Abstract
Polyphenols of plant origin with wide range of antiradical activity can prevent diseases caused by oxidative and inflammatory processes. In this study, we show using ESR method that the purified water-soluble extract from leaves of Rhus typhina L. containing hydrolysable tannins and its main component, 3,6-bis-O-di-O-galloyl-1,2,4-tri-O-galloyl-β-d-glucose (C55H40O34), displayed a strong antiradical activity against the synthetic 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) in homogenous (solution) and heterogeneous systems (suspension of DPPH containing liposomes) in the range of 1–10 μg/ml. The C55H40O34 and extract at 1–30 μg/ml also efficiently, but to a various degree, decreased reactive oxygen and nitrogen species (RONS) formation induced in erythrocytes by oxidants, following the sequence: tert-butyl hydroperoxide (tBuOOH) > peroxynitrite (ONOO−) >hypochlorous acid (HClO). The explanation of these differences should be seen in the specificity of scavenging different RONS types. These relationships can be represented for C55H40O34 and the extract by the following order of selectivity: O.− 2 ≥ NO· > ·OH > 1O2. The extract exerted a more pronounced antiradical effect in reaction with DPPH and ROS in all models of oxidative stress in erythrocytes in comparison with C55H40O34. The redox processes between the extract components and their specificity in relation to RONS can underlie this effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, much attention has been paid to alternative medicine and search for available sources for obtaining of plant origin compounds which prevent diseases caused by oxidative and inflammatory processes. Such sources can be plants which produce tannins, i.e., compounds from the class of polyphenols. Among them, there are tannin-rich plants from genus Rhus (Anacardiaceae family). Since then, extracts from their fruit and leaves have long been used in traditional medicine as antimalarial, antibacterial, antidiarrheal, and antiinflammatory agents [1–3]. Some of them were patented as interferon-inducing compounds [4].
Tannins are polyphenoles with the molecular mass ranging between 500 and 3000 Da. On the basis of their structures, these compounds are classified into two groups: hydrolysable and condensed tannins. Hydrolysable ones are esters of sugars and phenol carbonic acids, and condensed tannins are polycondensates of catechins (flavan-3-ol). Hydrolyzable tannins are classified in turn into gallotannins and ellagotannins and contain gallic and ellagic acid, respectively [5]. Tannins are characterized by structural diversity and high chemical activity including scavenging radicals and interaction with proteins and lipids that make a basis for variety of biological effects, such as antitumor, antimutagen, antimicrobial, antiviral, antiplatelet, hypoglycemic, and antiinflammatory ones [6–9]. It should be emphasized that antiinflammatory effects of tannins are due to antioxidant properties, including antiradical activity, inhibition of Fenton reaction as a result of metals chelating, and the impact on the expression and activity of pro- and antioxidant enzymes [6, 10, 11].
Numerous phenol groups in the chemical structures of high-molecular tannins allow to reduce at a high rate free radicals compared to low-molecular flavonoids. As was shown, tannins exert little or no pro-oxidative effect in contrast to flavonols which can exhibit pro-oxidative properties due to formation of reactive oxygen species (ROS) via their oxidation products—O-quinones [12, 13]. Moreover, the size and degree of galloylation of the hydrolyzable tannins affect the solubility of compounds, which determines the efficiency of their antioxidant action in lipid and water phases. The antioxidant effects depend not only on the structure of a compounds itself but also on the type of oxidative stress inducers [14, 15].
Reactive oxygen and nitrogen species (RONS) are produced both as free radicals (O.− 2, ·OH, NO·) and non-radicals (1O2, H2O2) due to cellular metabolism and play roles of mediators and regulators of various physiologic processes in living organisms. RONS production is controlled by an antioxidant system including both antioxidant enzymes and low molecular weight antioxidants. However, exceeding physiologic concentration of RONS provokes enhanced oxidation of proteins, lipids and nucleic acids, and development of diseases caused by oxidative and inflammatory processes [16–18].
There are a number of diseases that are accompanied by increased oxidative stress of erythrocytes: neurodegenerative [19], diabetic [20], obesity [21], thyroid dysfunction [22], chronic kidney disease [23], and systemic lupus erythematosus [24]. Oxidative damage of cellular components in these cells gives rise to eryptosis, a specific form of erythrocyte apoptosis [25]. Enhanced eryptosis results in elimination of oxidized erythrocytes from blood, which, in turn, causes development of different forms of anemia [26–28]. In addition, under pathologic processes oxidized erythrocytes themselves become a source of RONS [29, 30], the so-called pro-oxidative bullets capable of causing oxidation of blood vessel cells. And, moreover, due to oxidative stress, erythrocytes begin to show glycated proteins named Advanced Glycation End Products (AGEs) which, while interacting with receptors on endothelial cells, induce oxidative stress in them and activate the nuclear factor kappaB (NF-κB) [29, 31].
Two lines can be observed in studies on biological activities of compounds of plant origin: investigation of isolated substances and/or whole extracts aiming at elucidation of the possibility of synergic action of their components.
In connection with the aforesaid, the goal of this study was to carry out a comparative investigation of the specificity of protection of erythrocytes by purified water-soluble extract from leaves of Rhus typhina L. containing more than 98 % hydrolysable tannins and its main component, 3,6-bis-O-di-O-galloyl-1,2,4-tri-O-galloyl-β-d-glucose (C55H40O34) against oxidative stress induced by different pro-oxidants (tert-butyl hydroperoxide [tBuOOH], peroxynitrite [ONOO−], and hypochlorous acid [HClO]) as well as to elucidate whether this peculiarity is due to the specificity of the compounds interaction with different RONS.
