Abstract
Aptamers are short and functional single-stranded oligonucleotide sequences selected from systematic evolution of ligands by exponential enrichment (SELEX) process, which have the capacity to recognize various classes of target molecules with high affinity and specificity. Various analytical aptamers acquired by SELEX are widely used in many research fields, such as medicine, biology, and chemistry. However, the application of this innovative and emerging technology to food safety is just in infant stage. Food safety plays a very important role in our daily lives because varieties of poisonous and harmful substances in food affect human health. Aptamer technique is promising, which can overcome many disadvantages of existing detection methods in food safety, such as long detection time, low sensitivity, difficult, and expensive antibody preparation. This review provides an overview of various aptamer screening technologies and summarizes the recent applications of aptamers in food safety, and future prospects are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
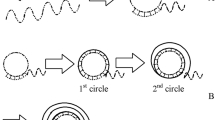
Aptamer is from the Latin word “aptus” meaning “to fit” [1]. Aptamers are functional nucleic acids binding to specific target molecules, including DNA or RNA aptamer [2]. The aptamer selection technology is called systematic evolution of ligands by exponential enrichment (SELEX) (Fig. 1), which is a screening technique that involves the progressive selection of highly specific ligands based on several repeated rounds of partition, elution, and amplification by the polymerase chain reaction (PCR) technology from a large random combinatorial nucleic acid library [3]. Due to the large capacity of a random library, in theory, the specific recognition aptamers for any target molecule can be selected by such an excellent method [4], such as amino acids [5], peptides [6], proteins [7], polysaccharides [8], and whole cells [9]. Aptamers can form many three-dimensional structures (G-quartet, bulge loop, pseudoknot, hairpin, etc.) through molecular interactions such as hydrophobic interaction, hydrogen bond, van der Waals force, and other molecular interactions, which enable aptamers to recognize the target specifically [10, 11]. The advantages of aptamers are as follows: (1) wide range of target molecules, ranging from small molecules to proteins and even whole cells, i.e., the aptamers binding to these molecules or cells can be selected; (2) high specificity and strong affinity: aptamers have higher specificity and affinity compared to antibody and other kinds of ligands and can discriminate a methyl from a hydroxyl in the molecular structure [12]. Aptamers have strong binding strength with target molecules, generally the dissociation constant (K d ) is between nanomolar and picomolar levels; (3) easy preparation and convenient modification [3]: aptamers have small molecular weight than antibody, so various necessary DNA sequences can be synthesized in vitro rapidly and flexibly with the chemical synthesis method. Moreover, exact site modification, such as biotin labeling and fluorescent labeling, can be realized, and the products have the advantages of low cost and easy to repeat. Since 1990, in order to obtain more target molecules rapidly and efficiently, many advanced SELEX technologies have been developed (Table 1).
To date, more than hundreds of aptamers have been selected out, which have high specificity and affinity with target molecule, such as growth factor, antibody, cell surface receptor, transcription factor, polypeptide, enzyme, antibiotic, protein, heavy metal ion, organic dye, and pathogen [13–17]. These aptamers have the features of strong molecular recognition and chemical stability; therefore, they have broad application value in biology and medicine [18–20]. In addition, they have great potential for applications in the analytical science field, especially for analysis of ion and small molecular compounds [21, 22].
Nowadays, food safety has aroused more and more public attention because of its close relevance to our health [23]. Since many kinds of poisonous and harmful substances could be existed in foods, the effective food analysis detection technology becomes more and more important [24]. In particular, the amount of harmful substances is extremely limited and is usually in the level of micrograms, nanograms, and even picograms; many analysis instruments have the disadvantages of expensive cost, complex operation, and long detection time. Thus, traditional analysis and detection methods cannot meet the demand of food safety testing, which should be very quick, convenient, and accurate [25]. Given the advantages of strong specificity, high sensibility, wide range of target molecules, low testing cost, easy synthesis in vitro, safety, and reliability, aptamers have promising applications in food safety testing (Table 2). Currently, various aptamer-based techniques were researched for the application in food safety testing, such as aptamer-nanoparticle colorimetry [26, 27], surface plasmon resonance (SPR) biosensor [28–30], dynamic light scattering (DLS) [31], fluorescent biosensor [32–34], electrochemical biosensor [35, 36], and solid-phase extraction(SPE) column [37, 38] (Table 2). In this paper, after briefly describing the screening technique of aptamers, we focus on the applications of aptamers in food safety, and future prospectives are also discussed.
Screening Technique of Aptamer (SELEX)
The conventional aptamer selection process with the SELEX technology is shown in Fig. 1. The selection of aptamers mainly involves the following five steps [39]:
-
1.
Designation and synthesis of the single-stranded random DNA or RNA oligonucleotide sequence library. Generally, the library contains 1014–1015 different sequences which fold into a vast array of different structures [5], and the length of the random sequence is about 20–80 nt
-
2.
Selection of library: co-culturing the library with target molecule under specified buffer system to form oligonucleotide complexes with the target molecule
-
3.
Partitioning of the nucleic acid sequence binding target molecules from nonbinding target molecules. The oligonucleotides binding the target molecule closely are separated from the library with various separation methods, such as magnetic cell sorting, capillary electrophoresis, column chromatography, and membrane filtration
-
4.
Amplification of sequences: conducting the PCR proliferation for the acquired specific binding nucleic acid sequence to provide a new nucleic acid pool. Steps 2 to 4 are repeated usually about 10–20 times
-
5.
Acquisition of individual aptamer: after the last round of SELEX, the acquired aptamers are cloned, sequenced, and tested for binding specificity and affinity.
The SELEX technology is characterized in that the molecule evolution course is conducted in vitro, which is often limited by the selection environment and the technology itself, leading to the decreased selection efficiency [11]. In the past decade, various improvements of the original SELEX method for different purposes have been reported (Table 1), such as capillary electrophoresis SELEX, toggling SELEX, tailored SELEX, whole-cell SELEX, FluMag-SELEX, Photo-SELEX, and subtractive SELEX, which have greatly facilitated the application of aptamer technology in many fields [4].
Application of Aptamers in Food Safety
Food safety problems such as the pollution of variety of pathogenic bacteria, biotoxin, antibiotics, and pesticide residue in food have caused more and more attention to all countries around the world [11]. The increasing presence of various contaminants in food, which are capable of causing diseases, toxication, or chronic illness although extremely low contents, has highlighted the need for analytical systems capable of rapid multianalyte measurements of complex food, animal feedstuff, or environmental samples [40, 41]. At present, the drawbacks for the detection of the poisonous and harmful substances in food include expensive equipment requirement, the high cost, the high false-positive rate of detection method, the complex testing process, long detection time, and other issues. Moreover, the existing analysis methods are unable to effectively detect some poisonous and harmful substances. Therefore, the development of new detection methods is attracting more and more researchers’ attention. Aptamer technology is a newly and rapidly developed technique in recent years. Currently, various aptamers have been selected in the field of food safety (Table 2) (Fig. 2).
