Abstract
A new solid-state fermentation (SSF) reactor was developed for the spore production of biocontrol agent Clonostachys rosea. The greatest spore production in the reactor, 3.36 × 1010 spores g DM−1 occurred with mixings, which was about 10 times greater than that in traditional tray reactor. The reactor provides about two times sporulation surface area for spore formation. Moisture content of the medium was adjusted to meet the spore production by changing the surface porosity. Two mixings were carried out during cultivation to make the medium loose, which resulted in a mass of new sporulation surface. The fermentation period shortened from 14–15 to 10–11 days. It is suggested that the new reactor has great potential in the mass production of spores of C. rosea and other fungal biocontrol agents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The non-pathogenic, saprophytic fungus Clonostachys rosea is one of the most powerful biological control agents (BCAs), as shown in the control of Botrytis cinerea on strawberry [1], tomato, rose and eucalyptus [2, 3]. The spores have been successfully produced by solid-state fermentation (SSF) processes using wheat grains as solid matrix [4, 5]. SSF enables fungi to produce aerial conidia, which are similar to those produced naturally on the surface of insect cadavers and are superior to mycelia and blastospores produced under submerged fermentation (SmF) conditions [6]. However, some disadvantages hinder the optimal application and scale-up of SSF processes. Heterogeneous composition of substrate, lack of free water and low conductivity of solid particles can give rise to difficulties in monitoring and controlling cultivation parameters such as temperature, pH, biomass and moisture [7]. Furthermore, indirect measurements of biomass in SSF have distracted the attention from growth kinetics modelling.
SSF involves the fermentation on solid substrates in situations where the continuous phase is gas, with little or no liquid water in the inter-particle spaces [8]. These processes are particularly attractive to industrial applications, supplying higher production yields and different expressions of microbial metabolites compared to SmF [9, 10]. SSF has obvious advantages in lower energy requirements, lower operating costs and investment outlays, less downstream processing and produces less waste water than SmF. Moreover, SSF is environmental friendly as it resolves the problem of solid wastes disposal [11].
SSF is prone to process failure due to channelling caused by evaporative cooling and the formation of an inter-particle mycelium network [12]. Therefore, mixing is needed to break the mycelium network and to avoid such failure. There are a few systematic studies of mixing in SSF. However, the available reports have shown conflicting results and do not provide clues on the mechanisms behind the reported success or failure. The mixing event in SSF with Aspergillus oryzae is needed to break mycelium to avoid aggregate formation in the grain bed, and to not distribute water added to compensate for evaporation losses or smooth out temperature gradients [12]. Weber et al. [13] demonstrated that mixing is needed in SSF with substrates that shrink upon drying and fungi that form abundant aerial mycelium. Some reports have indicated that the bed of solid substrate can be mixed continuously without detrimental effects [14, 15]. In contrast, Han et al. [16] investigated the effect of intermittent mixing on enzyme production during cultivation of Rhizopus on soybeans and concluded that intermittent mixing may negatively affect enzyme production.
Only the achievement of economical, mass production of spores will bring successful development of fungal BCAs. Reactor is regarded as the core of fermentation process. Therefore, it is necessary to develop a new solid-state fermentation reactor and investigate methods for enhancing the spore yield. In the present research, a new SSF reactor was developed to enhance the spore yield of Clonostachys rosea. This new reactor provides two times exposure surface area for sporulation compared to the traditional tray bioreactor. It is worth mentioning that the moisture content and the air permeability of the solid medium in the new reactor can be controlled by changing the surface porosity. Moreover, two mixings were performed in order to increase spore production markedly during the fermentation.
Materials and Methods
Inoculum Preparation
Clonostachys rosea strain CRW010 from rose debris was isolated using the pre-colonized plate method as previously described [17]. A mutant strain CRM-16 was selected for its great spore production and antibacterial activity. It was maintained on potato dextrose agar (PDA) medium at 4 °C. A 0.5-cm agar plug was transferred from PDA slant cultures to the centre of each PDA plate, which was incubated at 24 °C under constant fluorescent light (20 W) for 10 days. Spore suspensions were obtained from 10-day-old cultures by flooding the PDA plates with 30-ml sterile distilled water containing Tween 80 (0.1 % v/v) and simultaneously scraping the surface with a sterile spatula, and then filtering it through two layers of cheesecloth to remove hyphal fragments. Spores counts were conducted microscopically [18]. Spore numbers per gram dry matter (DM), is defined as spore production or yield. Conidial viability was examined by streaking each suspension onto two replicate PDA plates and measuring percentage germination after 24 h.
