Abstract
Lectins have captured the attention of a large number of researchers on account of their various exploitable activities, including antitumor, immunomodulatory, antifungal, as well as HIV reverse transcriptase inhibitory activities. A mannose/glucose-specific lectin was isolated from green split peas (a variety of Pisum sativum) and characterized. The purification step involved anion-exchange chromatography on a DEAE-cellulose column, cation-exchange chromatography on an SP-Sepharose column, and gel filtration by fast protein liquid chromatography (FPLC) on Superdex 200. The purified lectin had a native molecular mass of around 50 kDa as determined by size exclusion chromatography. It appeared as a heterotetramer, composed of two distinct polypeptide bands with a molecular mass of 6 and 19 kDa, respectively, in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The N-terminal sequence of green split pea lectin shows some degree of homology compared to lectins from other legume species. Its hemagglutinating activity was inhibited by glucose, mannose, and sucrose, and attenuated at pH values higher than 12 or lower than 3. Hemagglutinating activity was preserved at temperatures lower than 80 °C. The lectin did not show antifungal activity toward fungi including Fusarium oxysporum, Botrytis cinerea, and Mycosphaerella arachidicola. Green split pea lectin showed a mitogenic effect toward murine splenocytes and could inhibit the activity of HIV-1 reverse transcriptase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lectins are a group of proteins (or glycoproteins), other than antibodies and enzymes, that bind specifically and reversibly to carbohydrates, particularly the sugar moiety of glycoconjugates, resulting in cell agglutination and precipitation of glycoconjugates. Lectins are translators of the sugar code [1]. There have been various definitions for lectin since its discovery, yet in general, a more update definition of lectin was given by Peumans and Van Damme [2], in which lectins are referred to as proteins possessing at least one non-catalytic domain that binds reversibly to a specific mono- or oligosaccharide [2, 3].
Lectins are widely distributed in nature and could be derived from plants [4–13], fungi [14–17], animals [11, 18–20], and humans [21]. There are various ways of classification of lectins. One of the most common ways of classification is according to their structures and subunits [3]. Another common method to distinguish lectins is by their carbohydrate-binding specificity apart from their structure.
Lectins are believed to have multifunctional roles in living organisms. The physiological functions of lectins in plants include storage of proteins [22], protection against insects [8, 23], defense [11], cell wall extension [22], mitogenic stimulation [22, 24], transport of carbohydrates [22], and packaging and/or mobilization of storage materials [22]. Over the last few decades, interest in lectins has been growing due to its wide variety of biological properties and applications, including clinical, biochemical, and agricultural aspects.
Green split peas, known as the green pea, field pea, or garden pea, belongs to the Leguminosae family. Split peas are dried, peeled, and split seeds of Pisum sativum. The appearance of the pea is round, shiny, and dark green in color with a creamy white interior. The peas are sweet with a low starch content and commonly used as an ingredient in cuisine or as a manure crop. Green split peas are believed to originate from the eastern rim of the Mediterranean, which then later spread to Europe, India, and China. It is a starchy, large-seeded variety of pea (P. sativum var. medullare). There are only a few publications on split peas [25–29]. Although a lectin has been isolated from P. sativum cv. sugar snap [30], in view of the fact that lectins from different cultivars of Phaseolus vulgaris exhibit differences in N-terminal sequences and biological activities [31–35], we undertook the present study to isolate a lectin from green split peas.
