Abstract
Chitosan is an exciting alternative for the development of coating-surfaces due to its large action spectrum against pathogenic microorganisms. However, to produce a stable coating with effective antibacterial action, a compromise between deacetylation degree (DD) and molecular weight (MW) is essential. Four chitosan samples were characterized regarding Mw and DD and correlated with the minimum and bactericide concentrations against E. coli, P. aeruginosa, and S. aureus. CHI80MW (79.7% DD and 7.0 × 105 Da) showed the best antibacterial effect and was selected to functionalize polytetrafluoroethylene (PTFE) surfaces by plasma. CHI80MW was grafted onto the PTFE surfaces using two different spacer molecules: poly(ethylene glycol) bis (carboxymethyl) ether (PEG) and poly(ethylene-alt-maleic anhydride) (PA). PTFE-Plasma-PA-CHI80MW exhibited a coating with more attached chitosan and better antibacterial action if compared to PTFE-Plasma-PEG-CHI80MW: after 8 h, PTFE-Plasma-PEG-CHI80MW presented a bacterial reduction of 25-30% for the three bacterial strains, and PTFE-Plasma-PA-CHI80MW reduced them to 77-90%. Moreover, cytotoxicity tests showed that PTFE-Plasma-PA-CHI80MW samples were compatible with human fibroblasts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite the considerable progress in the treatment of microbial infections in healthcare environments, bacterial contaminations still cause serious concerns. Nosocomial infections or health-care-associated infections (HAIs) are usually contracted in hospital environments and can lead to severe socio-economic problems.1,2,3 HAIs can lead to implant rejection, disease transmission, and they are the primary cause of morbidity and mortality, as well as a significant financial burden.4 This issue is particularly relevant when considering the surface of a biomedical device that must be implanted or used in direct contact with human body tissues, such as cardiovascular stents, urinary stents, contact lens, pin, and screw for the bone fixation, etc.1,2,3 The contamination of biomedical devices and surgical tools is considered a significant issue in medical interventions and, consequently, the healthcare environment. In most cases (about 90%) of microbial contamination, the microorganisms are bacteria that can adapt to different conditions, colonize the material surface, and form a resistant biofilm.
Different approaches can be employed to overcome the infection problem, such as altering the surface of the medical device in a way that bacterial growth on the surface material and the host tissue is inhibited. Three strategies are often used: antibacterial agent release coatings, antiadhesive surfaces, and contact-killing surfaces. This goal is achieved, making the surface unfavorable for bacterial growth, which can contribute to decreasing both the antimicrobial resistance and contamination in these devices.4
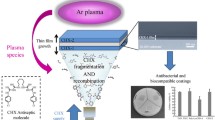
Plasma is a fast and versatile technology that offers several advantages since the technique allows surface modification without changing the inherent properties of the material (bulk properties). Moreover, this technique is eco-friendly and has an excellent industrial importance in modifying polymer surfaces. It can perform in many different ways without replacing the whole plasma system, for example, simple replacing gas, or allowing different applications because of the tunable properties of the surfaces.5,6 One of the main challenges of achieving “smart” materials is increasing surface adhesion in order to obtain a durable coating. In order to avoid the short durability of the coating, the interfacial interactions between the material surface and the coating could be effectively improved by the introduction of new chemical groups to the substrate surface, followed by the grafting.
Chitosan is an exciting alternative for the development of new products, bio-product from sea companies with low-cost, high availability, purity, biocompatibility, with a weak inflammatory response capable of interacting with cells and inhibit the action of microorganisms.7,8,9,10 The primary mechanism proposed for antimicrobial activity is based on the interactions between the microbial cell wall and the cationic groups present in the polymer’s structure, which increases the permeability of the negatively charged cell membrane, causing its disruption and release of intracellular compounds. In this regard, this study aimed to evaluate different types of chitosan to develop a nontoxic and antibacterial chitosan-based coating using a plasma grafting technique for applications in the medical devices field. In that sense, four chitosan samples were initially characterized about their degree of deacetylation (DD) and molecular weight (MW) and, biologically, about their minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC). These results indicated the potential chitosan sample with the best balance of properties to produce chitosan coatings. As polytetrafluoroethylene (PTFE) is widely used as a material for prosthetic devices,6 flat samples of PTFE were used as substrates. In that sense, plasma surface treatment was employed to functionalize PTFE surface with NH2 groups. Moreover, according to our previous studies,6 the use of the spacer molecules provides uniform coats to insert new chemical groups into PTFE substrates. That is why two different anchor molecules, poly(ethylene glycol) bis (carboxymethyl) ether (PEG) and poly(ethylene-alt-maleic anhydride) (PA) were used to produce a chitosan coating which remains covalently adherent to the PTFE surface, which has a long-lasting antibacterial action, and which is not cytotoxic.