Microorganisms in Food
The pollution of food microorganism being in the meat, poultry, eggs, and other foods is influencing the human health. Traditional testing method to the food microbial usually is plate count, which has the disadvantages of fussy detection process and long detection cycle [42]. The development of aptamers technology has laid the foundation for the new, quick, and efficient testing method of food microbial [43–46]. Detection of antigen by aptamers is a new technology developed in recent years, the principle is that one end of the aptamer is cross-linked to solid-phase carrier for the capture of target material of the specimen, and the other 5′ end of the aptamer is marked corresponding indicator, such as fluorescent element, biotin, radioactive isotopes, or gold particles [36, 47, 48]. The signal will appear when the aptamer binds to the target molecules in the corresponding sample, which achieves the purpose of testing [23].
Salmonella are important foodborne pathogens that pose a serious threat to food safety [45]. The traditional detection method of Salmonella generally takes 4–5 days, is cumbersome to operate, the pretreatment process is trivial, and the detection cycle is long, which cannot meet the requirements of fast, easy, and high accuracy in food testing. In recent years, a lot of rapid detection approaches for Salmonella have been set up, such as enzyme-linked immunosorbent assay, gene chip technology, PCR technology, and instrumental analysis technology [49, 50]. With the increasing importance in food safety, it will be the main direction to develop portable, low-cost, simple-operation, and rapid detection of Salmonella for future research. Aptamer is an emerging technology applied to the rapid detection of Salmonella. Pan et al. [46] have reported the direct selection of aptamers for Salmonella enteric serovar Typhi, and an RNA aptamer specific for type IVB was acquired to decrease the entry of the piliated strain into human cells. Joshi et al. [45] have selected and assessed the DNA aptamers for the capture and detection of Salmonella enteric serovar Typhimurium. The aptamer 33 they selected was bound to magnetic beads and used for the capture of Salmonella enteric serovar Typhimurium seeded into the whole carcass chicken rinse samples, and the study indicated the applicability of Salmonella-specific aptamers for preanalytical sample processing as applied to complex sample matrices. Hyeon et al. [3] examined the potential of RNA aptamers in detecting Salmonella enteritidis and observed that the selected aptamer can specifically bind to Salmonella enteritidis without any cross-reactivity to other Salmonella serovars. Using a Salmonella-specific recognition aptamer, Ma et al. [51] prepared an electrochemical biosensor for Salmonella detection, with a detection limit as low as 3 CFU/mL. Through ten rounds of Cell-SELEX, Park et al. [52] identified single-stranded DNA (ssDNA) aptamers that can specifically bind to Salmonella enteritidis and Salmonella typhimurium, and the dissociation constants (K d ) were in the range of nanomolar to submicromolar levels. These studies provided the technical support for possible application of aptamer to the detection of Salmonella species in food, clinical, and environmental samples by using aptasensor.
In addition, many scholars have made a lot of research studies for the rapid detection of other food pathogens. Using plastic-adherent DNA aptamer-magnetic bead, Bruno et al. [43] developed a quantum dot (QD)-based sandwich assay for Campylobacter jejuni detection, with detection limits of as low as 10–250 CFU in various food matrices. Ohk et al. [53] used an aptamer and an antibody-functional optical fiber biosensor for the detection of Listeria in foods such as chicken and beef, and the detection limit was 103 CFU/mL. Applying SELEX technique to a biotin-labeled ssDNA combinatorial library, Suh and Jaykus [54] separated an aptamer, Lbi-17, with binding specificity to Listeria spp., which could be used to detect the pathogen at concentrations <60 CFU/500 μL buffer in the presence of a heterogeneous cocktail of non-Listeria bacterial cells. Furthermore, using a whole-cell SELEX, Suh et al. [55] identified a total of tem unique aptamers specific for Listeria species, six associated with log phase cells and four with stationary phase cells. This is the first study in which SELEX targeted bacterial cells at different growth phases, which could increase the analytical sensitivity of future capture and detection assays. Lee et al. [56] established a sensitive method to test Escherichia coli by using aptamers and RT-PCR amplification technology. Wu et al. [57] developed an aptamer-nanoscale polydiacetylene based biosensor for rapid detection of E. coli at concentration of 105 CFU/mL. Ikanovic et al. [47] developed a novel assay for the detection of Bacillus thuringiensis (BT) spores, based on the fluorescence observed after binding an aptamer-quantum dot conjugate to BT spores, and the method is specific, semi-quantitative, and can detect BT spores at concentrations of about 103 CFU/mL. He et al. [58] employed aptamer-based surface-enhanced Raman spectroscopy and designed an innovative label-free platform for detection and discrimination of Bacillus anthracis spores in orange juice at concentrations of 104 CFU spores. Using aptamer recognition and fluorescent silica nanoparticles, He et al. [59] developed a sensitive and specific determination method for Staphylococcus aureus detection, which allowed for detection of as low as 7.6×102 cells/mL S. aureus in spiked milk. In particular, Wu et al. [60] fabricated a highly sensitive and specific aptasensor for simultaneous detection of three target bacteria S. aureus, Vibrio parahemolyticus, and Salmonella typhimurium, with the limits of detection 25, 10, and 15 CFU/mL, respectively. These aptamer-based technologies will play an important role in the rapid detection of pathogen microorganisms in the future [61].
Heavy Metal Ions
Industrial emissions like cadmium, mercury, lead, and other heavy metal ions entered into the soil and rivers can cause pollution of the environment; then they could accumulate in crops, sea foods, and other food raw materials to enter into the human food chain, causing acute or chronic food poisoning of human and damaged the human health [62]. Heavy metal ions can cause harm to human health through a variety of ways. The sources of heavy metal ions in food mainly come from the use of pesticide, industrial pollution, the pollution of raw materials, additives, and packaging materials in food processing. For example, lead oxide and some low-quality food additives added in preserved egg processing can cause food lead contamination. Lead has different degrees of damage in many organs in vivo, especially in hematopoietic system, nervous system, and kidney. The main way for mercury to enter into the human body is through contaminated fish and shellfish, and the accumulation of mercury in human body cause damage to liver, kidney, and other organs [33]. The rapid testing technology for heavy metal ions is urgently needed in food samples. However, to date, few applications in food analysis are reported.
Using aptamer-functionalized colloidal photonic crystal hydrogel (CPCH) films, Ye et al. [63] developed a robust method for the visual detection of heavy metal ions Hg(2+) and Pb(2+). These aptasensors could be used in the screening of a broad range of metal ions in food, drugs, and the environment, with high selectivity and reversibility. Employing aptamer-based fluorescence polarization (FP) method, Shen et al. [64] prepared a sensitive and selective sensor for Hg(2+) detection in canned fish samples, and the limit of detection was 0.49 nM. Notably, the proposed biosensor can be reused for six cycling times.
