Abstract
The phytase of the yeast Pichia anomala is a histidine acid phosphatase based on signature sequences and catalytic amino acids identified by site-directed mutagenesis. Among modulators, N-bromosuccinimide and butanedione inhibit phytase, while Ca2+ and Ni2+ stimulate slightly. Vanadate exhibits competitive inhibition of phytase, making it bifunctional to act as haloperoxidase. Molecular docking supports vanadate to share its binding site with phytate. The T 1/2, activation energy (E a ), temperature quotient (Q 10), activation energy of thermal inactivation (Ed), and enthalpy (ΔH 0d ) of the enzyme are 4.0 min (80 °C), 27.72 kJ mol−1, 2.1, 410.62 kJ mol−1, and ∼407.8 kJ mol−1 (65–80 °C), respectively. The free energy of the process (ΔG od ) increases from 49.56 to 71.58 kJ mol−1 with rise in temperature, while entropy of inactivation (ΔS 0d ) remains constant at ∼1.36 kJ mol−1 K−1. The supplementation of whole wheat dough with rPPHY resulted in 72.5 % reduction in phytic acid content of bread. These characteristics confirm that the phytase has adequate thermostability for its applicability as a food and feed additive.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytic acid [myo-inositol 1,2,3,4,5,6-hexakisdihydrogen phosphate (IP6)] is one of the organic forms of phosphorus in foods and feeds. In monogastrics, phytic acid acts as an antinutrient factor (ANF) by chelating cations, forming insoluble complexes with proteins and starch which have lower digestibility and inhibiting enzymes involved in digestion. The excreted phytic acid present in animal manure leads to eutrophication of aquatic environments [1]. Phytases are hydrolytic enzymes belonging to the subclass phosphatases, a diverse class of enzymes catalyzing the cleavage of monophosphoester bonds in phytic acid and several other organic phosphates. The phytases belonging to histidine acid phosphatases (HAP) share a unique and conserved active site heptapeptide motif (RHGXRXP) and the catalytic dipeptide HD [2]. Phytases are synthesized in bacteria, yeast, filamentous fungi, and plants [3]. There has been an undue focus on developing recombinant enzymes for bulk production. Phytases are mainly used in animal feeds and foods for ameliorating phosphorus availability and assimilation. As per the current estimates, the global feed enzyme market is valued at US$725 million and could amount to over US$1 billion by 2017 (http://www.feedinfo.com/console/PageViewer.aspx/page=3803488). The development of thermostable enzymes is one of the prime tasks of current enzyme biotechnology because they are sturdy and withstand harsh process conditions. There are several reports on thermodynamic characterization of amylases [4], xylanases [5], and lipases [6], but such reports on phytases are scanty. A phytase suitable for applicability in feed and food industry must have adequate thermostability to withstand feed pelleting step of feed preparation and acid stability to retain activity in the acidic environment of stomach. Although there are reports on heterologous expression and production aspects of microbial phytases, a detailed investigation on biochemical and thermodynamic characteristics is lacking. In this communication, we present inhibition kinetics and thermodynamics of phytase of the yeast Pichia anomala.
Materials and Methods
Identification of Active Site Residues
Amino acid sequence of rPPHY was aligned with sequences of other yeast (Debaryomyces castellii, Cyberlindnera fabianii, Yarrowia lipolytica CLIB122) phytases with the aid of BioEdit software (http://www.mbio.ncsu.edu/bioedit/bioedit.html). Along with sequence alignment, PPHY sequence was processed using ExPASy PROSITE software (http://prosite.expasy.org/) to identify catalytically important conserved residues.
Confirmation of Identity of Catalytic Sites by Site Directed Mutagenesis
rPPHY-pPICZαA, where PPHY (GenBank FN641803.1) was cloned in pPICZαA, was used as a template to construct mutants of PPHY. Three pairs of overlapping primers (Table 4) were designed to generate mutant constructs rPPHY (R70A)-pPICZαA, rPPHY(R74A)-pPICZαA, and rPPHY(D344A)-pPICZαA. QuikChange lightning site-directed mutagenesis kit (Agilent, USA) was used to create aforementioned constructs. Manufacturer’s protocol was followed for the amplification of the mutant constructs in a Thermocycler (Bio-Rad, USA). DpnI provided with the kit was used to degrade template plasmid. Template free amplified plasmid was transformed into chemically competent E. coli XL10-Gold cells. The mutation in the plasmid constructs isolated from the transformants were confirmed by sequencing at sequencing facility (CIF, UDSC, New Delhi) for confirming the mutated nucleotide(s). The mutated constructs were used to transform P. pastoris X33 cells. The recombinant P. pastoris integrants were analyzed for mutated the production of proteins (muteins). Muteins were purified and analyzed for phytase activity according to as described earlier [7].
