Abstract
Cationic polyacrylate latex has attracted extensive attention due to its special properties and potential applications in wood primers, metal coatings, and fabric treatments, etc. However, cationic polyacrylate latex has poor durability and stability, which limits its use in practical applications. In this study, a functional cationic monomer (3-chloro-2-hydroxypropyl) dimethyl [2-[(2-methyl-1-oxoallyl)oxy]ethyl] ammonium chloride (DEAC) was combined with two conventional monomers of methyl methacrylate (MMA) and butyl acrylate (BA) to prepare cationic polyacrylate latex by a semi-continuous emulsion polymerization. The obtained latexes showed typical cationic character with zeta potential in the range of + 40 to + 65 mV. The effect of DEAC on the stability of latex was studied and good alkali resistance could be gained at high DEAC content. Water resistance and mechanical properties of the film before and after crosslinking are studied in detail. The results showed that the water absorption reduced from 60.04% to a minimum of 9.18%, and the tensile strength increased from 3.9 to 8.4 MPa, which was comparable to solvent-based polyacrylate films. This work provides a new strategy for the preparation of waterborne cationic polyacrylate latex with improved water resistance and good mechanical properties, etc., which can be applied in papermaking, textiles, wood primers, and other potential fields.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Waterborne polyacrylate has excellent film-forming properties, mechanical properties, weather resistance, and low volatile organic compounds,1,2,3 and can be used in furniture industry, ink, architectural coatings, and other fields.4,5 At present, anionic waterborne polyacrylate has been widely studied and applied. On the other hand, cationic waterborne polyacrylate latex has received more and more attention since it has a better performance when employed in paper,6,7 textiles, oil drilling, wood primers, and other special fields.8,9,10,11 Cationic polyacrylate has characteristics of a positive charge and low toxicity.12,13 However, at the same time, waterborne cationic polyacrylate has some problems in stability, water resistance, and compatibility, and has other shortcomings. Few relevant studies have focused on cationic latex, which hinders its practical application.
To endow the latex cationic character, cationic monomers are generally introduced and combined with conventional polyacrylates. The monomer containing quaternary ammonium has the characteristics of low price and good stability, and is usually used as the cationic component.14 However, the quaternary ammonium structure can cause problems, such as poor water resistance.15 In order to expand the application range, it is necessary to improve the water resistance of cationic polyacrylate and to reduce the water absorption of latex film. To solve this problem, hydrophobic groups16 and crosslinked groups17,18,19,20,21 can be introduced into the main chain of the polyacrylate. Zhou et al.22 prepared a two-component hydrophobic fluorosilicone polyacrylic polyurethane coating based on a vinyl-terminated silicone compound and an acrylate monomer containing hydroxyl, carboxyl and fluorinated functional groups. By introducing F and Si groups with strong hydrophobicity, the coatings show good surface hydrophobicity, thermal stability, and adjustable mechanical properties. However, the combustion of fluoro-silicon materials will produce toxic gas, which is harmful to the environment. Alternatively, a crosslinked network structure can resist the entry of water molecules into the interior of the latex film and effectively improve its mechanical properties.23,24
Crosslinkable monomers, such as epoxy functional groups,25,26,27 have been introduced into waterborne polyacrylates to improve the film properties of polyacrylate latex. Wang et al.28 synthesized a polyurethane/polyacrylate composite containing epoxy functional groups. With the increase of crosslinkable monomer glycidyl methacrylate, the water resistance and tensile resistance were improved. Yao et al.29 developed a waterborne epoxy/polyacrylate composite anticorrosive coating using a microemulsion polymerization method. The crosslinking by a primary amine could enhance the barrier performance of the composite coating.
In our previous work, it was found that the cationic latex prepared by quaternary ammonium cationic monomer is stable14 and that a functional monomer, like a crosslinkable monomer, has mostly been selected to endow the latex functionality by a copolymerization process. To facilitate the synthetic process, a functional cationic monomer (3-chloro-2-hydroxypropyl) dimethyl [2-[(2-methyl-1-oxoallyl)oxy]ethyl] ammonium chloride (DEAC) with both quaternary ammonium structure and epoxy functional group was selected in this work.25,30 The functional cationic monomer DEAC, synthesized by 2-(dimethylamino)ethyl methacrylate (DMAEMA) and epichlorohydrin (EPI), was copolymerized with methyl methacrylate (MMA) and butyl acrylate (BA), and the effects of different concentrations of DEAC were compared. The results showed that the addition of DEAC significantly promoted the alkali resistance of the latex. The structure and properties of cationic latex and its films were compared before and after crosslinking by an amine curing agent and characterized by Fourier-transform infrared (FTIR), dynamic light scattering (DLS), a contact angle goniometer and tensile tests.