Materials and Methods
Green split peas (P. sativum) (140 g) from the USA and purchased from City Super supermarket in Hong Kong were soaked in distilled water at 4 °C overnight. The peas were then homogenized in distilled water and centrifuged (14,000×g) at 4 °C for 40 min. The supernatant was collected, followed by the addition of Tris–HCl buffer (pH 7.8) until the final Tris concentration attained 10 mM. The pea extract was then loaded onto a DEAE-cellulose column (5.5 cm × 17 cm) which had previously been equilibrated with 10 mM Tris–HCl buffer (pH 7.8). The unbound portion of the crude extract was eluted with 10 mM Tris–HCl buffer (pH 7.8) till the absorbance reading of the eluate fell below 0.1. Stepwise concentration gradient of NaCl (0.2 and 1 M) in 10 mM Tris–HCl buffer (pH 7.8) was used to elute the adsorbed proteins from the column after complete removal of unbound proteins. The eluted fractions were assayed for hemagglutination activity and the lectin-containing fraction (unbound fraction) was dialyzed against distilled water at 4 °C overnight for the next purification step. The dialyzed unbound fraction from the DEAE-cellulose column was dissolved in 10 mM ammonium acetate (NH4OAc) buffer (pH 4.5) until the final concentration of 10 mM was reached. The fraction was then applied to a cation-exchanger SP-Sepharose column (GE Healthcare) (2.5 cm × 17 cm) which had been equilibrated with 10 mM NH4OAc buffer (pH 4.5). The same buffer was applied to elute unbound proteins until OD 280 nm fell below 0.1. Stepwise gradients of different NaCl concentrations (0.2, 0.5, 1 M) in 10 mM NH4OAc buffer (pH 4.5) were applied to the column to elute adsorbed proteins from the column. A lectin-enriched fraction with hemagglutinating activity was found in the 0.2 M NaCl eluate, which was dialyzed against distilled water at 4 °C overnight to remove salt for the next purification step.
The dialyzed fraction from the SP-Sepharose column was dissolved in 10 M Tris–HCl buffer (pH 7.8) containing 200 mM glucose. The addition of glucose served to block the binding sites on the column as P. sativum lectin is mannose- and glucose-specific, and could bind to the Superdex column. The sample mixture was then applied on an FPLC-Superdex 200 HR 10/30 column (GE Healthcare) which had been previously equilibrated with 10 mM Tris–HCl buffer (pH 7.8). The sharp peak eluted in fractions 13 and 14 constituted purified lectin.
Assay of Hemagglutinating Activity
Rabbit blood was first collected and red blood cells were washed with phosphate-buffered saline (PBS, pH 7.2) to remove broken cells. The mixture was then centrifuged for 1500 rpm at 4 °C, and the supernatant was discarded. Repeated washing was preformed until the supernatant was clear. The red blood cells were resuspended in PBS to a final concentration of 2 %. The red blood cell suspension was kept at 4 °C and used within 1 week.
For the hemagglutinating assay, a serial twofold dilution of 50 μl lectin sample was preformed with an equal volume of PBS in a 96-well U-shaped microtiter plates. Fifty microliters of a 2 % (v/v) erythrocyte suspension in PBS was then added to each well at room temperature. The mixtures were allowed to incubate at room temperature for 1 h. The extent of hemagglutination was examined visually with PBS as a negative control. A diffuse mat of agglutinated erythrocytes could be observed in case of red blood cell agglutination. A clear red dot sedimented at the bottom of the well indicated negative results.
The hemagglutinating activity was expressed as a titer, which is defined as the reciprocal of the highest dilution of the test samples exhibiting visible hemagglutination activity. One hemagglutination unit (HAU) is defined as the amount of test sample which causes complete hemagglutination under the aforementioned conditions. The specific hemagglutinating activity was recorded as the number of hemagglutination units per microgram of protein [14].
Determination of Protein Concentration
This was conducted as described by Bradford [36].
Molecular Mass Estimation by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Gel electrophoresis was performed to determine the molecular masses of the lectin subunits, according to the method of Laemmli et al. [37].
Molecular Mass Determination by Size Exclusion Chromatography on a FPLC-Superdex 200 Column
Molecular mass of the purified P. sativum lectin was determined by gel filtration on FPLC-Superdex 200 column that had been calibrated with molecular mass standards (GE Healthcare) using 10 mM Tris–HCl buffer (pH 7.8) containing 200 mM glucose.
N-terminal Amino Acid Sequencing
Sequencing was carried out by Edman degradation using a Hewlett-Packard 1000A protein sequencer equipped with a high performance liquid chromatography system.
Hemagglutination Inhibition by Various Sugars
Saccharides with a high affinity toward the lectin could block the binding site of lectin from red blood cell glycoproteins, thus inhibiting hemagglutination. The sugar inhibition assay was performed in a manner analogous to the hemagglutination test. Serial twofold dilutions of 25 μl of various sugar samples (250 mM) were prepared in PBS in a 96-well U-shaped microtiter plate. Afterward, 25 μl of lectin sample with 4 hemagglutination units was added to the solution mixture. The plate was then incubated in room temperature for 30 min followed by the addition of 50 μl of 2 % rabbit erythrocyte suspension to each well. The number of wells which showed hemagglutination was counted and compared to the control, in which PBS was added instead of sugar solution. The minimum concentration of sugar required to inhibit hemagglutinating activity was calculated from the reduction in number of wells showing hemagglutination [14].