Molecular Docking of Substrate and Inhibitors
A three-dimensional model of rPPHY was generated using SWISS-MODEL which is an automated homology modeling server (http://swissmodel.expasy.org/workspace). PPHY sequence exhibited 50 % identity with the template 2gfiA (crystal structure of the phytase chain A from Debaryomyces castellii in PDB). PDB files of sodium phytate, vanadate, and tartrate were downloaded from (http://xray.bmc.uu.se/hicup/). Ligands and PPHY were docked using Patch Dock server (http://bioinfo3d.cs.tau.ac.il/PatchDock/) which resulted in multiple solutions. Best solution was validated using structural analysis and verification server (http://nihserver.mbi.ucla.edu/SAVES/).
Purification of rPPHY
Recombinant phytase was produced and purified according to Joshi and Satyanarayana [7]. The culture supernatant was concentrated by lyophilization followed by anion exchange chromatography (DEAE-sepharose CL-6B) and size exclusion chromatography (Sephacryl S-300 26/60 column) to homogeneity.
Effect of Various Additives on Phytase Activity
The purified rPPHY was exposed to various concentrations of metal ions (1, 5, and 10 mM) for 30 min at room temperature, and the residual enzyme activities were determined under standard assay conditions according to Heinonen and Lahti [8]. Similarly, rPPHY was also exposed to various modulators such as chaotropic agents (10 and 100 mM), inhibitors (1, 5, and 10 mM), detergents (0.1 and 0.5 %), and organic solvents (5 and 10 %), and the residual activities were measured.
Inhibition Kinetics
The purified rPPHY was exposed to various concentrations of sodium vanadate and sodium orhtovanadate and L(+)-tartrate (Sigma, St. Louis, USA) for 15 min. The residual phytase activities were determined [9]. Vanadate-treated enzyme was also used to derive kinetic parameters K m , V max, K cat , and K i using different concentrations of calcium phytate (0.1 to 1 mM) at pH 4 and 60 °C. Graphical method (Lineweaver–Burk plot) was used for identifying the mode of inhibition [10]. Competitive inhibition was assessed using the Michaelis-Menten approach. The additional parameters appear in the Lineweaver–Burk transformation of the equation:
where I = concentration of inhibitor; K i = dissociation constant of EI complex, as mentioned above and illustrated in the equation and graph, V max is unaltered by competitive inhibition, but K m is increased by a factor as follows:
Since the K m in the presence and absence of inhibitor may be determined graphically, the K i may be calculated from the following relationship:
where K P is the apparent K m in the presence of a competitive inhibitor.
Qualitative Assay of Haloperoxidase Activity
The haloperoxidase activity of the rPPHY was demonstrated by a qualitative assay according to Hunter-Cevera and sotos [10]. One milliliter of the reaction mixture contained 500 μL enzyme, 300 mM potassium phosphate buffer (pH 6), 20 mM KBr, 10 μm metavanadate, 50 μL of 0.3 % H2O2, and 100 μL 0.2 % phenol red (prepared in 95 % ethanol). The reaction was carried out at 37 °C while observed intermittently for change in color from red-orange to blue-violet.
Melting Point (T m )
The melting temperature of the purified PPHY and rPPHY was determined by using circular dichroism (CD). The enzyme was incubated in 50 mm acetate buffer (pH 4.0), and thermal denaturation was carried out as a function of increasing temperature using JASCO-815 Spectropolarimeter equipped with in-built peltier controlled thermostat cell holder (CDF-423S). The data were analyzed using the following equation:
where f is fraction of folded protein, Y is molar ellipticity at 222 nm at temperature T, Y min is molar ellipticity at 222 nm at 20 °C, and Y max is molar ellipticity at 222 nm at 100 °C. A curve between f value and temperature was plotted to obtain melting temperature of the native and recombinant phytases.
Activation Energy and Temperature Quotient
The first-order rate constant (k) for rPPHY was determined by the following equation:
Thermal activation plots were plotted from the regression of log k vs 1/T. The activation energy (E a ) of the purified enzyme was calculated using the following equation:
where E a represents activation energy, R is the gas constant, T 1 is the temperature 1 in K, T 2 is the temperature 2 in K, K 1 is the rate constant at T 1, and K 2 is the rate constant at T 2.
