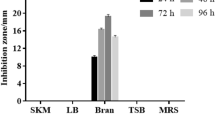
Abstract
Lactic acid bacteria are known to produce numerous antimicrobial compounds that are active against various pathogens. Here, we have purified and characterized a novel low-molecular-weight (LMW) antimicrobial compound produced by Lactobacillus and Pediococcus isolated from fermented idly and uttapam batter. The LMW compound was extracted from cell-free supernatant using ice-cold acetone, purified by gel permeation and hydrophobic interaction chromatography. It exhibited antimicrobial activity against Gram-positive and Gram-negative pathogenic bacteria sparing the probiotic strains like Lactobacillus rhamnosus. The molecular weight of the LMW compound was identified as 204 Da using LC-MS-ESI. In addition, the structure of the compound was predicted using spectroscopic methods like FTIR and NMR and identified as 2-hydroxyl indole-3-propanamide. The LMW compound was differentiated from its related compound, tryptophan, by Salkowski reaction and thin-layer chromatography. This novel LMW compound, 2-hydroxyl indole-3-propanamide, may have an effective application as an antibiotic which can spare prevailing probiotic organisms but target only the pathogenic strains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lactic acid bacteria (LAB) are a group of generally recognized as safe (GRAS) organisms which produce several low-molecular-weight (LMW) compounds acting as antimicrobials and/or antifungals and flavoring agents that enhance the stability of food products. They produce several metabolites like diacetyl, acetoin, butanediol, flavone, organic acids, and various volatile components depending on the sources of fermentation [1]. During fermentation process, they metabolize certain flavonol glycoside in the plant materials into 4-hydroxybenzoic acid [2] and gallic acid [3] that exhibited antimicrobial activity [2] and antioxidant activity [3]. Some Lactobacillus spp. and Pediococcus spp. were known to produce 2-pyrrolidone-5-carboxylic acid that exerts antimicrobial activity against spoilage bacteria [4]. Also, other LMW compounds like benzoic acid, glycyl-l-leucine, methyl hydantoin, mevalonolactone [5], 5-hydroxyl ferulic acid [6], 3-phenyllactic acid [7], 3-hydroxydecanoic acid [2], hydroxyl fatty acid [8], and more exhibited antimicrobial activity.
Reuterin, chemically known as β-hydroxypropionaldehyde produced by Lactobacillus reuteri, was a non-peptide broad-spectrum antimicrobial compound synthesized from glycerol exhibiting broad-spectrum antimicrobial activity against prokaryotes and eukaryotes [9]. Reuterin as an analogue of d-ribose also inhibits B1 subunit of ribonucleotide reductase and thioredoxin, suggesting its ability to inhibit sulfhydryl enzymes [10]. Although it was first identified from the Lactobacillus reuteri, it was also produced by other LAB like Lactobacillus brevis, Lactobacillus buchneri, Lactobacillus collinoides, and Lactobacillus coryniformis [11]. In addition, bacteriocins are well-known proteinaceous antimicrobial compounds widely studied for their use as food preservatives [12]. However, other antimicrobial compounds produced by LAB have not been evaluated extensively, as they are not produced in high concentrations in the medium. In this study, a successful attempt was made in isolation and purification of low-molecular-weight (LMW) antimicrobial compound produced by Pediococcus pentosaceus VJ13 [13], Lactobacillus plantarum JJ18, and Lactobacillus plantarum subsp. plantarum JJ60 [14] isolated from fermented idly batter, and Lactobacillus pentosus SJ65 [15] and P. pentosaceus PJ7 isolated from fermented uttapam batter. All these isolates produced the same compound which was identified by spectroscopic methods, and its antimicrobial activity was evaluated against various pathogens and probiotic organisms.
Materials and Methods
Chemicals and Media
All the chemicals and media were procured from HiMedia, Mumbai, while indole-3-lactic acid was purchased from Sigma, Bangalore, and dl-tryptophan from SRL, Mumbai. The solvents procured for HPLC, NMR, and FTIR are from Merck India, Mumbai.