New Solid-State Fermentation Reactor
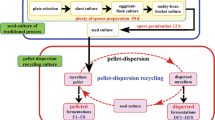
A novel solid-state fermentation reactor (Fig. 1) [18] is designed for the production of spores by Clonostachys rosea mutant strain CRM-16. This reactor is light transparent and ventilated both at the top and the bottom, which differs from traditional tray bioreactor with only light penetration at the top. Compared to the traditional one, it provides two times sporulation area for spore growth. The new reactor is composed of four frames (2.0 × 0.5 m for each) where plastic mesh is placed to support solid culture medium. Each frame can be loaded about 25 kg of the medium. The solid medium is covered both at the top and the bottom by high-density polyethylene membrane with appropriate surface porosity. The surface porosity (SP) of the plastic membrane, defined as the ratio of the area of pores over the total area of membrane, was varied from 0.31 to 2.89 %, as shown in Table 1. The plastic membrane also played an important role in reducing the risk of bacterial contamination, given that the direct exposure of the bed surface to the surrounding air was reduced significantly.
Spore Production Experiments
The spore production experiments of Clonostachys rosea mutant strain CRM-16 were carried out aseptically in the above new reactor. The initial moisture (wet basis) of the solid medium was adjusted by adding distilled water to dry solid substrates (wheat bran/maize meal = 3:1, w/w). After that, the moist media were sterilized at 121 °C for 65 min. Each treatment contained 5 kg of sterile medium inoculated with 7.5 × 107 spores g DM−1 using spore suspension previously prepared. The open sides of the polyethylene membranes were sealed with a clip. The reactor was incubated at 24 °C for 24 h in the dark period and then, under constant fluorescent light, (2 × 20 W for each frame) for 18 days.
For each treatment, samples were picked up aseptically from the different regions including the centre and the four sides of the substrate bed. Ten-gram samples used for determining spore yield were obtained from the mixture of five sampling points. Spores were extracted by suspending 10-g samples in 200 ml Tween 80 (0.1 % v/v) solution in a 500-ml beaker using a mechanic stirrer (280 rpm) for 5 min. After appropriate dilutions, spores were counted under the microscope using a counting chamber. The spore yield is based on the maximum value during the 19-day fermentation period. Samples used for determining the moisture content of medium (MC) were collected every day until the end of the fermentation. The MC was estimated by drying 20 g of sample to constant weight at 105 °C for 2 h. The temperature of solid substrate was examined using a temperature sensor and monitored online. Temperature gradients are ignored in the substrates and isothermal cultivation is expected to take place.
It must be pointed out that only two mixings were carried out during the course of the 19-day fermentation. The two mixings were performed on the fourth and on the sixth day. On the fourth day, the solid substrates were mixed round and milled for 2–3 min using a paddle mixer, and on the sixth day, the solid substrates were mixed for about 1 min. The experimental conditions are as follows: initial moisture content (IM) from 51–71 %, surface porosity of the plastic membrane (SP) from 0.31–2.89 %, bed temperature (T) from 20–28 °C, and medium thickness (MT) from 2–8 cm (Table 1).
Results and Discussion
Spore Production Profiles
Table 1 gave the results of 20 runs. The maximum spore production in the new reactor, 3.36 × 1010 spores g DM−1 occurred in run 6, which was about 10 times greater than that in traditional tray reactor. The tray bioreactor has no plastic membrane covering both at the top and the bottom of the solid medium, and no mixing during the fermentation. Based on our previous experiment (data not shown), the spore production obtained from the traditional reactor is about 0.2 − 3.0 × 109 spores g DM−1. The remarkable increase in spore production may be attributed to the following three reasons. Firstly, SSF is a contact process limited by available exposure surface where spores are produced. The new reactor can provide two times sporulation surface area or sporulation surface area. Secondly, moisture content can be adjusted by changing the surface porosity to meet the spore production in the new reactor. Finally, two mixings carried out at the fourth and sixth day, respectively, make the medium loose and result in a mass of new sporulation surface area.