The sugars tested included glucose, sucrose, mannose, galactose, mannitol, fructose, rhamnose, melibiose, raffinose, xylose, arabinose, sorbitol, and galacturonic acid.
Effect on pH on Hemagglutinating Activity
Solutions at various pH values (pH 1–14) were prepared by titrating 1 M HCl against 1 M NaCl. The lectin sample was added and incubated at different pH value for 30 min. Afterwards, the solution mixtures were neutralized to pH 7 by addition of acidic or alkaline buffer to prevent occurrence of hemolysis at an adverse pH. Assay of hemagglutinating activity was then carried out [14].
Effect of Temperature on Hemagglutinating Activity
To examine thermostability, lectin solutions were incubated in a water bath or an electronic thermostat at various temperatures ranging from 0 to 100 °C, with 10 °C increase at each step. The solutions were taken at defined periods of time and then cooled down on ice before hemagglutinating activity was assayed.
The lectin solution was boiled to 100 °C for different durations (10, 20, 30, 60, 90, 120, 150, 180, 210 s). The solutions were then cooled down on ice before hemagglutinating activity was assayed [14].
Mitogenic Activity
Three BALB/C mice (20–25 g) were sacrificed by cervical dislocation and the spleens were aseptically removed. Splenocytes were isolated from the spleen by pressing the tissue through a sterilized 100-mesh stainless steel sieve, and resuspended to 5 × 106 cells/ml in RPMI 1640 culture medium supplemented with 10 % fetal bovine serum and 100 units penicillin/ml. The splenocytes (5 × 105 cells/100 μl/well) were then seeded into a 96-well culture plate followed by the addition of green split pea (P. sativum) lectin in serial concentrations in 100 μl medium. Cells cultured in the absence of lectin served as negative control. Concanavalin A (Con A), which is specific for glucose and mannose, was used as a positive control due to its high potency. Incubation of the splenocytes was then performed at 37 °C in a humidified atmosphere of 5 % CO2 for 72 h, 10 μl radioactive [methyl-3H]-thymidine (0.25 μCi, GE Healthcare) was added, and the splenocytes were incubated for a further 6 h under the same conditions. The splenocytes were then harvested with an automated cell harvester onto a glass fiber filter. The thymidine incorporation (radioactivity) of each well was assessed using a Beckman model LS 6000SC scintillation counter. All samples were run in triplicate and the reported values were the means of triplicate samples [38, 39].
Anti-proliferative Activity
Hepatoma (HepG2) cell line from American Type Tissue Collection was first suspended in RPMI medium at a cell density of 2 × 104 cells/ml. The cell suspension (100 μl) was seeded on a 96-well culture plate, followed by incubation at 37 °C in a humidified atmosphere of 5 % CO2 for 24 h. A series of twofold serial dilution of 100 μM green split pea (P. sativum) lectin solution in 100 μl complete RPMI medium was added to the wells. The plate was further incubated under the same conditions for 72 h. Afterward, 20 μl of a 5 mg/ml MTT formazan 7[1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan] solution in PBS was added to the wells, followed by a further incubation of 4 h under the same condition. The plate was then centrifuged at 2500 rpm for 5 min. The supernatant was removed carefully and 150 μl of dimethyl sulfoxide was added to dissolve the MTT formazan at the bottom of the wells. The plate was read at 590 nm by using a microplate reader after 15 min [40].