Microbial Strains
The indicator strains used in this study were procured from Microbial Type Culture Collection (MTCC), Institute of Microbial Technology, Chandigarh, India. All LAB were maintained and propagated in de Man, Rogosa, and Sharpe (MRS)/Brain Heart Infusion (BHI) whereas pathogenic organisms in Tryptone Soy Broth (TSB)/Luria Bertani (LB). The cultures and isolates were stored at −20 °C with 20 % glycerol.
Accession Numbers
The isolates P. pentosaceus VJ13 [13], Lactobacillus plantarum JJ18, and Lactobacillus plantarum subsp. plantarum JJ60 [14] isolated from idly batter and Lactobacillus pentosus SJ65 [15] and P. pentosaceus PJ7 isolated from uttapam batter. The 16S rRNA gene sequences of these isolates from batter were deposited in GenBank (www.ncbi.nlm.nih.gov/genbank/) whose accession numbers were JN573609, JN573601, JN573602, JN573623, and KC439681.
Extraction and Purification of LMW Antimicrobial Compound
The isolates were grown in 1 L of de Man, Rogosa, and Sharpe (MRS) broth with 0.5 % of inoculum of overnight culture for 36 h at 37 °C under static condition, and the cell-free supernatant (CFS) was collected by centrifugation at 8000 × g for 15 min at 4 °C. The CFS was concentrated to 10-fold (100 mL) in rotavapor (Buchi, Flawil, Switzerland), and ice-cold acetone was added to attain 70 % (v/v) saturation initially. The supernatant was collected by centrifugation at 8000 × g for 20 min at –4 °C and was further subjected to 85 % (v/v) saturation with ice-cold acetone and incubated at –4 °C. After 2 h of incubation, the supernatant was collected by centrifugation at 8000 × g for 10 min at –4 °C. The acetone in the supernatant was removed completely using rotavapor (Buchi, Flawil), and the extract was dissolved in 9 mL of 5 mmol L−1 of acetate buffer, pH 5.2 (i.e., approximately 111-fold concentration of original CFS), which was then passed through Sephadex G25 (100-cm × 2-cm column) pre-equilibrated with 5 mmol L−1 acetate buffer, and the fractions were collected at the flow rate of 0.5 mL min−1 with same buffer. The fractions were monitored at 280 and 260 nm and evaluated for antimicrobial activity against Staphylococcus aureus and Listeria monocytogenes by agar well diffusion assay [16]. The active fractions were pooled and passed through Sephadex G15 (50-cm × 1.8-cm column) pre-equilibrated with 5 mmol L−1 of acetate buffer, pH 5.2, to obtain a single homogenous peak and eluted at the flow rate of 0.5 mL min−1. The fractions from Sephadex G15 were further purified by solid-phase extraction technique for spectroscopic studies. The sample was passed through Sep-Pak C18 (Waters, Bangalore) cartridge pre-equilibrated with water having 0.1 % trifluoroacetic acid (TFA) and was washed with 10 % acetonitrile (ACN) having 0.1 % TFA to remove the contaminants while the fraction eluted with 60 % ACN containing 0.1 % TFA after removal of solvent using SpeedVac (Christ, Osterode) concentrator showed antimicrobial activity against S. aureus and Listeria monocytogenes by agar well diffusion assay. This active fraction was lyophilized and used for further studies.
Antimicrobial Spectrum of LMW
The LMW fraction obtained from Sephadex G15 was evaluated for further antibacterial activity against various organisms as indicated in Table 3 using agar well diffusion method [16].