Material and Methods
Material and Chemicals
Leaves of R. typhina L. were collected in Tashkent environs (Uzbekistan) and taxonomically indentified in the Institute of Botanic of Academy of Sciences.
The Isolation of Total Polyphenols and Main Component
Total polyphenols from the leaves R. typhina L. were isolated according to the Islambekov [32]. Briefly, 300 g of ground aerial-dry leaves was extracted at first by chloroform and then dried raw material in undergone to triple extraction by hot 70 % aqueous acetone (module 1/10). Aqueous acetone fraction was filtrated, condensed, and was treated in sequence chloroform (three times) and ethyl acetate (five times) in solvent: fraction ratio 1:3 (v/v). The ethyl acetate extract was concentrated and sum of polyphenols was precipitated by fourfold volume of hexane. The extract contains the following: 3,6-bis-O-di-O-galloyl-1,2,4-tri-O-galloyl-β-d-glucose (74.05 %), rutin (1.15 %), 2,3-di-O-galloyl-β-d-glucose (2.15 %), 2-O-galloyl-β-d-glucose (2.10 %), 3-O-galloyl-β-d-glucose (2.20 %), 6-O-galloyl-β-d-glucose (2.05 %), 1,4,6-tri-O-galloyl-β-d-glucose (5.10 %), 1,2,3,4,6-penta-O-galloyl-β-d-glucose (10.05 %), quercetin (0.05 %), kaempferol (0.05 %), and gallic acid (1.05 %).
To isolate the main components of the extract, 10 g of the polyphenol sum was chromatographed through a column with hide powder using chloroform and mixture methanol-chloroform (2:8). Four fractions of phenol compounds were obtained. Fraction containing hydrolysable tannins was re-chromatographed on a column with silica gel (L40/100) in a solvents system containing diethyl ether-ethyl acetate at a ratio from 2:8 to pure ethyl acetate.
The main compound is an amorphous light brown powder. The substance is well soluble in water, ethanol, DMSO and methanol; soluble in ethyl acetate; and not soluble in chloroform and hexane. The structure as 3,6-bis-O-di-O-galloyl-1,2,4-tri-O-galloyl-β-D-glucosewas confirmed by NMR and MS spectra (Fig. 1).
3,6-bis-O-di-O-galloyl-1,2,4-tri-O-galloyl-β-D-glucose—С55Н40O34, [α]20 D -+22.880 (сa. 0.85, Me2СO), melting temperature 216–218 °С. MSm/z 1243 [M-H]-; 13С-NMR-(50 МHz, Me2СO–d6 + D2O, ppm) δ 96.5 (C-1), 74.2 (C-2), 73.0 (C-3), 69.6 (C-4), 75.1 (C-5), and 63.4 (C-6) glucose; 120.4(С-1), 110.1 (С-2, C-6), 145.9 (С-3, C-5), 139.3 (С-4), and 168.8 (С-7)—galloyl group; 115.8 (C-1), 126.4 (C-2), 108.1 (C-3), 145.8 (C-4), 136.4 (C-5), 144.5 (C-6), 168.0 (C-7), 115.5 (C′-1), 125.8 (C′-2), 107.8 (C′-3), 145.8 (C′-4), 136.6 (C′-5), 144.4 (C′-6), and 167.6 (C′-7)—digalloyl groups.
1,1-Diphenyl-2-picrylhydrazyl radical (DPPH), 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), nitroblue tetrazolium (NBT), nicotinamide adenine dinucleotide (NADH), phenazine methosulfate (PMS), and tBuOOH were from Sigma-Aldrich, St. Louis, MO, USA. The fluorescent probes 2′-7′-dichlorodihydrofluorescein diacetate (DCFH-DA) was from Molecular Probes, Eugene, OR, USA. All other reagents were purchased from POCH (Poland).
DPPH Radicals ESR Measurements
The DPPH free radical assay was carried out to the method described by Oszmianski et al. with modification [33]. The tested compounds in different concentration (0,5-10μg/ml) were added to 500 μl ethanol solution of DPPH, mixed, and then transferred in capillaries. The measurement was started after 3 min of antioxidant adding. The electron spin resonance (ESR) experiments were run on a spectrometer “Technical University of Wroclaw” with the following typical settings: power 50 mW, magnetic field 331 mT, slow scan range 10 mT, sweep time 64 s, time constant 1 s, modulation amplitude 1.25 × 10−1 mT, and microwave frequency 9.25 GHz. Typical ESR spectrum of DPPH in organic solvents is presented by five resolved peaks (Fig. 2a). For estimation of antioxidant activity of tested compounds in reaction with DPPH, the intensity of the strong peak in the center of the spectrum (h) of the control sample was taken for 100 %. Results are shown as dependence of DPPН signal intensity on concentration of the test compound.
ESR spectra of DPPH in the absence (control) and presence of C55H40O34 and extract (a). The dose-dependent scavenging effect of C55H40O34 and extract on DPPH in ethanol solution (b). The data presented are the means ± SE (n = 9). The effects of extract and C55H40O34 were statistically significant according to one-way ANOVA test (*p < 0.05; ***p < 0.001)
Preparation of DPPH Containing Liposomes
Liposomes were prepared according to Gabrielska [34]. The thin film of egg yolk phosphatidylcholine (PC) was formed on the bottom of a round flask (50 ml) by removing chloroform in rotary evaporator under vacuum. The film was hydrated in buffer solution (20 mM Tris-HCl, 125 mM KCl, pH = 7.4) and was vortexed to obtain a milky suspension of multilamellar vesicles. The obtained suspension was freeze/thawed thrice The final concentration of lipids in the suspension was 5 mg/ml. DPPH was added to prepared liposomes at a final concentration of 500 μM. Suspension was evaporated and resuspended in 1 ml of buffer 20 mM Tris-HCl, 125 mM KCl, and pH = 7.4.