Materials and methods
Materials
Chemicals
Four chitosan samples (CHI80LW–80% DD and low molecular weight, CHI75IW–75% DD and intermediate molecular weight, CHI85I–85% DD and intermediate molecular weight and, CHI80MW–80% DD and medium molecular weight) and fluorescein isothiocyanate isomer I (FIT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Acetic acid, poly(ethylene glycol) bis (carboxymethyl) ether (PEG; Mw = 600 Da), poly(ethylene-alt-maleic anhydride) (PA; Mw = 100-500 kDa), buffer 2-(N-morpholino)ethanesulfonic acid hydrate (MES), and N-(3-dimethyl aminopropyl)-N’-ethyl carboidiimide hydrochloride (EDAC) were obtained from Sigma-Aldrich (Oakville, ON, Canada). All reagents were analytical grade and were used without further purification and solutions were prepared using Milli-Q® water. Polytetrafluoroethylene (PTFE) films (Goodfellow, Mississauga, ON, Canada) with a thickness of 250 μm were used as substrate.
Biologicals
Microorganisms
For the bacterial tests, the microorganisms Escherichia coli (ATCC 11229), Pseudomonas aeruginosa (ATCC 27853), and Staphylococcus aureus (ATCC 25923) were purchased from the Collection of Cultures of Laboratório de Microbiologia Ambiental (LAMAB) at the Federal University of Ceara (Fortaleza, Brazil). Broth and agar Mueller-Hinton (MH) medium were purchased from Medix (Chicago, IL, USA).
Cells
Human Dermal Fibroblasts (HDF) such as primary neonatal fibroblasts from human skin (ATCC, Manassas, VA, USA) were selected for a cytotoxicity screening. Fetal bovine serum (FBS), penicillin/streptomycin, glucose, NaHCO3, and HEPES used in Dulbecco’s modified Eagle’s (DMEM) medium, were purchased from Sigma-Aldrich (St. Louis, MO, USA) and, 4',6-diamidino-2-phenylindole DAPI was purchased from Molecular Probes (Eugene, OR, USA).
Methods
Physicochemical and microbiological characterization: chitosan powder samples
Deacetylation degree (DD)
Nuclear magnetic resonance spectroscopy (1H NMR) was employed to determine the chitosan DDs. Chitosan solutions (CHI80LW, CHI75IW, CHI85IW, and CHI80MW) with a concentration of 25 mg/mL were prepared in D2O/DCl (0.96:0.04; v/v). NMR spectra were recorded on a Bruker AC 300/P spectrometer (Bremen, Germany) at 70°C. The peaks were integrated using the Topspin software (Bruker Inc. Bremen, Germany) and equation (1) was used to obtain the relative percentage of DD for each sample, according to the procedure described by Lavertu and co-workers.11
where \(H1D\) is deacetylated monomer and \(HAc\) is acetyl groups.
Molecular weight (M w)
The molecular weight of chitosan samples was determined by size exclusion chromatography (SEC) employing a Viscotek Chromatographic System (Houston, TX, USA) containing a column SB806M HQ (Shodex Pak, Milford, MA, USA), with refractometer and RALS detectors. The mobile phase used was acetic acid 0.33 mol/L set up at pH = 3.9 ± 0.2 with NaOH 0.1 mol/L and at a flow rate of 0.8 mL/min at 40ºC. For analysis, chitosan samples were dissolved in the mobile phase (2 mg/mL) with subsequent filtration of the mixture through a syringe filter with a pore diameter of 0.22 µm (Merck Millipore, Burlington, MA, USA). The calibration of the column was done indirectly using Dextran (Malvern PANanalytical Products, Westborough, MA, USA) standards with a molecular weight between 103–106 Da.
Determination of minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) for chitosan powders in aqueous solution
Minimal inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs) were performed by the broth microdilution method in Mueller-Hinton broth (MHB), following the guidelines of the Clinical and Laboratory Standards Institute CLSI.12 Standard inocula of Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus were prepared in Mueller-Hinton (MH) broth and cultivated until the log phase with a concentration of 1×108 colony-forming unit (CFU)/mL (0.5 McFarland). Serial dilutions were performed to obtain bacterial solutions with different concentrations (starting from 2,048 μg/mL). Microdilutions were performed using ≤ 10 μL of inocula in 0.1 mL of antibacterial agent solution [chitosan aqueous solution with different concentrations (w/w), were prepared in acetic acid 1% (v/v). These solutions were stirred at room temperature for 24 h]. Acetic acid 1 % (v/v) was used as a negative control and MH broth as a positive control. The plates were incubated at 37±2ºC for 24 h.
Functionalization and coating of PTFE substrates with chitosan
Preparation and functionalization of PTFE substrates
PTFE films were cut with a dimension of 3 cm × 3 cm. Samples were cleaned in acetone (≥99.5%), water (100%), and methanol (≥99.5%) in ultrasonic baths for 10 min in each solution, then dried with particle-free compressed air before use. PTFE was used in this study because it is a wide polymer used in the biomedical field.
