Abstract
One of the main drawbacks of drying fruits is the degradation of their quality by oxidative processes. In this study, antioxidant pectin coatings were prepared and applied onto kiwi slices before the drying process to evaluate their effect on drying kinetics, on maintaining nutritional quality and antioxidant capacity of the cut fruit, and during 31 days of storage. Pectin antioxidant coating formulations have been developed by adding antioxidant extracts from fruits or antioxidant acids. Uncoated dehydrated fruits and those coated with pectin without antioxidants were used as control. Convective drying was carried out, desorption isotherms (30, 50 and 60°C) and drying kinetics (50 and 60°C) were analyzed, and the obtained experimental data were adjusted to the GAB and Page models, respectively. The dried kiwi slices were evaluated for their retention of antioxidants (vitamin C and phenolic compounds), total antioxidant activity (DPPH), mechanical properties, and color. The coatings had a slight influence on the isotherms and drying kinetics at the studied temperatures, mainly for the one performed at 30°C. However, the drying rate at 60°C decreased more rapidly and the Page model fit well with the experimental data. The antioxidant coatings preserved the thermosensitive compounds during drying, and formulations with the acerola extract and antioxidant acids may be highlighted since, in addition to the antioxidant effect, they also nutritionally enriched the kiwi slices. Thus, the proposed methodology is a promising technique to reduce the oxidative effects inherent in the drying and storage processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The benefits derived from consuming fruits are attributed to the large number of biological compounds found in these foods. These compounds protect proteins, lipids, and DNA from oxidative damage due to their antioxidant capacity.1 Phenolic compounds, vitamin C, and carotenoids are the main bioactive phytochemicals present in fruits. They are responsible for the beneficial properties and reduce the risk of diseases associated with oxidative stress, such as cancer,2,3 cardiovascular diseases,4 Alzheimer’s,5 and diabetes.6
Kiwifruit (Actinidia deliciosa) is originally from Asia and has become popular around the world due to its sensorial and nutritional qualities. Different kiwi qualities are attributed to its fibers, minerals (P, K, Ca, Mg), and also to its bioactive compounds with good antioxidant properties, including ascorbic acid, carotenoids, and polyphenols.7,8 However, its shelf life decreases due to its softness and nutritional losses. Thus, the drying process can be used as a preservation method aiming to increase the fruit’s shelf life, expanding its availability in the market even beyond its seasonality.9,10 On the other hand, in addition to modifying taste, the drying process promotes a loss of volatile compounds and flavor, changes in color and texture, and can promote significant nutritional losses, especially with respect to vitamin C.9,11
An alternative method to minimize the undesirable effects of conventional drying processes is to apply edible coatings before the drying process. These coatings are efficient in preserving some volatile components and releasing the sugar content of dried fruits by controlling the gas exchange, the moisture transfer, and the oxidation processes. Moreover, the coatings have been shown to help to improve the sensorial quality and maintain the nutritional quality of fruit.11,12,13 Coatings enriched with antioxidants have demonstrated improvements in the quality and extension of the post-harvest shelf life of fresh and minimally processed fruits, as well as contributed to inhibit the darkening process, growth of microorganisms, and negative oxidative processes.14,15,16,17
In view of the above, this work aimed to evaluate the effect of a pectin coating enriched with isolated antioxidant compounds or fruit extracts on the quality of kiwifruit slices after drying and during storage.
Material and methods
Material
The fruits used in this experiment were purchased from the Supply Center (CEASA) in Campinas, SP-Brazil. Kiwis (A. deliciosa) which presented a firm texture, in a stage of physiological maturity, with no apparent injury, and uniform color and size were selected. Acerola berries (Malpighia punicifolia L.), cashew fruits (Anacardium occidentale L.), and strawberries (Fragaria vesca) were selected according to their ripeness degree for mature fruits. Table 1 (supplementary material) shows the centesimal composition for the kiwifruits used in this study.
Low-grade methoxylated pectin (GENU Pectin Type LM-102 AS-BNB), hydrated calcium chloride (USP pharmaceutical grade), and glycerol were used in the coatings.