Antifungal Activity
Antifungal activity was performed as described in [39] in which sterile petri plates (100 × 15 mm) containing 10 ml potato dextrose agar were used. At the center of each plate, fungal mycelium of the test species (Botrytis cinerea, Fusarium oxysporum, or Mycosphaerella arachidicola) was inoculated. The plates were incubated at room temperature for 3 days for mycelial colony development. Sterile blank paper disks (0.625 cm in diameter) were placed at a distance of 0.5 cm away from the rim of the mycelial colony. Green split pea (P. sativum) lectin (8 μl) in 10 mmol/l Tris–HCl buffer (pH 7.8) was added to a disk. Buffer (8 μl) served as negative control while 8 μl of 5 mM defensin [41] was used as the positive control. The petri plates were then further incubated at room temperature until mycelial growth had enveloped the peripheral disks and crescents had formed around the positive control. Inhibition of mycelial growth seen as crescents could be observed if there was antifungal activity.
HIV-1 Reverse Transcriptase Inhibitory Activity
The assay for the lectin’s ability to inhibit HIV-1 reverse transcriptase was assessed by using a non-radioactive enzyme-linked immunosorbent assay (ELISA) kit from Boehringer Mannheim (Germany) as described [39]. The assay makes use of the synthesized DNA as a parameter for detection in which instead of radio-labeled nucleotides, optimized ratio of digoxigenin- and biotin-labeled nucleotides were used in the incorporation to the same identical DNA molecule. According to the ELISA protocol, biotin-labeled DNA bound to the surface of microtiter plate modules that have been pre-coated with streptavidin. Subsequently, an antibody conjugated to peroxidase (anti-DIG-POD) toward digoxigenin bound to the digoxigenin-labeled DNA. Lastly, peroxidase substrate was added to produce a colored reaction by the reaction of the peroxidase enzyme. Absorbance at wavelength 405 nm was read using a microtiter plate (ELISA) reader which was directly proportional to the level of RT activity. Red kidney bean lectin (Ye et al. 2001) was used as a positive control of the experiment. A fixed amount (4–6 ng) of recombinant HIV-1 reverse transcriptase was used. The inhibitory activity of green split pea lectin was calculated as percent inhibition as compared to a control without the lectin [40].
Results
Purification
Anion Exchange Chromatography on DEAE-Cellulose Column
Anion exchange chromatography of the crude P. sativum extract yielded a large unadsorbed fraction (D1) and three adsorbed fractions (D2, D3, and D4) (Supplementary Fig. 1). Hemagglutination occurred only in fraction D1.
Cation-Exchange Chromatography on SP-Sepharose Column
The unadsorbed fraction (D1) from DEAE-cellulose column was resolved into one large unbound fraction (S1) and one large adsorbed (S3) with several minor (S2, S4, S5) adsorbed fractions (Supplementary Fig. 2). Hemagglutinating activity was enriched in fraction S3 which was eluted with 0.2 M NaCl in 10 mM NH4OAc buffer (pH 4.5).
Gel Filtration on Superdex 200 Column
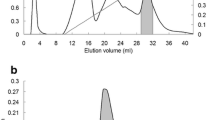
The unadsorbed fraction S3 from SP-Sepharose column was resolved into one large peak (F2) eluted at 14 ml as well as a small peak (F1) eluted at 9–10 ml (Supplementary Fig. 3). Hemagglutinating activity was concentrated in F2 which represented purified P. sativum lectin. Specific hemagglutinating activities and yields of chromatographic fractions are summarized in Table 1.
SDS-PAGE
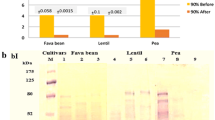
In SDS-PAGE, purified green split pea (P. sativum) lectin raised two distinct protein bands: one with a smaller molecular mass of 6 kDa (alpha chain), and the other one has a larger size of 19 kDa (beta chain) (Fig. 1).
N-terminal Amino Acid Sequencing
The sequences of P. sativum lectin alpha and beta subunits and other leguminous lectins are compared in Table 2.
Hemagglutination Inhibition by Various Carbohydrates
The lectin could be inhibited by 31.25 mM mannose or 125–250 mM glucose, sucrose, or fructose solution.
Effect of pH on Hemagglutinating Activity
The lectin showed 100 % activity from pH 3 to 12, and retained 42.9 % activity at pH 2 and 28.5 % activity at pH 13. On the other hand, at pH 1 or 14, the lectin lost its hemagglutinating activity completely.