The ESR measurement conditions as described for DPPH solution.
Hydroxyl Radicals ESR Assay
The concentration of ·OH radicals was measured by ESR method using spin trap DMPO according to Jang [35]. ·OH radicals were obtained in the mixture containing 300 μM ascorbic acid, 400 μM Fe3+, and 5 mM H2O2 according to Fenton reaction. Final concentration of DMPO was 5 mM. After 2 min incubation in the absence and the presence of tannins at the concentrations 10–40 μg/ml, the resulting adduct DMPO-·OH was measured by ESR. The measurement conditions were as described for DPPH. The typical four-line ESR spectrum of DMPO-·OH adduct is presented in Fig. 4a. To evaluate the efficiency of antioxidant activity of sumac tannins in relation to the concentration, the changes in intensity of the sum of two strong peaks of the spectrum (h 1 + h 2) were chosen (Fig. 4a).
Superoxide Radical Assay
The determination of superoxide radicals was carried out according to the method described by Nakagawa and Yokozawa with minor changes [36]. The superoxide radicals were obtained in non-enzymatic reaction using phenazine methosulfate–nicotinamide adenine dinucleotide (PMS/NADH) system, which reduced NBT to a purple formazan. The mixture (1.5 ml PBS, pH 7.4) containing 125 μM EDTA, 62 μM NBT, 38 μM NADH, and tested substances at different concentrations (2.5–15 μg/ml) was incubated for 5 min at room temperature. Then PMS at the final concentration 3.3 μM was added to probes and after 10 min incubation, the absorbance of mixture was measured at 540 nm. The results are presented as relative scavenging of superoxide radical (%) depending on concentration of tested compounds.
Detection of Nitric Oxide
The measurement of nitric oxide radicals was carried out according to the method described by Devi [37]. The various doses (2.5–15 μg/ml) of tested substances were incubated 150 min at 25 °C with 2 ml 10 mM sodium nitroprusside in PBS. Then 0.5 ml of probes was mixed with 1 ml 0.33 % sulfanilic acid in 20 % glacial acetic acid and allowed to stand for 5 min at the same temperature. Next, 1 ml of 0.1 % naphthylethylene diamide dihydrochloride was added into probes, mixed, and incubated for 30 min at 25 °C. The absorbance of probes was measured at 540 nm against blank sample. The results are presented as relative scavenging of nitric oxide (%) dependent on concentration of tested compounds.
Detection of Singlet Oxygen
Singlet oxygen was generated in non-enzymatic reaction between NaClO and H2O2, which was monitored by N,N-dimethyl-4-nitroaniline (RNO) bleaching according to the method described by Pedraza-Chaverri [38]. Mixture (2 ml 50 mM phosphate buffer, pH = 7.1) containing 10 mM histidine, 10 mM NaOCl, 10 mM H2O2, and 50 μM RNO as well as various doses (10–50 μg/ml) of tested substances were incubated for 40 min at 30 °C. Next absorbance of mixture was measured at λ = 440 nm against blank sample. The results are presented as relative scavenging of superoxide radical (%) dependent on concentration of tested compounds.
Measurement of RONS in Erythrocytes
Swine blood was collected in the presence of 3.8 % citrate as an anticoagulant (1:9). Then citrated blood was centrifuged (400 g, 15 min, 4 °C), and the plasma and buffy coat were removed by aspiration. The erythrocytes were washed twice with 0.9 % NaCl. RONS in erythrocytes was measured by the oxidation of DCFH-DA to fluorescent DCF in the presence of oxidants [39]. Erythrocytes (10 % suspension in PBS, pH = 7.4) were incubated with 40 μM DCFH-DA for 30 min at 37 °C in darkness. Next, incubation cells were washed twice and then resuspended in PBS. Then 0.5 % erythrocytes suspension was incubated with various doses of (1–30 μg/ml) of tested substances and different oxidants (10 mM tBuOOH, 1 mM HClO, or 400 μM ONOO−) for 5 min at 37 °C. The fluorescence of DCF was measured with excitation at λ = 498 nm and emission at λ = 522 nm. Fluorescence intensity of DCF in erythrocytes in the presence of hydrolysable tannins and oxidants was defined as a percentage of fluorescence in comparison with the oxidant’s one, which was taken for 100 %.
Statistical Analysis
The results are presented as mean ± SE. The level of significance was analyzed using one-way ANOVA test. P < 0.05 and below was accepted as statistically significant. The EC50 values were calculated by linear regression analysis. Statistical analysis was performed using Origin 8.5.1 (Microcal Software Inc., Northampton, MA) software.
Results and Discussion
Investigation of mechanisms of action of antioxidants in different oxidative stress models is important for working out a strategy against oxidative damage induced by various toxic factors. It is known that due to electron donor/acceptor properties, plant phenols and polyphenols manifest antiradical and antioxidant activities which depend on their structure, the presence of hydroxyl groups as well as their solubility in hydrophobic /hydrophilic phases [13, 40–45]. It is also know that to their structural diversity and properties, respectively, polyphenol-containing plant extracts often exert stronger antioxidant effects compared to isolated constituents [9, 46]. In this study, we show using ESR method that water-soluble extract from R. typhina L. leaves containing hydrolysable tannins and its main component, 3,6-bis-O-di-O-galloyl-1,2,4-tri-O-galloyl-β-d-glucose (C55H40O34), displayed strong, concentration-dependent, antiradical activity against the synthetic DPPH radical in solution (Fig. 2). On the basis of results obtained, amount of antioxidant necessary to decrease initial DPPH concentration by 50 % (EC50) was calculated by the method of linear regression.