Plasma treatment and grafting of spacer molecules
An atmospheric plasma equipped with a conventional parallel-plate dielectric barrier discharge (DBD) reactor on the grounded electrode was used. Gas flow (95% N2 + 5% H2) at 5 L/min was introduced directly between the electrodes through a diffuser. The frequency, applied voltage, gas gap, and treatment time were kept constant (3 kHz, 10 kV, 1 mm, and 45 s). After plasma treatment, the grafting process of spacer molecules (PA and PEG) was performed according to Vaz et al.6 PTFE films were immersed in acetone and 0.3 g/mL of PA was added three times at 0, 20, and 40 min. After 1 h of reaction, the films were washed three times with acetone (≥99.5%), air-dried, and stored under vacuum before use. For PEG grafting, PTFE films treated by plasma were immersed in 0.1 g/mL PEG solution (pH 4.75, MES buffer), previously activated with EDAC (3 mg/mL every 10 min for three times, the reaction was complete after 30 min). After 1 h of reaction, the films were washed three times with MES buffer, five times with deionized water, and then dried and stored under vacuum before use.
Preparation of chitosan solutions and grafting on PTFE surfaces
-
Chitosan (CHI80MW) solution: 2% (w/w) chitosan solution was prepared by dissolving 2 g of chitosan in 97 mL of distilled water, followed by addition of 3 mL of acetic acid (≥99%), and stirred at room temperature for 24 h.
-
Chitosan marker (FITCHI80MW) solution: to evaluate the homogeneity of chitosan coating on the PTFE surfaces obtained by grafting with different spacers (PEG and PA), chitosan sample was synthesized with a fluorescein isothiocyanate isomer I (FIT) marker as described.13 Briefly, 100 mL of a 1 g/L FIT in methanol solution (≥99.5%), was added to 100 mL of chitosan (1% w/v) dissolved in 0.1 acetic acid solution. The reaction was carried out for 3 h protected from light at 25°C. The pH of the solution was gradually increased to 10 to cause the precipitation of the modified chitosan. The unattached FIT and methanol residues were washed with water and separated (10000 rpm) until no fluorescence traces were detected in the supernatant. The chitosan pellet was then frozen and lyophilized for 48 h. The lyophilized FITCHI80MW was dissolved in an aqueous acetic acid solution (1% v/v) to reach a final polymer concentration of 2% (w/w) and covalently grafted on PTFE surfaces pre-functionalized by plasma and containing PEG or PA spacers.
-
Grafting of chitosan solutions on pre-treated PTFE surfaces: plasma-treated PTFE films grafted with PEG and PA were immersed in CHI80MW and FTICHI80MW chitosan solutions, as previously described, at room temperature for 3 h and under stirring in a rotary device. The samples were then washed five times with ultrapure water and then dried and kept under a vacuum.
Surface characterization
Evaluation of the chitosan coatings grafted with PEG and PA spacers on the PTFE surfaces
To evaluate the chitosan coating covalently linked with PEG and PA, the photoluminescence of chitosan marker (FTIC) samples was performed. It was excited by the focused 488 nm (2.54 eV) line of an Ar+ laser at 5 mW. The emitted light was collected with f/1 optics, filtered, dispersed in a 0.25 m spectrograph, and detected in a cooled thinned back-illuminated 1340 × 100 pixels Si CCD. Photoluminescence was measured in triplicate for each spacer with the samples at room temperature.14
Biological assays
Antibacterial tests: bacterial adhesion
Bacterial adhesion assays were performed based on the work of Hernandez-Montelongo and coworkers.13 PTFE samples (nontreated and coated with chitosan) were incubated in triplicate in an oven with air circulation at 37°C. After 4 h and 8 h, the culture medium was removed to stop growth. The samples were washed extensively with water to remove traces of the culture medium as well as poorly adhered bacteria on the surface of the sample. To count the microorganisms, present on the surface of the PTFE film, the samples were sonicated in phosphate-buffered saline (PBS) for 10 min to enable the bacteria to detach from the substrate to the buffer solution. From 0.1 mL of this solution, serial dilutions were made in 1:9 (v/v) PBS. Aliquots of 0.1 mL at dilutions were plated on agar Muller Hinton. After 24 h of incubation at 37°C in a forced circulation air oven, the number of bacterial colonies was counted, and the result, after multiplication by dilution factor, was expressed in colony-forming units per cm2 of the sample (CFU/cm2).