Experimental
Materials
MMA (99.5%), BA (99.5%), and DMAEMA (99%) were purchased from Shanghai Aladdin Biochemical Technology (Shanghai, China). MMA and BA were purified by vacuum distillation before use and stored in a 4°C refrigerator. Cetyltrimethyl ammonium bromide (CTAB, 99%) was purchased from Shanghai Zhanyun Chemical, an initiator, 2,2′-azobis[2-(2-imidazolin-2-yl)propane] dihydrochloride (AIBI, 98%), diethylenetriamine (DETA,99%), EPI (99%), and hydrochloric acid (HCl, 38%) were purchased from Shanghai Titan Scientific. Deionized water was used throughout the whole experiment and all commercially available chemicals were used as received.
Synthesis of DEAC
DEAC is a cationic monomer with a crosslinkable epoxy end group which was synthesized from DMAEMA and EPI.31 Typically, 44 g water and 21.6 g concentrated HCl were added to a three-necked flask. When the solution was cooled to 10°C, 33.8 g DMAEMA was slowly added. Then, 14 g EPI was directly added and stirred for 30 min in an ice bath. The reaction was carried out at room temperature for 24 h. After the reaction, the pH was adjusted to 3.5 with HCl. Purified quaternary ammonium salt DEAC was obtained by removing of the solvent using a rotary evaporator. Figure 1 shows the synthesis diagram of DEAC. The product (I) can be obtained by the reaction. When the pH value was lower than 6, the product (I) can exist stably and be preserved for a long time. When the pH value was neutral or basic, the proportion of product (II) will increase significantly. The product (II) was the required quaternary ammonium crosslinked monomer DEAC.32
In this study, two kinds of DEAC with different epoxidation were synthesized, DEAC72 (DMAEMA:EPI = 1:0.72 mol/mol) and DEAC97 (DMAEMA:EPI = 1:0.97 mol/mol).
H-NMR (DMSO) of product (I) (Fig. S1): 4.43 ppm (m, 1H, R–C–CH(OH)–C–Cl), 3.37 (s, 2H, C–NH2–C), 3.85 ppm (d, 2H, C–CH2–Cl), 3.56 ppm (d, 2H, N–CH2–C), 3.66 ppm (d, 2H, R–O–C–CH2–N), 1.91 ppm (s, 1H, C=CH–C=O), 3.37 ppm (s, 2H, C–NH2–C), 6.10 ppm (s, 1H, C–OH), 4.55 ppm (s, 2H, C–CH2–O–C=O), 6.18 ppm (s, 1H, CH=C=C=O–R), 6.22 ppm (d, 1H, CH=C=C=O–R).
Synthesis of cationic polyacrylate latex P(MMA-co-BA-co-DEAC)
Cationic polyacrylate latex was prepared by semi-continuous seeded emulsion polymerization. First, 0.7 g MMA, 0.7 g BA, 50 g DI water, and 0.2 g CTAB were added to the reaction vessel. The reaction was heated to 75°C in a water bath, protected in N2 atmosphere, and pre-emulsified for 15 min. Then, the initiator AIBI (0.1 g) and 8 g DI water were added, and the seed emulsion was obtained in 30 min. The remaining monomers (2.8 g MMA and 2.8 g BA) were added and reacted for another 1 h. Finally, the oil phase (3.5 g MMA and 4.5 g BA) and the water phase (0.13 g AIBI, 2.5 g DEAC, and 8 g DI water) were slowly added simultaneously. The final reaction was 1 h to ensure the polymerization was complete. Cationic polyacrylate latex CL72 (cationic latex containing DEAC72) and CL97 (cationic latex containing DEAC97) were prepared. The comprehensive flow chart is shown in Fig. 2.
Preparation of cationic polyacrylate film
The synthesized cationic latex CL72 and CL97 with or without the curing agent DETA was added to a Teflon mold, which was then dried at 40°C for 24 h and dried at 60°C for another 24 h. A thin film with a thickness of ~ 1 mm was obtained.
Characterization
The average particle size and size distribution of the latex particles were determined by DLS using a Nano-S90 instrument at 25°C. The samples were diluted to 0.8 wt% and measured three times. PSS Nicomp 380 was used to measure the zeta potential of latex at 25°C. The concentration of the latex used for determination was diluted to about 0.2 wt%.
FTIR was recorded between 4000 and 400 cm−1 using the attenuated total reflection (ATR) technique on an AVATAR370 spectrophotometer. Transmission electron microscopy (TEM; JEM-2100 Plus; JEOL) was carried out under 200 k. The sample solution was dropped onto a copper net with a pipette and dried at room temperature.
The monomer conversion rate and coagulum content were determined by a gravimetric method using:
where mc, m1 , and m2 are the weight of container, the weight of latex plus container, and the weight of latex plus container after drying at 50°C for 24 h. m0, m3, m4, and ma are the mass of the whole reaction system, and the initial amounts of the zeta potential monomer, emulsifier, and dried coagulant, respectively.
The latex film was soaked in deionized water at 25°C for 48 h, and then the water absorption (WA) calculated from:
where, w0 and w1 are the weight of the zeta potential latex film before and after soaking.
