Abstract
Ca2+ serves as a second messenger in plant responses to different signals, and salicylic acid (SA) has been recognized as a signal mediating plant responses to many stresses. We recently found that SA treatment led to the cytoplasmic acidification of Salvia miltiorrhiza cells and alkalinization of extracellular medium. Here, we demonstrate that SA can rapidly induce Ca2+ mobilization in protoplasts, but the induction can be blocked with a channel blocker of either plasma or organellar membranes. Following SA, A 23187, or 10 mmol/L Ca2+ treatment, rosmarinic acid (RA) accumulation reached the highest level at 16 h, whereas the peak was found at 10 h if plasma membrane channel blockers were used. By contrast, the highest accumulation of RA occurred at 16 h when organellar channels were blocked, exhibiting the same tendency with SA-induced cells. In agreement with these observations, both phenylalanine ammonia-lyase (PAL) activity and its gene expression detected by real-time PCR also showed the same patterns. These results indicate that SA treatment firstly results in calcium release from internal stores, which in turn leads to PAL activity increase, RA accumulation, and a large amount of Ca2+ influx from apoplast after 10 h of SA induction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants provide a wide variety of secondary metabolites useful to mankind, such as pharmaceuticals, food additives, and other industrial materials. Often, high-value secondary metabolites are found in low abundance in nature [1], such as tanshinone and rosmarinic acid (RA) and its derivative salvianolic acid B, in Salvia miltiorrhiza Bunge [2–4]. S. miltiorrhiza is a well-known medicinal plant, and its root has remarkable and reliable pharmaceutical functions [2, 4, 5]. Although several reviews have discussed how to increase the production of secondary metabolites from cell cultures [6, 7], the mechanism involved in the synthesis has generally not been elucidated.
Salicylic acid (SA), a phenolic compound broadly distributed across plant species, has been recognized as a regulatory signal mediating responses to many abiotic stresses [6]. Most research on SA has focused on its role in systemic acquired resistance [8–10] and in plant growth and development [6]. However, its role in secondary metabolites synthesis is poorly understood.
Calcium ion (Ca2+) is considered a versatile second messenger in responses to different stimuli like plant hormone, drought, or salt stresses [11]. The Ca2+ signal is effected by modulating activities of channels and transporters at different membranes and cell organelles [12–14]. While the functions of Ca2+ extrusion system are rather well understood in plant cells, the specific influx channels remain unknown [11].
Rosmarinic acid (RA), one of the water-soluble phenolic acids, has been found to have significant bioactivities including as an antioxidant and in anti-ischemia reperfusion [3]. RA is an ester of caffeic acid and 3,4-dihydroxyphenyl lactic acid (3,4-DHPLA), the biosynthesis of which involves both the phenylpropanoid pathway (for caffeic acid production) and a tyrosine-derived pathway (for 3,4-DHPLA production) [15]. However, the mechanism involved in RA accumulation is unknown as is the role of Ca2+ in SA responses in cell cultures. The aim of this investigation is to reveal the mechanism Ca2+ mobilization in SA-induced S. miltiorrhiza cell cultures and its effect on the accumulation of RA.
Materials and Methods
Cell Culture and Treatment
Fresh seeds of S. miltiorrhiza were inoculated on autoclaved Murashige and Skoog (MS) media that contained 30 g/L sugar and 5.5 g/L agar with pH 5.8 after sterilization and then cultured in illumination incubator under 2000–3000-lx light intensity at 25 ± 2 °C for 12–16 h per day. The axenic leaves collected from 2-month-old seedlings were cut into 0.5 × 0.5-cm segments and inoculated on the autoclaved MS media supplemented with 1 mg/L NAA, 1 mg/L 6-BA, 1 mg/L 2,4-D, 5.5 g/L agar, and 30 g/L sugar. The segments were grown at 24 ± 2 °C under a 12-h photoperiod for induction of calli [5].
