Abstract
In this study, Nostoc muscorum, a native cyanobacterial species isolated from a coal mining site, was employed to remove Cu(II), Zn(II), Pb(II) and Cd(II) from aqueous solution containing these metals in the mixture. In this multicomponent study, carried out as per the statistically valid Plackett-Burman design of experiments, the results revealed a maximum removal of both Pb(II) (96.3 %) and Cu(II) (96.42 %) followed by Cd(II) (80.04 %) and Zn(II) (71.3 %) at the end of the 60-h culture period. Further, the removal of these metals was attributed to both passive biosorption and accumulation by the actively growing N. muscorum biomass. Besides, the specific removal rate of these metals by N. muscorum was negatively correlated to its specific growth rate. For a better understanding of the effect of these metals on each other’s removal by the cyanobacteria, the results were statistically analyzed in the form of analysis of variance (ANOVA) and Student’s t test. ANOVA of the metal bioremoval revealed that the main (individual) effect due to the metals was highly significant (P value <0.05) on each other’s removal. Student’s t test results revealed that both Zn(II) and Pb(II) strongly inhibited both Cu(II) removal (P value <0.01) and Cd(II) removal (P value <0.02). All these results not only demonstrated a very good potential of the cyanobacteria in the bioremoval of these metals but also the effect of individual metals on each other’s removal in the multicomponent system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Contamination of the surface water is mainly due to the discharge of untreated industrial and agricultural effluents that ultimately find their way into the receiving water bodies. This raises a very high probability of heavy metal contamination in surface water that affects all living organisms by entering the food chain. This is particularly problematic because heavy metals are enduring and non-biodegradable in nature, and it tends to accumulate in various life forms [1]. Therefore, there is a constant search for economical, effective and environment-friendly sequestration technique for removing heavy metals from wastewater, which would ensure their presence in the environment below permissible limits [2].
Conventional treatment methods include chemical precipitation, ion exchange, electrochemical removal, etc. However, due to ineffectiveness, high costs or secondary sludge generation associated with these techniques, these methods are not preferred. Hence, there is an increased focus on the more effective biological methods for heavy metal removal [3]. Particularly, live or dead biomass of various organisms is increasingly being studied as these are ecologically sound, effective and technically feasible [4].
Several studies have examined the use of actively growing biomass like bacteria [5], bacterial consortium [6], fungi [7], etc. for heavy metal removal. However, the need for an additional organic carbon source severely limits their application potential [8]. On the contrary, photosynthetic blue green algae (cyanobacteria) require very low nutritional input. These organisms can also survive under stressed environmental conditions, thus making them ideal for bioremoval of heavy metals [9]. Extensive interest has been laid on cyanobacteria as they have been reported to efficiently scavenge heavy metals owing to their large surface area [10, 11]. Their greater mucilage volume as well facilitates more functional groups with high metal-binding affinity [12].
Pollutants such as heavy metals never occur singly in wastewater; it rather coexists with other cations and anions. This is important considering that microorganisms are selective in removing these metals from the mixture, which often results in reduced removal of the other metals in the mixture. Moreover, there could be a synergistic or antagonistic effect on the removal of each other’s metals in the mixture depending upon their interaction with microorganisms. Hence, it is of utmost importance that metal removal from multicomponent system be examined and analyzed in detail. Despite the numerous reports on single metal removal from aqueous solutions by biosorption and bioaccumulation using cyanobacteria [13, 14], metal removal from multicomponent system has not seen addresses so far in the literature.
Hence, the current study was aimed at investigating the bioremoval of heavy metals from multicomponent system by Nostoc muscorum, which has been previously studied for its very high removal of Cu(II), Pb(II), Zn(II) and Cd(II) from single metal solution [15]. In this multicomponent study, experiments were planned as per the statistically valid Plackett-Burman design of experiments to better understand the metal bioremoval from the mixture.