Biotoxins
Biology toxin is microbial metabolic products, have very strong toxicity, and mostly have carcinogenic, teratogenic, and mutagenic effects [65]. Therefore, it is very important to strengthen the test of biology toxin in order to prevent the entrance of toxin into food chain [66]. Aptamers specific for these particular targets, such as ricin, cholera toxin, staphylococcal enterotoxins, ochratoxin A (OTA), and abrin toxin, have been selected in the past years, and by using these aptamers, different detection systems have been developed [67]. Kirby et al. [68] published a toxin-related work based on aptamer arrays and reported the adaptation of a chip-based microsphere array to aptamer receptors. The results showed that the aptamer chips were useful for sensitively quantitating the biothreat agent ricin, and the detection limit was 320 ng/mL [68]. Haes et al. [69] have demonstrated the ability to detect sub-nanomolar concentrations of ricin using fluorescently tagged RNA aptamers, which have the power to simplify immunoassays using capillary electrophoresis. The research offers a promising method for the selective, rapid, and sensitive detection of ricin and other biowarfare agents. Bruno and Kiel [70] employed electrochemiluminescence and enzymatic methods to detect cholera toxin, with the detection limit of 40 ng/mL. Using a single DNA aptamer in combination with surface-enhanced Raman scattering technique, Lamont et al. [71] showed detection of 25 ng/mL of intact ricin. Similarly, by aptamer-based surface-enhanced Raman scattering technique, He et al. [72] detected 50–100 ng/mL of ricin in liquid foods. Tang et al. [73] established a highly selective and sensitive aptamer-based abrin assay by using a molecular light switching reagent [Ru(phen)2(dppz)]2+. The detection limit was 1 nM in the linear scope from 1 to 400 nM. This assay is also suitable to be operated in more complicated biological matrix, such as diluted serum.
OTA is a mycotoxin produced by several species of the genera Aspergillus and Penicillium and can be frequently found in many foods and beverages, including cereals, cocoa, coffee, spices, wine, beer, grape juice, and dried fruits. OTA is nephrotoxic and carcinogenic and poses a serious threat to the health of both humans and animals [74]. The International Agency for Research on Cancer (IARC) has considered OTA as a potential carcinogen. International and governmental agencies in many countries regulate the OTA content in a number of basic agro-products and foods [74]. Recently, OTA has been detected in various foods, agro-products, and feed sources. Several methods have been developed for the analysis of OTA in a variety of food commodities including cereals, cocoa, coffee, wine, beer, and dried fruits [74]. The European Commission (EC) required that the maximum level of OTA in raw cereal grains, cereal-derived products, and soluble coffee are at 5, 3, and 10 μg/kg, respectively (Commission Regulation No. 1881/2006). Therefore, it is of great importance to develop rapid and sensitive OTA detection methods for food safety and human health [26].
Many aptamer-based assays have been developed for OTA detection so far. Cruz-Aguado and Penner [75] selected OTA aptamers with high affinity and used the selected aptamers for the determination of parts per billion (ppb) quantities of OTA in the naturally contaminated wheat samples. Chen et al. [74] developed a rapid and reliable sensing platform for the detection of OTA based on the aptamer with target-induced structure-switching signaling. The whole detection and analysis process of OTA can be accomplished within 1 min, and the detection limit can be down to 0.8 ng/mL. De Girolamo et al. [37] described a procedure for preparing SPE columns, in which a stationary phase contained 24 nmol of a covalently bound DNA aptamer with high affinity and specificity to OTA. The aptamer-SPE columns showed a linear behavior in the range of 0.4–500 ng and were used for eliminating OTA from wheat samples. Additionally, De Girolamo et al. [76] also developed a DNA-ligand system using time-resolved fluorescence for the determination of OTA in wheat, and the entire procedure was performed in less than 0.5 h, including sample preparation, and allowed analysis of several samples simultaneously with a 96-well microplate reader. Kuang et al. [35] developed an ultrasensitive and rapid electrochemical sensor for the specific detection of OTA, and the sensitivity was as low as 30 pg/mL. Wang et al. [26] developed an aptamer-based chromatographic strip assay method for semi-quantitative detection of OTA, with the limit of detection 0.18 ng/mL. Duan et al. [77] applied an aptamer-based fluorescence assay for OTA detection, with limit of detection 10 nM. By enzyme-linked aptamer assays, Barthelmebs et al. [78] conducted rapid and sensitive detection of OTA in wine, with sensitivity of 1 ng/mL. Moreover, Barthelmebs et al. [79] employed an electrochemical DNA aptamer-based biosensor for OTA detection, and the limit of detection was down to 0.11 ng/mL. Recently, Lv et al. [80] developed a simple and rapid aptamer-based label-free approach for highly sensitive and selective fluorescence detection of OTA, and the limit of detection was as low as 1 ng/mL of OTA, and the assay exhibited high selectivity for OTA against two other analogs (N-acetyl-l-phenylalanine and zearalenone). The above works proved that the DNA-ligand system is simple, innovative, rapid, and can be used to screen large quantities of samples for OTA contamination, and DNA aptamers can be used to replace antibodies as binding reagents in commercial application for an economically important biotoxin analysis. Utilization of the proposed aptamer-based biosensor for quantitative determination of mycotoxins in food samples may provide significant improvements in quality control of food safety through a simple, rapid, and sensitive testing system for agricultural product monitoring.
Botulinum toxin (BTX) is a protein and neurotoxin produced by the bacterium Clostridium botulinum. BTX can cause botulism, a serious and life-threatening illness in humans and animals. For detection of botulinum neurotoxin, Wei and Ho [81] developed an aptamer-based electrochemical sensor to achieve sensitive detection, with a limit of detection at 40 pg/mL. Janardhanan et al. [82] designed a SPR-based RNA aptasensor for rapid detection of type A botulinum neurotoxin. The detection limits of the aptasensor in carrot juice and fat-free milk were 20.3 and 23.4 ng/mL, respectively. Most importantly, this aptasensor could effectively differentiate the natively folded toxin from denatured, inactive toxin.
Aflatoxins are secondary products metabolized by Aspergillus flavus and Aspergillus parasiticus [83]. Aflatoxins are a kind of carcinogen and highly toxic material. The US Food and Drug Administration (FDA) allows aflatoxins at low levels in nuts, seeds, and legumes. Therefore, it is urgently needed to find a fast and convenient detection of aflatoxins to guarantee the food quality and safety [84]. Very few papers have been published reporting applications of aptamer-based assays for the detection of aflatoxins so far. Using the immobilized aptamers as an affinity capture reagent, Nguyen et al. [85] prepared an electrochemical Fe3O4/polyaniline-based aptasensor for label-free and direct detection of aflatoxin M1 (AFM1), with the detection limit of 1.98 ng/L in the range of 6–60 ng/L. Guo et al. [86] selected an aflatoxin B1 (AFB1) aptamer as a molecular recognition probe and developed an ultrasensitive aptasensor for the detection of AFB1, with a limit of detection of 25 fg/mL in a wide linear range (5.0 × 10−5 to 5.0 ng/mL). This aptasensor can quantify AFB1 levels in infant rice cereal samples with satisfactory recoveries of 94–119 %.
Zearalenone (ZEN) is most commonly found as a contaminant in stored grain, which is a mycotoxin produced by several Fusarium spp. and has chronic estrogenic effects on mammals. Current methods of ZEN detection mostly rely on the use of low-stability antibodies or expensive equipment. Using the modified SELEX on magnetic beads, Chen et al. [87] identified a ssDNA aptamers recognizing ZEN. The best aptamer, 8Z31, with a dissociation constant (K d ) of 41 ± 5 nM, had the detection limit 7.85 × 10−10 M within linear range from 3.14 × 10−9 to 3.14 × 10−5 M, expecting to be used for the determination of ZEN in food and agricultural products.