Effect of Temperature on Hemagglutinating Activity
The hemagglutinating activity of the lectin remained unaltered after exposure to temperatures ranging from 0 to 80 °C for 30 min. At 100 °C, the lectin remained stable for 20 s. The hemagglutinating activity decreased drastically to 40 % of the original activity after 30 s and was lost completely after 50 s.
Antifungal Activity
The lectin did not exhibit any antifungal effect toward the three fungal species studied when 8 μg of the lectin was applied to a paper disk on the agar plate.
Mitogenic Activity
The maximum response was induced by green split pea (P. sativum) lectin at a concentration of 4.7 μg/ml (94 pM). On the other hand, Con A showed the strongest mitogenic activity on mouse splenocytes at a concentration of 3.9 μg/ml (32.5 pM). P. sativum lectin seemed to be less potent than Con A as a higher concentration of the former was required to induce maximum mitogenic response. However, the magnitude of the mitogenic response elicited by P. sativum lectin was greater than (about double) that induced by Con A (Fig. 2).
Anti-proliferative Activity
Figure 3 shows that the inhibition curve of P. sativum lectin started to level off beyond 50 μg/ml even with a continuous increase in concentration, implying that P. sativum lectin did not exhibit a strong anti-proliferative effect on HepG2 cells.
HIV-1 Reverse Transcriptase Inhibitory Activity
At 0.1 mg/ml, P. sativum lectin induced 64 ± 2.56 % inhibition of HIV-1 reverse transcriptase activity. The IC50 value was thus less than 2 μM.
Discussion
Green split pea (P. sativum) lectin is not adsorbed on anionic DEAE-cellulose but is adsorbed on cationic SP-Sepharose, reflecting that the lectin is basic in nature. Results of gel filtration on FPLC-Superdex 200 indicate that the lectin has a native molecular mass of 50 kDa. Nevertheless, in SDS-PAGE, two distinct protein bands could be observed with molecular mass of 6 kDa (alpha chain) and 19 kDa (beta chain), respectively, suggesting that the lectin is a heterotetrameric protein with two alpha and two beta subunits arranged into a molecular stricture of (αβ)2, making up a native molecular mass of 50 kDa. The two subunits dissociated in the presence of SDS and β-mercaptoethanol suggesting that the subunits interacted non-covalently. In general, many legume lectins are composed of four subunits and exist as homotetramers or heterotetrameters [41, 42].
The sequences of the first 15 N-terminal amino acids in alpha and beta subunits of split pea lectin are TETTS FLITK FSPDG and VTSYT LSDVV SLKDV, respectively. The alpha chain exhibits 100 % identity with pea lectin (Lathyrus sativus). On the other hand, the beta chain shows 100 % identity toward chickpea lectin (Cicer arietinum). Indeed, the two lectins were quite similar in their biological properties as both green split peas and chickpea possess mitogenic and anti-HIV reverse transcriptase activity. Nevertheless, chickpea lectin also possesses antifungal activity whereas green split pea lectin does not [39].
Many lectins possess mitogenic activity, such as GNL-1 (a lectin from great northern beans) haricot bean agglutinin, PHA-L4 (a lectin from kidney beans), red kidney bean lectin, pinto bean lectin, and Voodoo lily lectin [24, 34, 35]. Green split pea lectin is also capable of eliciting a mitogenic response from mouse splenocytes. The concentration of the lectin required to induce maximal response is higher than that of the positive control Concanavalin A, yet the magnitude of maximal response is even greater than its counterpart, a well known mitogen. Split pea lectin exerts significant effect at a relatively low dose of 4.7 μg/ml which suggests that it may be possible for the lectin to be further developed into a drug.
The hemagglutinating activity of split pea lectin could be inhibited by 31.25 mM mannose or 125–150 mM glucose or sucrose solution, implying that the lectin was glucose/mannose-specific. As lectins are protein in nature, they could be adversely affected by high temperatures or pH [43]. Nevertheless, from the results of the present study, split pea lectin retains its hemagglutinating activity upon exposure to a wide range of pH (pH 3–pH 12) or after incubation at 80 °C for 30 min. The high stability of split pea lectin may be due to the absence of disulfide linkages, absence of heat-sensitive tryptophan residues, or due to the presence of a heat-stable structure, suggesting a possibility of its application in industrial processing.