The static contact angle of the latex film was measured at 25°C according to the droplet state on the surface of the latex film by using the solid drop method and a microsyringe on the JCY contact angle tester. Each sample was measured three times to obtain an average value. The tensile strength was measured on a Shimadzu AGS-J instrument. The loading unit was 100 N and the crosshead speed was 50 mm/min. The sample was made into a dumbbell shape (length 75 mm, width 5 mm).
Results and discussion
Effect of DEAC on polyacrylate latex
The DEAC was characterized by 1H NMR and FTIR (Figs. S1, S2) which ensured the successful synthesis and was used as the comonomer with BA and MMA thereafter. The chemical composition of cationic latex was characterized by FTIR spectroscopy (Fig. 3), the spectra showing that all the prepared latex had strong absorption peaks at 2930 and 2870 cm−1, which indicates –CH3 and –CH2 stretching vibrations in polyacrylate.34 The peaks at 1730 cm−1 denotes C=O and at 1140 cm−1 corresponds to C–O, which is attributed to the ester group in DEAC, BA, and MMA. The absorption peak at 949 cm−1 is ascribed to the epoxy group in DEAC.35,36 The peak at 3400 cm−1 indicates –OH in DEAC, and the vibration peak of C=C at 1640 cm−1 disappeared,37 which proved that DEAC indeed participated in copolymerization and a cationic polyacrylate latex was obtained.
In the preparation process of the cationic polyacrylate latex, the introduction of the cationic monomer DEAC may affect the intrinsic properties of the polyacrylate latex, such as monomer conversion, coagulant content, zeta potential, particle size, and PDI, etc.14 Because of the poor stability of polyacrylate latex prepared by the DMAEMA precursor system, CL72 and CL97 cationic latex with different levels of quaternary ammonium and epoxy group were prepared to explore the influence of quaternization degree and epoxidation on the latex and film properties.
The influence of the dosage of the cationic monomer DEAC72 on the latex properties is shown in Table 1. With the increase of DEAC72 dosage, the monomer conversion decreased a little but maintained a high level above 96%. The coagulum content decreased obviously, especially at high DEAC72 dosage. The zeta potential was positive and fluctuated around + 50 mV. The high monomer conversion indicates a good polymerization degree of the cationic acrylic latex. The reduction in coagulum content was due to the increased latex stability brought by the cationic DEAC72, which could increase the electrostatic repulsion between neighboring latex particles. The obtained latex bears a positive charge and was independent of the DEAC72 dosage, demonstrating potential good storage stability for the polyacrylate latex with a DEAC72 component. The influence of the DEAC97 dosage on the properties of the cationic acrylic latex are shown in Table 2. Monomer conversion and coagulum content were basically consistent with that of DEAC72, while the zeta potential is 10 mV less than that of the DEAC72, which may be due to the high epoxidation of the end group of the DEAC97.
The effect of DEAC dosage (DEAC72, DEAC97) on particle size and polydispersity is shown in Fig. 4. With the increase of DEAC, the particle size gradually increases and the particle size distribution changed little. The addition of extra DEAC increased the monomer concentration, which contributes to the size increase of latex. The polydispersity index is around 0.1, indicating its monodispersity despite of the cationic component. The size change with DEAC97 dosage was also monitored and is shown in Fig. 4b. The size variation trend is close to that of DEAC72, demonstrating that the end group of the pendant has no effect on the size and size distribution.
It is reported that a hydrophilic monomer would affect the surface morphology of latex.14 For comparison, the TEM image of polyacrylate latex is shown in Fig. 5. The size of P(MMA-co-BA) without a DEAC component is uniform and spherical with an explicit periphery. The addition of DEAC not only increases the particle size but also makes the latex surface blur. Otherwise, the particle sizes of P(MMA-co-BA) and CL97 (15 wt%) observed by TEM are about 30 nm and 60 nm, respectively, while those detected by DLS are about 45 nm and 90 nm, respectively. The size difference may be due to the different characterization methods.38
Effect of DEAC on stability properties
Salt stability
The addition of salt ions will affect the size, charge, and stability of latex in solution. In order to verify the salt existence on cationic polyacrylate latex, a series of salt concentrations of different orders of magnitude were applied and the results are shown in Fig. 6. The size varies in two regions: low salt concentration and high salt concentration. At low salt concentration, the particle sizes of CL72 and CL97 are basically unchanged with the increase of salt concentration. When DEAC is 1.5 wt% and 5 wt%, the particle sizes of Cl72 and CL97 become larger at 50 mM, while the particle sizes of latex with 10 wt% and 15 wt% DEAC are basically unchanged. At higher salt concentration, the particle size of the latex began to increase, and the particle size of latex with DEAC (10 wt% and 15 wt%) increased significantly when the salt concentration was 500 mM. This indicates that the degree of electrostatic shielding between the cationic layers of the latex has reached the maximum, Cl− destroys the cationic charge balance from emulsifier and cationic component, and latex begins to form agglomerates. It is concluded that the higher the content of DEAC, the better the salt tolerance of the latex.