Staphylococcus aureus is a common foodborne pathogen capable of secreting a family of small, stable, and heat-resistant toxins, known as staphylococcal enterotoxins (SEs). The ingestion of as little as 100 ng of SE is sufficient to cause staphylococcal food poisoning (SFP) [88]. By electrochemiluminescence and enzymatic methods, Bruno and Kiel [70] performed the specific detection of staphylococcal enterotoxin B (SEB), and the limit of detection was 10 pg/mL. DeGrasse [89] isolated a novel aptamer, designated APT (SEB1), with affinity to staphylococcal enterotoxin B (SEB). Similarly, Huang et al. [90] characterized aptamers binding to Staphylococcus aureus enterotoxin C1 (SEC1) with high affinity and selectivity. Aptamer-based quantification of SEC1 in the food sample was implemented with a limit of detection of 6 ng/mL. These works set the foundation for future aptamer-based assay toward the entire family of SEs.
Antibiotics
Antibiotics are chemical substances which have the capacity to inhibit the growth of microorganism or destroy them [91]. Kinds of antibiotics are often added in aquaculture industry in order to cure animal diseases or promote animal growth, so there are antibiotic residues in many agricultural, animal, and plant products [92]. Accumulation of antibiotics in human body will cause very serious consequences [11]. Food quality and safety issues caused by excessive antibiotic residues have aroused more and more attention worldwide, and even some developed countries have taken strict measures to control the addition of antibiotics in food. Currently, liquid chromatography and mass spectrometry method and enzyme-linked immunosorbent assay are used in the antibiotics detection, which have disadvantages of complicated operation, expensive equipment, low detection sensitivity, and difficulty in preparing antibodies. Therefore, it is necessary to develop a new technology overcoming these shortcomings for detection of antibiotic residues.
Since its discovery in 1990, the aptamer technology has been used in the analysis of antibiotics [93–95]. Aptamers targeting to various antibiotics have been selected. Lato et al. [95] used in vitro selection to study the diversity of aminoglycoside-binding sites on RNA molecules. Sequence analysis experiments indicated that there are many different ways to form specific and tight aminoglycoside-binding sites. Fan et al. [93] designed a new aptamer biosensor for quick detection of penicillin. The detection limit was 2.81 nmol/L, and the detection time was 5 min. Niazi et al. [96, 97] identified ssDNA aptamers separately binding the oxytetracycline (OTC) and tetracycline (TET) with high affinity and specificity by using tosylactivated magnetic beads (TMB)-based SELEX technology, and exhibited the specificity to OTC, TET, and doxycycline (DOX). Zhou et al. [92] applied an electrochemical aptasensor for detection of TET with limit of detection 5 nM. Kim et al. [94] have developed an electrochemical sensing system for OTC detection by using gold-immobilized ssDNA aptamer interdigitated array (IDA) electrode chip. The result showed that the specificity of developed EC biosensor for OTC was highly distinguishable from its structural analogs, such as TET and DOX. Similarly, Kim et al. [98] developed an electrochemical aptasensor for the detection of TET using ssDNA aptamer that selectively binds to TET as target material. The minimum detection limit of TET by using this method was 10 nM, and the linear detection range was between 10 nM to 10 μM. The aptasensor showed higher selectivity for TET than the other structural analogs of OTC and DOX [99]. Recently, using ssDNA aptamers, Kim et al. [100] developed an indirect competitive assay-based aptasensor for detection of OTC in milk, with the limit of detection 49.8 μg/L and recovery rate of more than 90 %. Han et al. [101] identified specific and high-affinity RNA aptamers with 2′-fluoro-2′-deoxyribonucleotide modified pyrimidine nucleotides bound to danofloxacin, but not to TET, which could be used for the rapid and cost-effective detection and sensing of danofloxacin in foods or food products. By selecting a 76-mer ssDNA aptamer, Wang et al. [102] developed an indirect competitive assay-based aptasensor for highly sensitive detection of TET residue in honey. The limit of detection was 9.6 × 10−3 ng/mL with a linear working range from 0.01 to 100 ng/mL.
The characterization of a DNA aptamer of daunomycin was described by Wochner et al. [103], and the aptamer displayed high specificity and affinity to daunomycin. de-los-Santos-Álvarez et al. [104, 105] developed electrochemical sensor and SPR biosensor for detection of neomycin, and the limits of detection were 0.75 μM and 10 nM, respectively. As a synthetic fluoroquinolone with broad spectrum antibacterial activity, danofloxacin is used for the treatment of respiratory diseases in animal husbandry and is toxic to humans. For kanamycin detection, Sun et al. [106] prepared an aptasensor based on chitosan-gold nanoparticles, graphene-gold nanoparticles, and multiwalled carbon nanotubes-cobalt phthalocyanine nanocomposites. The aptasensor was successfully applied to the detection of kanamycin in real milk spiked samples, with high sensitivity, high specificity, and a low detection limit 5.8 × 10−9 M.
These methods can potentially be used for detection of antibiotics in contaminated food products, drinking water, and other fields. Generally, it needs to pretreat the food matrix because of its complexity and diversity in order to eliminate the background interference of the main ingredients [23]. For example, aptamer-based optical method detection of risk factors in actual sample, usually, solid-phase extraction technology is used to pretreat samples [34, 35]. Zhang et al. [107] developed a novel aptamer biosensor for fast direct determination of the tetracyclines in milk samples by immobilizing the tetracyclines aptamer on the surface of the glassy carbon (GC) electrodes without sample treatment. The sensitivity limit of the device is 1 ng/mL, and the detection time is 5 min. The method provides great reference value for rapid detection of other target in complex food samples.
Organic Dyes
In some food processing production, there are varieties of organic dyes added into food in order to reach the effect of dyeing, covering up bad food appearance, and corrosion, and so on. The most common additive in tightly rolled dried skin of bean milk, bean vermicelli, flour, and bamboo shoot is sodium formaldehyde sulfoxylate to play the roles in whitening, preservation, increasing the taste, and corrosion, which can cause harm to human kidney, liver, central nervous, immune function, and digestive system. Malachite green is a kind of poisonous triphenylmethane compounds, is widely used in aquaculture as insect repellent, and has carcinogenic effects, so the malachite green residue in aquatic products is very harmful to human health [108]. In addition, some kinds of azo alkaline industrial dyes are also illegally used in food processing, such as Basic Orange is used for dyeing of bean curd and fresh fish. It will cause acute and chronic poisoning when people absorb excessively. Therefore, it has brought a great deal of concern to establish a rapid and efficient detection method of organic dyes. At present, SELEX technology is rarely applied in the analysis and detection of organic dyes in food samples. Stead et al. [34] presented a robust screening assay employing SPE followed by a novel aptamer-based procedure for the rapid and effectual detection and semi-quantitation of malachite green in fish tissue. The study provided evidence that an RNA aptamer could be used as an alternative recognition element replacing conventional antibodies with application to detection of chemical residues in food.