Conventional screening techniques involve varying factor levels while maintaining the other factors at an unspecified constant level. In these methods, the combined effect of the factors is generally neglected; moreover, they are time-consuming and require a sufficiently larger number of experimental runs. These limitations of a conventional method can be eliminated by screening all the factors collectively encompassing statistical design of experiments and analysis. Plackett-Burman design is an efficient statistical experimental design that is employed mainly for investigating the individual main effects of factors on a given response [16]. These designs are highly useful for economically detecting large main effects by assuming that all interactions are negligible when compared with a few important main effects. Interpretation of the results is achieved through analysis of variance (ANOVA) and Student’s t test. In the present study, combination of low and high concentration levels of the four heavy metals Cu(II), Zn(II), Pb(II) and Cd(II) in the mixture was chosen employing the Plackett-Burman screening design. ANOVA and Student’s t test were then employed for statistical analysis of the results to understand the significance and effect of these metals on each other’s removal in the multicomponent system.
Materials and Methods
N. muscorum and Growth Conditions
The cyanobacterium N. muscorum was initially isolated from a heavy metal-contaminated site in Meghalaya, India [15]. The strain was grown in liquid blue green BG110 media using Erlenmeyer flasks with alternate light and dark periods of 16 and 8 h, respectively. Temperature and light intensity for the cyanobacteria growth were maintained at 25–30 °C and 3000–3500 lx (cool white light), respectively. Composition of the BG110 medium was (g/L): K2HPO4·3H2O, 40; MgSO4·7H2O, 75; CaCl2, 36; citric acid, 6; ferric ammonium citrate, 6; EDTA, 1; and Na2CO3, 2. The medium was supplemented with 1 ml/L trace metal solution containing (g/L) H3BO3, 2.86; MnCl2·4H2O, 1.81; Na2MoO4·2H2O, 0.390; ZnSO4·7H2O, 0.222; CuSO4·5H2O, 0.079; and Co(NO3)2·6H2O, 0.0494. The culture was regularly maintained by subculturing and washing with BG110 medium every 20 days.
Chemicals
All chemicals and reagents used in this study were of analytical grade and were obtained from either Merck India Ltd. or Himedia India Ltd.
Heavy Metal Removal from Multicomponent System by N. muscorum
A Plackett-Burman design comprising of 12 experimental runs with different combination of levels of Cu(II), Zn(II), Pb(II) and Cd(II) was employed to study their removal from multicomponent system by N. muscorum (Table S1). The low and high concentration levels of each of these metals were chosen based on our earlier study [15]. The stock solution (1000 mg/L) of each of these metals was prepared by dissolving 2.68 g of CuCl2·2H2O, 4.40 g of ZnSO4·7H2O, 2.29 g of CdSO4·3H2O and 1.60 g of Pb(NO3)2 respectively in 1000 ml of deionized water. These stock solutions were initially diluted with BG110 medium to achieve a desired concentration of the heavy metals in each experimental run (Table S1). All the experiments in this multicomponent study were performed using 250 ml Erlenmeyer flask with 50 ml working volume. Following inoculation, the flasks were incubated at 27 °C temperature and under ambient light conditions, as mentioned earlier. During the experiment, 2 ml sample was drawn every 12 h for the analyses of residual metal concentration and biomass. Results were expressed as % metal removal as given in Eq. 1, where C o and C e are the initial and final metal concentrations in solution (mg/L), respectively.
For determining the amount of metals internalized or bioaccumulated by N. muscorum, the biomass was collected at the end of the experiment by centrifugation at 5000×g for 5 min, washed with EDTA and digested using concentrated HNO3 at 85 °C until the solution turned colourless [16]. For statistical analysis of the results, the statistical software Minitab (version 16, PA, USA) was used.