Alkali resistance stability
Because of the positive charge of latex particles in the cationic latex, it is generally considered that the alkali resistance of cationic latex is poor. The effect of DEAC dosage on the alkali resistance of cationic latex is shown in Fig. 7, from which it can be seen that the alkali resistance of latex with low DEAC content is poor, which is consistent with the alkali resistance of unmodified cationic latex. After adding NaOH solution, a large number of floccules immediately appeared in the latex with low DEAC content. However, with the increase of DEAC content, the alkali resistance was improved when the DEAC content reached 10 wt%, and was significantly improved when the DEAC content reached 15 wt%. After adding NaOH solution and standing for 48 h, no flocculation occurred. When the DEAC72 content increased to 10 wt%, little flocculation occurred after standing for 48 h. When the DEAC content was 15 wt%, there was almost no flocculation, but CL97 (10 wt%) showed obvious flocculation. Through the above phenomenon, adding a certain amount of DEAC into the system can significantly improve the alkali resistance of the cationic emulsion, and the alkali resistance of CL72 is better than that of CL97.
When DEAC was introduced into the cationic polyacrylate, the latex had a positive charge, which can shield a part of the negative charge in the alkaline solution, to ensure the stability of the latex. With the increase of DEAC content, the stability of the latex was enhanced. However, CL72 has a better alkali resistance than CL97, which may be due to the introduction of an epoxy terminal group after quaternization of DEAC in CL97. Because of the presence of the electrically neutral epoxy end group, the positive charge of the quaternary ammonia structure cannot be directly exposed to the surface of the latex. This is also confirmed by the lower zeta potential of CL97 as shown in Tables 1 and 2.
Film properties
In the practical application of cationic polyacrylate latex, it is usually used as an adhesive or wood primer, in the form of film. Therefore, it is of great significance to study the film properties of the obtained cationic polyacrylate latex. Among the various film properties of waterborne latex, water resistance is the most important but generally poor water resistance is obtained and should be improved by crosslinking or other strategies. Mechanical properties are also considerable, to endure external operations like pressure. Hence, water resistance before and after curing, and the mechanical properties are studied thereafter.
Effect of DEAC on water resistance of films
It is known that latex or waterborne resin has poor water resistance and needs further treatment to enhance this for practical applications. In Fig. 8, the latex film shows a white appearance after soaking in water in 24 h. Especially, the water bleaching phenomenon is more obvious when the DEAC content is high, which is ascribed to the fact that DEAC contains the hydrophilic groups of quaternary ammonium. Upon contact with water, a large number of water molecules enter the film structure and swelling occurs. It is reported that latex film is sensitive to water bleaching.39
When the DEAC content is greater than 5%, the latex film gradually turns white. The water absorption rate of the latex film was studied, and the results are shown in Fig. 9. As can be seen from Fig. 9a, water absorption of CL72 increased from 3.5% to 59.4% as the dosage of DEAC72 increased from 1.5% to 15 wt%. In the case of CL97, the corresponding water absorption rate increased from 2.5% to 60.4%, as shown in Fig. 9b. The more DEAC amount in the cationic latex, the higher water absorption and the worse the water resistance of the film, which is a disadvantage in practical applications. Both CTAB and DEAC containing a quaternary ammonium structure result in poor water resistance of the latex film.
To figure out the mechanism of water adsorption under distinct DEAC content, the water contact angle of the latex film was tested, which could confirm the hydrophilicity of the latex film. The larger the contact angle, the higher the hydrophobicity, which can reflect the surface performance of the latex film.40 In Fig. 9c and d, the change trend of the contact angle is opposite to that of water absorption. With the increase of DEAC (DEAC72, DEAC97) dosage, the water absorption increased, while the contact angle decreased. It was also found that, when the contact angle is less than 90°, the water absorption increases rapidly and is greater than 20%, suggesting that the film has poor water resistance and strong hydrophilicity on its surface.41
Therefore, it is concluded from the above analysis that the hydrophilicity of the film surface determines the whitening and water adsorption of the films. When the contact angle is controlled above 90°, low water adsorption and less whitening could be achieved, indicating a good water resistance performance of the films.
Effect of curing agent on water resistance of films
With the increase of the DEAC content, the water resistance of the film decreases, especially when the DEAC content is high (10 wt% and 15 wt%). On this basis, an amine curing agent was selected to optimize the properties of the film. The amine curing agent DETA is mostly used to enhance the film properties for epoxy-containing polymers.42 Epoxy content will increase in the DEAC when the pH increases (formula II in Fig. 1). The crosslinking reaction diagram of amine hardeners is shown in Fig. 10.43,44
Samples with high DEAC content (10 wt% and 15 wt%) were then selected to be treated further with the DETA curing agent. As shown in Fig. 11a, 11c, 11e, and 11g, when DETA is applied, the water absorption rate of CL latex film first decreases and then increases with the increase of DETA content, while the water adsorption always decreased after the introduction of DETA regardless of its dosage. When the DETA content is low (2.5 wt%), the degree of crosslinking increased a little to hinder the abundant water adsorption, while the crosslinking level was low, which is not enough to reduce the water adsorption in a large content. In CL72 (10 wt% and 15 wt%) and CL97 (10 wt% and 15 wt%), the water absorption of the latex film was the lowest when the amount of DETA was 5 wt% and 10 wt%, respectively. However, when the content of DETA was high (25 wt%), the water absorption increased again, indicating that extra DETA that does not participate in the crosslinking reaction leading to increased water absorption.