Pesticide Residue
Carbamate pesticide is a broad spectrum and effective pesticide [109]. However, because of its abuse, the toxicating phenomenon of human and livestock occurred frequently [110]. Therefore, carbamate pesticide has become the key testing species in pesticide residue of vegetables [13]. At present, the main detection methods of pesticide residue are chromatography, spectral analysis, enzyme inhibition method, and immune analysis, and other fast detection methods, which have the disadvantages of complex operation, time-consuming, difficulty in maintaining the enzyme activity, and inability to detect various kinds of pesticide residue at the same time [110]. Wang et al. [110] established a simple new method of screening specific aptamers for pesticide molecules. With the principle of the structure changing of nucleic acids from DNA-DNA duplex to a DNA-target complex, the specific aptamers could be obtained, and after nine rounds of selection, the screening efficiency increased from 0.70 to 13.32. There have many studies for isolation and identification of aptamers targeting to some kinds of pesticides. For example, He et al. [13] selected aptamers targeting acetamiprid by SELEX technology and successfully obtained an acetamiprid-specific aptamer with the dissociation constant estimated to be 4.98 μm. Wang et al. [109] isolated ssDNA aptamers against four organophosphorus pesticides (phorate, profenofos, isocarbophos, and omethoate), which offered application potential in the analysis and neutralization of the residues of the four organophosphorus pesticides. Indeed, using a single aptamer-based surface-enhanced Raman scattering method, Pang et al. [111] realized the detection and discrimination of four specific pesticides (isocarbophos, omethoate, phorate, and profenofos), with the limits of detection 3.4, 24, 0.4, and 14 μM, respectively. This method was validated in apple juice, demonstrating the great potential of aptamer-based sensors as analytical tools for rapid detection and discrimination of multipesticides.
Allergens
Food allergy is an abnormal immune-mediated reaction occurred in susceptible individuals after consumption of a certain food or food ingredient. Generally, the foodstuff containing allergens include milk, eggs, peanuts, tree nuts, soy, wheat, fish, shellfish, etc. Most allergens are proteins, and immunoassays are frequently developed for detection of allergens [112]. Recently, there are a few aptamers selected to bind allergenic proteins. Using capillary electrophoresis (CE)-SELEX, Tran et al. [113] reported the selection of aptamers against one of the most important peanut allergens, Ara h 1. The dissociation constants of the selected aptamers were in the nanomolar range, which specifically recognized Ara h 1 and did not significantly bind with other proteins, including another peanut allergen Ara h 2. By in vitro screening, high affinity DNA aptamers to the food allergen Lup an 1, ß-conglutin, were selected, which were highly specific, showing no cross-reactivity with other flour ingredients or with other conglutin fractions of lupin [114]. Furthermore, through comparing the predicted secondary structures of the aptamers, a high-affinity 11-mer DNA aptamer containing a G-quadruplex against Lup an 1 (β-conglutin) was obtained, with an apparent equilibrium dissociation constant (K d ) of 1.7 × 10−9 M [115]. Especially, using such a truncated 11-mer aptamer against β-conglutin, a fluorescence resonance energy transfer (FRET)-based method was developed for selective and sensitive Lup an 1 allergen detection, with a detection limit of 150 pM [116]. This holds a great potential for the direct detection of the toxic β-conglutin subunit in foodstuffs. A major hurdle in the effective management of celiac disease is the sensitivity of the available methods for assessing gluten contents in food. Amaya-González et al. [117] successfully selected aptamers binding to celiac disease-triggering hydrophobic proteins gliadins. Using aptamers as specific receptors, a highly sensitive approach for gluten analysis was developed, which allowed the measurement of as low as 0.5 ppb of gliadin standard (0.5 ppm of gluten), six times higher sensitivity than reference immunoassay.
Other Compounds
Natural or synthetic hormones are frequently used in meat and dairy industries to increase productivity. Such as, 17β-estradiol is endocrine disruptors that may affect the reproduction of humans and animals and induce tumors. Its routine monitoring in water samples is required. Yildirim et al. [118] developed an aptamer-based optical biosensor for rapid and sensitive detection of 17β-estradiol in water samples, with a detection limit of 0.6 ng/mL. Similarly, based on anti-17β-estradiol aptamer, Fan et al. [119] developed a novel and ultrasensitive photoelectrochemical (PEC) sensor for detection low level of 17β-estradiol in water samples, with a detection limit of 33 fM.
Melamine is widely reported as a food adulterant, and melamine-induced nephrotoxicity is now a global concern [120]. Melamine is commonly used in plastics that may be in contact with food. On the other hand, sometimes it is added in dairy product for infants in China due to its high nitrogen content to replace proteins. So, its migration or addition levels in food products have to be monitored. Using aptamer-modified nanosilver probe, Jiang et al. [121] realized the detection of trace melamine in the range of 0.02–1.06 μg/L. Moreover, Liang et al. [122] prepared an aptamer-nanogold probe for detection of melamine, with a detection limit of 0.98 μg/L.
As an endrocrine disruptor too, bisphenol A (BPA) is used in polycarbonate plastic products, such as in baby bottles. There are major concerns regarding BPA exposure to fetuses, infants, and young children. BPA often present at low levels in various water sources, so it is very important to develop a fast, cost-effective, and sensitive method for on-site detection of BPA. By adding a high selective anti-BPA aptamer to gold nanoparticles, Mei et al. [123] prepared a label-free aptasensor for BPA detection, and the limit of visual detection was 0.1 ng/mL. Kuang et al. [124] presented a sensitive plasmonic chirality-based aptasensor for BPA detection. In the range 0.02–5 ng/mL, a low limit of detection of 0.008 ng/mL was obtained. Yildirim et al. [125] prepared a portable, evanescent, wave fiber-optic aptasensor for rapid, on-site detection of BPA, with a low detection limit of 1.86 nM. In particular, based on gold nanoparticles dotted graphene modified glassy carbon electrode, Zhou et al. [126] fabricated an electrochemical aptasensor for label-free detection of bisphenol A in milk samples, with the detection limit of 5 nM.
Prospects
With the globalization of food market, food safety issue has become serious problem to be solved by the world [25]. Although many countries make strict food safety regulations and standards, there are still various food safety accidents happened in the world. Nowadays, food safety faces many challenges, such as illegal addition, spoofing, and pollution. It will inevitably lead to serious public health problems for excessive or illegal addition of some additives into foods, such as adding plasticizing agent in drinks, adding tonyred in foods, adding other vegetable oil in peanut oil, adding melamine in milk, and adding drainage oil in edible oils. [23]. In addition, varieties of food pollution such as pesticide residues, chemical raw materials, industrial dye, organic pollutants, and toxins produced naturally in food (aflatoxin) can cause food safety crises easily. As an important guarantee of food safety, the detection is playing an important role in all food production chains. Therefore, it is urgently needed to develop scientific and effective detection methods for more and more food safety problems.
However, the detection of food safety is facing huge challenges, because the existing detection methods used for food safety have many disadvantages, which include expensive cost analysis instruments, low sensitivity, complicated operation, time-consuming detection, and difficulties of monitoring on line, detecting multicomponent samples, and preparing antibody, and so on [23]. Therefore, it needs to develop new rapid and effective detection methods with high sensitivity, low detecting limit, low prices, and uncomplicated operation. Aptamer technology is a newly developed detection and analysis method in food safety, environment monitoring, life science, chemistry, and other fields, caused more and more attentions and studies of home and abroad researchers.