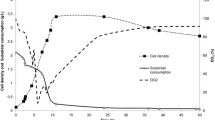
As can be seen from Fig. 2, there was a 4-day lag during the initial stage of growth in the spore production profile of run 6. A similar phenomenon occurred in other runs including runs 1, 7 and 5. The sporulation rate, then, accelerated slowly from the fourth day to the fifth day; finally, a period of rapid acceleration from the fifth day to the tenth day followed by a stabilization phase appears. Variation in spore production was negligible until the end of the experiment in the stationary phase. The spore production had reached maximum at the 10th–11th day (Fig. 2). Therefore, the optimum fermentation period for spore production is about 10–11 days. The fermentation period using the new reactor shortened from 14–15 to 10–11 days compared to the traditional reactor.
Profile of spore production of C. rosea CRM-16 in a new solid-state fermentation reactor designed in the present study. Squares the spore production of run 6 (T 24 °C, IM 64.8 %, MT 4 cm, SP 0.31 %); triangles the spore production of run 7 (T 24 °C, IM 64.8 %, MT 6 cm, SP 2.89 %); circles the spore production of run 15 (T 26 °C, IM 64.8 %, MT 8 cm, SP 0.74 %); diamonds spore production of run 1 (T 20 °C, IM 61.74 %, MT 3 cm, SP 0.31 %)
Profiles of Moisture Content of Solid Medium
Moisture content of the medium is an important factor which has a significant effect on spore production. Only at an appropriate range of moisture content can a better spore production be obtained. One of the advantages of the new reactor is that moisture content can be adjusted by changing the surface porosity to meet the need of spore production. As shown in Fig. 3, the SP of the plastic membrane had a significant effect on MC. The MC decreased from 71 to about 23.7 % for a SP of 2.89 % (run 20). However, a tendency of minor increasing in MC was observed during the course of fermentation in run 6, which had a SP of 0.31 %. The MC increased from an initial value of 64.8 to 71.6 % on the 7th day, then slowly decreased to 66.8 % on the 19th day. For a SP of 0.74 % (run 8), the variation of the MC was negligible until the 17th day. At the 19th day, the MC decreased to 60.1 %, which was only slightly lower than the initial MC of 64.8 %.
Profile of moisture content of medium during the solid-state fermentation of C. rosea CRM-16. Triangles the moisture content of run 6 (T 24 °C, IM 64.8 %, MT 4 cm, SP 0.31 %); squares the moisture content of run 8 (T 24 °C, IM 64.8 %, MT 4 cm, SP 0.74 %); circles the moisture content of run 20 (T 24 °C, IM 71.0 %, MT 4 cm, SP 2.89 %)
Weber et al. [19] reported that Coniothyrium minitans is very sensitive to reduced water activity (a w): 50 % inhibition of respiration is found at a w 0.95, spore formation is completely inhibited at a w 0.97. They considered that it is essential to keep a w at about 1.0 during SSF. The a w is highly dependent on the water-binding properties of the substrate. The a w remains high over a wide range of MC, thus large amounts of water can be evaporated without influence on spore production before the a w declines to critical values. The growth and sporulation of Mucor bacilliformis were performed on an inert solid support under different conditions of moisture content and nutrient concentration by Lareo et al. [20]. Maximum biomass (0.59 g/ginert support) and spore production (6 × 108 spores/g inert support) are obtained for the highest moisture content (90 % w/w) and the most concentrated medium studied.
As shown in Fig. 3, the MC of run 20 declined to about 45–50 % at the tenth day; after that, the spore production did not increase until the end of the experiment. In fact, the spore production kept no change and the maximum spore production was only 2.69 × 1010 (Table 1). With 2.89 % of the surface porosity, the profiles of runs 7 and 19 were similar to that of run 20, their maximum spore productions were only 2.30 × 1010 and 2.85 × 1010, respectively, because the moisture contents in these runs were lower. It implied that MC should be maintained higher than 45–50 % for sporulation of Clonostachys rosea in our new solid-state fermentation reactor.
Variation of moisture content during fermentation mainly depends on two factors: water losses due to evaporation and water production due to microorganism metabolism. The greater the surface porosity is, the more water is lost due to evaporation. Therefore, the moisture content is lower when the surface porosity is greater. Moreover, lower surface porosity will limit the oxygen diffusion, because oxygen is indispensible for the fermentation of Clonostachys rosea. Therefore, an appropriate surface porosity is important for spore production. Only in the optimum surface porosity and the appropriate initial moisture content can the MC be in an optimum range for growth and sporulation of Clonostachys rosea.