Cell behavior
Primary neonatal fibroblasts from human skin were selected for a cytotoxicity screening. Cells were cultured with Dulbecco’s modified Eagle’s (DMEM) medium, containing 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, 4500 mg/L glucose, 3.7 g/L NaHCO3, and 15 mM HEPES. The cultures were kept at a temperature of 37°C in a humidified 5% CO2 atmosphere and were routinely passaged at preconfluency using 0.25% trypsin and 0.01% EDTA (Invitrogen, Waltham, USA). Samples were sterilized through exposure to UV-C light for 15 min per side.15
Viability assay
The indirect in vitro toxicity was assessed by exposing human dermal fibroblasts (HDFs) to degradation products of the films and subsequently determining their cell metabolic function by using 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazo-lium bromide (MTT) tetrazolium salt. An extract media was obtained by immersing each film in a culture medium (24 h, 37ºC, 5% CO2) at a ratio of 1 mL per 6 cm2 area of dry material, as preconized by EN ISO 10993:12. Concomitantly, HDFs were seeded into a 96-well plate with a density of 6×103 cells/cm2 and cultured for 24 h to attain cell adhesion. After this, cells were washed once with PBS and covered with 100% extract solutions for 24 h, remaining under an appropriate atmosphere. Cultures exposed to the standard culture medium were used as a negative control, and those exposed to 1% (v/v) Triton-X100 (Sigma-Aldrich) in PBS for 5 min were used as a positive control of toxicity. Posteriori, the cells were washed twice with PBS, and then an MTT (Sigma-Aldrich) solution (0.5 mg/mL in standard medium) was added and remained in contact for 4 h (37°C and 5% CO2). After this, the medium was carefully removed and the formazan crystals were dissolved in dimethyl sulfoxide. The absorbance was read at 570 nm using a spectrophotometer SpectraMax Microplate Reader (Molecular Devices, San Jose, USA). Absorbance of the negative control was used as 100% viability. As a complement, both optical and fluorescence microscopies were performed. After 24 h of treatment, the cells were washed once with PBS and were fixed with 4% paraformaldehyde in PBS (2-8ºC, 10 min). The cells then received 0.1% Triton-X100 (Sigma-Aldrich) in PBS for 30 min, working as a permeabilization buffer. The cytoskeleton (F-actin) marking was performed using Phalloidin-FITC fluorescein isothiocyanate (Invitrogen) for 40 min, at 2-8ºC, in a light-free environment. Fluorescence micrographs (×200 magnification) were captured using a BX-50 (Olympus, Tokyo, Japan) microscope coupled with a 460-490 nm band-pass excitation filter.16
Cell adhesion
A direct contact assay was used to evaluate the cell adhesion potential of the HDFs to chitosan films. Glass coverslips were used as a control to compare films with a known adhesion model. Briefly, 6×103 HDFs/cm2 were seeded on film samples and maintained under incubation for 1 h. Next, the cells were washed twice with PBS, then fixed and permeabilized, as previously described. The remaining cells were stained using 300 nM 4',6-diamidino-2-phenylindole DAPI (Molecular Probes, Eugene, USA) in PBS, at 2-8ºC, for 30 min, in a dark environment. Fluorescence micrographs of cell nuclei (× 40) were captured using a BX-50 device equipped with 358/461 nm filters. The quantification of DAPI-stained nuclei was performed using the ImageJ software (NIH, Bethesda, USA) after imputing the required spatial calibrations.17
Statistical analysis
The data were analyzed through the one-way or two-way ANOVA followed by Bonferroni’s or Tukey’s multiple comparisons test, using the Graph Pad Prism 6 software (La Jolla, CA, USA). The results are expressed as mean value ± standard deviation. The differences were considered significant when the p-value was 0.05 or less.
Results and discussion
Characterization: chitosan powder samples
As the properties of chitosan are related to its chemical structure, four chitosan samples were characterized by deacetylation degree (DD) and molecular weight (MW).
Deacetylation degree (DD) and molecular weight (M w)
The determination of chitosan samples by 1H NMR is direct, using the integrals of the peaks.11 Four chitosan samples exhibited similar NMR spectra, with very close chemical shifts of the D-glucosamine (proton H1 of deacetylated monomer—H1D) and of the peak of the N-acetyl-D-glucosamine (three protons of acetyl group—HAc). Results of the deacetylation degree (DD) for chitosan samples are presented in Table 1 and they agree with the results presented in various works.6,7,14,18,19 CHI85IW presents a higher DD when compared to the other chitosan samples, 85% of DD. CHI75IW presents the lower DD of 75% and CHI80LW and CHI80MW showed a similar DD, 80%, respectively.