The effect of temperature on the rate of reaction is frequently expressed in terms of temperature coefficient (Q 10), which is a factor by which the rate increases when the temperature is raised by 10 °C. Q 10 was calculated by the following equation:
Thermal Inactivation of rPPHY
The thermal stability of rPPHY and native phytase was investigated at four different temperatures (65, 70, 75, and 80 °C). Purified rPPHY was incubated at aforementioned temperatures. The enzyme solution was placed in a prewarmed tube at the specified temperature, and aliquots were drawn at desired time intervals, cooled and residual activities were measured. The stability of the enzyme was expressed as the residual activity. The incubation was carried out in sealed vials to prevent change of volume of the sample, and hence, the enzyme concentration due to evaporation. The data obtained from the thermal stability profile were used to analyze thermodynamic parameters related to the rPPHY activity. The experimental points were plotted according to the equation given below:
where A 0 is the initial activity, A is the residual activity after heat treatment, k d is thermal inactivation rate constant (min−1), and t is the exposure time (min). The half-life of the rPPHY (t 1/2, min−1) was determined from the following relationship:
The D values (decimal reduction time or time required to preincubate the enzyme at a given temperature to maintain 10 % residual activity) was calculated from the following relationship:
The z value (temperature rise necessary to reduce D value by one logarithmic cycle) was calculated from the slope of graph between log D versus T (°C) using equation:
The activation energy for denaturation (E d ) of rPPHY was determined by an Arrhenius plot of log denaturation rate constants (ln k d ) versus reciprocal of the absolute temperature (K) using the equation:
The change in enthalpy (ΔH od , kJ mol−1), free energy (ΔG od , kJ mol−1), and entropy (ΔS od , J mol−1 K−1) for thermal denaturation of rPPHY were determined using the following equations:
where E d is the activation energy for denaturation, T is the corresponding absolute temperature (K), R is the gas constant (8.314 J mol−1 K−1), h is the Planck constant (6.626 × 10−34 J s), k B is the Boltzman constant (1.38 × 10−23 J K−1), and k d is the deactivation rate constant (min−1).
Applicability of rPPHY in Dephytinization of Wheat Bread
Wheat flour (1 kg) was mixed with activated yeast (25 g), 1.5 % NaCl, 4.0 % sucrose, 20 mL sunflower oil and recombinant phytase (500 U) + Bradzyme (Tushar Nutritives Food P Ltd, New Delhi, India), blended with 600 mL water, and mixed mechanically for 30 min to make dough and cut into four equal sized parts. Dough was allowed to undergo proofing by fermentation for an hour, followed by baking at 220 °C for 25 min, shaping and cutting. The bread prepared without phytase was considered as the control. The breads were assessed for phytate content, soluble inorganic phosphate, reducing sugars, and soluble protein contents.
Statistical Analysis
All experiments have been performed in triplicate and the average values are presented as ± standard deviation.
Results and Discussion
Upon alignment of the amino acid sequence of rPPHY of the phytase of P. anomala with other yeast phytases, a conserved heptapaptide (RHGERYP) and a dipeptide (HD) motif have been detected (Fig. 1).
Catalytic heptapeptide and dipeptide of P. anomala phytase. Multiple sequence alignment of P. anomala phytase (GenBank ID FN641803.1) with phytases from Debaromyces castelli (PDB 2GFI_A), Cyberlindnera fabianii (GenBank ID BAH58739.1), Yarrowia lipolytica CLIB122 (GenBank ID CR382129.1) (a); consensus in heptapeptide containing region—[LIVMF]-x-[LIVMFAG]-{T}-x-[STAGI]-H-D-[STANQ]-{V}-[LIVM]-x(2)[LIVMFY]-x(2)-[STA] where H is an active site residue (b); consensus in HD region—[LIVM]-x(2)-[LIVMA]-x(2)-[LIVM]-x-R-H-[GN]-x-R-x-[PAS] where H is the phosphohistidine (c). Purified rPPHY and muteins R70A, R74A and D334A (d); zymogram of purified rPPHY and muteins R70A, R74A, and D334A (e)
Among various classes of phytases, only HAP phytases are known to possess a conserved heptapeptide sequence (RHGXRXP) and a catalytically active dipeptide, HD [2]. According to Ostanin et al. [11], HAPs employ two-step dephosphorylation mechanism to hydrolyze phytic acid: first, a nucleophilic attack on the phosphorous atom by the histidine (H) of the active site motif which is followed by hydrolysis of the resulting phospho-histidine intermediate in the second step. The positive charge of the guanido group of the arginine (R) residue in the conserved tripeptide (RHG) directly interacts with the phosphate group in the substrate, and thus, making it more susceptible to nucleophilic attack. The histidine residue serves as a nucleophile in the formation of covalent phosphohistidine intermediate, while the aspartic acid residue (D) protonates the group leaving the substrate. In site-directed mutagenesis, when arginine (R) of heptapeptide and aspartate (D) of dipeptide were substituted with alanine, there was complete loss of activity in the mutant rPPHY (supplementary data, Table S1). The purified muteins did not exhibit phytase activity (Fig. 1), which confirmed that these residues are critically important for phytase activity. Not only crystal structure [12] but also SDM aids in identifying catalytically important amino acid residues. Osman et al. [13] identified the active site residues and substrate binding sites of the beta-propeller phytase of Bacillus sp. by site directed mutagenesis. Using molecular modeling approach, Tomshchy et al. [14] were able to show that the arginine residue (R297) of the phytase of Aspergillus niger T213 directly interacts with the phosphate group of phytic acid.