Effect of Mixing on Spore Production
In the present study, two different mixing strategies were performed. It can be seen from Fig. 4 that mixing strategy 1 was more favourable for sporulation than mixing strategy 2. Furthermore, two mixings were good enough for sporulation. More mixings did not make further efforts to increase the spore production. Therefore, two mixings were performed in our study. One mixing was carried out at the fourth day, as results in a looser solid substrate with the particles size ranging from 3–12 mm. Another one was carried out at the sixth day, and this mixing was easy because the solid substrate was looser as a result from the first mixing. Therefore, more mixing was not needed.
Effect of mixing action on the spore production of C. rosea CRM-16 in solid-state fermentation process. Mixing strategy 1: one mixing was performed at the fourth day; two mixings were performed at the fourth and sixth day, respectively; three mixings were performed at the fourth, sixth and seventh day, respectively; four mixings were performed at the fourth, sixth, seventh and ninth day, respectively. Mixing strategy 2: one mixing was performed at the third day; two mixings were performed at the third and fifth day, respectively; three mixings were performed at the third, fifth and seventh day, respectively; four mixings were performed at the third, fifth, seventh and ninth day, respectively
Our result (Fig. 4) indicates that mixing operation at the appropriate time is very important for obtaining great spore production. Less and excessive mixings are both unfavourable for sporulation. It is appropriate to carry out the first mixing operation at the fourth day and the second mixing at the sixth day. Moreover, an appropriate mixing degree is also very important because sporulation may decrease due to excessive disruption of mycelia resulting from excessive mixing. Compared with both continuous and discontinuous mixing, two mixings are more exercisable and economical and reduces the risk of bacterial contamination. Flodman et al. [21] observed similar results in experiments performed to determine the influence of mixing on spore production in the solid-state fermentation of wet corn distiller grains with Trichoderma reesei. The researchers found that high mixing frequencies may not be desirable when spore production is the primary objective. The reforming and breaking of the hyphal network between particles may have caused an increase in injury-induced conidiation, resulting in higher spore production for mixing frequencies of 1–2 /day compared to 3–6/day.
By mixing the dense network of mycelium, agglomeration was broken up. In other words, the mixing operation has an obvious crushing effect on solid substrate. As a result, new exposure surface area, available for new sporulation, was formed by mixing. The structure of the solid substrate became loose and remained loose during fermentation through mixing, because the mycelium’s network was broken up and was no longer able to agglomerate substrate particles. Therefore, mixing may be essential in SSF because it can provide greater exposure surface area or sporulation surface area. Casas-Flores et al. [22] found conidiation caused by mechanical injury to mycelia for Trichoderma atroviride. Injury-induced conidiation may be the primary contributing factor to the increased spore production for mechanically mixed fermentations.
Mixing is necessary in SSF for solid substrate particles that have small exposure surface area, shrink upon drying and fungi that form abundant aerial mycelium. Agglomeration of substrate particles, due to mycelium growing, results in a very small exposure surface area, which leads to a remarkable decrease in spore production. Mixing can convert substrate agglomerates into small particles ranging from 3–12 mm, thus, new exposure surface or sporulation surface are formed. Aerial hyphae did not stopping growing until the agglomeration of substrate particles due to the formation of hyphal bonds. It is unfavourable for sporulation due to small exposure of surface area. The substrate is mixed to provide a mass of new exposure surface area, therefore, SSF with mixing action may be preferred. This is in agreement with the findings by Schutyser et al. [12]. They performed several discontinuously mixed solid-state fermentations in the drum fermentor and measured the number and size of grain aggregates remaining after the first mixing action. Their results showed that the first mixing event in SSF with A. oryzae was needed to break mycelium to avoid aggregate formation in the grain bed. They concluded that SSF with discontinuous mixing was an attractive operating strategy and the first mixing event during their fermentation experiments with A. oryzae on wheat was necessary to disrupt the mycelium network. Based on the loose medium and a mass of sporulation surface area resulted from two mixings and other advantages mentioned above, it is believed that the mixed bioreactor with two mixings are superior to a non-mixed system for large-scale production of Clonostachys rosea spores.
Conclusions
A new solid-state fermentation (SSF) reactor was developed, and the spore production of Clonostachys rosea in the reactor achieved 3.36 × 1010 spores g DM−1, which was about 10 times greater than that in traditional tray reactor. The reactor provides two times sporulation surface area for spore formation. Moisture content of the medium was adjusted to meet the spore production by changing the surface porosity. Two mixings were carried out during cultivation to make the medium loose, which resulted in a mass of new sporulation surface. It is concluded that the new reactor has great potential in mass production of spores of Clonostachys rosea and other fungal biocontrol agents.