These values are presented in the Table 1 in comparison with ЕС50 of Trolox, a well-known antioxidant that was used as a positive control. According to the results, activity of the extract has been established to be 1.44 times higher than Trolox in the reaction with DPPH, while the activity of C55H40O34 was 1.8 times lower. However, it should be emphasized that activity of the extract in this reaction was 2.61 times as high as that of C50H40O34.
Obtained data indicate a high antiradical activity of tannins under investigation against DPPH in the homogenous phase. As tannins are water-soluble compounds, it was of interest to study of their antiradical properties in protein-free cell model using suspension of liposomes containing of DPPH.
Comparison of EC50 values for the extract, C55H40O34 and Trolox (Table 1), showed that a series of efficiency of DPPH reduction in liposomes is analogous to the obtained one in the reaction with free radical in ethanol solution and can be represented by the following order: extract > Trolox > C55H40O34. In a heterogeneous system (water/lipid), i.e., in a reaction with DPPH embedded in liposomes, the EC50 for C55H40O34, and the extract were higher than for free DPPH, which seems to be due to slighter solubility of tannins in the lipid phase or shielding of their OH groups because of binding to lipid.
Our data on high antiradical activity of tannins in relation to DPPH in model systems pose a question of this activity realization in cellular models of oxidative stress induced by different prooxidant, where tannins are partly bound by as lipids as protein. The ability of tannins to bind with proteins and, to some extent with lipids, is well known. Tannins via their aromatic groups interact with hydrophobic sites of proteins and form hydrogen bonds between polyphenol hydroxyl groups and protein acceptor sites [47, 48]. For lipids, it is suggested that polyphenols form hydrogen bonds between phenolic groups and carbonyl and phosphorous groups of phospholipid and hydrophobic interaction between the aromatic groups and the hydrocarbon chain of phospholipids [49, 50]. Shielding of tannin hydroxyl groups, as result interaction with proteins and lipids, may cause greatly change in their antiradical activity. Therefore, we examined the ability of sumac tannins to scavenge RONS generated by various oxidants in erythrocytes. Erythrocytes are especially susceptible to oxidative stress due to high tension of oxygen and presence of hemoglobin iron. The used oxidants were short-chain hydroperoxide—tert-butyl hydroperoxide (tBuOOH), an analog of the lipid hydroperoxides, as well as peroxynitrite (ONOO−) and hypochlorous acid (HClO), which are generated in the organism and transformed in enzymatic and non-enzymatic reactions to RONS [51–57].
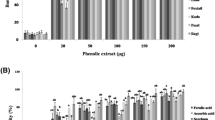
Our research showed that in all the models of oxidative stress, induced in erythrocytes by tBuOOH, ONOO−, and HClO, the extract and C55H40O34 considerably decreased RONS formation in a concentration-dependent way in the range of 1–30 μg/ml (Fig. 3a, b). The results obtained are consistent with the existing data that protein-bound tannins possess the sufficiently high antiradical activity and enhance the total antioxidant activity of blood [58–60].
Dose-dependent effects of C55H40O34 (a) and extract (b) on RONS in erythrocyte induced by 1 mM HClO, 400 μM ONOO−, and 10 mM tBuOOH. The data presented are the means ± SE (n = 9). The effects of C55H40O34 and extract were statistically significant according to one-way ANOVA test (*p < 0.05; **p < 0.01; ***p < 0.001)
However, it should be noted that the efficiency of antioxidant activity of sumac tannins depends on the oxidant used. According to findings, C55H40O34 exerts higher activity concerning tBuOOH and 2.1- and 2.8-fold lower one regarding ONOO− and HClO, respectively (Table 2). Similar sequence was found for extract too. It should be also noted that as compared to C55H40O34, the extract exhibited higher activity with statistical significance (p < 0.01). For instance, in the case of the oxidant tBuOOH, the scavenging effect of extract was higher by 1.2-fold, whereas in the case of ONOO− and HClO, the effect was increased by 1.3- and 1.4-fold, respectively (Table 2).
The analysis of the results obtained indicates that the efficiencies of the scavenging activities of studied tannins toward erythrocyte RONS generated by different pro-oxidant considerably varied and can be represented by following order: tBuOOH > ONOO− > HClO.
The explanation of these differences seems to be sought in types of RONS generated by these oxidants in erythrocytes as well as in the specificity of scavenging of their by tannins. In the case of tBuOOH, interacting with hemoglobin and methemoglobin causes the formation of O.− 2 and H2O2 [51]. In erythrocytes, ONOO− produces O.− 2, ·NO2, ·OH, and NO− 3 [52–54], while HClO contributes to generation of ·OH and 1O2 [55, 56]. Moreover, oxidative stress induced by these oxidants provokes an increase in erythrocyte Ca2+ level, which results in activation of Ca/CaM-dependent NOS, thus contributing to an increase of NO· level [51, 57].
In order to test our hypothesis, we detected a specific scavenging activity of C55H40O34 and extract against RONS, such as O2 .−, 1O2, ·OH, and NO· which are formed in the erythrocytes in presence of studied pro-oxidants.
