively. An enzyme blank (same enzyme loading without substrate in the same volume as the tested samples) was also prepared, and the sugar in the enzyme solution was deducted from the sugar concentrations measured in the test flasks.
The glucan and xylan digestibility were calculated as follows:
where 0.9 is the conversion factor of glucose to equivalent glucan.
where 0.88 is the conversion factor of xylose to equivalent xylan.
Analytical Method
Carbohydrates (monomeric sugars), lignins (acid insoluble lignin (AIS)), and acid soluble lignin (ASL), ash, and extractives of the samples were quantified following NREL-LAP [12]. In brief, a two-step extractives analysis using deionized water and ethanol successively in a Soxhlet extractor (for 8 and 24 h, respectively) was used to determine the extractives of a sample. The extractive-free sample solids were then subjected to two-stage acid hydrolysis for determination of carbohydrate and lignin [12].
The sugars (glucose, xylose, galactose, arabinose, and mannose) were determined and quantified using a high-pressure liquid chromatograph (HPLC; model LC-10A, Shimadzu, Inc., Japan) equipped with a Bio-Rad Aminex HPX-87P/HPX-87H column with a refractive index detector (RID-10A, Shimadzu, Inc., Japan). Insoluble lignin and ash contents were quantified from the solid residues using a gravimetric analysis method, after two-stage acid hydrolysis using 72 and 4 %, respectively. The acid soluble lignin in the liquid hydrolysate was determined using a UV spectrophotometer (UV-mini 1240, Shimadzu, Inc., Japan) at 320-nm wavelength.
Ammonia Analysis
Quantification of ammonia remaining on the LMAA treated samples after evaporation was performed as follows. Approximately 0.3 g of solid sample was placed into a screw capped Pyrex media bottle and soaked with 80 mL 1 % boric acid solution for 24 h at 80 °C. This liberates ammonium ions from the sample structure. After 24 h of incubation, the bottles were taken out of the oven, allowed to cool to room temperature, and then filtered to separate the solids from the liquid filtrate. The filtrate was tested for ammonia content using a calibrated ISE meter (Accumet® XL-250, Fischer Scientific, cat. No. 13–636-XL-250, Hampton, NH, USA) equipped with an ammonia ion selective electrode (Fischer Scientific, cat. no. 13-620-509). The mechanism of detection is similar to that of the ammonia analyzer (TL-200, Timberline Instruments, Boulder, CO, USA) used in a previous study [7] and is based on the diffusion-conductivity principle. The ammonia electrode is capable of measuring the ammonia concentration in the aqueous solution without interference from the sample turbidity and color. First, trapped ammonia in the borate solution is liberated by adding a strong base (10 N NaOH) until pH >11. At this pH, ammonium ions are liberated and form ammonia gas. Then, the liberated ammonia gas diffuses through a hydrophobic membrane permeable only to ammonia gas. The amount of ammonia that passes through the membrane in the electrode has a certain conductivity (mV) response, which is proportional to the ammonia concentration in the solution.