The calli were transferred to a 100-ml flask and cultured on liquid hormone-free MS media at 25 °C in darkness on an orbital shaker with shake speed 125 rotations per minute (rpm). Each flask contained 30-ml MS media and 1.3-g fresh calli collected from 20-day-old flask cultures. The calli were inoculated to new MS media every 20 days for subculturing for three times (60 days), when the morphology and growth speed of calli were stable. At that time, the calli were collected from culture media through filtration to harvest fresh weight after blotting dry with paper towels. The dry weight was obtained by drying them at 47 °C in a vacuum oven till to constant weight.
Stock solutions of SA (Sigma, USA), A 23187 (calcium ionophore from apoplast to cytoplast, Sigma, USA), verapamil and LaCl3 (blockers of calcium channel on plasma membrane, Sigma, USA), 2-APB (IP3R antagonist, which can be against the reaction of IP3 with IP3R, and thus inhibiting calcium release mediated by IP3, Sigma, USA), LiCl (inhibitor of IP3 cyclase, which can block IP3 synthesis, and thus inhibiting calcium release mediated by IP3, Sigma, USA), and CaCl2·2H2O (exogenous calcium, Sigma, USA) were prepared in distilled water and then sterilized after filtration through 0.22-μm membrane. Each inhibitor was added separately to the cell cultures 30 min before SA treatment. All experiments were performed with three replications.
Determination of Intracellular Free Calcium
The cellulose, pectase, and macerozyme were dissolved in a mixture solution including 0.4 mol/L mannitol, 20 mmol/L KCl, and 20 mmol/L 2-morpholinoethanesulfonic acid (MES) and maintained at 1.5, 0.3, and 0.5 %, respectively. The pH value of this mixture was modulated to 5.7 by using 1 mol/L KOH. Ten milliliters of mixed solution was incubated at 55 °C in a water bath for 10 min, followed by filtration by 0.45-μm membrane after cooling to room temperature and sterilization. Fresh cell cultures (1.5 g) were added to the sterilized solution and cultured at 25 °C on an orbital shaker in darkness with a rotation speed of 40 rpm for 12 h. The mixture was centrifuged at 80 rpm for 1 min, followed by filtration by 600-mesh (23 μm) nylon net. The filtrate was collected in a 50-ml centrifuge tube, to which equal volume of W5 solution (154 mmol/L NaCl, 125 mmol/L CaCl2, 5 mmol/L KCl, 2 mmol/L MES-K, pH 5.7) was added. The mixture was centrifuged at 600 rpm for 5 min, and another 10-ml W5 solution was added to the deposit to repeat centrifugation at 600 rpm for 5 min. Two-milliliter W5 solution was added to the deposit, and the new mixture was put on ice for 30 min, followed by centrifugation at 600 rpm for 3 min. Another 1.5-ml W5 solution (0.4 mol/L mannitol was added) was added to the deposit, and the purified protoplast of S. miltiorrhiza was obtained.
Before observation under a laser scanning confocal microscope, the purified protoplast was fixed on a cover glass that was coated with 0.02 g/ml poly-l-lysine hydrobromide with 300,000 molecular weight (Sigma, USA). At first, Fluo-3/AM, a visible wavelength calcium probe, was dissolved in dimethylsulfoxide (DMSO) to obtain 1 mol/L stock solution, and it was stored at −20 °C. The purified protoplast was incubated in Fluo-3/AM working solution (20 μmol/L) for 1 h, which was obtained from dilution of stock solution by using W5 solution (containing 0.4 mol/L mannitol). After washing twice by W5 solution, the protoplast was put at room temperature for 1 h, followed by fixation on the cover glass after different treatments. Fluorescence intensity was determined and photographed every 2 s within the 20-min duration period after that treatment was carried out. The excitation and emission wavelengths were 488 and 510 nm, respectively. The control did not add any inhibitors, calcium, or calcium ionophore except deionized water.