Heavy Metal Removal Kinetics
To account for the heavy metal removal by biosorption process, which generally precedes its bioaccumulation by the cyanobacterium N. muscorum, the removal kinetics of Cu(II), Zn(II), Cd(II) and Pb(II) was examined at the initial concentration of the metals used in the multicomponent system. The concentrations were chosen based on tolerance and removal of these heavy metals by N. muscorum in our previous single component system study [15]. For studying the heavy metal removal kinetics by N. muscorum, the metal removal data was fitted to the first- and second-order kinetic models reported in the literature. The kinetics of metal removal by the actively growing N. muscorum biomass was also compared with that due to the resting N. muscorum biomass. One of the earliest and most widely used kinetic equations so far for the sorption of a solute from liquid solution is Lagergren’s pseudo first-order equation, given as follows:
where k 1 is the rate constant of pseudo first-order model (min−1) and Qe and Qt denote the amounts of metal ions sorbed at equilibrium and at time t (mg g−1), respectively. A plot of log (Qe – Qt) versus t will give a straight line to confirm the applicability of this kinetic model. In a true first-order process, log (Qe) should be equal to the intercept of a plot of log (Qe – Qt) against t. The pseudo second-order rate expression is used to describe chemisorption involving valency forces through the sharing or exchange of electrons between the adsorbent and adsorbate as covalent forces and ion exchange. A pseudo second-order equation is given by the following form:
where k 2 (g mg−1 min-1) is the rate constant of the pseudo second-order sorption. A plot of t/Q t versus t should give a linear relationship for second-order kinetics. The values of the first- and second-order kinetic rate constants (k 1 and k 2) and the coefficients of determination (R 2) due to these models were calculated from the corresponding plots of the respectively linearized model equations.
For correlating the heavy metal removal kinetics by N. muscorum with its growth, the specific growth rate and specific heavy metal uptake rate by N. muscorum were estimated as per the following Eqs. (4 and 5):
where μ is the specific growth rate (h−1), q M is the specific metal removal rate (h−1), x and C are the concentrations (mg/L) of biomass and heavy metals (mg/L) corresponding to the time t (h) in the multicomponent system.
Analytical Methods
For determining the concentration of heavy metals, samples were centrifuged at 4000×g for 5 min, and the resultant supernatant was analyzed using an Atomic Absorption Spectrophotometer (AAS) (VARIAN, AA 240, Australia). The range of detection limits of the AAS for the different heavy metals was: Cu(II) 0.2–2 mg/L, Zn(II) 0.03–2 mg/L, Cd(II) 0.02–3 mg/L and Pb(II) 0.1–15 mg/L. For the analysis of the cyanobacterial biomass, a gravimetric method, as reported in our earlier study [15], was followed.
Results
Heavy Metal Removal from Multicomponent System
Figure S1(a–d) shows the removal of Cu(II), Zn(II), Pb(II) and Cd(II) by N. muscorum in the multicomponent system, which reveals that the metal removal efficiency varied depending upon the combination level of these metals in the respective mixtures. A maximum removal of both Pb(II) (96.3 %) and Cu(II) (96.42 %) was achieved followed by Cd(II) (80.04 %) and Zn(II) (71.3 %) at the end of the 60-h incubation period. The removal of Cu(II), Pb(II) and Cd(II) was maximum at a low concentration combination (5 mg/L) of these metals, except for Cu(II) which showed a high removal at its high initial level (10 mg/L) in the mixture (Table S1). Zn(II) removal, on the contrary, was maximum when all the four heavy metals were set at their respective low levels (5 mg/L) (Table S1). These results clearly indicate dependence of metal bioremoval by N. muscorum on the metals and their concentration combination in the mixture. An overall removal efficiency of more than 70 % for each metal was achieved in the multicomponent system.
Figure S2 depicts the N. muscorum biomass versus time profile in all the 12 experimental runs. These profiles are similar to the heavy metal removal patterns in the respective runs. The figure also shows that a maximum biomass amount of 212.5 mg/L was achieved in the experimental run no. 5, whereas the heavy metal removal results were better in the experimental runs 3 and 10 compared to those obtained in the other experimental runs.