(a) CL72 (10 wt%) water absorption diagram; contact angle diagram of (b) CL72 (10 wt%), (c) CL97 (10 wt%) water absorption diagram; contact angle diagram of (d) CL97 (10 wt%); (e) CL72 (15 wt%) water absorption diagram; contact angle diagram of (f )CL72 (15 wt%), (g) CL97 (15 wt%) water absorption diagram; contact angle diagram of (h) CL97 (15 wt%)
In CL72 (15 wt%) and CL97 (15 wt%), the latex film with 10 wt% DETA content had the lowest water absorptions, which were 8.0% and 9.18%, respectively. The water absorption rate decreased significantly from ~ 60% to less than 10%, as shown in Fig. 11e and 11g. When the DEAC (DEAC72, DEAC97) content was 15 wt%, the hydrophilicity increased, while it decreased significantly after crosslinking, which is another key factor in addition to hydrophilicity. When more hydrophilic components were introduced, the water absorption increased to a high level, which could then decrease sharply when the high crosslinked network was formed.
As can be seen from Fig. 11, with the increase of DETA dosage, the contact angle of CL first increases and then decreases. When DETA dosage was 5 wt%, CL72 (10 wt%) contact angle was 84.82°, and CL97 (10 wt%) contact angle was 92.92°. When DETA dosage was 10 wt%, the contact angle of CL72 (15 wt%) was 104.45°, and that of CL97 (15 wt%) was 114.16°, which is consistent with the above phenomenon. Compared with the CL72 latex film with DETA, CL97 has a larger contact angle and better water resistance. The DEAC in CL97 has more epoxy end groups, which brings a denser network structure, resistant to the entry of water molecules into the film.
Mechanical properties
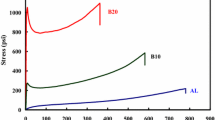
The mechanical properties of the cationic polyacrylate latex film are shown in Fig. 12a and 12b. As the amount of DEAC72 increased from 1.5 to 15.0 wt%, the tensile strength of the film gradually increased from 1.9 to 4.2 MPa, and the elongation at break decreased from 312% to 201%. As for the case with DEAC97, the tensile strength increased from 2.3 to 3.9 MPa, and the elongation at break decreased from 358% to 233%. The elongation at break of CL97 latex film is higher than that of CL72 latex film no matter how much DEAC is added, which indicates that CL97 latex film has better flexibility. This may be brought about by the longer pendants in DEAC97, which provide more free volume, resulting in a softer latex film.
The mechanical properties of CL after adding DETA are shown in Fig. 12c and 12d. When the DETA content was 10 wt%, the tensile strength of CL72 and CL97 films reached the maximum (5.8 MPa and 8.4 MPa, respectively), but part of the elongation at break was sacrificed (143% and 131%). Crosslinking enhanced the tensile strength, but reduced the elongation when the curing agent increased.
With the increase of the amount of DETA, compared with the film without DETA, the tensile strength of the film first increases and then decreases, and the elongation at break decreases gradually. When the amount of DETA was 10 wt%, the tensile strength of CL72 and CL97 reached the maximum. This may be due to the high degree of crosslinking of the film and the tight connection between the polymer chain groups, resulting in greater tensile strength. When the content of DETA is too high (25 wt%), the mechanical properties of the latex film are reduced, which is attributed to the fact that DETA contains some flexible groups, resulting in the mechanical properties of the film being reduced. The mechanical property of CL97 is stronger than that of CL72, and the maximum tensile strength reaches 8.4 MPa. DEAC72 has less crosslinking sites than that of DEAC97. After adding the curing agent, the degree of curing is also lower. The above phenomena indicate that the addition of DETA can improve the mechanical properties of cationic polyacrylate latex film, and that the mechanical properties are the best when 10 wt% DETA is added to DEAC97 (15 wt%) latex.
Conclusions
A waterborne cationic polyacrylate was prepared by semi-continuous seed emulsion polymerization from conventional monomers, MMA and BA, and a cationic monomer, DEAC. With the increase of the DEAC dosage, the size of the latex increases and the size distribution narrows. The zeta potential of the latex ranged from + 40 to + 65 mV, and the alkali resistance stability of the latex improved gradually with the increase of DEAC dosage. The water absorption of CL (15 wt%) latex films decreased after the addition of an amine curing agent, DETA, for crosslinking. When the DETA content was 10 wt% in CL97 (15 wt%), the water absorption of latex film decreased from 60.04% to 9.18%, and the contact angle reached the maximum of 114.16°. The formation of a crosslinked structure effectively improves the water resistance of latex films. It was found that the addition of DETA improves the tensile strength of the films, but sacrifices the elongation at break. The elongation at break of CL97 latex film is slightly larger than CL72 due to more flexible DETA existing in CL97. When 10 wt% DETA was added to CL97 (15 wt%) latex, the tensile strength reached a maximum value (8.4 MPa), and the latex film showed good mechanical properties. In conclusion, the crosslinkable waterborne cationic polyacrylate latex has good stability, water resistance, and mechanical properties, and has potential application prospects in a variety of fields.