Mode of Inhibition of rPPHY by Vanadates and Tartarate
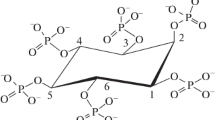
Vanadate is a phosphate analog and is generally considered to bind as a transition state analogue to the phosphoryl transfer enzymes that it inhibits, since it can easily adapt a trigonal bipyramidal structure [15]. Vanadate has a wide variety of effects on biological systems [16]. The fungal PhyA protein, which was first identified as an acid optimum phosphomonoesterase (EC 3.1.3.8), could also serve as a vanadate haloperoxidase (EC 1.11.1.10) provided that the acid phosphatase activity is shut down by vanadate [17]. In case of phytases, a detailed investigation on the mechanism of inhibition of phytases by vanadates has not yet been carried out, and therefore, the kinetic analysis of inhibition has been attempted. The active site of haloperoxidase shows close similarity with histidine acid phytases [18–20]. Inhibition of phytase by vanadate can make the enzyme behave as a vanadate-dependent haloperoxidase provided phosphoesterase activity of the enzyme is shut down by the vanadate [17]. The vanadate exists as an anion at pH 3.0 and possibly binds to the active site cleft of phytase, which has a cluster of positively charged amino acids arginine, lysine, and histidine below the isoelectric point (pI) of the enzyme [17]. Upon molecular docking of metavanadate with the rPPHY, it was observed to interact with the same amino acid residues of the catalytic site, with which substrate interacts. Both inhibitor and substrate might sit into the catalytic cleft of the enzyme (Fig. 2) which is placed between conserved α/β-domain and a variable α-domain of rPPHY. When bonding of the substrate/inhibitor was analyzed, it was found to form bonds with ariginine (R70), arginine (R74), and aspartate (D344) (Fig. 2).
Prediction of interactions of sodium phytate, vanadate, and tartrate with rPPHY structure by in silico molecular docking. Position occupied by sodium phytate in the substrate binding cleft and its bonding with amino acid residues of conserved heptapeptide (a, b); similarly, c and d show position and bonding of vanadate while e and f show the position and bonding of tartrate in the substrate binding pocket
In vitro experiments revealed that vanadate exhibits competitive inhibition on rPPHY activity (Fig. 3) as V max at all varying concentrations of inhibitor remained almost constant (∼78.13 η kat mg−1 s−1). With increase in the concentration of inhibitor from 0 to 500 μM, the apparent K m increased from 200 to 1000 μM. K i values for metavanade were 333, 200, and 1.2 μM at 50, 100, and 500 μM inhibitor concentrations, respectively (Table 1).
The inhibition of phytase by metavanadate suggests the applicability of rPPHY as haloperoxidase. The rPPHY changed the color of phenol red from red-orange to blue-violet in the presence of metavanadate ions, H2O2 (Supplementary data, Fig. S1). Vanadate haloperoxidases find applications in the field of nuclear medicine for the production of radiolabeled monoclonal antibodies, diagnostics, quantitation of chloride in liquid samples and as antimicrobial agents [21–23]. In view of multifarious applications of vanadate haloperoxidases, detailed investigations are needed on the utility of rPPHY as haloperoxidase.
Effect of Various Modulators on rPPHY Activity
Ag2+, Al2+, Pb3+, and Sn2+ completely inhibited rPPHY activity at 5 mM concentration (Table 2), while the lower degree of inhibition was recorded by Ba2+, Cu2+, Fe2+, Zn2+, while Hg2+. Mn2+ and Na+ did not affect rPPHY. The cations Ca2+ and Ni2+ slightly stimulated the rPPHY activity. In the presence of metal ions, the inhibition of rPPHY could be due to the formation of insoluble substrate–metal complex in the reaction mixture that leads to decrease in the availability of the substrate [24]. The chelating agent EDTA did not inhibit enzyme activity. There have been the reports of variable effects of metal ions on phytase activity [25, 26].
The reducing agent β-mercaptoethanol (β-ME) did not affect rPPHY activity significantly, suggesting sulfhydryl groups (−SH) are not involved in catalysis or lack of freely available –SH group in the protein. Most of the phytases have been reported to possess even number of cysteine residues which might be involved in the formation of disulphide bridges as in Aspergillus niger [27]. The enzyme is insensitive to phenylmethanesulphoonyl fluoride (PMSF), a well-known serine protease inhibitor [28]. N-ethylmaleimide (NEM) had no observable effect on the enzyme activity.