Determination of Molecular Mass of LMW
The lyophilized LMW was dissolved in 0.5 mL of water, and 50 μL was applied to HPLC (Waters 600, Millipore Corp., Milford, MA) connected with reverse-phase column C18 (Luna 5 μm, 100 Å, 30 × 2 mm), pre-equilibrated with 3 % of ACN having 0.1 % TFA. Fractions were eluted using water having 0.1 % TFA (A) and ACN having 0.1 % TFA (B) in gradient of 0 to 15 min with 3 % B and 16 to 40 min with 60 % B. The flow rate was maintained at 1.5 mL min−1, and fractions were monitored at 215 nm. The mass of the compound was identified on a liquid chromatography equipped with diode array (Waters 600, Millipore Corp., Milford, MA) connected to time-of-flight mass spectrometer using QStar pulsar (Applied Biosystems, Foster City, CA). The compound was applied (10 μL) to the C18 column and eluted using the above-mentioned mobile phase and gradient at the flow rate of 1.5 mL min−1. The liquid chromatography (LC)-MS/time-of-flight (TOF) parameters used were as follows: end plate offset voltage, 500 V; capillary voltage, 4500 V; nebulizer pressure, 1.2 bar; dry gas flow, 6 L min−1; dry temperature, 200 °C; collision energy, 18 eV/z; transfer time, 100 μs; and collision RF, 400 Vpp.
UV Absorption Spectrum of the LMW
The lyophilized LMW was dissolved in water and scanned for absorption spectrum at UV region using UV/vis spectroscopy (Shimadzu, Japan) from 200 to 300 nm.
FTIR Spectroscopy
The Sep-Pak purified fraction was lyophilized, and 1 mg of sample was grounded with 100 mg of KBr to get a fine powder and made as pellet. The FTIR spectrum was recorded in the frequency range of 4000 to 400 cm−1 using Nicolet 6700 FTIR spectrometer (Thermo, Madison, WI).
NMR Spectroscopy
The NMR spectrum was recorded with Bruker 400-MHz spectrophotometer at 295 K. The lyophilized Sep-Pak purified sample was dissolved in D2O for deuterium exchange and analyzed for 1H and 13C NMR. The CH, CH2, and CH3 were identified by distortionless enhancement by polarization transfer (DEPT) analysis. For the identification of hydroxyl group, the lyophilized LMW was dissolved in DMSO and OH exchange was done with a drop of D2O. The spectrum was recorded before and after exchange with D2O. The chemical shift was recorded using tetramethylsilane (TMS) as internal reference.
Salkowski Reaction
The LMW, tryptophan, and indole-3-lactic acid (5 mg mL−1) were treated with Salkowski reagent and incubated for 30 min to differentiate the indole derivatives [17].
Thin-Layer Chromatography
The TLC plate was prepared with the uniform slurry of silica gel GF in water (2:5) and poured in glass plates with the thickness of 2 mm. After activation at 100 °C for 2 h, the LMW sample (having molecular mass similar to tryptophan) and tryptophan (5 mg mL−1) was applied on the TLC plate with applicator. After air-drying, the plate was developed in chamber saturated with acetone/ammonia/isopropanol (0.45:1.25:5.6) and the spots were identified using iodine vapors.
Results and Discussion
The demand for antimicrobial compounds is constantly growing with regard to the threat caused by pathogenic strains; hence, purification and characterization of novel antimicrobial compounds produced by LAB are currently being focused. In recent years, many organic compounds produced by LAB that were able to inhibit other microbes were identified [18]. Idly and uttapam batter are indigenous south Indian fermented food sources from which various organisms were isolated and characterized [13–15] that exhibited effective antimicrobial and probiotic properties [19–21].