Results and Discussion
Effects of Pretreatment Temperature and Time on Biomass Composition
The effects of pretreatment temperature were evaluated by maintaining the ammoniation conditions at ambient temperature for 10 min. With the LMAA-treated corn stover under this ammoniation condition, the treated corn stover contained 0.1 g-NH3/g-biomass. For pretreatment of ammoniated corn stover, six different temperatures were used (60, 90, 120, 130, 140, or 150 °C) in combination with four different pretreatment times (72, 96, 120, and 144 h). The compositional change of corn stover with LMAA pretreatment under the various reaction conditions is summarized in Fig. 1a. It was observed that LMAA pretreatment in the lower temperature range (60–120 °C) did not result in significant changes to the chemical composition in the treated solid. The degradation of glucan and other sugars was marginal, and the lignin content was not changed substantially. Overall, this is in accord with the previous study by Yoo et al. [7], except for treatment at 120 °C for 144 h, in which increased lignin (∼3 %) and decreased sugar (∼5 %) were noted. On the other hand, corn stover pretreated in the higher temperature range (130–150 °C) showed a greater change in xylan + galactan (XG) and lignin (AIL + ASL) content in the treated solids. As the pretreatment temperature increased from 130 to 150 °C, the lignin content increased from 22.6 to 28.2 % (for 72 h), and from 23.7 to 31.9 % (for 144 h), respectively (Fig. 1a). However, the ash content remained unchanged over the entire temperature range, as well as for all tested reaction times. Unlike for the 60–120 °C range, the pretreatment temperature at 130–150 °C substantially affected XG and arabinan. These are the components of hemicellulose. The arabinan in the solids treated at 150 °C for 72–144 h was found to have almost disappeared (0.2 %) and the XG content decreased to 16.0–19.3 % at high temperature (≥140 °C) and was assumed to have degraded at high temperature. Among the pretreatment temperatures (140 or 150 °C) and reaction time (Fig. 1a), it was probable that the pretreatment time affected XG degradation more significantly than did the pretreatment temperature. This is because the changes in XG concentration at the two different pretreatment temperatures (140 or 150 °C) were observed to be about the same if the pretreatment time was the same. Moreover, a similar degree of XG degradation in the corn stover that heated for 72 at 144 h was observed at both temperatures (1.5 and 2.1 % decrease of XG contents at 140 and 150 °C, respectively). This phenomenon can be attributed to xylan decomposition to furfural and other components at certain high temperatures (130–190 °C) [9, 13, 14]. There are three main components (cellulose, hemicellulose, and lignin), which can decompose at certain temperatures and each of them behave distinctly in such process. Cellulose, which has the structure of a chain polymer of glucose, was reported to degrade at 240–350 °C, and lignin exhibits thermal decomposition at 280 to 500 °C [14]. This is the reason that, compared to glucose and lignin, xylose and arabinose are most likely to degrade first under prolonged exposure to heat.
Effects of pretreatment temperature and time with on a solid compositions and b 72-h enzymatic digestibility. Note: ammoniation of corn stover with 50 % moisture content and 0.1 g-NH3/g-biomass at ambient temperature; pretreatment at 60–150 °C for 72–144 h. Data in the graph shows the mean value (n = 2, SE ≤2.5 %)
Effects of Pretreatment Temperature and Time on Enzymatic Digestibility
With the aforementioned LMAA-treated solids in Fig. 1a ammoniated at ambient temperature for 10 min followed by pretreatment at four different temperatures (60, 90, 120, or 150 °C) in combination with four different pretreatment times (72, 96, 120, and 144 h), the enzymatic digestibility was tested using LMAA-treated solids in duplicate. Although the enzymatic digestibility generally increases after 72 h of enzymatic hydrolysis, the 72-h enzymatic digestibility was taken and used for evaluation of the pretreatment effect in this study. The 72-h enzymatic digestibility of pretreated corn stover was compared and presented in Fig. 1b. The highest level of digestibility (79.9 %) of LMAA-treated corn stover was 2.9 times higher that of untreated corn stover (29.8 %) after 72-h digestion. Among the tested conditions, corn stover pretreated at 90 °C for 120 h achieved the highest glucan digestibility (81.0 %). As the pretreatment temperature increased, the digestibility of LMAA-treated (90 °C) corn stover increased until 120 h of pretreatment time and then it decreased from 81.0 to 71.8 %. It was observed that the enzymatic digestibility of 60 °C pretreated corn stover was not considerably affected by the extended pretreatment time. The most dramatic changes occurred in the 120 °C pretreated corn stover, which gradually reached the highest level (71.2 %) and then dropped to 38.2 % after 120 h pretreatment time and to 13.2 % at 144 h pretreatment time. Moreover, the 150 °C pretreated corn stover resulted in consistently lower enzymatic digestibility (9.4–15.9 %) regardless of pretreatment time. This was even lower (29.8 %) than that of untreated corn stover. The reason for this is unclear, but it indicated that the reaction temperature of 120 °C, a relatively moderate temperature, combined with the extended period of pretreatment time (>96 h) was the critical factor that affected the enzymatic digestibility of the LMAA-treated corn stover.