The rPPHY is significantly inhibited by 2,3-butanedione suggesting the possible role of arginine in catalysis which is similar to the effect on wild-type phytase of P. anomala [29] and Sporotrichum thermophile [30]. The modification of arginine residue of the active site by 2,3-butanedione results in loose binding and lower amount of product. According to Tang et al. [31], HAPs exhibit higher degree of inhibition by 2,3-butanedione than sodium molybdate. A similar inhibition pattern had been displayed by rPPHY.
Among various organic solvents tested, the phytase is tolerant to glycerol, acetone, hexane, benzene, and toluene, while isopropanol, ethanol, amyl alcohol, and chloroform exerted inhibitory effect. The stabilizing effect by hydrophobic organic solvents can be explained by the presence of hydrophobic surface residues and hydrophobic clusters in the rPPHY as analyzed by the ExPASy tool Drawhca (draw a hydrophobic clusters analysis) [32] (Supplementary data, Fig. S2). In organic solvents, the flexibility of the protein molecule declines, and thus, the dense packing of the enzyme molecule can create an effective nanospace for improved catalysis [33]. The phytase of S. thermophile [30] was also stabilized by hexane and acetone while slightly inhibited by benzyl alcohol, ethanol, and toluene.
Among different detergents tested, nonionic Tween 20, Tween 80, and Triton X-100 stabilized the rPPHY activity. Even low concentrations of anionic detergents [sodium dodecyl sulfate (SDS) and N,N,N,N-cetyltrimethylammonium bromide (CTAB)] strongly inhibited the enzyme. The detergents can bind to the protein and cause structural changes in it. In case of nonionic ones, these changes had stabilizing effect, but inhibitory effect in the presence of SDS and CTAB. Chaotropic agents like urea and guanidinium hydrochloride (Gdn-HCl) inhibited phytase activity suggesting the role of noncovalent linkages such as H-bonds and van der Waal’s interactions in maintaining the active conformation of the enzyme [34, 26]. N-Bromosuccinimide (NBS) completely inhibited the rPPHY activity at 10 mM, suggesting a possible role of trytophan in maintaining the secondary structure and coordinating rPPHY activity.
T m of PPHY
Melting temperature of the native phytase was 73 °C, while that of the recombinant phytase was 70 °C. This observation suggests that the equilibrium of the folded and unfolded fractions reaches early in rPPHY than the native (Fig. 4). High melting temperature (T m ) of both the native and recombinant phytases indicates their higher thermostability. T m is considered one of the best indicators of protein thermostability [35]. Subtle difference in the T m of the native and recombinant phytases could be due to differences in pattern and extent of the glycosylation. The glycosylation of proteins in P. pastoris is known to alter the properties of recombinant proteins [36, 37].
Activation Energy (E a ) and Temperature Quotient (Q 10)
Thermal activation plot of the regression of log (k) versus 1/T (K−1) (Supplementary data, Fig. S3) indicated the activation of phytase up to 60 °C followed by inactivation afterward. The activation energy of phytase is 27.72 kJ mol−1 and the temperature quotient is 2.1 (60/50 °C). The Arrhenius activation energy for the hydrolysis of sodium phytate by phytases from different sources ranged between 25 and 53 kJ mol−1 [24, 30, 38, 39].
Thermal Inactivation of rPPHY
To understand the stability properties of an enzyme at different temperatures, half-life (T 1/2) determinations are accurate and reliable. The residual activity of rPPHY versus time of incubation at particular temperature and Log K d versus 1000/T (K−1) were plotted to compute various thermodynamic parameters of thermal irreversible inactivation of rPPHY (Fig. 5).
Thermal denaturation of rPPHY. Pseudo-first-order plots for irreversible thermal denaturation of recombinant phytase of P.anomala. The pure enzyme was preincubated at different temperatures (60, 65, 70, 75 and 80 ºC). Phytase activity was determined at the desired time intervals at 60 °C in sodium acetate buffer (pH 4.0) (a). Arrhenius plot for determining activation energy (E d ) of irreversible thermal denaturation of rPPHY using K d = −E d /RT where R (gas constant) = 8.314 kJ mol−1 (b)
The thermal inactivation of rPPHY follows first-order kinetics. Inactivation rate constant (K d ) increased with the increase in temperature and a sharp decline in T 1/2 values was recorded with increase in temperature (Table 3). The T 1/2 values of rPPHY at 65, 70, 75, and 80 °C are 3600, 300, 210, and 4.02 min, respectively. The decrease in the T 1/2 value with increase in temperature occurs as a result of irreversible thermal inactivation of the enzyme as the heat increases with the rise in temperature. Decrease in D value was also observed with the increase in first-order thermal deactivation rate constants (K d ) (Table 3).
