Abstract
The callus growth kinetics allows identifying the appropriate moment for callus pealing and monitoring the accumulation of primary and secondary metabolites. The physic nut (Jatropha curcas L.) is a plant species used for biofuel production due to its high oil content; however, this plant presents a great amount of bioactive compounds which can be useful for industry. The aim of this research was to establish a calli growth curve and to evaluate the fatty acid profile of crude oil extracted from callus. The callus growth kinetics presented a sigmoid standard curve with six distinct phases: lag, exponential, linear, deceleration, stationary, and decline. Total soluble sugars were higher at the inoculation day. Reducing sugars were higher at the inoculation day and at the 80th day. The highest percentage of ethereal extract (oil content) was obtained at the 120th day of culture, reaching 18 % of crude oil from the callus. The calli produced medium-chain and long-chain fatty acids (from 10 to 18 carbon atoms). The palmitic acid was the fatty acid with the highest proportion in oil (55.4 %). The lipid profile obtained in callus oil was different from the seed oil profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The physic nut (Jatropha curcas L.) is a perennial plant that belongs to Euphorbiaceae family and currently is in domestication process [1, 2]. It presents high oil content, drought tolerance, and easy cultivation, and its oil is used for biofuel production. Moreover, this plant is an important candidate for the development of new products such as biopesticides, anthelmintics, molluscicides, and fungicides. The main base for these products are the phorbol esters, which have molluscicidal [3–5], insecticidal [6], and fungicidal activities [5]. Such properties have been demonstrated in lab-scale experiments. On the other hand, the compound responsible for the anthelmintic activity remains unknown. However, other compounds were also identified for molluscicidal activity, such as curcusone D and jatropholone B [4].
Several compounds present in the physic nut have a great potential to be used for the development of new pharmaceutical drugs. Noteworthy, for cancer disease, some compounds already identified which present antitumor activities, as the jatrophone [7], curcacycline A [8], curcin [9] jatrophine, jatropham, and curcain [10]. Moreover, this species presents also antimicrobial activities, as well as for some fungi [5]. Curcin at 5 μg mL−1 inhibited hyphal growth and spore formation in Pyricularia oryzae, Pestalotia funereal, Rhizoctonia solani, and Sclerotinia sclerotiorum [11]. The seed oil can be applied to treat eczema and skin diseases and to soothe rheumatic pain [12].
The plant tissue culture is an important tool that allows the production of primary and secondary metabolites in environmental controlled conditions, independent of climatic conditions. Diverse approaches for metabolites production involving plant tissue culture can be performed, such as calli culture, cell suspension culture, hairy root, and multiple shoots. Moreover, the culture in bioreactors can promote a higher production of biomass than the conventional culture using flasks [13]. Calli induced from endosperm of physic nut can be used to produce curcin [14]. However, the knowledge of the metabolite production and its biosynthesis in physic nut calli is insufficient. Nevertheless, the production of oils from callus can be an interesting strategy to study the biosynthesis of fatty acids and other metabolites including industrial applications.
The aim of this research was to establish calli growth kinetics and to evaluate the fatty acid profile of crude oil extracted from callus.
Material and Methods
Plant Material and Culture Conditions
Seeds of a toxic variety of physic nut (J. curcas L.) were purchased from Carol Company, Dourados City, Mato Grosso do Sul State, Brazil. In order to establish the seedlings (explant donors) in vitro, seeds were decoated and immersed in 70 % ethanol for 1 min and immersed in 2.5 % sodium hypochlorite for 10 min (all solutions were agitated manually in laminar flow cabinet during seed immersion), and after the disinfection, the seeds were washed four times with distilled and autoclaved water. The seeds were sowed on MS medium [15] with 30 g L−1 sucrose and solidified with 6 g L−1 agar (VetecTM). The pH was adjusted to 5.8 [16]. All explants were maintained in a growth chamber under light (photoperiod of 16 h), at a temperature of 25 ± 2 °C and under a light intensity of 40 μM m−2 s−1 produced by white fluorescent lamps.
Callus Kinetics Growth
In order to induce calli formation, hypocotyl explants were excised from seedlings, and after 15 days, they germinated with approximately 1.0-cm length. The inoculation of explants was carried out on MS medium containing 3 % sucrose, 0.7 % agar, pH adjusted to 5.8, and supplemented with 0.5 mg L−1 2,4-D (2,4-dichlorophenoxyacetic acid) [17]. The hypocotyl weight was discounted from the calli weight. There was no subculture during the experimental period. Fresh weight, dry weight, total soluble sugars, reducing sugars, total protein, and amino acids were analyzed each 10 days after the inoculation of the explants, until the 180th day.