Another critical parameter regarding the chitosan structure and properties is molecular weight (MW). The values acquired for the molecular weight for the four types of chitosan were in a range of 1×105 Da to 7x105 Da, increasing from CHI80LW to CHI80MW (Table 1). According to the literature, CHI80LW can be classified as low molecular weight chitosan (in the range of 100,000 Da); CHI80MW is chitosan with medium molecular weight (in the range of 500,000 Da), and CHI75IW and CHI85IW as chitosan with intermediated molecular weight.17
Determination of minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) for chitosan powders in aqueous solution
The minimal inhibitory concentration (MIC) can be defined as the lowest concentration of an antimicrobial agent necessary to inhibit the visible growth of the microorganism after 24 h of incubation. MIC has been useful as an indicator of the activity of drugs and natural extracts, for instance, against a selected pathogenic micro-growth.20 In this study, the MICs of CHI80LW, CHI75IW, CHI85IW, and CHI80MW were determined in acetic acid 1% (v/v) against two Gram-negative bacteria (E. coli and P. aeruginosa), and one Gram-positive (S. aureus); they ranged from 256–128 μg/mL. These results are presented in Fig. 1.
Regarding the inhibitory action of CHI80LW and CHI80MW chitosans against E. coli, it is possible to remark that MW was the primary factor that influences the MICs results when chitosan samples have the same DD. CHI80MW, chitosan with higher MW (7.0±0.3 ×105 Da), presented a more effective action when compared to CHI80LW, chitosan with lower molecular weight (1.4±0.1 ×105 Da). However, for CHI75IW and CHI85IW, which presented an intermediate MW (2.1±0.2 ×105 and 3.0±0.2×105 Da, respectively), it is possible to note that DD was the principal factor to contribute to the behavior of these chitosans against E. coli. CHI85IW with 84.8±0.9 DD presented as more effective against E. coli when compared to CHI75IW with 75.4±0.5 DD. A study by Liu and coworkers21 evaluated the antibacterial action of chitosan with different molecular weights on E. coli. It was observed that chitosans with higher MW showed better bactericidal action compared to chitosans with lower molecular weight.
Results for P. aeruginosa bacterium showed that MW is the primary factor that influences the MICs results for this microorganism. While for CHI80LW, CHI75IW and CHI85IW, chitosans with low and intermediate MW, it was necessary up to 256 μg/mL to inhibit the growth of this bacterium. However, for CHI80MW with a higher MW, just 128 μg/mL was necessary. Analyzing the inhibitory action of chitosans against S. aureus, it is possible to remark that DD was the primary factor that influences the MICs results. CHI80LW, CHI85IW, and CHI80MW, which process a DD between 80-85%, presented a more effective action when compared to CHI75IW, chitosan with lower DD (75%).
The minimal bactericidal concentration (MBC) is a complementary technique to the MIC; whereas the MIC test demonstrates the lowest level of antimicrobial agent that inhibits growth, the MBC demonstrates the lowest level of antimicrobial agent that results in microbial death. The MBC is identified by determining the lowest concentration of antibacterial agent that reduces the viability of the initial bacterial inoculum by ≥99.9%. MBCs of CHI80LW, CHI75IW, CHI85IW, and CHI80MW were determined in aqueous solution against E. coli and P. aeruginosa (both Gram-negative), and S. aureus (Gram-positive). These results are presented in Fig. 2.
In a general way, the MBCs tests presented the same trend observed in MICs tests. MW was the primary factor that influenced the MICs results for E. coli when chitosan samples have the same DD (CHI80LW and CHI80MW). For CHI75IW and CHI85IW, DD was the principal factor contributing to the behavior of these chitosans against E. coli. CHI85IW, with 85% DD, was more effective against E. coli when compared to CHI75IW with 75% DD. Results of MBC for P. aeruginosa bacterium showed that MW of chitosan is the primary factor that influences the action of chitosan against this microorganism.
Analyzing the inhibitory action of chitosans against S. aureus bacterium, it is possible to remark that DD was the primary factor that influences the MBCs results. CHI80LW, CHI85IW, and CHI80MW, which process a DD between 80-85%, presented a more effective action when compared to CHI75I chitosan, with a lower DD (75%).
When compared to the action of chitosan against Gram-negative and Gram-positive bacteria, chitosan showed more pronounced inhibitory and bactericidal effects for Gram-positive bacteria than for Gram-negative bacteria. The antimicrobial activity of chitosan may be influenced by some factors such as microorganism’s stirps, molecular weight (MW), degree of deacetylation (DD), concentration, and pH of the chitosan solution. DD and MW directly influence the solubility of chitosan in solution, as well as its interaction with the cell walls of target microorganisms. In our case, the combination of both parameters showed the best antibacterial effect, the CHI80MW chitosan, with the high values of MW and DD; it exhibited higher antimicrobial behavior in both techniques, MIC and MBC.