References
Bao, Y, Ma, J, Zhang, X, Shi, C, “Recent Advances in the Modification of Polyacrylate Latexes.” J. Mater. Sci., 50 6839–6863. https://doi.org/10.1007/s10853-015-9311-7 (2015)
Liao, W, Teng, H, Qu, J, Masuda, T, “Fabrication of Chemically Bonded Polyacrylate/Silica Hybrid Films with High Silicon Contents by the Sol–Gel Method.” Prog. Org. Coat., 71 376–383. https://doi.org/10.1016/j.porgcoat.2011.04.008 (2011)
Yilmaz, O, “A Hybrid Polyacrylate/OMMT Nanocomposite Latex: Synthesis, Characterization and Its Application as a Coating Binder.” Prog. Org. Coat., 77 110–117. https://doi.org/10.1016/j.porgcoat.2013.08.008 (2014)
Miklečić, J, Blagojević, SL, Petrič, M, Jirouš-Rajković, V, “Influence of TiO2 and ZnO Nanoparticles on Properties of Waterborne Polyacrylate Coating Exposed to Outdoor Conditions.” Prog. Org. Coat., 89 67–74. https://doi.org/10.1016/j.porgcoat.2015.07.016 (2015)
Hu, J, Peng, K, Guo, J, Shan, D, Gloria, B, “Click Cross-Linking-Improved Waterborne Polymers for Environment-Friendly Coatings and Adhesives.” ACS Appl. Mater. Interfaces, 8 17499–17510. https://doi.org/10.1021/acsami.6b02131 (2016)
Yan, X, Ji, Y, He, T, “Synthesis of Fiber Crosslinking Cationic Latex and Its Effect on Surface Properties of Paper.” Prog. Org. Coat., 76 11–16. https://doi.org/10.1016/j.porgcoat.2012.08.003 (2013)
Liu, QX, Xu, WC, Yin, YN, “Study on Cationic Polyacrylate Emulsion to Paper Printing Performance.” Appl. Mech. Mater., 200 282–286. https://doi.org/10.4028/www.scientific.net/AMM.200.282 (2012)
Zhou, J, Chen, X, Ma, J, “Synthesis of Cationic Fluorinated Polyacrylate Copolymer by RAFT Emulsifier-Free Emulsion Polymerization and Its Application as Waterborne Textile Finishing Agent.” Dyes Pigm., 139 102–109. https://doi.org/10.1016/j.dyepig.2016.11.035 (2017)
Patrizi, ML, Diociaiuti, M, Capitani, D, Masci, G, “Synthesis and Association Properties of Thermoresponsive and Permanently Cationic Charged Block Copolymers.” Polymer, 50 467–474. https://doi.org/10.1016/j.polymer.2008.11.023 (2009)
Zhang, W, Wang, X, Wang, Y, Xiong, Y, Duan, M, Fang, S, “The Effect of Cationic Unit Structure on Reverse Demulsification and Air Flotation Performance of Cationic Polyacrylate.” J. Environ. Chem. Eng., 10 108766. https://doi.org/10.1016/j.jece.2022.108766 (2022)
Yin, X, Li, Z, Chai, F, Tian, Y, Tan, Y, Wang, M, “Synthesis of a Micro-crosslinked Polyacrylamide Flocculant and Its Application in Treatment of Oily Produced Water.” Energy Fuels, 35 18396–18405. https://doi.org/10.1021/acs.energyfuels.1c02213 (2021)
Fu, X, Zhang, Y, Jia, X, Wang, Y, Chen, T, “Research Progress on Typical Quaternary Ammonium Salt Polymers.” Molecules, 27 1267. https://doi.org/10.3390/molecules27041267 (2022)
Wang, B, Wang, F, Kong, Y, Wu, Z, Wang, RM, Song, P, He, Y, “Polyurea-Crosslinked Cationic Acrylate Copolymer for Antibacterial Coating.” Colloids Surf. A., 549 122–129. https://doi.org/10.1016/j.colsurfa.2018.04.012 (2018)
Hua, C, Chen, K, Wang, Z, Guo, X, “Preparation, Stability and Film Properties of Cationic Polyacrylate Latex Particles with Various Substituents on the Nitrogen Atom.” Prog. Org. Coat., 143 105628. https://doi.org/10.1016/j.porgcoat.2020.105628 (2020)
Ma, Z, Pu, Y, Huang, Y, Liu, L, Yao, Y, “Improvement in Interface and Mechanical Properties of CF/Poly(Ether-Imide) and CF/Poly(Ether-Ether-Ketone) by Aqueous Sizing of Reactive Cationic Poly(Amide-Imide) with Catechol Pendant Groups on CF.” Compos. Sci. Technol., 230 109754. https://doi.org/10.1016/j.compscitech.2022.109754 (2022)