Biochemical Analysis
In order to prepare the analysis, three samples containing 500 mg calli were homogenized in a grail with 5 mL 0.1 M potassium phosphate buffer (pH 7.5). The total protein content and amino acids were determined using the methodology described by Bradford [18] and Stein and Moore [19], respectively. The levels of soluble sugars were determined using the method described by Yemm and Willis [20]. The reducing sugars were determined using the methodology described by Miller [21]. In order to determine the ethereal extract from callus tissue, the callus oil was extracted using petroleum ether (55–60 °C) in a soxhlet extractor for 5 h at 30.2 ± 1 °C of condensation temperature. The condenser has 30-cm length.
Analysis of Fatty Acid Composition
The methyl esters were prepared from the total lipids (crude oil from callus tissues) by the method of AOAC [22]. These fatty acid methyl esters were analyzed by a gas chromatography coupled with a mass spectrometry detector (GC-MS, Varian brand), model 3800 CP/Saturn 2000 equipped with the CP-Sil CB 8 column (30 M × 0.25 mm). The initial temperature was 60 °C with an elevation rate of 3 °C min−1 until 250 °C. The split was 1/200. The scanning range was from 30 to 500 m/z. The electron impact mode (EI) was set up at 70 eV. The identification was carried out in comparison with the NIST 98 MS Library (Varian [23]) in addition to the specific literature [24]. The linear retention index and the theoretical retention confirmed the identifications. A standard fatty acid methyl ester mixture was run, and the retention times were used to identify the sample peaks. Fatty acid levels were estimated as area percent of total peak area of methyl esters.
Statistical Analysis
The experimental design was a completely randomized design with three replicates; 10 test tubes were used per replicate. The data was submitted in a normality analysis for Lilliefors test and submitted to the analysis of variance (ANOVA) followed by regression analysis. All statistical analyses were done following the procedures of the software SOC [25].
Results and Discussion
Calli Growth Kinetics
The calli originated in this experiment had a compact appearance, and no visual changes in its appearance were observed until the end of the experiment. The growth curve determined by the observed points (i.e., the curve plotted using points obtained by measurements), for the dry and fresh calli presented a sigmoid standard curve with six distinct phases: lag, exponential, linear, deceleration, stationary, and decline (Fig. 1a). The lag phase occurred until 10 days after the experiment installation, and during this period, no morphogenic event was visually verified. The exponential phase began at the 10th day and finished at the 60th day. During this period, calli induced from hypocotyls began to proliferate. The linear phase occurred between the 60th and the 100th days. During this period, an intense cellular proliferation was observed resulting in a fast growth of calli. The deceleration phase occurred after the 100th day and finished at the 120th day, when the higher yield of callus biomass was obtained, an average of 93.9 g. The stationary phase was verified from the 120th day until the 130th day, followed by a decline phase that began at the 130th day (Fig. 1a). The most suitable mathematical model to describe the data was the logistic model, used to estimate both fresh and dry calli growth kinetics. Both the models presented a determination coefficient of 0.98 (Fig. 1a).
Callus growth kinetics originated from hypocotyls of physic nut (Jatropha curcas L.) cultivated on MS medium supplemented with 0.5 mg L−1 2,4-dichlorophenoxyacetic acid (2,4-D) until 180 days of in vitro culture. a Dry and fresh weight of callus, b Total protein content from callus tissues, and c amino acid content from callus tissues.
The calli growth kinetics is important to decide the management of calli culture, mainly to decide when these calli must be pealed and to determine when a great amount of secondary metabolites will be produced. According to Smith [26], in the deceleration phase (in our study, it occurred between the 100th and the 120th days), it is recommended the calli pealing for another new culture medium due to nutrient reduction, loss of water from culture medium, and toxic substances accumulation. The ideal period for extraction of secondary metabolites is the stationary phase (in our study, it occurred between the 120th and the 140th days), when the production of the primary metabolites practically ceases and the accumulation of secondary metabolites is beginning. However, studies of different secondary metabolites must be carried out to establish their kinetics and to evaluate the factors involved in their production. The dry calli growth kinetics is important for the management involving industrial applications, whereas dry biomass is more stable than the fresh biomass; moreover, it is necessary to know the dry biomass yield because it is used in industrial process. The largest yield of dry calli biomass had an average of 6.2 g at the 120th day of culture, representing 6.6 % of the original fresh biomass of callus.