Analysis of PAL Activity
Fresh calli (2 g) were collected to put in a precooled mortar at 4 °C after being washed by deionized water and removing water on the surface by suction filtration. The calli were homogenized with 5 ml extraction buffer (0.05 mol/L boric acid buffer, 5 mmol/L mercaptoethanol, 1 mmol/L EDTA-2Na, 5 % glycerol, PVP, pH 8.8) that was precooled at 4 °C. The homogenate was filtrated through four layers of cheesecloth after grinding on ice. The filtrate was centrifuged at 4 °C for 15 min with 4000 rpm. The supernatant was crude enzyme, which was used to determine the phenylalanine ammonia-lyase (PAL) activity. The 3-ml reaction mixture contained 0.5 ml crude enzyme, 16 mmol/L l-phenylalanine, 50 mmol/L of Tris-HCl buffer (pH 8.9), and 3.6 mmol/L of NaCl, which was incubated at 37 °C for 60 min. The reaction was stopped by using 500 μl 6 mol/L HCl. The reaction mixture was then centrifuged for 10 min at 12,000×g. The absorbance was measured at 290-nm wavelength, and one enzyme activity unit (U) was defined when the OD value varied 0.01 within 1 min. The formula of calculating PAL activity was as follows:
where V T represented total volume of enzyme (ml), FW represented fresh weight (g), V s represented the volume of enzyme used to determine activity (ml), v represented total volume of reaction mixture (ml), and t represented reaction time (h).
Accumulation of RA
The dried cells (0.05 g) were extracted ultrasonically with 3 ml of methanol-water solution (7:3 v/v) for 45 min. The mixture was centrifuged at 8000 rpm for 10 min, and the supernatant was filtered through a 0.45-μm membrane. The content of RA was quantified by HPLC analysis that was performed using Shimadzu (Japan) system with UV/visible absorbance detector (DAD). The 5-μm C18 reverse-phase column of 4.6 × 250-mm dimension was used with column temperature of 30 °C. The mobile phase was acetonitrile, distilled water, and phosphoric acid (25:75:0.1, v/v/v) with flow rate 1.0 ml/min. RA was detected at 285 nm and quantified by co-chromatography with a RA standard. The injection volume was 10 μl. RA content was calculated based on the formula, C = 3.399x × 10−8 / 0.05, in which C represented RA content (mg/g DW), x represented the peak area of RA, and 0.05 was the dry weight of cells.
Total RNA was extracted from 200-mg cell cultures by using TRIzol method (Qiagen, Beijing) according to the protocol provided by the manufacturer. The integrity and quantity of RNA were detected by protein nucleic acid analyzer (BioPhotometer Plus, Eppendorf, Germany). First-strand complementary DNA (cDNA) was synthesized from 2 μg RNA using RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, USA).
Primers used to amplify PAL for real-time quantitative PCR are listed in Table 1, and the actin gene was employed as control. The qPCR amplification was performed in a total 20-μl reaction system, which contained appropriate cDNA, 0.2 μmol/L of each primer, 10 μl 2× qPCR Mix (SYBR Green II was included), and RNase-free water. Negative control missing cDNA template was also included in this experiment. qPCR was performed with the condition as 1 cycle of 94 °C for 1 min, 35 cycles of 94 °C for 10 s, 60 °C for 20 s, and 82 °C for 30 s [16]. The products of qPCR were detected on 1.5 % agarose gel electrophoresis, and the equal-sized band was observed as predicted. Quantification of PAL gene expression was done with comparative CT method. Each data represents the average of three replications.
PAL Gene Expression
Total RNA was extracted from 200-mg cell cultures by using TRIzol method (Qiagen, Beijing) according to the protocol provided by the manufacturer. The integrity and quantity of RNA were detected by protein nucleic acid analyzer (BioPhotometer Plus, Eppendorf, Germany). First-strand cDNA was synthesized from 2 μg RNA using RevertAid First-Strand cDNA Synthesis Kit (Thermo Scientific, USA).
Primers used to amplify PAL for real-time quantitative PCR are listed in Table 1, and the actin gene was employed as control. The qPCR amplification was performed in a total 20-μl reaction system, which contained appropriate cDNA, 0.2 μmol/L of each primer, 10 μl 2× qPCR Mix (SYBR Green II was included), and RNase-free water. Negative control missing cDNA template was also included in this experiment. qPCR was performed with the condition as 1 cycle of 94 °C for 1 min, 35 cycles of 94 °C for 10 s, 60 °C for 20 s, and 82 °C for 30 s [17]. The products of qPCR were detected on 1.5 % agarose gel electrophoresis, and the equal-sized band was observed as predicted. Quantification of PAL gene expression was done with comparative CT method. Each data represents the average of three replications.