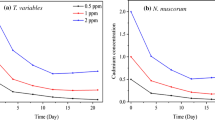
The amount of heavy metals internalized or accumulated by N. muscorum biomass was analyzed in all the experimental runs following the 72-h contact period and is shown in Fig. 1. It can be clearly seen that bioaccumulation of the different heavy metals in the different experimental runs was similar to that of their total removal pattern. Maximum internalization was observed in the experimental run 3 in the case of Pb(II) (1.19 mg/g of biomass). The Pb(II) accumulation value is also higher than the other three metals in the multicomponent system.
Heavy Metal Removal Kinetics and Its Modelling
Figure S1 shows that a maximum removal of the metals in the mixture took place within the first 24–30 h. Although some variation in percentage removal of the heavy metals was observed, the pattern of heavy metal removal by N. muscorum is observed to be initial and quick biosorption followed by their bioaccumulation. For a better understanding of the heavy metal sorption kinetics by N. muscorum, the experimental results were fitted to two kinetic models found in the literature, i.e. Lagregen’s first-order and Ho’s second-order kinetics [17]. The values of the estimated kinetic model parameters and the coefficient of determination (R 2) due to these models, obtained using both actively growing and resting biomass of N. muscorum, are presented in Table 1. These results reveal that, in general, the heavy metal removal by N. muscorum followed the pseudo second-order kinetics more accurately than the pseudo first-order kinetics (Table 1), which is in agreement with the literature [12, 13]. Further, the pseudo second-order kinetic constant (K 2) values were found highest for Pb(II) at both 5 and 10 mg/L initial concentrations in the multicomponent system. Furthermore, the values were slightly lower than those obtained using the actively growing cells (Table 1). These results, therefore, indicate that passive biosorption and active bioaccumulation by living cells of N. muscorum were involved in the heavy metal removal process. Moreover, the value of q e , which is the amount of metal ions removed per unit weight of the biomass, indicates a good removal of Pb(II) and Zn(II) by N. muscorum. The removal efficiency of these metals is also high for their low initial concentration as revealed by the value of the estimated pseudo second-order kinetic parameters (Table 1).
The removal of the four heavy metals by N. muscorum was correlated to its biomass growth in the multicomponent system by comparing the estimated specific growth rate and specific metal removal rate in the different experimental runs, as shown in Fig. 2. A maximum specific growth rate of 0.0134 h−1 was observed in the experimental run no. 7, which also showed the least specific removal rate value of all the metals in the multicomponent system (Fig. 2). Furthermore, the N. muscorum-specific growth rate decreased with an increase in the specific metal removal rate, particularly due to that of Cu(II) (experimental run nos. 1, 3 and 10 in Fig. 2). Thus, it could be posited that uptake of these metals in the multicomponent system delayed the N. muscorum biomass growth [13].
Statistical Analysis
For a better understanding and evaluation of the relative significance of each heavy metal in the multicomponent system, the metal removal results were analyzed statistically in the form of analysis of variance (ANOVA) and Student’s t test. Table 2 presents ANOVA of metal removal in the multicomponent system in which a high Fisher’s F value and a low probability P value of the regression model indicate its accuracy in explaining the variations in the results. The values of the statistical parameters, namely Fisher’s P, S, R 2 and adjusted R 2, collectively describe if the level means are significantly different from each other or not. These parameter values also indicate goodness of fit of the regression model used to describe the experimental results [17, 18]. The parameter S is known as both the standard error of the regression model and the standard error of the estimate. It is expressed in the same units as that of the response variable and represents the standard distance between the experimental and the model predicted values. Thus, a lower value of S indicates a better accuracy of the model in predicting the experimental data. On the other hand, R 2 and adjusted R 2 describe the amount of variation in the observed response values that is explained by the predictors. Thus, a minimum S value and a maximum R value indicate an accurate prediction ability of the model.