Aptamers have wide application due to the advantages of low molecular mass, relative stability, and simplicity for synthesis and modification [46]. In theory, almost any target molecules can be selected with high affinity and specificity by using SELEX technology [127]. It is especially notable that aptamers have the high recognition and binding capability to low molecular mass substances [128], which provide an ideal tool for separation, purification, analysis, and detection of low molecular mass substances. The aptamer technology is still under evolution and investigation due to its complexity and stability [67]. The utilization of the DNA or RNA aptamer in any applications requires the aptamer to be stable and resistant to the enzymatic activities of nucleases [2]. Aptamers are more stable at high temperature, and they can be regenerated easily and quickly after denaturation, and aptamers are in general more stable than antibodies, so they have a longer shelf life than antibodies. RNA molecules have the drawback of susceptibility to enzymatic degradation, so that the DNA aptamer is advantageous over the RNA aptamer due to its well stability with the absence of the hydroxyl group at the 2′-end of the DNA molecule, making it widely used in a number of applications [2]. It is noted that the lackness of RNA stability can be overcome by modifying the 2′ position of the ribose ring in the RNA backbone with amino, fluoro, or O-methyl functional groups used in the SELEX process [128]. de-los-Santos-Álvarez et al. [104, 105] opened up the way to use the modified-RNA aptamer-based sensor for competitive impedimetric assay and SPR assay of neomycin B, respectively, which demonstrated the feasibility of detecting small molecules using RNA aptamers with high sensitivity and stability. In addition, many research studies have been performed to explore the binding mechanism and interaction between aptamer and target and the changes of secondary structure in different conditions, such as ion intensity, temperature, and acidity-alkalinity [62, 129, 130]. Therefore, it is possible to enhance the stability and improve other molecular characteristics of aptamers by developing their resistance to ion intensity, temperature, and acidity-alkalinity. Different applications of aptamer-based assays have been already described for diagnostics, environmental or food quality testing, with analytical performances rivaling those of antibody-based assays. The analytical studies are now demonstrating that some of the limitations of many conventional assays can be circumvented by alternative recognition reagent such as aptamers [2]. Although the application of aptamers to poisonous and harmful materials in food is now in the initial stage, existing studies and findings provide theoretical basis and technical support for the actual application of aptamer technology (represented by the aptamer-based technology in Table 2) in the detection of food.
This paper summarized various aptamer screening technologies and comprehensively reviewed the recent applications of selected aptamers in food safety (Table 2, Fig. 2) compared with other papers published. For example, Liang et al. [23] highlighted the applications of aptamers in heavy metals, small molecular, and biotoxins; Palchetti and Mascini [130] focused on electrochemical aptasensors. Especially, the present paper updated very recent works in the past 2 years.
The future works are to select more food-related aptamers by using advanced SELEX technologies. For example, emulsion PCR-based SELEX [131], with this method, the products formation increased significantly and the by-products formation decreased tremendously to an undetectable level; single-primer-limited amplification (SPLA)-based SELEX [132], in which by-products and nonspecific products are at undetectable levels. Additionally, even non-SELEX technique is a good choice too, such as nonequilibrium capillary electrophoresis of equilibrium mixtures (NECEEM) method [133], which is a highly efficient affinity method and allows the clones with suitable binding parameters are sequenced only; hence, the screening time is significantly shortened, 1 week for NECEEM versus several weeks for conventional SELEX protocols. Especially, Yu and Yu [134] conducted a mathematical analysis of the selective enrichment in NECEEM, which is good for further improvement in the efficiency of partitioning target-bound ligands from free ligands. After selecting specific aptamers, the subsequent task is to apply them to food safety detection, to improve the stability and other performance of DNA or RNA aptamers under different conditions (i.e., ion intensity, temperature, and acidity-alkalinity) by using chemical modification. At last, it is to develop novel sensors or kits for the rapid and efficient detection of food safety by combining aptamer technology with other technology like nanotechnology, enzyme-linked technology, plasma resonance technology, and electrochemical technology.
References
Ellington, A. D., & Szostak, W. I. (1990). Nature, 346(6287), 818–822.
Marimuthu, C., Tang, T. H., Tominaga, J., et al. (2012). Analyst, 137(6), 1307–1315.
Hyeon, J. Y., Chon, J. W., Choi, I. S., et al. (2012). Journal of Microbiological Methods, 89(1), 79–82.
Yang, Y., Yang, D. L., Schluesener, H. J., et al. (2007). Biomolecular Engineering, 24, 583–592.
Omidinia, E., Shadjou, N., & Hasanzadeh, M. (2014). Applied Biochemistry and Biotechnology, 172(4), 2070–2080.
Mie, M., Kai, T., Le, T., et al. (2013). Applied Biochemistry and Biotechnology, 169(1), 250–255.
He, C., Xu, Z., Sun, T., et al. (2014). Applied Biochemistry and Biotechnology, 172(2), 1018–1026.
Sun, W., Du, L., & Li, M. (2010). Current Pharmaceutical Design, 16(20), 2269–2278.
Dastjerdi, K., Tabar, G. H., Dehghani, H., et al. (2011). Biotechnology and Applied Biochemistry, 58(4), 226–230.
Pestourie, C., Tavitian, B., & Duconge, F. (2005). Biochimie, 87, 921–930.
Xu, D. M., Wu, M., Zou, Y., et al. (2011). Chinese Journal of Analytical Chemistry, 39(6), 925–933.
Hamula, C. L. A., Guthrie, J. W., Hongquan, Z., et al. (2006). TrAC, Trends in Analytical Chemistry, 25(7), 681–691.
He, J., Liu, Y., Fan, M., et al. (2011). Journal of Agricultural and Food Chemistry, 59(5), 1582–1586.
Liang, A. H., Zhou, L. P., Qin, H. M., et al. (2011). Journal of Fluorescence, 21(5), 1907–1912.
Tanaka, Y., Akagi, K., Nakamura, Y., et al. (2007). Oligonucleotides, 17(1), 12–21.
Xu, S. M., Yuan, H., Chen, S. P., et al. (2012). Analytical Biochemistry, 423(2), 195–201.
Zeng, X. D., Zhang, X. L., Yang, W., et al. (2012). Analytical Biochemistry, 424(1), 8–11.
Deisingh, A. K. (2006). Handbook of Experimental Pharmacology, 173, 341–357.
Phillips, J. A., Lopez-Colon, D., Zhu, Z. Y., et al. (2008). Analytica Chimica Acta, 621(2), 101–108.
Tombelli, S., & Mascini, M. (2010). Combinatorial Chemistry and High Throughput Screening, 13(7), 641–649.
Huang, C. C., & Chang, H. T. (2008). Chemical Communications, 12, 1461–1463.
Zuo, X. L., Song, S. P., Zhang, J., et al. (2007). Journal of the American Chemical Society, 129(5), 1042–1043.
Liang, M., Liu, R., Su, R. X., et al. (2012). Progress in the Chemistry, 24(7), 1378–1387.
Havelaar, A. H., Brul, S., de Jong, A., et al. (2010). International Journal of Food Microbiology, 139(Suppl1), 79–94.