Results
Calcium Mobilization Induced by SA in Protoplast
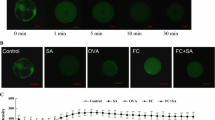
SA (22 mg/L) significantly promoted calcium mobilization in protoplasts of S. miltiorrhiza. The increase began from 43 s after SA treatment and reached the highest level at 12 min (Fig. 1), at which point mobilization was 4.3-fold of H2O control. After that time, the calcium fluorescence intensity decreased gradually until 20 min post-treatment, at which point intensity was the same as at the beginning of the experiment.
The compounds 2-APB (15 μmol/L) and LaCl3 (0.5 mol/L) were used to inhibit calcium mobilization mediated by IP3 and on the plasma membrane, respectively. Both could significantly inhibit the calcium mobilization induced by SA, and LaCl3 showed stronger and significant inhibition from the fourth minute when compared with 2-APB (Figs. 1 and 2). The calcium fluorescence intensity in LaCl3-treated protoplasts was equal to or lower than that of H2O (Figs. 1 and 2).
The fluorescence photograph collected at the time point of 11 min based on the intensity tendency exhibited in Fig. 1 after being treated with different membrane channel blockers or calcium ionophore. The protoplasts were cultured in the medium treated with (A) H2O, (B) SA, (C) LaCl3 + SA, (D) 2-APB + SA, (E) A 23187 + Ca2+, (F) exogenous Ca2+, and (G) LaCl3 + 2-APB + SA. Those protoplasts from a to g were controls corresponding with treated protoplasts from A to G
Exogenous calcium (CaCl2) was used to determine the capacity of A 23187 (calcium ionophore from apoplast to cytoplast). A 23187 addition did not cause calcium mobilization in protoplasts if no calcium was added to its culture media (Fig. 3). Meanwhile, if protoplasts were cultured in the media containing 10 mmol/L CaCl2, A 23187 would significantly increase the calcium in the protoplast (Fig. 2). The calcium fluorescence intensity reached the strongest level at 11 min, which was 3.5-fold higher than that of A 23187 treatment without calcium in culture media.
Effects of SA, A 23187, and Exogenous Calcium on RA Accumulation in Cell Cultures
To investigate the effects of calcium mobilization on RA accumulation in cell cultures, treatments including SA, A 23187 and exogenous calcium were employed. From 10 h post-treatment, RA content in SA-, A 23187- and exogenous calcium-treated cells was significantly higher than that of H2O-treated controls (Fig. 4). This situation lasted for 14 h. RA accumulation reached the highest level at 16 h post-treatment, when its content was 11.025 mg/g in SA-elicited cells and was 84.7 % higher than that of H2O-treated controls.
Effect of Calcium Mobilization Inhibited by Plasma Membrane Channel Blockers on RA Accumulation
Both LaCl3 (0.5 mmol/L) and verapamil (0.5 mmol/L) were added separately to cell cultures in order to inhibit calcium influx from extracellular culture media. Their effects on RA accumulation were evaluated, and no significance was found between each of them and H2O (data not shown), indicating that such concentrations of both inhibitors were appropriate for subsequent treatments. RA contents in LaCl3 + SA and verapamil + SA treatments were significantly lower than that of SA-induced cells, which began from 16 to 24 h (Fig. 5). At the time point of 10 h, RA accumulation reached the highest level in both LaCl3 + SA and verapamil + SA treatments, while it increased to the peak till to 16 h in SA-induced cells.
Effect of Calcium Mobilization Inhibited by Organellar Channel Blockers on RA Accumulation
Both 2-APB and LiCl were used to inhibit calcium release mediated by IP3, and the effects of both on RA accumulation were also evaluated. It was found that 15 μmol/L 2-APB and 7 mmol/L LiCl did not significantly affect accumulation of RA. However, RA contents in both 2-APB + SA and LiCl + SA treatments were significantly lower than that of SA-induced cells from the 10 h and lasted until 16 h (Fig. 6).