Three models have been proposed for the mechanism of antibacterial action of chitosan, with the most acceptable interaction between positively charged chitin/chitosan molecules and membranes of negatively charged microbial cells. In this model, interaction is mediated by the electrostatic forces between protonated NH3+ and negative residue groups, presumably by competition with Ca2+ for sites on the electronegative membrane surface. This electrostatic interaction results in double interference: i) by promoting changes in membrane properties, wall permeability thus causes internal osmotic imbalances and consequently inhibits the growth of microorganisms and ii) by hydrolysis of the peptidoglycans in the wall of the microorganism, leading to leakage of intracellular electrolytes such as potassium and other low molecular weight protein constituents (e.g., proteins, nucleic acids, glucose, and lactate dehydrogenase). The third mechanism is metal chelation, suppression of spore elements and binding to the essential nutrients for microbial growth. It is well known that chitosan has excellent metal bonding capabilities, where amine groups in chitosan molecules are responsible for the absorption of metal cations by chelation.6,22
Surface characterization: concentration of chitosan on the functionalized PTFE surfaces by plasma-grafting
To verify the ability of the PTFE substrates to bind chitosan on the surface, PTFE samples were functionalized by plasma, and PEG and PA spacers were covalently grafted on the surface of the substrate. As CHI80MW chitosan exhibited the best antibacterial effect in MIC and MBC techniques, this one was chosen to be immobilized. CHI80MW was prepared with a FIT marker (called FITCHI80MW) and covalently grafted on PTFE-Plasma-PEG and PTFE-Plasma-PA surfaces and fluorescence images were taken.
These analyses were performed, aiming to determine the best linking-arm to be able to covalently graft a greater quantity of chitosan samples to produce antibacterial, noncytotoxic PTFE surfaces. Figure 3 shows that PEG and PA linking-arms were able to anchor chitosan on PTFE surfaces; however, the fluorescence intensity of PTFE-Plasma-PA-CHI80MW was higher than control (nontreated PTFE) and PTFE-Plasma-PEG-CHI80MW. This observation can be explained due to the higher density of anhydrides groups of PA when compared to PEG, which contains only one terminal carboxylic group in its repeating unit. Since these functional groups act as reactive sites to graft chitosan chains, PA presents a higher ability to covalently graft chitosan on the PTFE surface.6,23
Fluorescence intensity of chitosan marked with fluorescein isothiocyanate isomer I, 85% DD and medium Mw (FITCHI80MW) assembled on plasma-treated polytetrafluoroethylene (PTFE) substrates anchored by two linking-arms: poly(ethylene glycol) bis (carboxymethyl) ether (PEG, Mw = 600 Da) and poly(ethylene-alt-maleic anhydride) (PA, Mw = 100-500 kDa)
Biological assays
Antibacterial action of PTFE surfaces coated with chitosan
PTFE samples coated with chitosan were tested in contact with pathogenic human bacteria E. coli and P. aeruginosa and S. aureus aiming to evaluate their antibacterial action. For PTFE-Plasma-PEG-CHI80MW (Figs. 4a and 4b), the bacterial reduction decreased to 55%, 48%, and 62% for E. coli, P. aeruginosa, and S. aureus, respectively, after 4 h. These results are presented in relation to bare PTFE, used as a control. Results after 8 h of contact corroborate with the results previously shown, where a bacterial reduction of about 25-30% was presented.
The use of PA spacer allowed to confirm free amino groups derived from bio-polymers in higher quantities when compared to PTFE-Plasma-PEG-CHI80MW, which played a significant role in the antimicrobial effect. After 4 h, PTFE-Plasma-PA-CHI80MW (Fig. 5a) sample presented the best antibacterial action when it was tested against the S. aureus strain, reducing 95%. In the case of the other bacteria, the bacterial reduction for E. coli and P. aeruginosa was 93% and 90%, respectively. After 8 h, PTFE-Plasma-PA-CHI80MW (Fig. 5b) reduced 90% of S. aureus, and the bacterial reduction for E. coli and P. aeruginosa was 88% and 77%, respectively.
Coatings with CHI80MW had a better antibacterial response compared to the PTFE surfaces (control) due to a large quantity of positively charged amino groups responsible for the inactivation of the bacterium by rupturing the negatively charged wall of the microbial cell. It was perceived in these samples a significant influence of the type of anchor used, with PA, which has several points of anchorage, allowing the formation of a more homogeneous coating when compared to PEG.
Evaluating the type of bacteria, it was possible to observe that PTFE-Plasma-PA-CHI80MW presented the best results for S. aureus. This behavior can be explained due to the difference in bacterial cell wall composition, which is more straightforward for Gram-positive bacteria, which is also reported in the literature:18,19,24 the enhanced resistance of the Gram-negative bacteria against chitosan can be explained by the outer cell membrane, which protects the cell wall from the biocide contact of free ammonium groups. These results suggest that these coatings are more useful for Gram-positive bacteria.