Accuracy and precision of the models, in the form of determination coefficient (R 2), adjusted R 2, standard deviation (S) and predicted residual error sum of squares (PRESS), shown in Table 2, also suggest that the models were highly efficient in predicting the experimental results. Further, the results suggest that the individual effect of these metals on each other’s removal in the multicomponent system was statistically significant (Table 2).
To further understand which of the individual heavy metals in the multicomponent system played a significant role on their removal, Student’s t test was performed, which is used as a tool to check the significance of the regression coefficient of the parameters. The estimated coefficients of individual effect of these metals are presented in Table S2 in which the associated t and P values were used to indicate their significance. Thus, Pb(II) exhibits a highly significant negative effect with a P value less than 0.01 on its own removal (Table S2). Zn(II) also showed a significant inhibitory effect on its own removal in the mixture, which was smaller than the effect due to Pb(II) (P < 0.001). Similarly, in the case of Cu(II) removal, the influence due to Zn(II) and Pb(II) was strong, with P values less than 0.01 each (Table S2). Both Zn(II) and Pb(II) again showed significant negative effect on Cd(II) removal with a P value of <0.02 each. All these observations on the effect of different heavy metals on each other’s removal in the multicomponent system are better depicted in the form of Pareto charts, as illustrated in Fig. 3a–d. Horizontal bars in these charts represent the effects due to the individual metals, and the effects which extend past the reference line (vertical line on the chart) denote the significant ones (α = 0.05). Overall, it can be seen that an increase in Pb(II) and Zn(II) concentration level in the mixture inhibited not only their own removal by N. muscorum but also the removal of other metals in the multicomponent system, except in the case of Pb(II) removal, for which no significant inhibitory effect due to Zn(II) was observed.
Discussion
Mechanism of metal removal in a multicomponent system is complex and unique which depends on the concentration combination of the metals as well as the uptake capacity of the microbial strain involved in the process. Therefore, single component study involving these same metals and microorganism cannot predict for their removal in the multicomponent system [6] which aptly correlates in the current study.
In the removal of heavy metals by living cells, different factors, such as metals, their concentration and the type of organism involved, play a critical role [19–22]. The metal removal by N. muscorum in this study involves initially quick sorption onto the cell surface followed by a successive slow uptake of the metals inside the cells (Fig. S1). Thus, both biosorption and bioaccumulation seem to play a role on metal bioremoval by N. muscorum [22, 23]. However, uptake of the metals depended upon their concentration combination in the mixture (Table S1), and the order of removal of metals is observed to be: Pb(II) > Cu(II) > Cd(II) > Zn(II). Pb(II) showed better removal compared with the other three metals in this mixture study. In addition, its removal was unaffected due to the presence of the other metals (Table S2 and Fig. 2). A similar high removal efficiency of Pb(II) compared with other metals was observed in our previous single component study [15]. On the contrary, Pb(II) showed a strong negative effect on the removal of all other metals in the mixture (Table S2 and Fig. 2). This could be explained based on its strong interaction with surface functional groups on the biomass compared with the interaction due to other metals in the mixture. The presence of more non-specific binding sites for Pb(II) on N. muscorum cell surface can also be attributed to its negative effect on the removal of other metals in the multicomponent system. Hydrated ionic radius of metals is another important parameter to consider for its binding as the smaller the hydrated radius, the higher is the affinity of its binding. It has been reported that Pb(II) has the smallest radius among the four metals examined in this study, which is 4.01 Å compared with 6.0, 4.19 and 4.26 Å of Zn(II), Cu(II) and Cd(II), respectively [24]. Besides, stereochemical hindrance, difference in metal class behaviour, etc. can be attributed to the negative effect of Pb(II) on the removal of other metals in the mixture [25].