Effect of Calcium Mobilization Inhibited by Both Channel Blockers of Plasma and Organellar Membranes on RA Accumulation
The LaCl3 and 2-APB treatments were used simultaneously to inhibit calcium mobilization on plasma membrane and calcium release mediated by IP3. RA content in LaCl3 + 2-APB treatment was not significantly different from that of LaCl3 + 2-APB + SA-treated cells during all treatment times. But, RA contents in these two combined treatments were significantly lower than that of SA-induced cells from 10 to 48 h (Fig. 7). These results indicate that calcium mobilization was modulated by SA, which was also the key factor to regulate downstream genes to accumulate RA.
Effects of SA, A 23187, and Exogenous Calcium on PAL Activity
SA, A 23187, and exogenous calcium promoted RA accumulation in cell cultures (Fig. 4). PAL is a key enzyme at the beginning of the phenylpropanoid pathway. Therefore, the enzymatic activity of PAL was determined in response to calcium-mobilizing treatments. The enzyme activity was highest from the 10 to 16 h following treatment, and no significant difference was found among SA, A 23187, and exogenous calcium treatments (Fig. 8).
Effect of Calcium Mobilization Inhibited by Both Channel Blockers of Plasma and Organellar Membranes on PAL Enzyme Activity
Both LaCl3 and verapamil were used to block calcium mobilization on plasma membrane, and 2-APB and LiCl were employed to block calcium release mediated by IP3. PAL activities in LaCl3 + SA and verapamil + SA treatments were significantly lower than that of SA-induced cells from 10 to 48 h (Fig. 9). The highest PAL activities in these two combined treatments occurred at 10 h. PAL activities in LiCl + SA and 2-APB + SA treatments continuously increased from the treatment beginning to 16 h when both reached the highest level, but no significance difference was found compared to SA-induced cells.
When LaCl3 and 2-APB were applied simultaneously to inhibit calcium mobilization on plasma membrane and calcium release mediated by IP3 at the same time, PAL activities in both LaCl3 + 2-APB and LaCl3 + 2-APB + SA treatments were significantly lower than that of SA-induced cells from 6 to 48 h (Fig. 10).
Effect of Calcium Mobilization on PAL Gene Expression in A 23187 and Exogenous Calcium-Treated Cells
The real-time PCR result showed that both A 23187 and exogenous calcium significantly promoted PAL gene expression when compared with its expression in those cells cultured in deionized H2O. Meanwhile, the expression level in A 23187 treatment was not significantly different than that exogenously Ca2+-treated cells (Fig. 11).
Effect of Calcium Mobilization Inhibited by Both Channel Blockers of Plasma and Organellar Membranes on PAL Gene Expression
PAL gene expression levels in two combined treatments, LaCl3 + SA and 2-APB + SA, were significantly lower than that of SA-induced cells, but significantly higher than both in LaCl3 + 2-APB + SA and H2O treatments (Fig. 12).
Effects of calcium mobilization on phenylalanine ammonia-lyase (PAL) gene expression when plasma and organellar membrane channel blockers, LaCl3, 2-APB, and combination of both, were added to salicylic acid (SA)-induced cell cultures. Different lowercase letters over columns represented significance at 0.05
Discussion
SA has been identified as a key component of the systemic acquired resistance (SAR) signal transduction pathway [18]. Upon pathogen attack, SA accumulates at the infection site to a high level, binds, and inactivates catalase activity [19], thereby leading to an increase of H2O2 release, which serves as a secondary messenger to induce the expression of genes and proteins [5, 20–23]. This stimulates the accumulation of secondary metabolites, such as indole glucosinolate in Arabidopsis [24], anthraquinone phytoalexin in Rubia cordifo cultures [25], and alkaloids in hairy root cultures of Brugmansia candida [26]. However, it is still not understood how SA stimulates ion transport and thus leads to accumulation of secondary metabolites.