It is clear from the observations that the enzyme is less stable at high temperatures as the high rate constant is an indicator of lower thermostability [40]. The z value of rPPHY, calculated from the slope of graph between log D versus temperature (T °C), is 5.55 (Table 4). In general, high z value indicates more sensitivity to the duration of heat treatment, while lower z value indicates high sensitivity to the increase in temperature [41].
The activation energy of the thermal inactivation (E d ) of rPPHY is 410.62 kJ mol−1. The activation energy of denaturation of rPPHY is very high as compared to that of the activation energy of catalysis (E a ). The higher value of E d in comparison to E a indicates that a higher amount of energy is required to initiate denaturation as compared to catalysis [41]. Apart from this, activation energy for thermal inactivation (E d ) is higher for rPPHY as compared to that of the wild-type PPHY suggesting that the recombinant phytase exhibits better thermal adaptation than that of wild-type PPHY. The enthalpy of denaturation (ΔΗ οd ) for rPPHY is ∼407.8 kJ mol−1 at 65–80 °C. The high values of ΔΗ οd recorded for the thermal inactivation of rPPHY indicate that the enzyme undergoes a considerable change in conformation during denaturation [42]. The high and positive values of free energy of thermal denaturation (ΔG οd ) have been recorded for rPPHY. Entropy of inactivation (ΔS οd ) was almost uniform and remained close to ∼1.36 kJ mol−1 K−1. As the value of entropy of inactivation (ΔS οd ) was positive, there are no significant processes of aggregation, since negative values have been associated with such processes [42]. Lower values of ΔS οd are indicative of the formation of highly ordered enzyme–substrate transition complex [43]. It is also worthwhile to mention that ΔG οd values, which measure the spontaneity of the inactivation processes, are lower than the ΔΗ οd values. This is due to the positive entropic contribution during the thermal inactivation process [19].
Applicability of rPPHY in Dephytinization of Wheat Bread
Wheat flour contains up to 4 mg g−1 phytic acid [44]. Phytate present in cereals lowers bioavailability of minerals. The reduction of phytic acid content can lead to improvement in mineral availability, and thus mitigate antinutrient effects of phytic acid. The supplementation of dough with rPPHY increased the inorganic phosphate, reducing sugars and soluble protein content in bread as compared to bread prepared with commercial enzymes. The addition of rPPHY resulted in 72.5 % reduction in phytic acid content (Table 5). There was no collapse of bread crust, while texture of the bread remained as good as that of the test bread. Singh and Satyanarayana have reported a 39.2 % reduction in phytic acid content by supplementing the dough with the phytase of Sporotrichum thermophile along with the α-amylase of Geobacillus thermoleovorans [45]. The rPPHY treatment has recently been shown to dephytinize soy protein and fractionation of allergenic glycinin [7], suggesting that rPPHY is useful in ameliorating the nutritional value of foods.
Conclusions
The phytase (rPPHY) of P. anomala possesses signature heptapeptide and dipeptide sequences of HAP phytases, where R70, R74, and D344 are involved in catalysis. The activation energy of its inactivation is higher than the activation energy of thermal denaturation, and thus confirming rPPHY to have adequate thermostability for its applicability as a feed additive. The inhibition of rPPHY by vanadate suggests its potential as a candidate for generating haloperoxidase, which has multifarious applications. A considerable reduction in phytic acid content of bread as a result of supplementation of whole wheat dough with rPPHY confirms its applicability as a food additive.
References
Smil, V. (2002). Phosphorus in the environment: natural flows and human interferences. Annual Review of Energy and the Environment, 25, 53–88.
Van Etten, R. L., Davidson, R., Stevis, P. E., MacArthur, H., & Moore, D. L. J. (1991). Covalent structure, disulfide bonding, and identification of reactive surface and active site residues of human prostatic acid phosphatase. Journal of Biological Chemistry, 266, 2313–2319.
Mullaney, E. J., Daly, C. B., & Ullah, A. H. (2000). Advances in phytase research. Advances in Applied Microbiology, 47, 157–199.
Mehta, D., & Satyanarayana, T. (2013). Biochemical and molecular characteristics of recombinant acidic and thermostable raw starch hydrolyzing α-amylase from an extreme thermophile Geobacillus thermoleovorans. Journal of Molecular Catalysis B: Enzymatic, 85–86, 229–238.
Haq, U., Hussain, Z., Khan, M. A., Muneer, B., Afzal, S., Majeed, S., & Akram, F. (2012). Kinetic and thermodynamic study of cloned thermostable endo-1,4-β-xylanase from Thermotoga petrophila in mesophilic host. Molecular Biology Reports, 39, 7251–7261.