The metal sorption kinetics followed the pseudo second-order kinetic model (Table 1), suggesting that the initial metal sorption by N. muscorum is reaction controlled, involving chemisorption, i.e. binding of heavy metals to surface functional group on N. muscorum [26]. The estimated model parameter value of Q e , which is the amount of metal ions removed per unit weight of the biomass, indicated a high removal of these metals by N. muscorum. The estimated kinetic parameters of heavy metal removal using both actively growing and resting biomass of N. muscorum (Table 1) indicated that both passive biosorption and active bioaccumulation by living cells of N. muscorum were involved in the heavy metal removal process. Thus, it could be well said that N. muscorum is highly efficient in the removal of metals, particularly Pb(II) and Cu(II), from constituent wastewater.
Conclusions
Among the different heavy metals examined for their bioremoval by N. muscorum in this multicomponent study, Pb(II) was removed with a high efficiency followed by Cu(II), Cd(II) and Zn(II). Pb(II) also showed a strong inhibitory effect on the removal of other metals by N. muscorum, which was attributed to its small hydrated ionic radius compared with these metals. Analysis of the metal removal data showed that the initial quick sorption onto N. muscorum followed the chemisorption-based pseudo second-order kinetics. The estimated sorption capacity values obtained for all these metals proved a very good potential of the cyanbocaterial biomass for their removal from contaminated wastewater.
References
Smejkalova, M., Mikanova, O., & Boruvka, L. (2003). Effects of heavy metal concentrations on biological activity of soil microorganisms. Plant, Soil and Environment, 49(7), 321–326.
Ahmet, S., & Mustafa, T. (2008). Biosorption of cadmium (II) from aqueous solution by red algae (Ceramium virgatum): equilibrium, kinetic and thermodynamic studies. Journal of Hazardous Materials, 157, 448–454.
Won, S. W., Kotte, P., Wei, W., Areum Lim, A., & Yun, Y. S. (2014). Biosorbents for recovery of precious metals. Bioresource Technology, 160, 203–212.
Babu, B. V., & Gupta, S. (2008). Adsorption of Cr (VI) using activated neem leaves as an adsorbent: kinetic studies. Adsorption, 14, 85–92.
Kotrba, P., Doleckova, L., Lorenzo, V., & Ruml, T. (1999). Enhanced bioaccumulation of heavy metal ions by bacterial cells due to surface display of short metal binding peptides. Applied and Environmental Microbiology, 65(3), 1092–1098.
Gikas, P. (2008). Single and combined effects of nickel (Ni (II)) and cobalt (Co (II) ions on activated sludge and on other aerobic microorganisms: a review. Journal of Hazardous Materials, 159, 187–203.
Acikel, U., & Alp, T. (2009). A study on the inhibition kinetics of bioaccumulation of Cu (II) and Ni (II) ions using Rhizopus delemar. Journal of Hazardous Materials, 168(2–3), 1449–1458.
Prasanna, R., Jaiswal, P., & Kaushik, B. D. (2008). Cyanobacteria as potential options for environmental sustainability—promises and challenges. Indian Journal of Microbiology, 48, 89–94.
Cain, A., Vannela, R., & Woo, L. K. (2008). Cyanobacteria as a biosorbent for mercuric ion. Bioresource Technology, 99, 6578–6586.
Bender, J., & Phillips, P. (2004). Microbial mats for multiple applications in aquaculture and bioremediation. Bioresource Technology, 94, 229–238.
Roeselers, G., Loosdrecht, M. C. M., & Muyzer, G. (2008). Phototrophic biofilms and their potential applications. Journal of Applied Phycology, 20, 227–235.
Roy, D., Greenlaw, P. N., & Shane, B. S. (1993). Adsorption of heavy metals by green algae and ground rice hulls. Journal of Environmental Science and Health, Part A: Environmental Science and Engineering & Toxic and Hazardous Substance Control, 28, 37–50.
Saeed, A., Iqbal, M., & Waheed, A. (2005). Removal and recovery of lead (II) from single and multimetal (Cd, Cu, Ni, Zn) solutions by crop milling waste (black gram husk). Journal of Hazardous Materials, 117(B), 65–73.