In Fig. 2, the effect of 72-h glucan digestion of LMAA-treated corn stover on its lignin content is presented. As shown in Fig. 1a, the lignin content of the LMAA-treated corn stover varied with its pretreatment temperature. Within the range of tested pretreatment temperatures (60–150 °C), the LMAA pretreatment in the range of 60–90 °C (19.3–20.2 % lignin content) enhanced the enzymatic digestibility most significantly (68.9–85.3 % at 72-h hydrolysis time). On the other hand, a pretreatment temperature of 130–150 °C resulted in higher lignin content (22.4–31.9 %), which did not improve the enzymatic digestibility (10.3–26.6 %).
According to the data in Figs. 1a and 2, it was observed that the LMAA pretreatment at higher temperature (120–150 °C) caused changes in the lignin and carbohydrate contents. Hemicellulose (xylan, galactan, and arabinan) decreased while the lignin content increased. It was speculated that the high temperature treatment was capable of changing the lignin content in the pretreated biomass, which could affect the enzymatic hydrolysis. Lignin is an aromatic polymer (polyphenol) that has amorphous structure and is composed mostly of monolignols. The structure of lignin varies among different plant species, and lignin tends to adsorb enzymes preventing them from achieving effective hydrolysis of cellulose fibers [14–16]. Lignin also hinders enzymatic hydrolysis by surrounding some fibers (carbohydrates) [17].
Effects of Ammoniation Time and Temperature on Biomass Composition and Enzymatic Digestibility
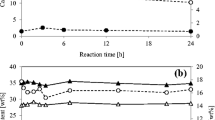
Ammoniation is the process by which anhydrous ammonia is introduced into steeped corn stover. In the aforementioned tests, ammoniation was conducted at ambient temperature and pretreatment temperature was varied, but it was also expected that the ammoniation rate and effectiveness could be improved at an elevated temperature. To evaluate the effect of ammoniation temperature, five different ammoniation temperatures (70, 90, 110, 130, and 150 °C) were tested. Each was followed by pretreatment under a fixed condition (90 °C for 48 h). The composition changes and 72-h glucan digestibility (enzymatic digestibility at 72 h of hydrolysis) of LMAA-treated solids at different ammoniation temperatures are presented in Fig. 3. Both glucan and xylan were well preserved at the elevated ammoniation temperatures. Lignin components (AIL and ASL) were only slightly affected. As the ammoniation temperature increased from ambient to 150 °C, the AIL content in the LMAA-treated solids decreased from 17.8 to 16.1 %, while the ASL contents proportionally increased from 2.4 to 3.9 %. This was because ammonia has high selectivity for lignin depolymerization; therefore, a large portion of the lignin molecules was depolymerized into smaller molecular size lignin, which is a soluble form [10].
Effects of ammoniation temperatures on composition and enzymatic digestibility (72-h). Note: ammoniation of corn stover with 50 % moisture content and 0.1 g-NH3/g-biomass at 60–150 °C for 20 min; pretreatment condition 90 °C, 48 h. Enzymatic hydrolysis: 15 FPU/ g-glucan loading, 0.05 M citrate buffer, 50 °C, and 150 rpm. Data in the graph shows the mean value (n = 2, SE ≤3.0 %)
Glucan digestibility of the LMAA-treated solids at various ammoniation temperatures was also measured with 15 FPU/g-glucan loading (Fig. 3). The 72-h digestibility of 70 °C-ammoniated LMAA-treated solids was 84.1 %, which was substantially better than the 29.8 % of untreated solid and 60.4 % of ambient temperature-ammoniated solid, LMAA-treated solids. It is interesting that among all tested ammoniation temperatures, ammoniation at 70 °C improved results most significantly and that the digestibility (at 72-h hydrolysis) dropped to 79.4 % as the ammoniation temperature increased to 150 °C. It is probable that, as temperature increases, water (moisture) in the biomass tends to vaporize, which makes it difficult to retain ammonia in the biomass.