Total Protein and Amino Acids
In order to adjust the regression equations for total protein content from callus tissue, two models were adjusted to explain different situations from different periods of kinetics. The best model to describe the data from the installation of the experiment to the 79th day was the exponential model, and from the 80th to the 180th, the quadratic model was the best one. The determination coefficient was 0.92 referring to all curve points (Fig. 1b).
During the lag phase (until the 10th day), the total protein accumulation was extremely low and its increment was negligible, but increased considerably at exponential phase until the 80th day, in which occurred the largest production of total proteins in callus tissue and resulted in 0.15 μg g−1 calli fresh weight, which corresponded to linear phase, characterized by active cellular growth. After the 80th day, the total protein accumulation started to decrease progressively in successive phases (deceleration, stationary, and decline). The lower value obtained by total protein was observed at the 160th day and continued stable until the end of the experiment (Fig. 1b).
The increment in protein content suggests changes in cellular metabolism, mainly due to the necessity of specific proteins to perform different biological functions; also, many enzymes (proteins) must be produced to accompany the cellular growth and its maintenance; moreover, many of these proteins play structural roles in the cells. When the callus becomes mature (i.e. the primary growth is almost complete), next to the deceleration phase, the protein content start to decrease, mainly because some proteins can be used as energy source, whereas the carbon source (i.e., sucrose) of the culture medium can be almost exhausted at this point.
In order to adjust the equations for the kinetics of total amino acid content from callus tissue, two models were adjusted to explain the different periods. The cubic model presented the best adjustment from the installation of the experiment to the 89th day, while the multiple multiplicative factor model presented the best adjustment from the 90th day to the 180th day. The determination coefficient was 0.89 referring to all curve points (Fig. 1c).
During the lag phase and the beginning of exponential phase (until the 20th day), there was a great increment in total amino acid content from callus tissue (Fig. 1c). This great and fast amino acids accumulation can be associated with the efficient use of the nitrogen sources presents in culture medium. From the 20th day to the 60th day, there was a relative stability which corresponds to the exponential phase, and it is hypothesized that the amino acids have been used in biosynthesis of proteins, whereas in this phase, there was an increment in protein accumulation in callus tissue (Fig. 1b). There was an increase in amino acids content from the 60th day to the 90th day. Almost at the end of linear phase, the amino acid content began to decrease until the end of the experiment (Fig. 1c). The decrease of amino acid content coincided with the beginning of deceleration phase and followed decreasing in successive phases, and it is hypothesized that the amino acids have been used as nitrogen source, similarly to what happened with proteins, due to shortage of the nitrogen source of culture medium.
The lowest value for total amino acids was 0.91 μg g−1 of calli fresh weight and occurred at the beginning period of the culture, and this reflect the nutritional status of the explant (hypocotyls). Different results were obtained in Byrsonima intermedia, in which the initial explants (i.e., leaf explants) at the start of culture presented the highest content of amino acids, what was possibly caused by the nutrition state of the explant before the excision [27].
Total Soluble Sugars and Reducing Sugars
The most suitable mathematical model to describe the data for the total soluble sugar kinetics was the linear model. The determination coefficient was 0.87 (Fig. 2a). Total soluble sugars were higher at the installation of the experiment and decreased linearly until the end of the experiment. Similar results were found in Bertholletia excelsa and in Garcinia brasilisiensis in which the total soluble sugars were higher on the inoculation day and decreased during the growth kinetics [28,29]. Higher sugar contents at the inoculation day can be associated to the energetic reserves of the explant and were consumed to supply the energetic necessity of all metabolic processes of the calli. This result suggests a difficulty of the callus uptakes the sucrose presents in culture medium.