We used DMPO spin trap to quantify the amount of ·OH generated in the Fenton reaction (Fe2+ + H2O2). As Fig. 4b shows, the hydroxyl radical (OH)-scavenging activities of C55H40O34 and extract were dose dependent.
ESR spectra of DMPO-·OH in the absence (control) and presence of C55H40O34 and extract (a). The dose-dependent scavenging effect of C55H40O34 and extract on DMPO-·OH (b). The data presented are the means ± SE (n = 9). The effects of extract and C55H40O34 were statistically significant according to one-way ANOVA test (*p < 0.05; ***p < 0.001)
The dose-dependence curves for 1O2, O.− 2, and NO· scavenging activity of C55H40O34 and extract are presented in Fig. 5, and EC50 values are listed in Table 3.
Dose-dependent scavenging effect of extract and C55H40O34 on singlet oxygen (a), superoxide radical (b), and nitric oxide (c). The data presented are the means ± SE (n = 9). The effects of extract and C55H40O34 were statistically significant according to one-way ANOVA test (*p < 0.05; **p < 0.01; ***p < 0.001)
According to these findings, C55H40O34 exerts approximately identical activity concerning NO· and O.− 2 and 3.3- and 4.0-fold lower one toward ·OН and 1O2, respectively. Similar efficiency was found for extract too. However, as it appears from the data presented in Table 3, the activity of extract was slightly higher (but significantly p < 0.05) compared with C55H40O34 concerning ROS. No apparent difference was noted in NO· scavenging activity between C55H40O34 and extract. The findings demonstrate that both the C55H40O34 and the extract are specific to RONS. The sequence of the C55H40O34 and extract toward RONS can be represented in the following order: O.− 2 ≥ NO· > ·OH > 1O2. Taking into consideration these data, we can explain the following sequence of efficiency of the antiradical activity of studied tannins toward RONS generated in erythrocytes by the oxidants (tBuOOH > ONOO− > HClO). The tannins scavenged RONS generated by tBOOH (O.− 2, NO·) to a greater extent compared to RONS generated by ONOO and HClO (·OH, 1O2).
However, it should be emphasized that as in the reaction with DPPH, the extract was more effective than C55H40O34 in the reaction with all the ROS studied. Antioxidant activity of an extract is generally believed to be represented by a sum of activities of its constituent components. However, there is also another point of view according to which a synergic action of extract components provides the basis for realization of antioxidant activity of the extract [61–63].
The mechanism of the synergic effect of the components mixture suggests formation of redox pairs among them. As a result of their interaction, antioxidant oxidizing in the reaction with radical is reduced by other components of the mixture which play a role of reducers. This enables the antioxidant to participate in reactions repeatedly [64]. It is possible that the extract, which consists 98 % of tannins with different structures and 2 % of flavonoids, acts in a similar way.
It should be pointed out that compared with C55H40O34, the extract also exerted a more pronounced antiradical effect in the models of oxidative stress in erythrocytes. However, in this case, the difference in activity was more pronounced when the concentrations of the compounds were higher. It is possible that the spatial location of extract components in the membrane hampers their interaction at a low concentration. We cannot also exclude the fact that the location of the extract components between the hydrophilic and hydrophobic regions of the membrane also plays an important role and can also affect the manifestation of their activities. This suggestion was confirmed by the literature data. It was found earlier that only a mixture of antioxidants inhibited both oxidative degradation of proteins and deformation of erythrocytes induced by various oxidants [65]. The authors suggest this to be related to the specificity of the location of mixture components in the membrane and the interaction with different types of radicals in hydrophobic and hydrophilic areas.
Conclusion
This study demonstrated that hydrolysable tannins (C55H40O34 and extract) isolated from leaves of R. typhina L. are characterized by high and specific antiradical activity both in the model systems and in the erythrocytes under oxidative stress induced by various oxidants such as tBuOOH, ONOO, and HClO.
The high antiradical activity of C55H40O34 and the extract against RONS indicate that the substances can function as both interventive and preventive antioxidants. It was found that in both cell-free and cellular models (liposomes, erythrocytes), the extract showed higher antiradical activity as compared to isolated C55H40O34. The redox processes between the extract components and their specificity in relation to RONS may underlie this effect. Hydrolysable tannins, from R. typhina leaves, being water-soluble, with low toxicity and highly efficient antioxidants, can be used as nutraceuticals in preventing the development of diseases associated with oxidative stress.
Abbreviations
- DMPO:
-
5,5-Dimethyl-1-pyrroline-N-oxide
- DCFH-DA:
-
2′-7′-dichlorodihydrofluorescein diacetate
- DPPH:
-
1,1-Diphenyl-2-picrylhydrazyl radical
- EDTA:
-
Ethylenediaminetetraacetic acid
- ESR:
-
Electron spin resonance
- HClO:
-
Hypochlorous acid
- NBT:
-
Nitroblue tetrazolium
- NO:
-
Nitric oxide
- 1O2 :
-
Singlet oxygen
- O.− 2 :
-
Superoxide radical anion
- OH:
-
Hydroxyl radical
- ONOO− :
-
Peroxynitrite
- PMS:
-
Phenazine methosulfate
- RNO:
-
N,N-Dimethyl-4-nitroaniline
- RONS:
-
Reactive oxygen and nitrogen species
- tBuOOH:
-
tert-butyl hydroperoxide.
References
Rayne, S., & Mazza, G. (2007). Biological activities of extracts from sumac (Rhus spp.): a review. Plant Foods for Human Nutrition, 62, 165–175.