Split pea lectin is devoid of antifungal activity against B. cinerea, F. oxysporum, and M. arachidicola. Some lectins have antifungal activity [11, 44], but other lectins lack antifungal activity [31, 33, 35]. In addition, P. sativum lectin does not exhibit pronounced anti-proliferative activity toward HepG2 cells. Previously, a pea lectin has been shown to suppress growth of Ehrlich ascites carcinoma cells [45], but some lectins are devoid of anti-proliferative activity toward cancer cells [45]. It remains to be seen whether split pea lectin inhibits other cancer cells.
Similar to some leguminous lectins such as red kidney bean lectin and knife bean lectin [33, 39], positive results are achieved by split pea lectin in the assay for HIV-1 reverse transcriptase inhibitory activity. The mechanism involved is probably protein-protein interaction [46].
Lectin from the sugar snap variety of P. sativum was unadsorbed on Affi-gel Blue gel, adsorbed on Q-Sepharose, and unadsorbed on SP-Toyopearl. Sugar snap lectin was identical in N-terminal sequences of its alpha- and beta subunit to split pea lectin and almost identical to lectin from P. sativum L. var. Feltham First except for the 19th N-terminal residue of the beta subunit. The molecular weights of the alpha- and beta subunits of lectins from the different cultivars are similar. Sugar snap lectin was devoid of antifungal activity, similar to split pea lectin. Hence, unlike lectins from different cultivars of Phaseolus vulgaris which may manifest differences, lectins from different cultivars of P. sativum are very similar to each other.
References
André, S., Kaltner, H., Manning, J. C., Murphy, P. V., & Gabius, H. J. (2015). Lectins: getting familiar with translators of the sugar code. Molecules, 20, 1788–1823.
Peumans, W. J., & Van Damme, E. J. (1995). Lectins as plant defense proteins. Plant Physiology, 109, 347–352.
Van Damme, E. J., Peumans, W. J., Pusztai, A., & Bardocz, S. (1998). Handbook of plant lectins: properties and biomedical applications. England: Wiley.
Van Damme, E. J. (2014). History of plant lectin research. Methods in Molecular Biology, 1200, 3–13.
Bhattacharyya, L., Brewer, C. F., Brown, R. D., 3rd, & Koenig, S. H. (1985). Preparation and properties of metal ion derivatives of the lentil and pea lectin. Biochemistry, 24, 4974–4980.
Biswas, S., & Kayastha, A. M. (2002). Thermal stability of Phaseolus vulgaris leucoagglutinin: a differential scanning calorimetry study. Journal of Biochemistry and Molecular Biology, 35, 472–475.
Bohlool, B. B., & Schmidt, E. L. (1974). Lectins: a possible basis for specificity in Rhizobium-legume root module symbiosis. Science, 188, 296–271.
Macedo, M. L., Oliveira, C. F., & Oliveira, C. T. (2015). Insecticidal activity of plant lectins and potential application in crop protection. Molecules, 20, 2014–2033.
Kenmochi, E., Kabir, S. R., Ogawa, T., Naude, R., Tateno, H., Hirabayashi, J., & Muramoto, K. (2015). Isolation and biochemical characterization of apios tuber lectin. Molecules, 20, 987–1002.
Singh, S. S., Devi, S. K., & Ng, T. B. (2014). Banana lectin: a brief review. Molecules, 19, 18817–18827.
Dias, R. O., Machado, L. D., Migliolo, L., & Franco, O. L. (2015). Insights into animal and plant lectins with antimicrobial activities. Molecules, 20, 519–541.
Van Holle, S., & Van Damme, E. J. (2015). Distribution and evolution of the lectin family in soybean (Glycine max). Molecules, 20, 2868–2891.
Schlick, K. H., Udelhoven, R. A., Strohmeyer, G. C., & Cloninger, M. J. (2005). Binding of mannose-functionalized dendrimers with pea (Pisum sativum) lectin. Molecular Pharmaceutics, 2, 295–301.
Zhang, W., Tian, G., Geng, X., Zhao, Y., Ng, T. B., Zhao, L., & Wang, H. (2014). Isolation and characterization of a novel lectin from the edible mushroom Stropharia rugosoannulata. Molecules, 19, 19880–19891.