Sulaymon, A.H., Ebrahim, S.H., and M-Ridha, M.J. (2003) Competitive biosorption of Pb (II), Cr (III), and Cd (II) from synthetic wastewater onto heterogeneous anaerobic biomass in single, binary and ternary batch systems. Desalin. Water. Treat. 1–10 doi: 10.1080/19443994.2013.813008.
Hazarika, J., Pakshirajan, K., Sinharoy, A., and Syiem, M.B. Bioremoval of Cu (II), Zn (II), Pb (II) and Cd (II) by Nostoc muscorum isolated from a coal mining site. J. Appl. Phycol. DOI: 10.1007/s10811-014-0475-3.
Flouty, R., & Estephane, G. (2012). Bioaccumulation and biosorption of copper and lead by a unicellular algae Chlamydomonas reinhardtii in single and binary metal systems: a comparative study. Journal of Environmental Management, 111, 106–114.
Haider, A. M., & Pakshirajan, K. (2007). Screening and optimization of media constituents for enhancing lipolytic activity by a soil microorganism using statistically designed experiments. Applied Biochemistry and Biotechnology, 141, 377–390.
Ho, Y. S., & McKay, G. (1998). Sorption of dye from aqueous solution by peat. Chemical Engineering Journal, 70(2), 115–124.
Mehta, S. K., & Gaur, J. P. (2005). Use of algae for removing heavy metal ions from wastewater: progress and prospects. Critical Reviews in Biotechnology, 25, 113–152.
Puranik, P. R., & Paknikar, K. M. (1999). Influence of co-cations on biosorption of lead and zinc—a comparative evaluation in binary and multimetal systems. Bioresource Technology, 70, 269–276.
Fraile, A., Penche, S., Gonza’lez, F., Bla’zquez, M. L., Mun˜oz, J. A., & Ballester, A. (2005). Biosorption of copper, zinc, cadmium and nickel by Chlorella vulgaris. Chemistry and Ecology, 21, 61–75.
Sheng, P. X., Ting, Y. P., & Chen, J. P. (2007). Biosorption of heavy metal ions (Pb, Cu, and Cd) from aqueous solutions by the marine alga Sargassum sp. in single- and multiple-metal systems. Industrial and Engineering Chemistry Research, 46, 2438–2444.
Gupta, V. K., & Rastogi, A. (2008). Biosorption of lead (II) from aqueous solutions by non-living algal biomass Oedogonium sp. and Nostoc sp.—a comparative study. Colloids and Surfaces B Biointerfaces, 64, 170–178.
Chen, S. B., Ma, Y. B., Chen, L., & Xian, K. (2010). Adsorption of aqueous Cd2+, Pb2+, Cu2+ ions by nano-hydroxyapatite: single- and multi-metal competitive adsorption study. Geochemical Journal, 44, 233–239.
Tsezos, M., & Remoundaki, E. (1997). Recent advances in the mechanistic understanding of metals mobility and interaction with microbial biomass. Research in Microbiology, 148, 515–517.
Plazinski, W., Rudzinski, W., & Plazinska, A. (2009). Theoretical models of sorption kinetics including a surface reaction mechanism: a review. Advances in Colloid and Interface Science, 152, 2–13.
Acknowledgments
The authors thank the Department of Biotechnology, Government of India (BT/216/NE/TBP/2011) for providing the necessary financial support for this research work. They also thank Mr. M. Gopi Kiran, Centre for the Environment, IIT Guwahati, India, for his help in heavy metal analyses of the samples.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roy, A.S., Hazarika, J., Manikandan, N.A. et al. Heavy Metal Removal from Multicomponent System by the Cyanobacterium Nostoc muscorum: Kinetics and Interaction Study. Appl Biochem Biotechnol 175, 3863–3874 (2015). https://doi.org/10.1007/s12010-015-1553-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1553-y