El Hasasna, H., Saleh, A., Samri, H. A., Athamneh, K., Attoub, S., Arafat, K., Benhalilou, N., Alyan, S., Viallet, J., Dhaheri, Y. A., Eid, A., & Iratni, R. (2016). Rhus coriaria suppresses angiogenesis, metastasis and tumor growth of breast cancer through inhibition of STAT3, NFκB and nitric oxide pathways. Scientific Reports, 6, 21144. doi:10.1038/srep21144.
Salimi, Z., Eskandary, A., Headari, R., Nejati, V., Moradi, M., & Kalhori, Z. (2015). Antioxidant effect of aqueous extract of sumac (Rhus coriaria L.) in the alloxan-induced diabetic rats. Indian Journal of Physiology and Pharmacology, 59, 87–93.
Salikhov, Sh., ,Mavlyanov, S., Karamov, E., Abdullajanova, N. (2012). The remedy possessing anti-influenza activity. Bull. N 7, UZ IAP 04524.
Mavlyanov, S. M., Islambekov, S. Y., Ismailov, A. I., Dalimov, D. N., & Abdulladzhanova, N. G. (2001). Vegetable tanning agents. Chemistry of Natural Compounds, 37, 1–24.
Koleckar, V., Kubikova, K., Rehakova, Z., Kuca, K., Jun, D., Jahodar, L., & Opletal, L. (2008). Condensed and hydrolysable tannins as antioxidants influencing the health. Mini Reviews in Medicinal Chemistry, 8, 436–447.
Serrano, J., Puupponen–Pimia, R., Dauer, A., Aura, A. M., & Saura–Calixto, F. (2009). Tannins: current knowledge of food sources, intake, bioavailability and biological effects. Molecular Nutrition & Food Research, 53, 310–329.
Heber, D. (2008). Multitargeted therapy of cancer by ellagitannins. Cancer Letters, 269, 262–268.
Cryan, L. M., Bazinet, L., Habeshian, K. A., Cao, S., Clardy, J., Christensen, K. A., & Rogers, M. S. (2013). 1,2,3,4,6-Penta-O-galloyl-β-D-glucopyranose inhibits angiogenesis via inhibition of capillary morphogenesis gene2. Journal of Medicinal Chemistry, 56, 1940–1945.
Fraga, C. G., & Oteiza, P. I. (2011). Dietary flavonoids: role of (−)-epicatechin and related procyanidins in cell signaling. Free Radical Biology & Medicine, 51, 813–823.
Larrosa, M., Garcia-Conesa, M. T., Espin, J. C., & Tomas-Barberan, A. T. (2010). Ellagitannins, ellagic acid and vascular health. Molecular Aspects of Medicine, 31, 513–539.
Hagerman, A. E., Riedl, K. M., Jones, G. A., Sovik, N. K., Ritchard, N. T., Hartzfeld, P. W., & Riechel, T. L. (1998). High molecular weight plant polyphenolics (tannins) as biological antioxidants. Journal of Agricultural and Food Chemistry, 46, 1887–1892.
Bors, W., Michel, C., & Stettmaier, K. (2000). Electron paramagnetic resonance studies of radical species of proanthocyanidins and gallate esters. Archives of Biochemistry and Biophysics, 374, 347–355.
Hapner, C., Deuster, P., & Chen, Y. (2010). Inhibition of oxidative hemolysis by quercetin, but not other antioxidants. Chemico-Biological Interactions, 186, 275–279.
Lu, L., Hackett, S. F., Mincey, A., Lai, H., & Campochiaro, P. A. (2006). Effects of different types of oxidative stress in RPE cells. Journal of Cellular Physiology, 206, 119–125.
Valko, M., Leibfritz, D., Moncol, J., Cronin, M., Mazur, M., & Telser, J. (2007). Free radicals and antioxidants in normal physiological functions and human disease. The International Journal of Biochemistry & Cell Biology, 39(2007), 44–84.
Silva, J. P., & Coutinho, O. P. (2010). Free radicals in the regulation of damage and cell death—basic mechanisms and prevention. Drug Discoveries & Therapeutics, 4, 144–167.
Panieri, E., Gogvadze, V., Norberg, E., Venkatesh, R., Orrenius, S., & Zhivotovsky, B. (2013). Reactive oxygen species generated in different compartments induce cell death, survival, or senescence. Free Radical Biology & Medicine, 57, 176–187.
Nakagawa, K., Kiko, T., Miyazawa, T., Sookwong, P., Tsuduki, T., Satoh, A., & Miyazawa, T. (2011). Amyloid β-induced erythrocytic damage and its attenuation by carotenoids. FEBS Letters, 585, 1249–1254.
Augustyniak, K., Zavodnik, I., Palecz, D., Szosland, K., & Bryszewska, M. (1996). The effect of oxidizing agents and diabetes mellitus on the human red blood cell membrane potential. Clinical Biochemistry, 29, 283–286.
Sola, E., Vaya, A., Martinez, M., Moscardo, A., Corella, D., Santaolaria, M. L., Espana, F., & Hernaadnez-Mijares, A. (2009). Erythrocyte membrane phosphatidylserine exposure in obesity. Obesity, 17, 318–322.
Messarah, M., Saoudi, M., Boumendjel, A., Boulakoud, M. S., & Feki, A. E. (2011). Oxidative stress induced by thyroid dysfunction in rat erythrocytes and heart. Environmental Toxicology and Pharmacology, 31, 33–41.