In this study, a novel antimicrobial compound produced by the LAB isolates was extracted from cell-free supernatant (CFS) with 85 % acetone, purified, and characterized. The antimicrobial compounds from crude 85 % supernatant were purified by passing through gel filtration chromatography Sephadex G25 followed by Sephadex G15, which gave a Gaussian peak with the elution volume (Ve) of 34 mL with no contaminants of higher molecular weight (data not shown). Further purification by hydrophobic interaction chromatography yielded a pure compound as depicted by LC profile (Fig. 1a). In this study, the 85 % supernatant after acetone precipitation was considered as crude LMW fraction, as CFS from LAB culture in MRS broth has bacteriocin as the major antimicrobial compound [22–24]. This LMW was present in very low concentration in the CFS as reflected by absence of antimicrobial activity after protease treatment of CFS. The supernatant obtained after precipitation of bacteriocin by acetone (85 %) and concentrated to 9 mL (111-fold concentrate of original CFS) showed antimicrobial activity (taken as crude). This upon further purification resulted in a yield of 11 % (Supplementary file S1). The purified LMW compound was active at a limited range of pH, up to pH 5.5 while the activity was lost at pH 6.5. A similar observation was made in an earlier study where a fatty acid derivative lost its antimicrobial activity at the pH of 6.0 [25]; however, certain fatty acid derivative compounds exhibited antifungal activity [8] while LMW in the present study did not exhibit any antifungal activity.
The physiochemical properties of the purified compound are given in Table 1. The compound was readily soluble in polar solvents like water, methanol, and acetone but insoluble in chloroform and benzene.
The Sep-Pak purified sample had retention time of 23 min. The ESI-MS showed a base peak at 205.61 Da [M + H] with fragmentation pattern of m/z 188 (M + H) and 146 (M + H) (Fig. 1b). The fragmentation pattern corresponds to loss of NH3 (17 Da), ethanone (42 Da), and CO (28 Da,) suggesting that the compound exhibited fragmentation except indole ring (117 Da)and correlate with the structure of the compound. The determined molecular weight of the LMW was 205.614 Da (M + H) using TOF-MS-ES+, and the calculated molecular mass was 205.225 Da (M + H). Although the reason for the difference in the molecular mass is not known exactly, it might be of the limitations of instrument and its resolution. Since further spectroscopic studies have confirmed the structure of the LMW compound, high-resolution MS for the compound was not performed.
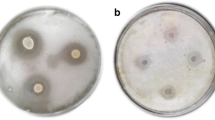
The LMW isolated from all isolates exhibited absorption maximum (λmax) in H2O at 287.6 and 279.2 nm (Fig. 2) which was also similar to tryptophan, suggesting that the compound might be a derivative of tryptophan. The FTIR spectra of LMW compounds from all isolates have shown similar pattern as depicted in Fig. 3, suggesting the presence of similar functional groups, and the data are correlated in Table 2. Thus, LMW compounds produced by all isolates were same. The 13C NMR of LMW showed presence of 11 carbons of which 8 are in aromatic region. The 13C NMR report was (100 MHz, D2O) δ (174.44, 136.27, 126.58, 124.96, 122.07, 119.40, 118.38, 111.88, 107.42, 55.01, 26.33) (Supplementary file S2). The proton NMR analysis report was 1H NMR (400 MHz, deuterium oxide) δ 7.73 (dd, J = 8.0, 1.2 Hz, 1H), 7.57–7.50 (m, 1H), 7.33–7.15 (m, 3H), 4.05 (dd, J = 8.1, 4.8 Hz, 1H), 3.53–3.43 (m, 1H), and 3.30 (dd, J = 15.3, 8.1 Hz, 1H) (Supplementary file S3). The DEPT 135 analysis showed inverted peak at δ −26.3, and the peak at δ −55 confirmed the presence of CH2 and CH in the alkyl carbon (Supplementary file S4). The FTIR analysis showed the presence of amide and hydroxyl group coupled with CH. The 13C NMR confirmed the presence of 11 carbons in the LMW, out of which 8 are in heterocyclic ring indicating an indole derivative. The peaks in the range of 7 to 8 and 100 to 140 in 1H NMR and 13C NMR, respectively, correspond to the heterocyclic ring. The presence of aliphatic CH stretch is confirmed in 1H NMR by the peaks in the range of 3.2 to 3.5 and the peaks in the range of 4.0 corresponding to –CH (OH)– stretch. In 13C NMR, the presence of carbonyl and its derivatives was determined by the presence of peak in 174, carbon linked with hydroxyl group by the peak at 55 [26], and the presence of CH2 in aliphatic chain is determined by the peak at 26. To further confirm the hydroxyl group in LMW, after recording in DMSO, the hydroxyl exchange was done with addition of a few drops of D2O into DMSO and the spectrum recorded again. The peak at δ 8.19 in DMSO disappeared after addition of D2O, suggesting the presence of hydroxyl group, while that at δ 11.04 suggesting the presence of –NH– group, thus confirming the compound (Supplementary file S5). Thus, the 1H, 13C, and HMBC spectra (Supplementary files S2 to S6) have established the structure of LMW as 2-hydroxyl indole-3-propanamide and are depicted in Fig. 4. The tryptophan, indole-3-lactic acid (ILA), and the LMW were differentiated with Salkowski reagent (Fig. 5a). The LMW gave orange color, while tryptophan gave yellow color. Further differentiation of tryptophan and the LMW was achieved by thin-layer chromatography (TLC) using acetone/ammonia/isopropanol (0.45:1.25:5.6) (Fig. 5b).