Ion fluxes are immediate responses of plant cells in response to elicitors or stress, among which Ca2+ influx is regarded as one of the most significant events, since Ca2+ is a key second messenger for many diverse physiological changes and cellular processes [23, 27, 28]. In this paper, the Ca2+ influx in protoplasts increased drastically from 42 s after SA induction to 12 min when the level reached the highest (4.3-fold of H2O, Fig. 1). It has been found that the increased Cacyt stimulates NAD(P)H oxidase activity, leading to extracellular O2 − release and H2O2 production [29, 30]. The Ca2+ spiking not only mediates the subsequent accumulation of secondary metabolites [31], but also further amplifies Ca2+ signaling through Ca2+-modulated production of H2O2 [32]. The Ca2+ influx/Cl− efflux accompanied with K+/H+ exchange, derived from the depolarization of plasma membrane, leads to cytoplasmic acidification and alkalinization of extracellular medium [33–35]. Cytoplasmic acidification is regarded as an essential step in signal transduction, leading to the oxidative burst and accumulation of secondary metabolites [36]. The alkalinization of the culture medium also can cause an increase in phenylalanine ammonia-lyse (PAL) activity, accompanied by accumulation of isofavone glycosides [37], lignin [38], or phytoalexin [33].
Elevation of Cacyt in response to signals is due to Ca2+ influx from apoplast and/or Ca2+ release from intracellular stores (vacuoles, endoplasmic reticulum, mitochondria, chloroplasts, and nucleus), during which different signals use distinct Ca2+ mobilization means in elevating Cacyt. Ca2+ increase is inhibited by plasma membrane channel blockers in cold-induced tobacco cells but is not affected by organellar channel blockers [39]. In parsley cells, elicitor-induced Cacyt increase is also primarily due to the influx of extracellular Ca2+ [40]. However, internal Ca2+ stores contribute to wind- and chitosan-induced elevation of Cacyt [16, 41]. ABA-induced changes in Cacyt are found to be attributed to both Ca2+ release from internal stores and Ca2+ influx from apoplast [42], which is similar to SA-induced cells in this paper. In SA, A 23187, or 10 mmol/L Ca2+-treated cells, RA accumulation reached the highest level at the 16th hour (Fig. 4), whereas the peak was found at 10 h if plasma membrane channel blockers were used (Fig. 5). By contrast, the highest accumulation of RA occurred at the 16th hour when organellar channels were blocked, exhibiting the same tendency with SA-induced cells (Fig. 6). These results indicate that SA induction firstly results in calcium release from internal stores, which in turn leads to PAL activity increase (Fig. 8) and a large amount of Ca2+ influx from apoplasts after 10-h SA induction.
Conclusions
SA can rapidly induce Ca2+ mobilization in protoplasts, but the induction can be blocked with either channel blocker of plasma or organellar membrane. In SA, A 23187, or 10 mmol/L Ca2+ treatment, rosmarinic acid (RA) accumulation reached the highest level at the 16th hour, whereas the peak was found at the 10th hour if plasma membrane channel blockers were used. By contrast, the highest accumulation of RA occurred at the 16th hour when organellar channels were blocked, exhibiting the same tendency with SA-induced cells. Both PAL activity and its gene expression also show the same tendency.
References
Chandra, S., & Chandra, R. (2011). Engineering secondary metabolite production in hairy roots. Phytochemistry Review, 10, 371–395.
Kai, G., Xu, H., Zhou, C., Liao, P., Xiao, J., Luo, X., You, L., & Zhang, L. (2011). Metabolic engineering tanshinone biosynthetic pathway in Salvia miltiorrhiza hairy root cultures. Metabolic Engineering, 13, 319–327.
Yan, Q., Shi, M., Ng, J., & Wu, J. Y. (2006). Elicitor-induced rosmarinic acid accumulation and secondary metabolism enzyme activities in Salvia miltiorrhiza hairy roots. Plant Science, 170, 853–858.
Zhang, Y., Yan, Y. P., Wu, Y. C., Hua, W. P., Chen, C., Ge, Q., & Wang, Z. Z. (2014). Pathway engineering for phenolic acid accumulation in Salvia miltiorrhiza by combinational genetic manipulation. Metabolic Engineering, 21, 71–80.
Dong, J., Wan, G., & Liang, Z. (2010). Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. Journal of Biotechnology, 148, 99–104.