Yu, D, Zhao, Y, Li, H, Qi, H, Li, B, Yuan, X, “Preparation and Evaluation of Hydrophobic Surfaces of Polyacrylate-Polydimethylsiloxane Copolymers for Anti-Icing.” Prog. Org. Coat., 76 1435–1444. https://doi.org/10.1016/j.porgcoat.2013.05.036 (2013)
Lü, T, Qi, D, Zhang, D, Liu, Q, Zhao, H, “Fabrication of Self-Cross-Linking Fluorinated Polyacrylate Latex Particles with Core–Shell Structure and Film Properties.” React. Funct. Polyme., 104 9–14. https://doi.org/10.1016/j.reactfunctpolym.2016.04.020 (2016)
Meng, L, Qiu, H, Wang, D, Feng, B, Di, M, Shi, J, Wei, S, “Castor-Oil-Based Waterborne Acrylate/SiO2 Hybrid Coatings Prepared via Sol–gel and Thiol-ene Reactions.” Prog. Org. Coat., 140 105492. https://doi.org/10.1016/j.porgcoat.2019.105492 (2020)
Huang, K, Liu, Y, Wu, D, “Synthesis and Characterization of Polyacrylate Modified by Polysiloxane Latexes and Films.” Prog. Org. Coat., 77 1774–1779. https://doi.org/10.1016/j.porgcoat.2014.06.001 (2014)
Parvate, S, Mahanwar, P, “Advances in Self-crosslinking of Acrylic Emulsion: What We Know and What We Would Like to Know.” J. Dispers. Sci. Technol., 40 519–536. https://doi.org/10.1080/01932691.2018.1472012 (2018)
Bückmann, AJP, Chen, Q, Overbeek, GC, Stals, PJM, Van der Zwaag, D, “Polymeric Aziridines as Benign Crosslinkers for Water-Based Coating Applications.” J. Coat. Technol. Res., 19 1345–1355. https://doi.org/10.1007/S11998-022-00626-W (2022)
Zhou, Y, Liu, C, Gao, J, Chen, Y, Yu, F, Chen, M, Zhang, H, “A Novel Hydrophobic Coating Film of Water-Borne Fluoro-Silicon Polyacrylate Polyurethane with Properties Governed by Surface Self-Segregation.” Prog. Org. Coat., 134 134–144. https://doi.org/10.1016/j.porgcoat.2019.04.078 (2019)
Wang, H, Yang, F, Zhu, A, Lu, T, Kong, F, Ji, L, “Preparation and Reticulation of Styrene Acrylic/Epoxy Complex Latex.” Polym. Bull., 71 1523–1537. https://doi.org/10.1007/s00289-014-1139-9 (2014)
Ruckerova, A, Machotova, J, Svoboda, R, Pukova, K, Bohacik, P, Valka, R, “Ambient Temperature Self-Crosslinking Latices Using Low Generation PAMAM Dendrimers as Inter-Particle Crosslinking Agents.” Prog. Org. Coat., 119 91–98. https://doi.org/10.1016/j.porgcoat.2018.02.027 (2018)
Oh, JS, Kim, MP, Kim, JH, Son, H, Kim, KH, Kim, SH, Yoo, JB, Lee, Y, Yi, GR, Nam, JD, “Diffusion-Assisted Post-Crosslinking of Polymer Microspheres Containing Epoxy Functional Groups.” Polymer, 133 110–118. https://doi.org/10.1016/j.polymer.2017.11.035 (2017)
Bohorquez, SJ, Mestach, D, “Film Formation and Crosslinking of Waterborne Two-Component Coatings Containing Quaternized Ammonium Groups.” J. Coat. Technol. Res., 16 1597–1607. https://doi.org/10.1007/s11998-019-00251-0 (2019)
Bian, F, Li, X, Zhao, J, Jiwen, H, Gui, X, Li, S, Lin, S, “One-Step Synthesis of Epoxy-Based Silicon Prepolymers and Its Application in UV-Curable Coating.” J. Coat. Technol. Res., 20 (1) 321–331. https://doi.org/10.1007/s11998-022-00672-4 (2022)
Wang, X, Shen, Y, Lai, X, “Micromorphology and Mechanism of Polyurethane/Polyacrylate Membranes Modified with Epoxide Group.” Prog. Org. Coat., 77 268–276. https://doi.org/10.1016/j.porgcoat.2013.09.013 (2014)
Yao, M, Tang, E, Guo, C, Liu, S, Tian, H, Gao, H, “Synthesis of Waterborne Epoxy/Polyacrylate Composites via Miniemulsion Polymerization and Corrosion Resistance of Coatings.” Prog. Org. Coat., 113 143–150. https://doi.org/10.1016/j.porgcoat.2017.09.008 (2017)
Pereira, CAA, Almeida, D, Moraes, J, “Effect of the Hardener to Epoxy Monomer Ratio on the Water Absorption Behavior of the DGEBA/TETA Epoxy System.” Polímeros, 26 30–37. https://doi.org/10.1590/0104-1428.2106 (2016)
Shih, YJ, Iovine CP, “Cationic Vinyl Ester Based Polymer Latices, Their Preparation and Use as Formaldehyde-Free Binders.” US Patent 5,39,038, 1983