For LMW and tryptophan having similar molecular mass, NMR and FTIR spectra were differentiated by TLC and Salkowski color reaction. Many indole compounds and their derivatives were well known to exhibit antimicrobial activity [27, 28]. Indole derivatives have been identified as secondary metabolites acting as potent antimicrobials from several sources especially from plants, microbes, and marine sources. The salient role of indole primarily involves signaling molecules in acid resistance, biofilm formation, and more [29]. Earlier studies in Bifidobacterium have demonstrated ILA, a metabolite from tryptophan exhibiting antifungal activity [30].
Indole propanamide derivative was known to exhibit antimicrobial and antiviral activity [27], and chemically derived indole-3-propanamide also acts as an immunosuppressive agent [31]. The LMW exhibited a wide spectrum of activity against various LAB and pathogens as summarized in Table 3. The LMW was not active against probiotic strains like Lactobacillus rhamnosus, Lactobacillus Plantarum, and Lactobacillus brevis. On the other hand, it exhibited effective inhibition against various food-borne pathogens like Listeria monocytogenes and S. aureus, while less effective against Escherichia coli and Aeromonas hydrophila. Although various compounds produced during fermentation by LAB were studied [2], this is the first to report production of 2-hydroxyl indole-3-propanamide exhibiting antibacterial activity. In recent years, some LAB were reported to produce certain other antimicrobial compounds like benzene acetic acid and 2-propenyl ester exhibiting antifungal activity [32]. This LMW isolated has effective antibacterial activity against Gram-positive and Gram-negative bacteria but spares the probiotic strains like Lactobacillus rhamnosus, suggesting that the compound can be used against clinical pathogens without harming the resident probiotics. The probable active moiety that exhibits antimicrobial activity of the LMW may be the amide group which is a strong nucleophile-attacking molecule. Even though, in the present study, the LMW compound exhibited its activity at the concentration of 50 μM, further substitutions in the side chains of indole might enhance the antimicrobial activity and stability of the compound in alkaline conditions.
Conclusion
Thus, 2-hydroxyl indole-3-propanamide from LAB strains isolated in this study showed broad-spectrum antibacterial activity against various Gram-positive and Gram-negative pathogenic organisms sparing the probiotic organisms like Lactobacillus rhamnosus, may have the potential as antibiotics, and play a significant role in maintenance of human health. As the molecular weight of LMW being 204 Da, it may be of use as antibiotic for several clinical applications. Further, using this compound as a lead molecule, additional quantitative structure–activity relationship (QSAR) studies, which quantitate structure and activity of a molecule, may yield a better understanding of molecule and its activity against other pathogenic organisms. Hence, the identified compound could have significant application in medicine.
References
Smid, E. J., & Kleerebezem, M. (2014). Production of aroma compounds in lactic fermentations. Annual Review of Food Science and Technology, 5, 313–326.
Broberg, A., Jacobsson, K., Strom, K., & Schnurer, J. (2007). Metabolite profiles of lactic acid bacteria in grass silage. Applied and Environmental Microbiology, 73, 5547–5552.