Tsuda, K. (2013). Chronic kidney disease predicts impaired membrane microviscosity of red blood cells in hypertensive and normotensive subjects. International Heart Journal, 54, 154–159.
Spengler, M. I., Svetaz, M. J., Leroux, M. B., Bertoluzzo, S. M., Carrara, P., Van Isseldyk, F., Petrelli, D., Parente, F. M., & Bosch, P. (2013). Erythrocyte aggregation in patients with systemic lupus erythematosus. Clinical Hemorheology and Microcirculation, 47, 279–285.
Lang, E., Qadri, S. M., & Lang, F. (2012). Killing me softly—suicidal erythrocyte death. The International Journal of Biochemistry & Cell Biology, 44, 1236–1243.
Fibach, E., & Rachmilewitz, E. (2008). The role of oxidative stress in hemolytic anemia. Current Molecular Medicine, 8, 609–619.
Amer, J., Ghoti, H., Rachmilewitz, E., Koren, A., Levin, C., & Fibach, E. (2006). Red blood cells, platelets and polymorphonuclear neutrophils of patients with sickle cell disease exhibit oxidative stress that can be ameliorated by antioxidants. British Journal of Haematology, 132, 108–113.
Chirico, E. N., & Pialoux, V. (2012). Role of oxidative stress in the pathogenesis of sickle cell disease. IUBMB Life, 64, 72–80.
Minetti, M., Agati, L., & Malorni, W. (2007). The microenvironment can shift erythrocytes from a friendly to a harmful behavior: pathogenetic implications for vascular diseases. Cardiovascular Research, 75, 21–28.
Nikolaidis, M. G., & Jamurtas, A. Z. (2009). Blood as a reactive species generator and redox status regulator during exercise. Archives of Biochemistry and Biophysics, 490, 77–84.
Somjee, S. S., Warrier, R. P., Thomson, J. L., Ory-Ascani, J., & Hempe, J. M. (2005). Advanced glycation end-products in sickle cell anaemia. British Journal of Haematology, 128, 112–118.
Islambekov, Y. S., Mavlyanov, S. M., Kamaev, F. G., & Ismailov, A. I. (1994). Phenolic compounds of sumac. Chemistry of Natural Compounds, 30, 37–39.
Oszmiański, J., Wolniak, M., Wojdylo, A., & Wawer, I. (2007). Comparative study of polyphenolic content and antiradical activity of cloudy and clear apple juices. Journal of the Science of Food and Agriculture, 87(2007), 573–579.
Gabrielska, J., Sekowski, S., Zukowska, I., Przestalski, S., & Zamaraeva, M. (2012). The modified action of triphenyllead chloride on UVB-induced effects in albumin and lipids. Ecotoxicology and Environmental Safety, 89, 36–42.
Jang, M. H., Kim, H. Y., Kang, K. S., Yokozawa, T., & Park, J. H. (2009). Hydroxyl radical scavenging activities of isoquinoline alkaloids isolated from Coptis chinensis. Archives of Pharmacal Research, 32, 341–345.
Nakagawa, T., & Yokozawa, T. (2002). Direct scavenging of nitric oxide and superoxide by green tea. Food and Chemical Toxicology, 40, 1745–1750.
Devi, K. P., Suganthy, N., Kesika, P., & Pandian, S. K. (2008). Bioprotective properties of seaweeds: in vitro evaluation of antioxidant activity and microbial activity against food borne bacteria in relation to polyphenolic content. BMC Complementary and Alternative Medicine, 8, 38. doi:10.1186/1472-6882-8-38.
Pedraza-Chaverrí, J., Barrera, D., Maldonado, P. D., Chirino, Y. I., Macías-Ruvalcaba, N. A., Medina-Campos, O. N., Castro, L., Salcedo, M. I., & Hernández-Pando, R. (2004). S-Allylmercaptocysteine scavenges hydroxyl radical and singlet oxygen in vitro and attenuates gentamicin-induced oxidative and nitrosative stress and renal damage in vivo. BMC Clinical Pharmacology, 4, 5. doi:10.1186/1472-6904-4-5.
Gomes, A., Fernandes, E., & Lima, J. L. (2005). Fluorescence probes used for detection of reactive oxygen species. Journal of Biochemical and Biophysical Methods, 65, 45–80.
Halliwell, B. (2011). Free radical and antioxidants—quo vadis? Trends in Pharmacological Sciences, 32, 125–130.
Foti, M. C. (2007). Antioxidant properties of phenols. The Journal of Pharmacy and Pharmacology, 59, 1673–1685.
Al-Sehemi, A. G., & Irfan, A. (2013). Effect of donor and acceptor groups on radical scavenging activity of phenol by density functional theory. Arabian Journal of Chemistry. doi:10.1016/j.arabjc.2013.06.019.
Nadour, M., Michaud, P., & Moulti-Mati, F. (2012). Antioxidant activities of polyphenols extracted from olive (Olea europaea) of chamlal variety. Applied Biochemistry and Biotechnology, 167, 1802–1810.
Jalal, T. K., Ahmed, I. A., Mikail, M., Momand, L., Draman, S., Isa, M. L., Abdull Rasad, M. S., Nor Omar, M., Ibrahim, M., & Abdul Wahab, R. (2015). Evaluation of antioxidant, total phenol and flavonoid content and antimicrobial activities of Artocarpus altilis (breadfruit) of underutilized tropical fruit extracts. Applied Biochemistry and Biotechnology, 175, 3231–3243.