Callus growth kinetics originated from hypocotyls of physic nut (Jatropha curcas L.) cultivated on MS medium supplemented with 0.5 mg L−1 2,4-dichlorophenoxyacetic acid (2,4-D) until 180 days of in vitro culture. a Total soluble sugars, b reducing sugars from callus tissues, and c ethereal extract (crude oil) from callus tissues
In order to adjust the regression equations for the kinetics of consumption of reducing sugars for callus tissue growth, two models were adjusted to explain different situations in different periods. The best model adjusted from the installation of the experiment to the 79th day was the rational model, and from the 80th day to the 180th day, the best one was the multiple multiplicative factor model. The determination coefficient was 0.99 referring to all curve points (Fig. 2b). Reducing sugars were higher at the inoculation day and at the 80th day (i.e. corresponding to linear phase) and then decreased until the end of the experiment (Fig. 2b). Reducing sugars are the first sugars consumed in the cellular metabolism due its higher reactivity than the non-reducing sugars [30]. The higher level of reducing sugars at the initial phase of the culture can be associated to the nutritional status of the initial explant (i.e., hypocotyl), and the second peak with higher level of reducing sugars that occurred at 80th day can be attributed to the glucose and fructose uptake originated from cleavage of sucrose presents in culture medium. The cleavage of sucrose in monosaccharides could have occurred due to instability in the culture medium influenced by putative pH changes or by explant exudation of putative compounds which could promote the sucrose cleavage.
Ethereal Extract
The highest percentage of ethereal extract (oil content) was obtained at the 120th day of culture, reaching 18 % of crude oil from the callus. For this characteristic, two mathematical models were proposed: one explaining the crude oil accumulation inside the callus tissues and the other explaining the oil consumption by the callus tissue. For oil accumulation, a quadratic model was adjusted and for the oil consumption a rational model was adjusted (Fig. 2c). An oil exudation could also be observed from callus tissues to the culture medium, suggesting that the amount of oil produced can be higher than 18 %. It is already known that oil plants need also fatty acids as carbon sources, as observed in Cocos nucifera embryos cultivated in vitro on lauric acid, wherein the lauric acid stimulated the plantlet growth and its development [31]. The oil yield in the calli was lower than the amount produced in seeds, whereas the oil content of seed kernel from 11 counties varied from 51.3 to 61.2 % [32]. Nevertheless, in vitro, it is possible to produce oil throughout the year, while the physic nut plants produce seeds once a year. In order to produce fatty acids from callus tissues, the calli must be harvest around the 120th day of in vitro culture.
Fatty Acid Composition
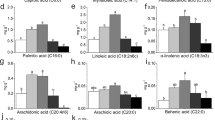
The calli produced medium-chain and long-chain fatty acids (from 10 to 18 carbon atoms) (Table 1). The medium-chain fatty acids were saturated (i.e., capric acid and lauric acid) while the long-chain fatty acids, produced in higher amounts (93.76 %), were saturated and unsaturated. The percentage of the unsaturated and saturated fatty acids was of 9.92 and 90.08 %, respectively. The oleic, stearic, and linoleic acids (C18) represented 13.77 % of the oil. The palmitic acid was the fatty acid with the highest proportion in oil (55.4 %), followed by pentadecanoic acid (11.63 %) and myristic acid (11.25 %) (Table 1). The palmitic acid was also the fatty acid found in higher concentration in calli of melon induced by different tissues such as hypocotyls, cotyledons, roots, leaves, and stems [33].
The lipid profile obtained in callus oil was different from the seed oil profile (Table 1). There are many differences in the oil produced in callus, such as the presence of fatty acids (capric, lauric, pentadecenoic, and pentadecylic) that are not present in seed oil. The callus oil had myristic acid, palmitic acid, and oleic acid in larger proportions than seed oil. The seed oil presents higher proportions of stearic acid and linoleic acid than callus oil (Table 1). These differences in lipid profile of callus and seed oil are probably due to differences in the gene expression of cells of different tissues (calli and embryos), which results in changes in the metabolism of these cells and tissues.
Similar results were found for the lipid profile obtained from cell suspension of physic nut which was different from the seed oil profile; however, there was a great variation on the lipid profile among different populations of physic nut, as well as qualitatively and quantitatively in these cell suspensions, being difficult to establish a profile for oil produced from cell suspension [34]. Consequently, it is possible that calli obtained from different populations of physic nut could present different lipid profile or other metabolites. In this case, appropriate genotypes must be selected according to the desired metabolite. However, the callus growth kinetics in the present study can be useful to carry out these researches.