Wen, S., Tan, T., & Zhao, H. (2012). Improving the thermostability of lipase Lip2 from Yarrowia lipolytica. Journal of Biotechnology, 164, 248–253.
Joshi, S., & Satyanarayana, T. (2014). Optimization of heterologous expression of the phytase (PPHY) of Pichia anomala in P. pastoris and its applicability in fractionating allergenic glycinin from soy protein. Journal of Industrial Microbiology and Biotechnology, 41, 977–987.
Heinonen, J. K., & Lahti, R. J. (1981). A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase. Analytical Biochemistry, 113, 313–317.
Segel, I. H. (1976). Biochemical calculations. Hoboken: Wiley.
Hunter-Cevera, J. C., & Sotos, L. (1986). Screening for a “new” enzyme in nature: haloperoxidase production by Death Valley dematiaceous hyphomycetes. Microbial Ecology, 12, 121–127.
Ostanin, K., Harms, E. H., Stevis, P. E., Kuciel, R., Zhou, M. M., & van Etten, R. L. (1992). Overexpression, site-directed mutagenesis, and mechanism of Escherichia coli acid phosphatase. Journal of Biological Chemistry, 267, 22830–22836.
Singh, H., Felts, R. L., Schuermann, J. P., Reilly, T. J., & Tanner, J. J. (2009). Crystal Structures of the histidine acid phosphatase from Francisella tularensis provide insight into substrate recognition. Journal of Molecular Biology, 394, 893–904.
Osman, A. A., Babu, P. R., Venu, K., Rao, K. V., & Reddy, V. D. (2012). Prediction of substrate-binding site and elucidation of catalytic residue of a phytase from Bacillus sp. Enzyme and Microbial Technology, 51, 35–39.
Tomschy, A., Wyss, M., Kostrewa, D., Vogel, K., Tessier, M., Hofer, S., Burgin, H., Kronenberger, A., Remy, R., van Loon, A. P., & Pasamontes, L. (2000). Active site residue 297 of Aspergillus niger phytase critically affects the catalytic properties. FEBS Letters, 472, 169–172.
Gresser, M. J., & Tracey, A. S. (1990). Vanadium in biological systems. Netherlands: Kluwer Academic Publishers.
Shechter, Y., Meyerovitch, J., Farfel, Z., Sack, J., Bruck, R., Bar-Meir, S., Amir, S., Degani, H., & Karlish, S. J. D. (1990). Vanadium in biological systems. Netherlands: Kluwer Academic Publishers.
Ullah, A. H., Sethumadhavan, K., & Mullaney, E. J. (2011). Vanadate inhibition of fungal PhyA and bacterial AppA2 histidine acid phosphatases. Journal of Agricultural and Food Chemistry, 59, 1739–1743.
Correia, L., Aksu, S., Adao, P., Pessoa, J. C., Sheldon, R. A., & Arends, I. W. C. E. (2008). Vanadate substituted phytase: immobilization, structural characterization and performance for sulphoxidations. Journal of Inorganic Biochemistry, 102, 318–329.
Tanaka, N., Dumay, V. R., Liao, Q., Lange, A. J., & Wever, R. (2002). Bromoperoxidase activity of vanadate-substituted acid phosphatases from Shigella flexneri and Salmonella enterica ser. typhimurium. European Journal of Biochemistry, 269, 2162–2167.
Van de Velde, F., Konemann, L., van Rantwijk, F., & Sheldon, R. A. (2000). The rational design of semisynthetic peroxidases. Biotechnology and Bioengineering, 67, 87–96.
Wever, R., Dekker, H. L., Van Schijndel, J. W. P. M., & Vollenbroek, E. G. M. (1995). Use of haloperoxidase enzymes-recombinant enzyme production for use in antifouling paint. PCT, W095/27-009.
De Boer, E., Plat, H., Tromp, M. G. M., Franssen, M. C. R., van der Plas, H. E., Meijer, E. M., Schoemaker, H. E., & Wever, R. (1987). Vanadium containing bromoperoxidase: an example of an oxidoreductase with high operational stability in aqueous and organic media. Biotechnology and Bioengineering, 30, 607–610.
Allen, R. C. (1992). Methods and compositions for the treatment of infection and control of flora using haloperoxidase, EP-0500387 A2.
Konietzny, U., & Greiner, R. (2002). Molecular and catalytic properties of phytate-degrading enzymes (phytases). International Journal of Food Science and Technology, 37, 791–812.
Casey, G., & Walsh, J. (2004). Identification and characterization of a phytase of potential commercial interest. Biotechnology, 110, 313–322.
Gulati, H. K., Chadha, B. S., & Saini, H. S. (2007). Production, purification and characterization of thermostable phytase from thermophilic fungus Thermomyces lanuginosus TL-7. Acta Microbiologica et Immunologica Hungarica, 54(2), 121–138.