Vicente, M. R. S., & Plasencia, J. (2011). Salicylic acid beyond defence: its role in plant growth and development. Journal of Experimental Botany, 62, 3321–3338.
Vijaya, S. N., Udayasri, P., Aswani Kumar, V. V. V., Ravi, B. B., Phani, K. Y., & Vijay, V. M. (2010). Advancements in the production of secondary metabolites. Journal of Natural Products, 3, 112–123.
Durrant, W. E., & Dong, X. (2004). Systemic acquired resistance. Annual Review of Phytopathology, 24, 185–209.
Shah, J. (2003). The salicylic acid loop in plant defense. Current Opinion on Plant Biology, 6, 365–371.
Vlot, A. C., Dempsey, D. M. A., & Klessing, D. F. (2009). Salicylic acid, a multifaceted hormone to combat disease. Nature Review of Phytopathology, 47, 177–206.
Batistič, O., & Kudla, J. (2012). Analysis of calcium signaling pathways in plants. Biochimica et Biophysia Acta, 1820, 1283–1293.
Dodd, A. N., Kudla, J., & Sanders, D. (2010). The language of calcium signaling. Annual Review of Plant Biology, 61, 593–620.
Kudla, J., Batistič, O., & Hashimoto, K. (2010). Calcium signals: the lead currency of plant information processing. Plant Cell, 22, 541–563.
Sanders, D., Brownlee, C., & Harper, J. F. (1999). Communicating with calcium. Plant Cell, 11, 691–706.
Petersen, M., & Simmonds, M. S. J. (2003). Rosmarinic acid. Phytochemistry, 62, 121–125.
Knight, M. R., Smith, S. M., & Trewavas, A. J. (1992). Wind-induced plant motion immediately increases cytosolic calcium. Proceedings of National Academy of Science of the United States of America, 89, 4967–4971.
Song, J., & Wang, Z. Z. (2009). Molecular cloning, expression and characterization of a phenylalanine ammonia-lyase gene (SmPAL1) from Salvia miltiorrhiza. Molecular Biology Reports, 36, 939–952.
Sticher, L., Mauch-Mani, B., & Métreaux, J. P. (1997). Systemic acquired resistance. Annual Review of Phytopathology, 35, 235–270.
Van Camp, W., Van Montagu, M., & Inzé, D. (1998). H2O2 and NO: redox signals in disease resistance. Trend in Plant Science, 3, 330–334.
Chen, H., Liu, L., Dong, J., & Xia, G. (2012). Hydrogen peroxide is involved in the signal transduction of salicylic acid-induced salvianolic acid B biosynthesis in Salvia miltiorrhiza cell cultures. Chinese Journal of Biotechnology, 28, 834–846.
Guo, B., Liang, Y. C., Zhu, Y. G., & Zhao, F. J. (2007). Role of salicylic acid in alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress. Environmental Pollution, 147, 743–749.
Taguchi, G., Yazawa, T., Hayashida, N., & Okazaki, M. (2001). Molecular cloning and heterologous expression of novel glucosyltransferases from tobacco cultured cells that have broad substrates specificity and are induced by salicylic acid and auxin. European Journal of Biochemistry, 268, 4086–4094.
Zhao, J., Davis, L. C., & Verpoorte, R. (2005). Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnology Advance, 23, 283–333.
Mikkelsen, M. D., Petersen, B. L., Glawischnig, E., Jensen, A. B., Andreasson, E., & Halkier, B. A. (2003). Modulation of CYP79 genes and glucosinolate profiles in Arabidopsis by defense signaling pathways. Plant Physiology, 131, 298–308.
Bulgakov, V. P., Tchernoded, G. K., Mischenko, N. P., Khodakovskaya, M. V., Glazunov, V. P., Radchenko, S. V., Zvereva, E. V., Fedoreyev, S. A., & Zhuravlev, Y. N. (2002). Effect of salicylic acid, methyl jasmonate, ethephon and cantharidin on anthraquinone production by Rubia cordifolia callus cultures transformed with rol B and rol C genes. Journal of Biotechnology, 97, 213–221.