Viability and cell adhesion of PTFE surfaces coated with chitosan
PTFE-Plasma-PA-CHI80MW sample, which presented the best antibacterial action, was placed in contact with human cell cultures (human dermal fibroblasts, HDFs) to evaluate its cytotoxicity and the influence on the viability of cells (Fig. 6). Evaluating the human fibroblast morphology, phase-contrast optical microscopy did not show substantial changes in the fibroblast morphology after 24 h, nor vacuolization, detachment, or differences in the cell monolayer confluence. Discrete intracytoplasmatic granules were observed after exposure to the different samples, similar to those only exposed to standard medium (Fig. 6a). Figure 6b shows the F-actin cytoskeleton organization of HDFs. Under treatment, these cells presented dorsal and ventral stress fibers distribution, similarly to the control. Therefore, none of the extract samples could modulate the F-actin cytoskeleton of HDFs that indirectly received their degradation products, showing the viability of cells when in contact with PTFE-treated films. Cell culture with standard supplemented culture medium was used as a negative control (DMEM supplemented with 10% FBS) and another with 1% (v/v) Triton-X100 (Sigma-Aldrich) in PBS as a positive control of toxicity (Fig. 6c). The results presented no statistical difference when compared to the negative control (DMEM supplemented with 10% FBS): HDF cultures exposed for 24 h to the PTFE-Plasma-PA-CHI80MW extract presented 115 ± 19.4% viability, followed by the PTFE-Plasma treatment with 92.4 ± 3.3% and, PTFE-plasma-PA with 88.8 ± 14%. In general, results from biological assays showed the biocompatibility of the PTFE substrate in every different step of the cascade process to obtain the final sample PTFE-Plasma-PA-CHI80MW. This guarantees the noncytotoxicity of every obtained surface on PTFE.
Cell viability for human dermal fibroblasts on the different samples studied: plasma-treated polytetrafluoroethylene (PTFE-Plasma), plasma-treated polytetrafluoroethylene anchored by poly(ethylene-alt-maleic anhydride) (PTFE-Plasma-PA), and plasma-treated polytetrafluoroethylene anchored by poly(ethylene-alt-maleic anhydride) grafted with chitosan 85% DD and medium Mw (PTFE-Plasma-PA-CHI80MW). (a) Human dermal fibroblasts (HDF) morphology after 24 h in contact with the samples. (b) HDF cytoskeleton in over 24 h in contact with the samples. (c) Maintenance of HDF viability after 24 h of indirect exposition with the samples and the negative control. Results assays represent mean ± standard deviation, statistically interpreted by analysis of variance (ANOVA). *** denotes a significant difference with a p-value ≤ 0.001
Conclusions
It was possible to develop, on PTFE substrates, a nontoxic and antibacterial chitosan-based coating using the plasma grafting technique. Based on the MIC and MBC results, chitosan with high DD and MW presented the higher Gram-negative antibacterial activity. In that sense, the most promising chitosan, CHI80MW (80% DD, 7×105 Da), was employed for the coating assembly on PTFE. To link CHI80MW on the PTFE surface, samples were treated with plasma and PEG, or PA, obtaining PTFE-Plasma-PEG-CHI80MW and PTFE-Plasma-PA-CHI80MW samples. Based on the fluorescence results and antibacterial tests against E. coli and P. aeruginosa, and S. aureus, the sample with PA functionalization presented the higher fluorescence and antibacterial effect, probably due to its chemical conformation and higher density of carboxylic groups’ terminal. Cytocompatibility assays using fibroblasts were performed on the final sample. Results showed the cytocompatibility of the PTFE substrate in each different step of the cascade process to obtain PTFE-Plasma-PA-CHI80MW. Based on these findings, the understating of chitosan properties in solution leads to better development of plasma-assisted coating for a wide range of applications, including the antimicrobial one, as demonstrated herein.
Data availability
All the data included in this study are available upon request by contacting the corresponding author.
References
Costerton, JW, Stewart, PS, Greenberg, EP, “Bacterial biofilms: A common cause of persistent infections.” Science, 284 1318–1322 (1999)
Borkow G, editor. Use of Biocidal Surfaces for Reduction of Healthcare Acquired Infections [Internet]. Springer International Publishing; 2014 [cited 2020 Mar 31]. https://www.springer.com/gp/book/9783319080567.