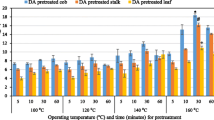
In order to study the effect of ammoniation time, LMAA was conducted at a fixed ammoniation temperature (70 °C) with 50 % moisture content and 0.1 g-NH3/g-ODW ammonia loading. Pretreatment conditions were also maintained at 90 °C and 48 h. The effects of various ammoniation times on the 72-h glucan and xylan enzymatic digestibility are shown in Fig. 4a. With 10 min of ammoniation time, the LMAA treatment produced digestibility of 68 and 53.9 %, for glucan and xylan, respectively. These increased from 83.7 to 84.1 % and from 71.0 to 73.6 % of glucan and xylan digestibility with extended ammoniation time (20–30 min). It was found that longer than 20 min of ammoniation provided no significant improvement of enzymatic digestibility. Therefore, the optimal ammoniation conditions selected were 70 °C for 20 min, with a ratio of 1:1 (S/L) with 0.1 g-NH3/g-biomass.
Effects of (1) ammoniation time and (2) pretreatment time on 72-h glucan digestibility. Note: (1) ammoniation of corn stover with 50 % moisture content and 0.1 g-NH3/g-biomass at 70 °C; pretreatment condition 90 °C, 48 h. (2) Ammoniation of corn stover with 50 % moisture content and 0.1 g-NH3/g-biomass at ambient temperature or 70 °C for 20 min; pretreatment condition 90 °C. Enzymatic hydrolysis 15 FPU/g-glucan loading, 0.05 M citrate buffer, 50 °C, and 150 rpm
Effects of Pretreatment Time on Enzymatic Digestibility
To evaluate the effects of pretreatment time, four different pretreatment times (0, 48, 96, and 120 h) were tested while maintaining the pretreatment temperature at 90 °C. Pretreatment was conducted in combination with two different ammoniation modes: (1) ammoniation at ambient temperature (LMAA) and (2) ammoniation at 70 °C (LMAA), keeping constant the other conditions of ammoniation (20 min, 50 % moisture content, and 0.1 g-NH3/g-biomass). The effects of pretreatment time for two different ammoniation operation modes, on glucan digestibility (at 72 h) are presented in Fig. 4b. Regarding ammoniation at ambient temperature, the 72-h glucan digestibility was gradually improved from 60.4 to 79.0 % as pretreatment time increased from 48 to 120 h. In contrast, pretreatment with ammoniation at 70 °C improved the 72-h glucan digestibility quickly to 84.1 % within 48 h of pretreatment time. After this, extended pretreatment time (>48 h) gave no significant increase of digestibility. This indicated not only that LMAA at the controlled and elevated temperature (70 °C) improved the enzymatic digestibility more significantly but also that it reduced the pretreatment time by 2.5 times, compared to that of LMAA pretreatment with ammoniation at ambient temperature.
Residual Ammonia Content vs. Enzymatic Digestibility
Removal and recovery of the excess ammonia used for the LMAA pretreatment are required to reduce the operating cost, but it is difficult to achieve complete removal of ammonia because this requires input of more energy for removal. The residual ammonia content in the LMAA-treated solids (after 12 h of evaporation in the air) was measured using an ammonia ISE meter. The correlation between the residual ammonia content and the 72-h glucan digestibility of LMAA-treated solids is shown in Fig. 5. It should also be noted that there are some positive effects from the remaining ammonia. For example, ammonia was found to be an effective preservation agent that can suppress the growth of bacteria and molds in the biomass [18]. Moreover, it serves as a nitrogen source for microbial fermentation [7]. Therefore, leaving some ammonia in the treated solid can be desirable, and Fig. 5 indicates that residual ammonia at a certain level (1–2 g-NH3/g-biomass) resulted in improved enzymatic digestibility, while the LMAA-treated solid with low (<1 g-NH3/g-biomass) or high (>2 g-NH3/g-biomass) residual ammonia content resulted in decreased digestibility.