Singh, S. S., Wang, H., Chan, Y. S., Pan, W., Dan, X., Yin, C. M., Akkouh, O., & Ng, T. B. (2014). Lectins from edible mushrooms. Molecules, 20, 446–469.
Mo, H., Winter, H. C., & Goldstein, I. J. (2000). Purification and characterization of a Neu5Acα2-6Galβ1-4Glc/GlcNAc-specific lectin from the fruiting body of the polypore mushroom Polyporus squamosus. Biological Chemistry, 275, 10623–10629.
Tronchin, G., Esnault, K., Sanchez, M., Larcher, G., Marot-Leblond, A., & Bouchara, J. P. (2002). Purification and partial characterization of a 32-kilodalton sialic acid-specific lectin from Aspergillus fumigatus. Infection and Immunity, 70, 6891–6895.
Watanabe, M., Nakamura, O., Muramoto, K., & Ogawa, T. (2012). Allosteric regulation of the carbohydrate-binding ability of a novel conger eel galectin by D-mannoside. The Journal of Biological Chemistry, 287, 31061–31072.
Liu, Y., Liu, J., Pang, X., Liu, T., Ning, Z., & Cheng, G. (2015). The roles of direct recognition by animal lectins in antiviral immunity and viral pathogenesis. Molecules, 20, 2272–2295.
García, M. A., Gutiérrez-Kobeh, L., & Vancell, R. L. (2015). Entamoeba histolytica: adhesins and lectins in the trophozoite surface. Molecules, 20, 2802–2815.
Mason, C. P., & Tarr, A. W. (2015). Human lectins and their roles in viral infections. Molecules, 20, 1229–2271.
Nathan, S., & Lis, H. (2004). History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology, 14, 53–62.
Al Atalah, B., Smagghe, G., & Van Damme, E. J. (2014). Orysata, a jacalin-related lectin from rice, could protect plants against biting-chewing and piercing sucking insects. Plant Science, 221–222, 21–28.
SinghBains, J., Singh, J., Nijjar, K. K., Agrewala, J. N., Kumar, V., Kumar, A., & Saxena, A. K. (2005). Mitogenic and anti-proliferative activity of a lectin from the tubers of Voodoo lily (Sauromatum venosum). Biochimica et Biophysica Acta - General Subjects, 123, 163–174.
Chapman, J. S., Jefferies, L. K., & Pike, O. A. (2010). Sensory and nutritional quality of split peas (Pisum sativum) stored up to 34 y in residential storage. Journal of Food Science, 75, S162–S166.
Drivelos, S. A., Higgins, K., Kalivas, J. H., Haroutounian, S. A., & Georgiou, C. A. (2014). Data fusion for food authentication. Combining rare earth elements and trace metals to discriminate “Fava Santorinis” from other yellow split peas using chemometric tools. Food Chemistry, 165, 316–322.
Bessey, W. C., & Woods, E. (1952). Effect of preparation method on thiamine availability in split-pea soup. Journal of the American Dietetic Association, 28, 235–237.
Klein, B. P. (1976). Isolation of lipoxygenase from split pea seeds, snap beans, and peas. Journal of Agricultural and Food Chemistry, 24, 938–942.
Dabai, F. D., Walker, A. F., Sambrook, I. E., Welch, V. A., Owen, R. W., & Abeyasekera, S. (1996). Comparative effects on blood lipids and faecal steroids of five legume species incorporated into a semi-purified, hypercholesterolaemic rat diet. The British Journal of Nutrition, 75, 557–571.
Ye, X., & Ng, T. B. (2001). Isolation of lectin and albumin from Pisum sativum var. macrocarpon ser. cv. sugar snap. The International Journal of Biochemistry & Cell Biology, 33, 95–102.
Ang, A. S., Cheung, R. C., Dan, X., Chan, Y. S., Pan, W., & Ng, T. B. (2014). Purification and characterization of a glucosamine-binding antifungal lectin from Phaseolus vulgaris cv. Chinese pinto beans with antiproliferative activity towards nasopharyngeal carcinoma cells. Applied Biochemistry and Biotechnology, 172, 672–686.