Kramer, A, Schwebke, I, Kampf, G, “How long do nosocomial pathogens persist on inanimate surfaces? A systematic review.” BMC Infect. Dis., 6 130 (2006)
Campoccia, D, Montanaro, L, Arciola, CR, “A review of the biomaterials technologies for infection-resistant surfaces.” Biomaterials, 34 8533–8554 (2013)
Ogino, A, Kral, M, Yamashita, M, et al. “Effects of low-temperature surface-wave plasma treatment with various gases on surface modification of chitosan.” Appl. Surf. Sci., 255 2347–2352 (2008)
Vaz J, Michel E, Chevallier P, et al. "Covalent grafting of chitosan on plasma-treated polytetrafluoroethylene surfaces for biomedical applications." J. Biomater. Tissue Eng., 4 (11), 915–924 (2014)
Kong, M, Chen, X, Xing, K, et al. “Antimicrobial properties of chitosan and mode of action: A state of the art review.” Int. J. Food Microbiol., 144 51–63 (2010)
Mourya, VK, Inamdar, NN, “Chitosan-modifications and applications: Opportunities galore.” React. Funct. Polym., 68 1013–1051 (2008)
Xu Y, Liu J, Guan S, et al. “A dual pH and redox-responsive Ag/AgO/carboxymethyl chitosan composite hydrogel for controlled dual drug delivery.” J. Biomater. Sci. Polym. Ed., 31 (13) 1706–1721 (2020)
Fan Z, Cheng P, Wang D, et al. “Design and investigation of salecan/chitosan hydrogel formulations with improved antibacterial performance and 3D cell culture function.” J. Biomater. Sci. Polym. Ed., 31 (17) 2268–2284 (2020)
Lavertu, M, Xia, Z, Serreqi, AN, et al. “A validated 1H NMR method for the determination of the degree of deacetylation of chitosan.” J. Pharm. Biomed. Anal., 32 1149–1158 (2003)
Clinical and Laboratory Standards Institute, editor. “Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: M07-A10; approved standard.” 10th ed. Wayne, PA: Committee for Clinical Laboratory Standards; 2015.
Huang, M, Ma, Z, Khor, E, et al. “Uptake of FITC-chitosan nanoparticles by A549 cells.” Pharm. Res., 19 1488–1494 (2002)
Tokura, S, Ueno, K, Miyazaki, S, et al. “Molecular weight dependent antimicrobial activity by chitosan.” Macromol. Symp., 120 1–9 (1997)
Guerreiro, SG, Brochhausen, C, Negrão, R, et al. “Implanted neonatal human dermal fibroblasts influence the recruitment of endothelial cells in mice.” Biomatter., 2 43–52 (2012)
Varma SR, Sivaprakasam TO, Mishra A, et al. “Protective effects of triphala on dermal fibroblasts and human keratinocytes.” PloS One, e0145921 (2016)
Dee, GJ, Rhode, O, Wachter, R, “Chitosan: Multi-functional marine polymer.” Chitosan Multi-Funct. Mar. Polym., 116 39–44 (2001)
Hernandez-Montelongo, J, Lucchesi, EG, Gonzalez, I, et al. “Hyaluronan/chitosan nanofilms assembled layer-by-layer and their antibacterial effect: A study using Staphylococcus aureus and Pseudomonas aeruginosa.” Colloids Surf. B Biointerfaces, 141 499–506 (2016)
Vasilev, K, Cook, J, Griesser, HJ, “Antibacterial surfaces for biomedical devices.” Expert Rev. Med. Devices, 6 553–567 (2009)
Hernandez-Montelongo, J, Corrales Ureña, YR, Machado, D, et al. “Electrostatic immobilization of antimicrobial peptides on polyethylenimine and their antibacterial effect against Staphylococcus epidermidis.” Colloids Surf. B Biointerfaces, 164 370–378 (2018)
Liu, H, Du, Y, Wang, X, et al. “Chitosan kills bacteria through cell membrane damage.” Int. J. Food Microbiol., 95 147–155 (2004)
Zivanovic, S, Chi, S, Draughon, AF, “Antimicrobial activity of chitosan films enriched with essential oils.” J. Food Sci., 70 M45–M51 (2005)
Croisier, F, Jérôme, C, “Chitosan-based biomaterials for tissue engineering.” Eur. Polym. J., 49 780–792 (2013)
Dash, M, Chiellini, F, Ottenbrite, RM, et al. “Chitosan—A versatile semi-synthetic polymer in biomedical applications.” Prog. Polym. Sci., 36 981–1014 (2011)
Funding
This work was supported by the Sao Paulo Research Foundation (Grant No 2013/05135-1), Coordination for the Improvement of Higher Education Personnel (CAPES: Procad 88882.151600/2017-01, Proex 33003017034P8), and National Council for Scientific and Technological Development (CNPq 421039/2016-7).
Author information
Authors and Affiliations
Contributions
JMV and RSV conceived and designed experiments; LMCGF performed the characterization of chitosan powders and obtained the MIC and MBC values for the chitosan powders; JMV functionalized the PTFE surfaces with chitosan by plasma grafting; JHM performed the photoluminescence characterization; JMV performed the antibacterial tests; CR performed the cytocompatibility tests; JMV, CR, and JHM analyzed the data; MMB and RSV contributed reagents/materials/analysis tools; JMV, TBT, JHM, and RSV wrote the manuscript. All authors revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vaz, J.M., Taketa, T.B., Hernandez-Montelongo, J. et al. Antibacterial noncytotoxic chitosan coatings on polytetrafluoroethylene films by plasma grafting for medical device applications. J Coat Technol Res 19, 829–838 (2022). https://doi.org/10.1007/s11998-021-00560-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-021-00560-3