Yadav, R., Dwivedi, S., Kumar, S., et al. (2010). Food and Environmental Virology, 2(2), 53–63.
Wang, L., Ma, W., Chen, W., et al. (2011). Biosensors and Bioelectronics, 26(6), 3059–3062.
Zhang, J., Wang, L. H., Pan, D., et al. (2008). Small, 4(8), 1196–1200.
Golub, E., Pelossof, G., Freeman, R., et al. (2009). Analytical Chemistry, 81(22), 9291–9298.
Mayer, K. M., & Hagner, J. H. (2011). Chemical Reviews, 111(6), 3828–3857.
Yakes, B. J., Deeds, J., White, K., et al. (2011). Journal of Agricultural and Food Chemistry, 59(3), 839–846.
Yang, X. H., Huang, J. H., Wang, Q., et al. (2011). Analytical Methods, 3, 59–61.
Lin, Y. W., Liu, C. W., & Chang, H. T. (2011). Talanta, 84(2), 324–329.
Liu, C. W., Huang, C. C., & Chang, H. T. (2009). Analytical Chemistry, 81(6), 2383–2387.
Stead, S. L., Helen, A., Brian, H. J., et al. (2010). Analytical Chemistry, 82(7), 2652–2660.
Kuang, H., Chen, W., Xu, D., et al. (2010). Biosensors and Bioelectronics, 26(2), 710–716.
Lee, J., Jo, M., Kim, T. H., et al. (2011). Lab on a Chip, 11(1), 52–56.
De Girolamo, A., McKeague, M., Miller, J. D., et al. (2011). Food Chemistry, 127, 1378–1384.
McKeague, M., Bradley, C. R., Girolamo, A. D., et al. (2010). International Journal of Molecular Sciences, 11(12), 4864–4881.
Stoltenburg, R., Reinemann, C., & Strehlitz, B. (2007). Biomolecular Engineering, 24, 381–403.
Alocilja, E. C., & Radke, S. M. (2003). Biosensors and Bioelectronics, 18(5–6), 841–846.
Vassioukovitch, O., Orsini, M., Paparini, A., et al. (2005). Biotechnology Annual Review, 11, 335–354.
Nugen, S. R., & Baeumner, A. J. (2008). Analytical and Bioanalytical Chemistry, 391(2), 451–454.
Bruno, J. G., Phillips, T., Carrillo, M. P., et al. (2009). Journal of Fluorescence, 19(3), 427–435.
Cao, X. X., Li, S. H., Chen, L. C., et al. (2009). Nucleic Acids Research, 37(14), 4621–4628.
Joshi, R., Janagama, H., Dwivedi, H. P., et al. (2009). Molecular and Cellular Probes, 23, 20–28.
Pan, Q., Zhang, X. L., Wu, H. Y., et al. (2005). Antimicrobial Agents and Chemotherapy, 49(10), 4052–4060.
Ikanovic, M., Rudzinski, W. E., Bruno, J. G., et al. (2007). Journal of Fluorescence, 17, 193–199.
Li, F., Zhang, J., Cao, X. N., et al. (2009). Analyst, 134(7), 1355–1360.
Seo, H. K., Holt, P. S., Stone, H. D., et al. (2003). Journal of Food Microbiology, 87(1–2), 139–144.
Seo, H. K., Holt, P. S., Valentin-Bon, I. E., Brackett, R. E., et al. (2004). Journal of Food Protection, 67(5), 864–869.
Ma, X., Jiang, Y., Jia, F., et al. (2014). Journal of Microbiological Methods, 98, 94–98.
Park, H.C., Baig, I.A., Lee, S.C., et al. (2014). Appl Biochem Biotechnol. [Epub ahead of print].
Ohk, S. H., Koo, O. K., Sen, T., et al. (2010). TrAC, Trends in Analytical Chemistry, 109(3), 808–817.
Suh, S. H., & LA Jaykus, J. (2013). Biotechnology, 167(4), 454–461.
Suh, S. H., Dwivedi, H. P., Choi, S. J., et al. (2014). Analytical Biochemistry, 459, 39–45.
Lee, H. J., Kim, B. C., Kim, K. W., et al. (2009). Biosensors and Bioelectronics, 24(12), 3550–3555.
Wu, W. H., Chen, Y., & Jiang, L. Q. (2010). Chinese Journal of Laboratory and Medicine, 33(7), 587–593.
He, L., Deen, B. D., Pagel, A. H., et al. (2013). Analyst, 138(6), 1657–1659.
He, X., Li, Y., He, D., et al. (2014). Journal of Biomedical Nanotechnology, 10(7), 1359–1368.
Wu, S., Duan, N., Shi, Z., et al. (2014). Analytical Chemistry, 86(6), 3100–3107.
Edith, T. C., & Alocilja, E. C. (2009). Biosensors and Bioelectronics, 24(11), 3175–3182.
Lin, P. H., Chen, R. H., Lee, C. H., et al. (2011). Colloids and Surfaces B: Biointerfaces, 88(2), 552–558.
Ye, B. F., Zhao, Y. J., Cheng, Y., et al. (2012). Nanoscale, 4(19), 5998–6003.
Shen, T., Yue, Q., Jiang, X., et al. (2013). Talanta, 117, 81–86.
Chu, F. S. (1991). Mutation Research, 259(3–4), 291–306.
Bryden, W. L. (2007). Asia Pacific Journal of Clinical Nutrition, 16(Suppl1), 95–101.
Tombelli, S., Minunni, M., & Mascini, M. (2007). Biomolecular Engineering, 24, 191–200.
Kirby, R., Cho, E. J., Gehrke, B., et al. (2004). Analytical Chemistry, 76(14), 4066–4075.
Haes, A. J., Giordano, B. C., & Collins, G. E. (2006). Analytical Chemistry, 78(11), 3758–3764.
Bruno, J.G., Kiel, J.L (2002). Biotechniques 32(1):178–80,182-3.
Lamont, E. A., He, L., Warriner, K., et al. (2011). Analyst, 136(19), 3884–3895.
He, L. L., Lamont, E., & Veeregowda, B. (2011). Chemical Science, 2, 1579–1582.
Tang, J. J., Yu, T., Guo, L., et al. (2007). Biosensors and Bioelectronics, 22(11), 2456–2463.
Chen, J. H., Fang, Z. Y., Liu, J., et al. (2012). Food Control, 25(2), 555–560.
Cruz-Aguado, J. A., & Penner, G. (2008). Journal of Agricultural and Food Chemistry, 56(22), 10456–10461.
De Girolamo, A., Le, L., Penner, G., et al. (2012). Analytical and Bioanalytical Chemistry, 403, 2627–2634.
Barthelmebs, L., Jonca, J., Hayat, A., et al. (2011). Food Control, 22(5), 737–743.
Barthelmebs, L., Hayat, A., Limiadi, A. W., et al. (2011). Sensors and Actuators B, 156(2), 7932–7937.
Duan, N., Wu, S. J., & Wang, Z. P. (2011). Chinese Journal of Analytical Chemistry, 39(3), 300–304.
Lv, Z., Chen, A., Liu, J., et al. (2014). PLoS One, 9(1), e85968.
Wei, F., & Ho, C. M. (2009). Analytical and Bioanalytical Chemistry, 393(8), 1943–1948.