Kostrewa, D., Gruninger-Leitch, F., D’Arcy, A., Broger, C., Mitchell, D. B., & van Loon, A. P. G. M. (1997). Crystal structure of phytase from Aspergillus ficuum at 2.5 A resolution. Nature Structural Biology, 4, 185–190.
Joshi, S., & Satyanarayana, T. (2013). Biotechnology of cold-active proteases. Biology, 2, 755–783.
Vohra, A., & Satyanarayana, T. (2001). Purification and characterization of a thermostable and acid-stable phytase from Pichia anomala. World Journal of Microbiology and Biotechnology, 18, 687–691.
Singh, B., & Satyanarayana, T. (2009). Characterization of HAP-phytase from a thermophilic mould Sporotrichum thermophile. Bioresource Technology, 100, 2046–2051.
Tang, J., Leung, A., Leung, C., & Lim, B. L. (2006). Hydrolysis of precipitated phytate by three distinct families of phytases. Soil Biology and Biochemistry, 38, 1316–1324.
Eudes, R., Tuan, K. L., Delettre, J., Mornon, J. P., & Callebaut, I. (2007). A generalized analysis of hydrophobic and loop clusters within globular protein sequences. BMC Structural Biology, 7, 2.
Tejo, B. M., Salleh, A. B., & Pleiss, J. (2004). Structure and dynamics of Candida rugosa lipase: the role of organic solvent. Journal of Molecular Modelling, 10, 358–366.
Vats, P., & Banerjee, U. C. (2005). Biochemical characterisation of extracellular phytase (myo-inositol hexakisphosphate phosphohydrolase) from a hyper-producing strain of Aspergillus niger van Teighem. Journal of Industrial Microbiology and Biotechnology, 32, 141–147.
Kumar, S., Tsai, C. J., & Nussinov, R. (2000). Factors enhancing protein thermostability. Protein Engineering, 13, 179–191.
Bretthauer, R. K. (2000). Genetic engineering of Pichia pastoris to humanize N-glycosylation of proteins. Trends in Biotechnology, 2, 459–462.
Shental-Bechar, D., & Levy, Y. (2009). Folding of glycoproteins: toward understanding the biophysics of the glycosylation code. Current Opinion in Structural Biology, 19, 524–533.
Shimizu, M. (1993). Purification and characterization of phytase and acid phosphatase produced by Aspergillus oryzae K1. Bioscience Biotechnology and Biochemistry, 57, 1364–1365.
Kerovuo, J., Lauraeus, M., Nurminen, P., Kalkkinen, N., & Apajalahti, J. (1998). Isolation, characterization, molecular gene cloning, and sequencing of a novel phytase from Bacillus subtilis. Applied and Environmental Microbiology, 64, 2079–2085.
Marangoni, A. G. (2003). Enzyme kinetics: A modern approach. Hoboken: John Wiley and Sons.
Tayefi-Nasrabadi, H., & Asadpour, R. (2008). Effect of heat treatment on buffalo (Bubalus Bubalis) lactoperoxidase activity in raw milk. Journal of Biological Sciences, 8, 1310–1315.
Marin, E., Sanchez, L., Perez, M. D., Puyol, P., & Calvo, M. (2003). Effect of heat treatment on bovine lactoperoxidase activity in skim milk: kinetic and thermodynamic analysis. Journal of Food Science, 68, 89–93.
Kikani, B. A., & Singh, S. P. (2012). The stability and thermodynamic parameters of a very thermostable and calcium-independent α-amylase from a newly isolated bacterium, Anoxybacillus beppuensis TSSC-1. Process Biochemistry, 47, 1791–1798.
Garcia-Estepa, R. M., Guerra-Hernandez, E., & Garcia-Villanova, B. (1999). Phytic acid content in milled cereal products and breads. Food Research International, 32, 217–221.
Singh, B., & Satyanarayana, T. (2008). Phytase production by Sporotrichum thermophile in a cost-effective cane molasses medium in submerged fermentation and its application in bread. Journal of Applied Microbiology, 105(6), 1858–1865.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31, 426–428.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Acknowledgments
We are grateful to the University Grants Commission (UGC), Council of Scientific & Industrial Research (CSIR), Govt. of India, New Delhi, and to Mr. V.K. Gupta (Tushar Nutritives Food P Ltd, New Delhi, India) for providing financial assistance, awarding fellowship to SJ, and extending help in assessing the applicability of rPPHY in bread making, respectively, while carrying out this investigation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joshi, S., Satyanarayana, T. Characteristics and Applicability of Phytase of the Yeast Pichia anomala in Synthesizing Haloperoxidase. Appl Biochem Biotechnol 176, 1351–1369 (2015). https://doi.org/10.1007/s12010-015-1650-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1650-y