Processing
Obtaining antioxidant extracts
The antioxidant extracts from acerola, cashew and strawberry were obtained according to the methodology described by Eça et al.18 The fruit was ground using a mixer at 20,000 rpm for 2 min, homogenized with 95% ethanol in a 1:2 (w/w) ratio, and then the mixture was filtered. The residue was reextracted in the same conditions, and the combined extracts were concentrated in a rotary evaporator (40°C and 700 mm Hg). The extraction was performed at ambient temperature and under reduced light. The concentrate was conditioned in amber glasses at − 18°C until use (Table 1).
Preparation of the coatings and formulations used
The methodology for obtaining the coatings was based on Rojas-Graü et al.17 A low methoxylated pectin was dispersed in distilled water (2 g of pectin/100 mL distilled water) and mixed using a mechanical two-helice propeller (Fitasom, Brazil) until homogeneous. The solution was heated to 70°C under constant stirring (500 rpm), and glycerol was subsequently added as plasticizer (1.5 g glycerol/g pectin), and the solution was stirred for 15 min. Finally, 1% calcium chloride solution (crosslinking agent) was added to the suspension drop by drop to complete the pectin gel formulation (0.005 g CaCl2/g pectin). After, the pectin coating was cooled to 40°C (to reduce the effects of temperature on the nutrient degradation in the extracts), and the antioxidant additives were incorporated to compose the seven proposed coating formulations (0.5 g of antioxidant extract/g pectin) (as shown in Table 2), and the mixture was stirred for 10 min to ensure complete homogenization. The component concentrations of the coatings were fixed, and the differences among the formulations were based on the type of antioxidant added (Table 2).
Preparation of kiwi fruits and coating application
The kiwi fruits were cleaned, sanitized (533 ppm of acetic acid/3 min), peeled, and sliced across the longitudinal axis with a pre-established thickness of 5.0 ± 0.5 mm. Next, the kiwi slices were immersed in the different coating formulations for 60 s, and then placed in plastic crates to drain off the excess coating solution. Then, the fruit slices were dipped into a calcium chloride solution (2%) for 30 s, and finally into distilled water for 10 s to remove any excess salt. The coated fruit slices were then dried in an incubator chamber (Model MA-415UR, Marconi) at 23°C and 55% RH. Fresh fruit slices without coating were used as a control.
Desorption isotherms
Desorption isotherms were determined by the static gravimetric method according to Jowitt et al.19 with seven supersaturated aqueous solutions of salt (KOH, LiCl, MgCl2, NaBr, KI, NaCl, and KCl) with relative humidity in the range of 5.6–84% (Table 3) at 30, 50, and 60°C. The experiments were conducted in triplicate. After initial weighing, the samples were stored in hermetic glass jars, previously filled with the saline solutions, and then placed in a lab stove with air circulation on tripods. The samples were weighed regularly until they reached equilibrium and then dried in a vacuum oven at 70°C to determine the equilibrium moisture for each system. Equilibrium moisture and water activity curves were obtained, and the experimental data were adjusted by the GAB model [equation (1)].
In which: CGAB and KGAB stand for desorption constants related to the energy interactions between the monolayer molecules and the subsequent interactions at a given desorption site; Xe stands for the equilibrium moisture expressed as dry basis (g water/g dry solids); Xm is the moisture content at the molecular monolayer (g water/g dry solids); and aw is the water activity. These parameters were determined by Statistica 8.0 software (Statsoft Inc. Tulsa, OK, USA), and R2 was used as the criterion for choosing the best settings.
Drying process
Kiwi slices were laid in trays parallel to the air flow, and then dried at temperatures of 50 and 60°C with an air velocity of 1.7 m/s for approximately 7 h until reaching equilibrium humidity. The position of the trays was changed at predetermined times in order to minimize any possible interference between the samples during the drying process. The kinetic curves were presented in the dimensionless form of moisture versus time, and the empirical model of Page20 was used to adjust the data [equation (2)]. The drying process was performed in duplicate, and the curves were created from the mean values.
In which: k stands for the drying constant (min−n), n is the model parameter (dimensionless), and Y is the dimensionless content of moisture ratio, defined according to equation (3):
In which: \(\overline{X}_{t}\) is the mean mass fraction of water in the slice on dry basis; Xeq is the equilibrium moisture content; and X0 is the mass fraction at the initial conditions.
The analytical solution proposed by the second Fick Law was used, considering an infinite plane to determine the effective diffusivity [equation (4)]. The thickness used for the calculations considered the increase of the coating on the slice. The analytical solution for infinite plates integrated along the thickness is defined by Crank21:
In which: D is the effective diffusivity (m2/s); t stands for the time (s); and l is the semi-thickness of the kiwi slices (m). For this, five terms of the infinite series were used.