Janardhanan, P., Mello, C. M., Singh, B. R., et al. (2013). Talanta, 117, 273–280.
Sun, X. L., Zhao, X. L., Tang, J., et al. (2006). Food Control, 17(4), 256–262.
Mehle, N., Nikolić, P., Gruden, K., et al. (2013). Methods in Molecular Biology, 938, 269–281.
Nguyen, B. H., Tran, L. D., Do, Q. P., et al. (2013). Materials Science and Engineering C: Materials for Biological Applications, 33(4), 2229–2234.
Guo, X., Wen, F., Zheng, N., et al. (2014). Biosensors and Bioelectronics, 56, 340–344.
Chen, X., Huang, Y., Duan, N., et al. (2013). Analytical and Bioanalytical Chemistry, 405(20), 6573–6581.
Argudin, M. A., Mendoza, M. C., & Rodicio, M. R. (2010). Toxins, 2, 1751–1773.
DeGrasse, J. A. (2012). PLoS One, 7(3), e33410.
Huang, Y., Chen, X., Duan, N., et al. (2015). Food Chemistry, 166, 623–629.
Lalumera, G. M., Calamari, D., Galli, P., et al. (2004). Chemosphere, 54(5), 661–668.
Zhou, L., Li, D. J., Gai, L., et al. (2012). Sensors and Actuators B, 162(1), 201–208.
Fan, T., Zhang, J. K., Li, M., et al. (2011). Chinese Journal of Bioprocess Engineering, 9(3), 41–46.
Kim, Y. S., Niazi, J. H., & Gu, M. B. (2009). Analytica Chimica Acta, 634, 250–254.
Lato, S. M., Boles, A. R., & Ellington, A. D. (1995). Chemical Biology, 2(5), 291–303.
Niazi, J. H., Lee, S. J., Kim, Y. S., et al. (2008). Bioorganic and Medicinal Chemistry, 16(3), 1254–1261.
Niazi, J. H., Lee, S. J., Gu, M. B., et al. (2008). Bioorganic and Medicinal Chemistry, 16(15), 7245–7253.
Kim, Y. J., Kim, Y. S., Niazi, J. H., et al. (2010). Bioprocess and Biosystems Engineering, 33(1), 31–37.
Kim, Y. S., Kim, J. H., & Kim, I. N. (2010). Biosensors and Bioelectronics, 26(4), 1644–1649.
Kim, C. H., Lee, L. P., Min, J. R., et al. (2014). Biosensors and Bioelectronics, 51, 426–430.
Han, S. R., Yu, J., & Lee, S. W. (2014). Biochemical and Biophysical Research Communications, 448(4), 397–402.
Wang, S., Yong, W., Liu, J., et al. (2014). Biosensors and Bioelectronics, 57, 192–198.
Wochner, A., Menger, M., Orgel, D., et al. (2008). Analytical Biochemistry, 373(1), 34–42.
de-los-Santos-Álvarez, N., Lobo-Castañón, M. J., Miranda-Ordieres, A. J., et al. (2007). Journal of the American Chemical Society, 129(3), 3808–3809.
De-los-Santos-Álvarez, N., Lobo-Castañón, M. J., Miranda-Ordieres, A. J., et al. (2009). Biosensors and Bioelectronics, 24(8), 2547–2553.
Sun, X., Li, F., Shen, G., et al. (2014). Analyst, 139(1), 299–308.
Zhang, J. K., Zhang, B. B., Wu, Y., et al. (2010). Analyst, 135, 2706–2710.
Srivastava, S., Sinha, R., & Roy, D. (2004). Toxicology, 66(3), 319–329.
Wang, L., Liu, X. J., Zhang, Q., et al. (2012). Biotechnology Letters, 34(5), 869–874.
Wang, Y., He, J., Wang, L., et al. (2011). Journal of Nanjing Agricultural University, 34(3), 131–134.
Pang, S., Labuza, T. P., & He, L. (2014). Analyst, 139(8), 1895–1901.
Van Hengel, A. J. (2007). Analytical and Bioanalytical Chemistry, 389, 111–118.
Tran, D. T., Knez, K., Janssen, K. P., et al. (2013). Biosensors and Bioelectronics, 43, 245–251.
Nadal, P., Pinto, A., Svobodova, M., et al. (2012). PLoS One, 7(4), e35253.
Nadal, P., Svobodova, M., Mairal, T., et al. (2013). Analytical and Bioanalytical Chemistry, 405(29), 9343–9349.
Mairal, T., Nadal, P., Svobodova, M., et al. (2014). Biosensors and Bioelectronics, 54, 207–210.
Amaya-González, S., De-Los-Santos-Álvarez, N., Miranda-Ordieres, A. J., et al. (2014). Analytical Chemistry, 86(5), 2733–2739.
Yildirim, N., Long, F., Gao, C., et al. (2012). Environmental Science and Technology, 46(6), 3288–3294.
Fan, L., Zhao, G., Shi, H., et al. (2014). Environmental Science and Technology, 48(10), 5754–5761.
Rai, N., Banerjee, D., & Bhattacharyya, R. (2014). Nutrition, 30(4), 380–385.
Jiang, Z., Zhou, L., & Liang, A. (2011). Chemical communications (Cambridge), 47(11), 3162–3164.
Liang, A., Zhou, L., Qin, H., et al. (2011). Journal of Fluorescence, 21(5), 1907–1912.
Mei, Z., Chu, H., Chen, W., et al. (2013). Biosensors and Bioelectronics, 39(1), 26–30.
Kuang, H., Yin, H., Liu, L., et al. (2014). ACS Applied Materials & Interfaces, 6(1), 364–369.
Yildirim, N., Long, F., He, M., et al. (2014). Environmental Science Processes and Impacts, 16(6), 1379–1386.
Zhou, L., Wang, J., Li, D., et al. (2014). Food Chemistry, 162, 34–40.
Ulrich, H., Martins, A. H. B., & Pesquero, J. B. (2004). Cytometry. Part A, 59(2), 220–231.
Osborne, S. E., & Ellington, A. D. (1997). Chemical Reviews, 97(2), 349–370.
Neves, M. A. D., Reinstein, O., Saad, M., et al. (2010). Biophysical Chemistry, 153(1), 9–16.
Palchetti, I., & Mascini, M. (2012). Analytical and Bioanalytical Chemistry, 402(10), 3103–3114.
Shao, K., Ding, W., Wang, F., et al. (2011). PLoS One, 6(9), e24910.
He, C. Z., Zhang, K. H., Wang, T., et al. (2013). Analytical Biochemistry, 440(1), 63–70.
Berezovski, M. V., Musheev, M. U., Drabovich, A. P., et al. (2006). Nature Protocols, 1(3), 1359–1369.
Yu, X., & Yu, Y. (2014). Applied Biochemistry and Biotechnology, 173(8), 2019–2027.
Conflict of Interest
The authors report no declarations of interest.
Author contributions
Liu XF drafted the manuscript, and Zhang XW revised the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, X., Zhang, X. Aptamer-Based Technology for Food Analysis. Appl Biochem Biotechnol 175, 603–624 (2015). https://doi.org/10.1007/s12010-014-1289-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1289-0