Feng, H. L., Tian, L., Chai, W. M., Chen, X. X., Shi, Y., Gao, Y. S., Yan, C. L., & Chen, Q. X. (2014). Isolation and purification of condensed tannins from flamboyant tree and their antioxidant and antityrosinase activities. Applied Biochemistry and Biotechnology, 173, 179–192.
Williamson, E. M. (2001). Synergy and other interactions in phytomedicines. Phytomedicine, 8, 401–409.
Haslam, E. (2007). Vegetable tannins—lessons of a phytochemical lifetime. Phytochemistry, 68, 2713–2721.
Kosińska, A., Kamarać, M., Penkacik, K., Urbalewicz, A., & Amarowicz, R. (2011). Interaction between tannins and proteins isolated from broad seeds (Vicia faba Major) yield soluble and non-soluble complexes. European Food Research and Technology, 223, 213–222.
He, Q., Shi, B., & Yao, K. (2006). Interaction of gallotannins with proteins, amino acids, phospholipids and sugars. Food Chemistry, 95, 250–254.
Beretta, G., Artali, R., Caneva, E., & Facino, R. M. (2011). Conformation of the tridimensional structure of 1,2,3,4,6-pentagalloyl-β-D-glucopyranose (PGG) by 1H NMR, NOESY and theoretical study and membrane interaction in a simulated phospholipid bilayer: a first insight. Magnetic Resonance in Chemistry, 49, 132–136.
Deliconstantinos, G., Villiotou, V., & Stavrides, J. (1996). Tumour promoter tert-butyl-hydroperoxide induces peroxynitrite formation in human erythrocytes. Anticancer Research, 16, 2969–2980.
Romero, N., Denicola, A., & Radi, R. (2006). Red blood cells in the metabolism of nitric oxide-derived peroxynitrite. IUBMB Life, 58, 572–580.
Metere, A., Iorio, E., Pietraforte, D., Podo, F., & Minetti, M. (2009). Peroxynitrite signaling in human erythrocytes: synergistic role of hemoglobin oxidation and band 3 tyrosine phosphorylation. Archives of Biochemistry and Biophysics, 484, 173–182.
Rubbo, H., Trostchansky, A., & O’Donell, V. B. (2009). Peroxynitrite—mediated lipid oxidation and nitration: mechanism and consequences. Archives of Biochemistry and Biophysics, 484, 167–172.
Zavodnik, I. B., Lapshina, E. A., Zavodnik, L. B., Bartosz, G., Soszynski, M., & Bryszewska, M. (2001). Hypochlorous acid damages erythrocyte membrane proteins and alters lipid bilayer structure and fluidity. Free Radical Biology & Medicine, 30, 363–369.
Pennathur, S., Maitra, D., Byun, J., Sliskovic, I., Abdulhamid, I., Saed, G. M., Diamond, M. P., & Abu-Soud, H. M. (2010). Potent antioxidative activity of lycopene: a potential role in scavenging hypochlorous acid. Free Radical Biology & Medicine, 49, 205–213.
Özöyaman, B., Grau, M., Kelm, M., Merx, M. W., & Kleinbongard, P. (2008). RBC NOS: regulatory mechanisms and therapeutic aspects. Trends in Molecular Medicine, 14, 314–322.
Riedl, K. M., & Hagerman, A. E. (2001). Tannin-protein complexes as radical scavengers and radical sinks. Journal of Agricultural and Food Chemistry, 49, 4917–4923.
Koren, E., Kohen, R., & Ginsburg, I. (2010). Polyphenols enhance total oxidant—scavenging capacities of human blood by binding to red blood cells. Experimental Biology and Medicine, 235, 689–699.
Olchowik, E., Lotkowski, K., Mavlyanov, S., Abdullajanova, N., Ionov, M., Bryszewska, M., & Zamaraeva, M. (2012). Stabilization of erythrocytes against oxidative and hypotonic stress by tannins isolated from sumac leaves (Rhus typhina L.) and grape seeds (Vitis vinifera L.). Cellular & Molecular Biology Letters, 17, 333–348.
Mertens-Talcott, S. U., Talcott, S. T., & Percival, S. S. (2003). Low concentrations of quercetin and ellagic acid synergistically influence proliferation, cytotoxicity and apoptosis in MOLT-4 human leukemia cells. The Journal of Nutrition, 133, 2669–2674.
Intra, J., & Kuo, S. M. (2007). Physiological levels of tea catechins increase cellular lipid antioxidant activity of vitamin C and vitamin E in human intestinal caco-2 cells. Chemico-Biological Interactions, 169, 91–99.
Mikstacka, R., Rimando, A. M., & Ignatowicz, E. (2010). Antioxidant effect of trans-resveratrol, pterostilbene, quercetin and their combinations in human erythrocytes in vitro. Plant Foods for Human Nutrition, 65, 57–63.
Kowalewska, E., & Litwinienko, G. (2010). Phenolic chain-breaking antioxidants—their activity and mechanisms of action. Postepy Biochemii, 56, 274–283.
Yanai, N., Shiotani, S., Hagiwara, S., Nabetani, H., & Nakajima, M. (2008). Antioxidant combination inhibits reactive oxygen species mediated damage. Bioscience, Biotechnology and Biochemistry, 72, 3100–3106.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Olchowik-Grabarek, E., Mavlyanov, S., Abdullajanova, N. et al. Specificity of Hydrolysable Tannins from Rhus typhina L. to Oxidants in Cell and Cell-Free Models. Appl Biochem Biotechnol 181, 495–510 (2017). https://doi.org/10.1007/s12010-016-2226-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2226-1