Lewis, SN, Grove, W, Merritt RF, Washington, F, Emmoms, WD, Valley, H, “Unsaturated Quaternary Monnmers and Polymers.” US Patent 8,13,724, 1969
Lin, M, Chu, F, Guyot, A, Putaux, JL, Bourgeat-Lami, E, “Silicone–Polyacrylate Composite Latex Particles. Particles Formation and Film Properties.” Polymer, 46 1331–1337. https://doi.org/10.1016/j.polymer.2004.11.063 (2005)
Xu, X, Shi, Y, Sun, L, “Synthesis and Characterization of Poly(Methyl Methacrylate-Butyl Acrylate-Acrylic Acid)/Polyaniline Core–Shell Nanoparticles in Miniemulsion Media.” J. Appl. Polym. Sci., 123 1401–1406. https://doi.org/10.1002/app (2012)
Ai, D, Mo, R, Wang, H, Lai, Y, Jiang, X, Zhang, X, “Preparation of Waterborne Epoxy Dispersion and Its Application in 2K Waterborne Epoxy Coatings.” Prog. Org. Coat., 136 105258. https://doi.org/10.1016/j.porgcoat.2019.105258 (2019)
Xingqin, F, Chen, T, Wang, C, Zhang, Y, “Rheological Behavior of a Quaternary Ammonium Copolymer in the Presence of Inorganic Salts.” J. Macromol. Sci. Part B, 60 (1) 18–29. https://doi.org/10.1080/00222348.2020.1816886 (2020)
Piroonpan, T, Huajaikaew, E, Katemake, P, Pasanphan, W, “Surface Modification of SiO2 Nanoparticles with PDMAEMA Brushes and Ag Nanoparticles as Antifungal Coatings Using Electron Beam Assisted Synthesis.” Mater. Chem. Phys., 253 123438. https://doi.org/10.1016/j.matchemphys.2020.123438 (2020)
Zhou, J, Chen, X, Ma, J, “Cationic Fluorinated Polyacrylate Emulsifier-Free Emulsion Mediated by Poly(2-(Dimethylamino) Ethyl Methacrylate)-b-Poly (Hexafluorobutyl Acrylate) Trithiocarbonate via Ab Initio RAFT Emulsion Polymerization.” Prog. Org. Coat., 100 86–93. https://doi.org/10.1016/j.porgcoat.2016.03.016 (2016)
Machotová, J, Černošková, E, Honzíček, J, Šňupárek, J, “Water Sensitivity of Fluorine-Containing Polyacrylate Latex Coatings: Effects of Crosslinking and Ambient Drying Conditions.” Prog. Org. Coat., 120 266–273. https://doi.org/10.1016/j.porgcoat.2018.03.016 (2018)
Yang, XW, Shen, YD, Li, PZ, “Preparation and Water/Oil Repellency Properties of Waterborne Cationic Perfluorinated Polyacrylate.” Adv. Mater. Res., 602–604 643–647. https://doi.org/10.4028/www.scientific.net/AMR.602-604.643 (2012)
Machotova, J, Knotek, P, Cernoskova, E, Svoboda, R, Zarybnicka, L, Kohl, M, Kalendova, A, “Effect of Fluorinated Comonomer, Polymerizable Emulsifier, and Crosslinking on Water Resistance of Latex Coatings.” Coatings, 12 1150. https://doi.org/10.3390/coatings12081150 (2022)
Huang, C, Sun, X, Yuan, H, Song, C, Meng, Y, Li, X, “Study on the Reactivity and Kinetics of Primary and Secondary Amines During Epoxy Curing by NIR Spectroscopy Combined with Multivariate Analysis.” Vib. Spectrosc., 106 102993. https://doi.org/10.1016/j.vibspec.2019.102993 (2020)
Zhang, J, Qiu, Q, Yek, WY, Wang, F, Jia, Z, Guo, B, Jia, D, “Preparation and Application of a New Curing Agent for Epoxy Resin.” Int. J. Polym. Mater., 61 520–531. https://doi.org/10.1080/00914037.2011.593064 (2012)
Pramanik, M, Early, M, Wand, S, Gottschalk, D, Mendon, SK, Rawlins, JW, “Amidoamine: Synthesis, Disparity in Cure with Epoxy Resins Between Bulk and Solvent Systems, and Structure-Property Relationships of its Epoxy-Based Coatings.” Polym. Eng. Sci., 59 E69–E81. https://doi.org/10.1002/pen.24858 (2019)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Chen, K., Li, Z. et al. Preparation of crosslinkable cationic polyacrylate latex and its film properties. J Coat Technol Res 21, 341–354 (2024). https://doi.org/10.1007/s11998-023-00826-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-023-00826-y