Conclusions
The callus growth kinetics presented a sigmoid standard curve with six distinct phases: lag (0–10th days), exponential (10–60th days), linear (60–100th days), deceleration (100–120th days), stationary (120–130th days), and decline (after the 130th day). The highest percentage of ethereal extract (oil content) was obtained at the 120th day of culture, reaching 18 % of crude oil from the callus. The calli produced medium-chain and long-chain fatty acids (from 10 to 18 carbon atoms).The lipid profile obtained in callus oil was different from the seed oil profile. The palmitic acid was the fatty acid with the highest proportion in oil (55.4 %). The callus oils presented some fatty acids (i.e., capric, lauric, pentadecenoic, and pentadecylic) that are not present in seed oil. The callus oil had myristic acid, palmitic acid, and oleic acid in larger proportions than seed oil.
References
Costa, J. L., Lima, R. P., Silva, A. L. L., Scheidt, G. N., & Erasmo Lemus, E. A. (2011). Initial growth of plants of physic nut under shading in the Gurupi county, Tocantins State, Brazil. Journal of Biotechnology and Biodiversity, 2, 43–47.
Horbach, M. A., Malavasi, U. C., Malavasi, M. M., Ajala, M. C., Lima, P. R., & Schulz, D. G. (2014). Propagation methods for physic nut (Jatropha curcas). Advances in Forestry Science, 1(1), 53–57.
Goel, G., Makkar, H. P. S., Francis, G., & Becker, K. (2007). Phorbol esters: structure, occurrence and biological activity. International Journal of Toxicology, 26, 279–288.
Zhang, X. (2012). The molluscidal activities of different extracts from Jatropha curcas L. against Pomacea canaliculata. Journal of Anhui Agricultural University, 40, 3349–3350.
Devappa, R. K., Rajesh, S. K., Kumar, V., Makkar, H. P., & Becker, K. (2012). Activities of Jatropha curcas phorbol esters in various bioassays. Ecotoxicology and Environmental Safety, 78, 57–62.
Li, J., Yan, F., Wu, F. H., Yue, B. S., & Chen, F. (2004). Insecticidal activity of extracts from Jatropha curcas seed against Lipaphis erysimi. Acta Physica Sinica, 31(3), 289–293.
Kupchan, S. M., Sigel, C. W., Matz, M. J., Gilmore, C. J., & Bryan, R. F. (1976). Structure and stereochemistry of jatrophone, a novel macrocyclic diterpenoid tumor inhibitor. Journal of the American Chemical Society, 98, 2295–2300.
Van den Berg, A. J., Horsten, S. F., Kettenes van den Bosch, J. J., Kroes, B. H., Beukelman, C. J., Loeflang, B. R., & Labadie, R. P. (1995). Curcacycline A: a novel cyclic octapeptide isolated from the latex of Jatropha curcas Linn. FEBS Letters, 358, 215–218.
Lin, J., Yan, F., Tang, L., & Chen, F. (2003). Antitumor effects of curcin from seeds of Jatropha curcas. Acta Pharmacologica Sinica, 24, 241–246.
Reddy MP, Pamidimarri DS (2010) Biology and biotechnological advances in Jatropha curcas - a biodiesel plant. In: (Ramawat K. G. ed.) Desert plants. Springer Berlin Heidelberg. pp. 57-71.
Wei, Q., Liao, Y., Zhou, L. J., Zhou, J. X., Wang, S. H., & Chen, F. (2004). Antifungal activity of curcin from seeds of Jatropha curcas. Chinese Journal of Oil Crop Sciences, 26, 71–75.
Heller, J. (1996). Physic nut. International Plant Genetic Resources Institute, Rome: Jatropha curcas L. Promoting the conservation and use of underutilized and neglected crops.
Scheidt, G. N., Silva, A. L. L., Oliveira, Y., Costa, J. L., Biasi, L. A., & Soccol, C. R. (2011). In vitro growth of Melaleuca alternifolia Cheel in bioreactor of immersion by bubbles. Pakistan Journal of Botany, 43(6), 2937–2939.
Rong, F., & Wang, S. H. (2005). Identification of curcin by western blot in calli generated from explants of Jatropha curcas L. Journal of Sichuan University (Natural Science Edition), 42(1), 211–214.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiology, 15, 473–497.
Costa, J. L., Silva, A. L. L., Scheidt, G. N., Erasmo, E. A. L., & Soccol, C. R. (2010). In vitro establishment of seeds of physic nut (Jatropha curcas L.) - Euphorbiaceae. Caderno de Pesquisa Série Biologia, 22, 5–11.