Pitta-Alvarez, S. I., Spollansky, T. C., & Giulietti, A. M. (2000). The influence of different biotic and abiotic elicitors on the production and profile of tropane alkoids in hairy root cultures of Brugmansia candida. Enzyme and Microbiology Technology, 26, 252–258.
Peiter, E. (2011). The plant vacuole: emitter and receiver of calcium signals. Cell Calcium, 50, 120–128.
Trewavas, A. J., & Malho, R. (1998). Ca2+ signalling in plant cells: the big network! Current Opinion in Plant Biology, 1, 428–433.
Halliwell, B., & Gutteridge, J. (1999). Free radicals in biology and medicine (3rd ed., p. 936). Oxford: Oxford University Press.
Pei, Z., Murata, Y., Benning, G., Thomine, S., Kluesener, B., Allen, G. J., Grill, E., & Schroeder, J. I. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature, 406, 731–734.
Smith, C. J. (1994). Signal transduction in elicitation of phytoalexin synthesis. Biochemical Society Transactions, 22, 414–419.
Lecourieux, D., Mazars, C., Pauly, N., Ranjeva, R., & Pugin, A. (2002). Analysis and effects of cytosolic free calcium increases in response to elicitors in Nicotiana plumbaginifolia cells. Plant Cell, 14, 2627–2641.
Armero, J., & Tena, M. (2001). Possible role of plasma membrane H+-ATPase in the elicitation of phytolexion and related isoflavone root secretion in chickpea (Cicer arietinum L.) seedlings. Plant Science, 161, 791–798.
Boller, T. (1995). Chemoperception of microbial signals in plant cells. Annual Review of Plant Physiology and Plant Molecular Biology, 46, 189–214.
Liu, L., Wang, C., Dong, J., Su, H., Zhuo, Z. Q., & Xue, Y. X. (2013). Effect of calcium on medium alkalinization induced by salicylic acid in Salvia miltiorrhiza suspension cultures. Chinesis Journal of Biotechnology, 29, 986–997.
Sakano, K. (2001). Metabolic regulation of pH in plant cells: role of cytoplasmic pH in defense reaction and secondary metabolism. International Review of Cytology, 206, 1–44.
Hattori, T., & Ohta, Y. (1985). Induction of phenylalanine ammonia-lyase activation and isoflavone glucoside accumulation in suspension-cultured cells of red bean, Vigna angularis, by phytoalexins elicitors, vanadate and elevation of medium pH. Plant Cell and Physiology, 26, 1101–1110.
Hagendoorn, M. J. M., Traas, T. P., Boon, J. J., & van der Plas, L. H. W. (1990). Orthovanadate induced lignin production, in batch and continuous cultures of Petunia hybrida. Journal of Plant Physiology, 137, 72–80.
Van der Luit, A. H., Olivari, C., Haley, A., Knight, M. R., & Trewavas, A. J. (1999). Distinct calcium signaling pathways regulate calmodulin gene expression in tobacco. Plant Physiology, 121, 705–714.
Blume, B., Nurnberger, T., Nass, N., & Scheel, D. (2000). Receptor-mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell, 12, 1425–1440.
Vasconsuelo, A., Morelli, S., Picotto, G., Giulietti, A. M., & Boland, R. (2005). Intracellular calcium mobilization: a key step for chitosan-induced anthraquinone production in Rubia tinctorum L. Plant Science, 169, 712–720.
McAinsh, M. R., Brownlee, C., & Hetherington, A. M. (1992). Visualising changes in cytosolic Ca2+ during the response to stomal guard cells to abscisic acid. Plant Cell, 4, 1113–1122.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 31170274) and Academic Backbone Youth of Northwest A&F University (No. Z111020906).
Author information
Authors and Affiliations
Corresponding author
Additional information
Hongbo Guo and Nan Zhu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Guo, H., Zhu, N., Deyholos, M.K. et al. Calcium Mobilization in Salicylic Acid-Induced Salvia miltiorrhiza Cell Cultures and Its Effect on the Accumulation of Rosmarinic Acid. Appl Biochem Biotechnol 175, 2689–2702 (2015). https://doi.org/10.1007/s12010-014-1459-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1459-0