Residual ammonia content vs. 72-h glucan digestion of LMAA-treated corn stover. Note: ammoniation of corn stover with 50 % moisture content and 0.1 g-NH3/g-biomass at 60 ambient temperature for 20 min; pretreatment at 60–150 °C for 72–144 h. Enzymatic hydrolysis 15 FPU/g-glucan loading, 0.05 M citrate buffer, 50 °C, and 150 rpm. Data in the graph shows the mean value (n = 2, SE ≤3.0 %)
Eberhart et al. [19] reported that cellulase activity is significantly affected by pH and temperature. It was assumed that the LMAA-treated solid readily released ammonium ions into solution when the treated solids underwent enzymatic hydrolysis. Under this condition, ammonium salt can be formed. It was reported by Menon et al. [20] that the presence of ammonium salts significantly affected enzyme activity, which agrees with the observation in our study with high residual ammonia content (>2 g-NH3/g-biomass) (Fig. 5). Samples treated at 60 and 90 °C had average ammonia content of 1.5–1.6 %, whereas samples treated at 120 °C had residual ammonia content of 2.4 %. On the other hand, samples treated at 150 °C had low average ammonia content (0.05 %). It was speculated that moisture in a closed system (batch reactor) tends to be vaporized; hence, the chance of ammonia-water reaction during pretreatment is reduced; in other words, significant pretreatment effect did not occur, which was attributed to low enzymatic digestibility of 150 °C pretreated samples. The behavior shown in Fig. 5 relating residual ammonia to digestibility is of a second-order polynomial curve with a coefficient of determination (R 2) of 0.9617. This means that the extent of relationship is significant. Nevertheless, we cannot clearly assume that the digestibility results were directly related to the residual ammonia alone because other factors (such as pretreatment temperature and time) should also be considered.
Overall Mass Balance
Figure 6 summarizes the overall mass balance of the biomass-to-sugar conversion process using the LMAA pretreatment and enzymatic hydrolysis under the optimum conditions. Corn stover was subjected to moisturization where equal portions of deionized water were needed. Approximately 10 wt% of NH3 was loaded prior to pretreatment (90 °C for 48 h). During the evaporation step, about 85–90 % ammonia was removed along with 33 % water. The residual ammonia of the pretreated sample was around 1.0–1.5 % on the basis of oven-dried corn stover. With enzymatic saccharification using CTec2 enzymes alone, at 15-FPU with 1.0 % (w/v) glucan loading, the conversion process could produce 51.1 g of glucose + xylose from 100 g (ODW) of corn stover.
Conclusions
The main goal of this study was to evaluate the effects of ammoniation at elevated temperature (60–150 °C) on biomass composition and enzymatic saccharification. The most notable composition change occurred in lignin at high-temperature (130–150 °C), which was assumed to influence the digestibility of the LMAA-pretreated corn stover. In addition, maintaining the ammoniation temperature at 70 °C not only improved the digestibility most significantly but also reduced the pretreatment time to within 48 h. The correlation between the residual ammonia content and the digestibility indicated that the effect of the ammonia content is significant, yet other factors should still be considered.
References
Naylor, R. L., Liska, A. J., Burke, M. B., Falcon, W. P., Gaskell, J. C., Rozelle, S. D., & Cassman, K. G. (2007). The ripple effect: biofuels, food security, and the environment. Environment: Science and Policy for Sustainable Development, 49(9), 30–43.
Zilberman, D., Hochman, G., Rajagopal, D., Sexton, S., & Timilsina, G. (2013). The impact of biofuels on commodity food prices: assessment of findings. America Journal Agricultural Economics, 95(2), 275–281.
Zhao, L., Chang, S., Wang, H., Zhang, X., Ou, X., Wang, B., & Wu, M. (2015). Long-term projections of liquid biofuels in China: uncertainties and potential benefits. Energy, 83, 37–54.
Kumar, P., Barrett, D. M., Delwiche, M. J., & Stroeve, P. (2009). Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Industrial and Engineering Chemistry Research, 48(8), 3713–3729.