Soomro, R., & Memon, R. A. (2007). Establishment of callus and suspension culture in Jatropha curcas. Pakistan Journal of Botany, 39, 2431–2441.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Stein, W., & Moore, S. (1948). A modified ninhydrin reagent for photometric determination of amino acids and related compounds. The Journal of Biological Chemistry, 176, 367–372.
Yemm, E. W., & Willis, A. J. (1954). The estimation of carbohydrates in plant extracts by anthrone. The Biochemical Journal, 57(3), 508–514.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Biochemistry, 31, 426–428.
AOAC. (1990). Official methods of analysis of the Association of Official Analytical Chemists (15th ed., pp. 369–406). Association of Official Analytical Chemists: Washington.
Varian. NIst 98 MS Library Database, ver. 1.7.USA, 1998.
Adams, R. P. (2007). Identification of essential oil components by gas chromatography/mass spectrometry (4th ed.). Carol Stream: Allured Publishing Corporation.
EMBRAPA. (1990). Núcleo Tecnológico para Informática. Campinas: SOC–Software Científico.
Smith, R.H Plant tissue culture: techniques and experiments. San Diego: Academic, 1992. 171p.
Nogueira, R. C., Paiva, R., Lima, E. C., Soares, G. A., Oliveira, L. M., Santos, B. R., Emrich, E. B., & Castro, A. H. F. (2008). Curva de crescimento e análises bioquímicas de calos de murici-pequeno (Byrsonima intermedia A. Juss.). Revista Brasileira de Plantas Medicinais, Botucatu, 10(1), 44–48.
Serra, A. G. P., Paiva, R., & Paiva, P. D. O. (2000). Análises bioquímicas de calos formados de explantes foliares de castanha do Brasil (Bertholletia excelsa H.B.K.). Ciencia e Agrotecnologia, 24(40), 833–840.
Santos-Filho, P. R., Santos, B. R., Barbosa, S., Vieira, L. R., Freitas, N. C., Dias, D. F., & Santos, M. H. (2014). Growth curve, biochemical profile and phytochemical analyses in calli obtained from the procambium segments of Bacupari. Brazilian Archives of Biology and Technology, 57, 326–333.
Carvalho, D. C., Silva, A. L. L., Schuck, M. R., Purcino, M., Tanno, G. N., & Biasi, L. A. (2013). Fox grape cv. Bordô (Vitis labrusca L.) and grapevine cv. Chardonnay (Vitis vinifera L.) cultivated in vitro under different carbohydrates, amino acids and 6-benzylaminopurine levels. Brazilian Archives of Biology and Technology, 56(2), 191–201.
López-Villalobos A, Dodds PF, Hornung R (2011) Lauric acid improves the growth of zygotic coconut (Cocos nucifera L.) embryos in vitro.Plant Cell Tiss Organ Cult. 106, 317-327.
Li, Y. L., Zhang, P., & He, Y. (2006). Perspective of the development and application of Jatropha curcas in the dry-hot valley of Panzhihua. Guangxi Trop Agri., 2, 39–40.
Halder, T., & Gadgil, V. N. (1984). Comparison of fatty acid patterns in plant parts and respective callus cultures of Cucumis melo. Phytochemistry, 23(8), 1790–1791.
Correa, S. M., & Atehortúa, L. (2012). Lipid profile of in vitro oil produced through cell culture of Jatropha curcas. Journal of AOAC International, 95, 1161–1169.
Martínez-Herrera, J., Siddhuraju, P., Francis, G., Dávila-Ortíz, G., & Becker, K. (2006). Chemical composition, toxic/antimetabolic constituents, and effects of different treatments on their levels, in four provenances of Jatropha curcas L. from Mexico. Food Chemistry, 96(1), 80–89.
Acknowledgments
The authors thank CNPq (the National Council for Scientific and Technological Development) and CAPES (Coordination for the Improvement of Higher Level -or- Education - Personnel) for the Post-Doctoral, Ph. D., and M. Sc. fellowships. Moreover, the authors thank the Fundação Araucária for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Luz Costa, J., da Silva, A.L.L., Bier, M.C.J. et al. Callus Growth Kinetics of Physic Nut (Jatropha curcas L.) and Content of Fatty Acids from Crude Oil Obtained In Vitro. Appl Biochem Biotechnol 176, 892–902 (2015). https://doi.org/10.1007/s12010-015-1618-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1618-y