Duckstein, S. M., Lorenz, P., & Stintzing, F. C. (2012). Conversion of phenolic constituents in aqueous Hamamelis virginiana leaf extracts during fermentation. Phytochemical Analysis, 23, 588–597.
Yang, Z., Suomalainen, T., Mayra-Makinen, A., & Huttunen, E. (1997). Antimicrobial activity of 2-pyrrolidone-5-carboxylic acid produced by lactic acid bacteria. Journal of Food Protection, 60, 786–790.
Niku-Paavalo, M. L., Laitila, A., Mattila-Sandholm, T., & Haikara, A. (1999). New type of antimicrobial compounds produced by Lactobacillus plantarum. Journal of Applied Microbiology, 86, 29–35.
Knockaert, D., Raes, K., Wille, C., Struijsa, K., & Van Camp, J. (2012). Metabolism of ferulic acid during growth of Lactobacillus plantarum and Lactobacillus collinoides. Journal of the Science of Food and Agriculture, 92, 2291–2296.
Rodriguez, N., Salgado, J. M., Cortes, S., & Domínguez, J. M. (2012). Antimicrobial activity of D-3-phenyllactic acid produced by fed-batch process against Salmonella enterica. Food Control, 25, 274–284.
Sjogren, J., Magnusson, J., Broberg, A., Schnurer, J., & Kenne, L. (2003). Antifungal 3-hydroxy fatty acids from Lactobacillus plantarum MiLAB 14. Applied and Environmental Microbiology, 69, 7554–7557.
Chung, T. C., Axelsson, L., Lindgren, S. E., & Dobrogosz, W. J. (1989). In vitro studies on reuterin synthesis by Lactobacillus reuteri. Microbial Ecology in Health and Disease, 2, 137–144.
El-Ziney, M. G., Debevere, J. M., & Jakobsen, M. (2000). In A. S. Naidu (Ed.), Natural food antimicrobial systems (pp. 567–588). Florida: CRC Press LLC.
Ganzle, M. G., Zhang, C., Monang, B. S., Lee, V., & Schwab, C. (2009). Novel metabolites from cereal-associated lactobacilli—novel functionalities for cereal products? Food Microbiology, 26, 712–719.
Jeevaratnam, K., Jamuna, M., & Bawa, A. S. (2005). Biological preservation of foods-bacteriocins of lactic acid bacteria. Indian Journal of Biotechnology, 4, 446–454.
Vidhyasagar, V., & Jeevaratnam, K. (2012). Isolation and characterization of Pediococcus pentosaceus from idly batter: a traditional South Indian fermented food source. Biosciences, Biotechnology Research Asia, 9, 427–431.
Agaliya, P. J., & Jeevaratnam, K. (2013). Molecular characterization of lactobacilli isolated from fermented idli batter. Brazilian Journal of Microbiology, 44, 1199–1206.
Saraniya, A., & Jeevaratnam, K. (2012). Molecular characterization of bacteriocinogenic Lactobacillus species isolated from fermented Uttapam batter. Biosciences, Biotechnology Research Asia, 9, 417–421.
Parente, E., Brienza, C., Moles, M., & Ricciardi, A. A. (1995). A comparison of methods for the measurement of bacteriocin activity. Journal of Microbiological Methods, 22, 95–108.
Ehmann, A. (1977). The van Urk-Salkowski reagent—a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. Journal of Chromatography A, 1977, 267–276.
Stoyanova, L. G., Ustyugova, E. A., & Netrusov, A. I. (2012). Antibacterial metabolites of lactic acid bacteria: their diversity and properties. Applied Biochemistry and Microbiology, 48, 229–243.
Agaliya, P. J., & Jeevaratnam, K. (2012). Screening of Lactobacillus plantarum isolated from fermented idli batter for probiotic properties. African Journal of Biotechnology, 11, 12856–12864.
Saraniya, A. and Jeevaratnam, K. (2014) In vitro probiotic evaluation of phytase producing Lactobacillus species isolated from Uttapam batter and their application in soy milk fermentation. J Food Sci Technol, 1-10.