Fang, E. F., Lin, P., Wong, J. H., Tsao, S. W., & Ng, T. B. (2010). A lectin with anti-HIV-1 reverse transcriptase, antitumor, and nitric oxide inducing activities from seeds of Phaseolus vulgaris cv. extralong autumn purple bean. Journal of Agricultural and Food Chemistry, 58, 2221–2229.
Sharma, A., Wong, J. H., Lin, P., Chan, Y. S., & Ng, T. B. (2010). Purification and characterization of a lectin from the Indian cultivar of French bean seeds. Protein and Peptide Letters, 17, 221–227.
Ye, X. Y., Ng, T. B., Tsang, P. W., & Wang, J. (2001). Isolation of a homodimeric lectin with antifungal and antiviral activities from red kidney bean (Phaseolus vulgaris) seeds. Journal of Protein Chemistry, 20, 367–375.
Wong, J. H., Wong, C. T., & Ng, T. B. (2006). Purification and characterization of a galactose-specific lectin with mitogenic activity from pinto beans. Biochimica et Biophysica Acta, 1760, 808–813.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Laemmli, K., & Favre, M. (1973). Gel electrophoresis of proteins. Journal of Molecular Biology, 80, 575–599.
Chan, Y. S., Wong, J. H., Fang, E. F., Pan, W., & Ng, T. B. (2012). Isolation of a glucosamine binding leguminous lectin with mitogenic activity towards splenocytes and anti-proliferative activity towards tumor cells. PloS One, 7, e38961.
Wong, J. H., & Ng, T. B. (2005). Isolation and characterization of a glucose/mannose/rhamnose-specific lectin from the knife bean Canavalia gladiata. Archives of Biochemistry and Biophysics, 439, 91–98.
Lin, P., Wong, J. H., Ng, T. B., Ho, V. S., & Xia, L. (2013). A sorghum xylanase inhibitor-like protein with highly potent antifungal, antitumor and HIV-1 reverse transcriptase inhibitory activities. Food Chemistry, 141, 2916–2922.
Brinda, K. V., Mitra, N., Surolia, A., & Vishveshwara, S. (2004). Determinants of quaternary association in legume lectins. Protein Science, 13, 1735–1749.
Cavada, B. S., da Silva, L. I. M. M. M., Ramos, M. V., Galvani, F. R., Grangeiro, T. B., Leite, K. B., Assreuy, A., Cajazeiras, J. B., & Calvete, J. (2003). Seed lectin from Pisum arvense: isolation, biochemical characterization and amino acid sequence. Protein and Peptide Letters, 10, 607–617.
Mitra, N., Srinivas, V. R., Ramya, T. N., Ahmad, N., Reddy, G. B., & Surolia, A. (2002). Conformational stability of legume lectins reflect their different modes of quaternary association: solvent denaturation studies on concanavalin A and winged bean acidic agglutinin. Biochemistry, 41, 9256–9263.
Lam, S. K., & Ng, T. B. (2010). Isolation and characterization of a French bean hemagglutinin with antitumor, antifungal, and anti-HIV-1 reverse transcriptase activities and an exceptionally high yield. Phytomedicine, 17, 457–462.
Kabir, S. R., Nabi, M. M., Haque, A., Rokon, U., Reza, M. A., & Zaman, M. Z. H. (2013). Pea lectin inhibits growth of Ehrlich ascites carcinoma cells by inducing apoptosis and G2/M cell cycle arrest in vivo in mice. Phytomedicine, 20, 1288–1296.
Bottcher, M., & Grosse, F. (1997). Protease inhibits its homologous reverse transcriptase by protein-protein interaction. Nucleic Acids Research, 25, 1709–1714.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supp. Fig. 1
Elution profile of crude extract of green split peas on a DEAE-cellulose column (GIF 12 kb)
Supp. Fig. 2
Elution profile of Fraction D1 on an SP-Sepharose column (GIF 12 kb)
Supp. Fig. 3
Elution profile of Fraction S3 on an FPLC-Superdex 200 column (GIF 7 kb)
Rights and permissions
About this article
Cite this article
Ng, T.B., Chan, Y.S., Ng, C.C.W. et al. Purification and Characterization of a Lectin from Green Split Peas (Pisum sativum). Appl Biochem Biotechnol 177, 1374–1385 (2015). https://doi.org/10.1007/s12010-015-1821-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1821-x