References
Cota, L. V., Maffia, L. A., Mizubuti, E. S. G., Macedo, P. E. F., & Antunes, R. F. (2008). Biological Control, 46(3), 515–522.
Cota, L. V., Maffia, L. A., Mizubuti, E. S. G., & Macedo, P. E. F. (2009). Biological Control, 50(3), 222–230.
Morandi, M. A. B., Mattos, L. P. V., Santos, E. R., & Bonugli, R. C. (2008). Crop Protection, 27(1), 77–83.
Morandi, M. A. B., Maffia, L. A., Mizubuti, E. S. G., Alfenas, A. C., & Barbosa, J. G. (2003). Biological Control, 26(3), 311–317.
Sutton, J. C., Liu, W., Huang, R., & Owen-Going, N. (2002). Biocontrol Science and Technology, 12(4), 413–425.
Roberts, D. W., & St Leger, R. J. (2004). Advances in Applied Microbiology, 54, 1–70.
Auria, R., Morales, M., Villegas, E., & Revah, S. (1993). Biotechnology and Bioengineering, 41(11), 1007–1013.
8.Moo-Young, M., Moreira, A.R., & Tengerdy, R.P. (1983). Principles of solid substrate cultivation, vol. 2: Filamentous Fungi (Smith, J.E., Berry, D.R., Kristiansen, B., ed.), Edward Arnold, London, pp. 557–566.
Burke, R. M., & Cairney, J. W. G. (1997). Mycological Research, 101(9), 1135–1139.
Rodríguez-Fernández, D. E., Rodríguez-León, J. A., de Carvalho, J. C., Sturm, W., & Soccol, C. R. (2012). Bioresource Technology, 118, 603–606.
Lekanda, J. S., & Perez-Correa, J. R. (2004). Process Biochemistry, 39(11), 1793–1802.
Schutyser, M. A., de Pagter, P., Weber, F. J., Briels, W. J., Boom, R. M., & Rinzema, A. (2003). Biotechnology and Bioengineering, 83(5), 503–513.
Weber, F. J., Oostra, J., Tramper, J., & Rinzema, A. (2002). Biotechnology and Bioengineering, 77(4), 381–393.
Nagel, F. J. I., Tramper, J., Bakker, M. S. N., & Rinzema, A. (2001). Biotechnology and Bioengineering, 72(2), 219–230.
Oostra, J., Tramper, J., & Rinzema, A. (2000). Enzyme and Microbial Technology, 27(9), 652–663.
Han, B., Kiers, J. L., & Nout, R. M. J. (1999). Journal of Bioscience and Bioengineering, 88(2), 205–209.
Krauss, U., Marti′nez, A., Hidalgo, E., Hoopen, M., & Arroyo, C. (2002). Mycological Research, 106(102), 1449–1454.
Zhang, Y. Y., Liu, J. H., Zhou, Y. M., Zhang, Y. Y., Liu, Y., Gong, T. Y., & Wang, J. (2013). Process Biochemistry, 48(8), 1119–1125.
Weber, F. J., Tramper, J., & Rinzema, A. (1999). Biotechnology and Bioengineering, 65(4), 47–458.
Lareo, C., Sposito, A. F., Bossio, A. L., & Volpe, D. C. (2006). Enzyme and Microbial Technology, 38(3–4), 391–399.
Flodman, H. R., & Noureddini, H. (2013). Biochemical Engineering Journal, 81, 24–28.
Casas-Flores, S., Rios-Momberg, M., Bibbins, M., Ponce-Noyola, P., & HerreraEstrella, A. (2004). Microbiology, 150(11), 3561–3569.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (no. 21376129), the Qingdao Municipal Science and Technology Commission (Grant no. 09-1-1-89-nsh), China Postdoctoral Science Foundation (no. 2014M551871) and the Qingdao Municipal Human Resources and Social Security Bureau for its postdoctoral project start-up funding.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, Y., Liu, J., Zhou, Y. et al. Spore Production of Clonostachys rosea in a New Solid-state Fermentation Reactor. Appl Biochem Biotechnol 174, 2951–2959 (2014). https://doi.org/10.1007/s12010-014-1239-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1239-x