Zhang, Y. H. P., Ding, S. Y., Mielenz, J. R., Cui, J. B., Elander, R. T., Laser, M., Himmel, M. E., McMillan, J. R., & Lynd, L. R. (2007). Fractionating recalcitrant lignocellulose at modest reaction conditions. Biotechnology and Bioengineering, 97(2), 214–223.
Hendriks, A. T. W. M., & Zeeman, G. (2009). Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresource Technology, 100, 10–18.
Yoo, C. G., Nghiem, N. P., Hicks, K. B., & Kim, T. H. (2011). Pretreatment of corn Stover using low-moisture anhydrous ammonia (LMAA) process. Bioresource Technology, 102(21), 10028–10034.
Kim, T. H. (2013). Chapter in book: 6. Pretreatment of lignocellulosic biomass. In S. T. Yang, H. A. El-Enshasy, & N. Thongchul (Eds.), Bioprocessing technologies in biorefinery for sustainable production of fuels (pp. 91–1110). N.Y., USA: Wiley.
Kim, T. H., & Lee, Y. Y. (2005). Pretreatment and fractionation of corn stover by ammonia recycle percolation process. Bioresource Technology, 96(18), 2007–2013.
Kim, T. H., Kim, J. S., Sunwoo, C., & Lee, Y. Y. (2003). Pretreatment of corn stover by aqueous ammonia. Bioresource Technology, 90(1), 39–47.
Kim, T. H., & Lee, Y. Y. (2007). Pretreatment of corn stover by soaking in aqueous ammonia at moderate temperatures. Applied Biochemistry and Biotechnology, 137-140(1–12), 81–92.
NREL (National Renewable Energy Laboratory) in Golden, CO. (2008). LAP (Laboratory Analytical Procedure). Accessed 29 Feb 2016 http://www.nrel.gov/biomass/analytical_procedures.html.
Chen, W. H., & Kuo, P. C. (2011). Torrefaction and co-torrefaction characterization of hemicellulose, cellulose and lignin as well as torrefaction of some basic constituents in biomass. Energy, 36(2), 803–811.
Mohan, D., Pittman, C. U., & Steele, P. H. (2006). Pyrolysis of wood/biomass for bio-oil: a critical review. Energy & Fuels, 20(3), 848–889.
Palonen, H., Tjerneld, F., Zacchi, G., & Tenkanen, M. (2004). Adsorption of Trichoderma reesei CBH I and EG II and their catalytic domains on steam pretreated softwood and isolated lignin. Journal of Biotechnology, 107, 65–72.
Yang, B., & Wyman, C. E. (2006). BSA treatment to enhance enzymatic hydrolysis of cellulose in lignin containing substrates. Biotechnology and Bioengineering, 94, 611–617.
Rahikainen, J. L., Martin-Sampedro, R., Heikkinen, H., Rovio, S., Marjamaa, K., Tamminen, T., Rojas, O. J., & Kruus, K. (2013). Inhibitory effect of lignin during cellulose bioconversion: the effect of lignin chemistry on non-productive enzyme adsorption. Bioresource Technology, 133, 270–278.
Lancaster, E. B., Hall, G. E., & Brekke, O. L. (1974). Treating corn with ammonia—behavior of the corn-water-ammonia system. T. ASAE, 17(2), 331–0334.
Eberhart, B. M., Beek, R. S., & Goolsby, K. M. (1977). Cellulose of Neurospora crassa. Journal of Microbiology, 130, 181–186.
Menon, K., Rao, K. K., & Pushalkar, S. (1994). Production of β-glucosidase by Penicillium rubrum O stall. Indian Journal of Experimental Biology, 32, 706–709.
Acknowledgments
This work was supported by the R&D program (No. 20153010091990) and Human Resources Development program (No. 20134030200230) of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government Ministry of Trade, Industry and Energy (MOTIE).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cayetano, R.D.A., Kim, T.H. Effects of Low Moisture Anhydrous Ammonia (LMAA) Pretreatment at Controlled Ammoniation Temperatures on Enzymatic Hydrolysis of Corn Stover. Appl Biochem Biotechnol 181, 1257–1269 (2017). https://doi.org/10.1007/s12010-016-2282-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2282-6