Vidhyasagar, V., & Jeevaratnam, K. (2013). Evaluation of Pediococcus pentosaceus strains isolated from idly batter for probiotic properties in vitro. Journal of Functional Foods, 5, 235–243.
Agaliya, P. J., & Jeevaratnam, K. (2013). Characterisation of the bacteriocins produced by two probiotic Lactobacillus isolates from idli batter. Annals of Microbiology, 63, 1525–1535.
Saraniya, A., & Jeevaratnam, K. (2014). Purification and mode of action of antilisterial bacteriocins produced by Lactobacillus pentosus SJ65 isolated from Uttapam batter. Journal of Food Biochemistry, 38, 612–619.
Vidhyasagar, V., & Jeevaratnam, K. (2013). Bacteriocin activity against various pathogens produced by Pediococcus pentosaceus VJ13 isolated from Idly batter. Biomedical Chromatography, 27, 1497–1502.
Silva, M., Jacobus, N. V., Deneke, C., & Gorbachl, S. L. (1987). Antimicrobial substance from a human Lactobacillus strain. Antimicrobial Agents and Chemotherapy, 31, 1231–1233.
Breitmaier, E. (2002). Structure elucidation by NMR in inorganic chemistry: a practical guide (3rd ed.). England: Wiley Ltd.
Olgen, S., Altanlar, N., Karatayli, E., & Bozdayi, M. (2008). Antimicrobial and antiviral screening of novel indole carboxamide and propanamide derivatives. Zeitschrift Naturforschung C, 63, 189–195.
Rohini, R., Reddy, P. M., Shanker, K., Kanthaiah, K., Ravinder, V., & Hu, A. (2011). Synthesis of mono, bis-2-(2-arylideneaminophenyl) indole azomethines as potential antimicrobial agents. Archives of Pharmacal Research, 34, 1077–1084.
Lee, J. H., & Lee, J. (2010). Indole as an intercellular signal in microbial community. FEMS Microbiology Review, 34, 426–444.
Sajid, I., Shaaban, K. A., & Hasnain, S. (2011). Identification, isolation and optimization of antifungal metabolites from the Streptomyces malachitofuscus ctf9. Brazilian Journal of Microbiology, 42, 592–604.
Carbonnelle, D., Duflos, M., Marchand, P., Chauvet, C., Petit, J.-Y., & Lang, F. (2009). A novel indole-3-propanamide exerts its immunosuppressive activity by inhibiting JAK3 in T Cells. Journal of Pharmacol and Experimental Therapeutics, 331, 710–716.
Wang, H., Yan, Y., Wang, J., Zhang, H., & Qi, W. (2012). Production and characterization of antifungal compounds produced by Lactobacillus plantarum IMAU10014. PLoS ONE, 7, e29452. doi:10.1371/journal.pone.0029452.
Acknowledgments
The authors are thankful to ER&IPR, Defense Research & Development Organization, New Delhi, for funding this project and Defence R&D Establishment, Gwalior, for providing facilities for structural elucidation of this compound. Ms. Jayaprabha Agaliya is thankful for providing fellowship in this project, while Ms. Saraniya and Mr. Vidhyasagar are thankful to University Grants Commission, New Delhi, for providing fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kadirvelu Jeevaratnam, Venkatasubramanian Vidhyasagar, Perumal Jayaprabha Agaliya, Appukuttan Saraniya, and Muthukandan Umaiyaparvathy have equal contributions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 277 kb)
Rights and permissions
About this article
Cite this article
Jeevaratnam, K., Vidhyasagar, V., Agaliya, P.J. et al. Characterization of an Antibacterial Compound, 2-Hydroxyl Indole-3-Propanamide, Produced by Lactic Acid Bacteria Isolated from Fermented Batter. Appl Biochem Biotechnol 177, 137–147 (2015). https://doi.org/10.1007/s12010-015-1733-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1733-9