The retention of phytochemical compounds and total antioxidant activity (TAA) were determined according to equation (5):
In which: Cf is the final concentration of the compounds, and Ci is the initial dry basis concentration.
Analytical procedures
Determination of total phenolic compounds (TPC): The TPC content of the different antioxidant coating formulations as well as of the coated and dehydrated kiwi fruit slices was determined by the Folin–Ciocalteu colorimetric method according to Waterhouse.22 A sample (40 μL) was mixed with 3.16 mL of distilled water and 200 μL of the Folin–Ciocalteau reagent. Next, 600 μL of sodium carbonate solution (20%) was added after 3 min. Then, the absorbance was measured at 765 nm in a spectrophotometer (model SQ-2800 UV/VIS, UNICO brand, United Products & Instruments Inc., New Jersey, USA) after 2 h of reaction in the environment temperature in the absence of light. The TPC content was obtained based on a standard curve of gallic acid (0.5–100 μg/mL), and the result were expressed in mg of gallic acid equivalents (GAE) of dry sample.
Vitamin C content determination: The vitamin C content was determined by a titration process using 2,6-dichlorophenol-indophenol according to the method of AOAC No. 985.33.23
Total antioxidant activity (TAA): TAA of the different antioxidant coating formulations as well as of the coated and dehydrated kiwi fruit slices was conducted using the DPPH method, as described by Brand-Williams et al.24 A sample aliquot was homogenized with 0.3 mL of DPPH solution (0.5 mM/L) and 2.4 mL of ethanol (99.5%). The absorbance was then measured using a spectrophotometer (Model SQ-2800 UV/VIS, UNICO, USA) at 517 nm after 60 min at room temperature and under light. The results were obtained from a standard Trolox curve (0–2000 μM), and the results were expressed in mM Trolox g−1 dry sample.
Color: Color analyses were assessed on colorimeter (Hunter Lab, Color Quest II®) using a CIELab scale (L*, a*, b*) at 25°C with the D-65 illuminant and an observation angle of 10°. The equipment was calibrated using a black and white standard light trap. The influence of ambient light was controlled following the procedures described in the manual.
Stability study
The kiwi slices obtained using the 7 formulations proposed were evaluated in terms of retention of phytochemical compounds [“Determination of total phenolic compounds (TPC)” and “Vitamin C content determination” sections] and TAA [“Total antioxidant activity (TAA)” section], and the maintenance of these properties throughout storage to evaluate the stability of the formulations. Furthermore, the products were evaluated in terms of surface color (“Color” section). All the products resulting from the drying process at 50°C were analyzed. The samples were stored under controlled temperature and humidity in an air circulation chamber (Model MA-415UR, Marconi, Brazil) without packing for 31 days at 40°C during the stability study in order to study the effect of the antioxidant coating on the fruits. The dehydrated slices of kiwi fruit were analyzed at 0 (drying day), 7, 14, and 31 days after the drying process.
Statistical analysis
All analyses were performed in triplicate. The results obtained in the experiments were statistically processed by variance analysis (ANOVA) using statistical software Minitab version 16. The Tukey test was used assuming a 95% confidence interval to verify the significant difference between the samples.
Results and discussion
Desorption isotherms curves
A rise of equilibrium moisture associated with its corresponding Aw was observed at constant temperature, while there were lower values for the constant relative moisture at 50 and 60°C.
A high temperature promotes an increase in the kinetic energy associated with the water molecules, resulting in a decrease of the attraction forces, consequently reducing the moisture of the product.25 Lahsasni et al.26 and Moreira et al.27 found similar results for prickly pear fruit and quince fruit, respectively. The variation of the composition between the formulations had little influence on the results obtained at 30°C. A greater differentiation in the curves was noticed for other temperatures, however, without establishing any tendency (Fig. 1).
The GAB model is a good fit for all the data obtained and satisfactorily predicted the equilibrium moisture behavior for the kiwifruit, which reinforces the fact that the GAB model is able to make good adjustments for foods with an aw of 0.9.28 The curves are sigmoidal, Type II, which have two inflection regions due to changes in the magnitude of the chemical and physical effects: the accumulation of water multilayers and filling of the small pores can be noticed when aw is between 0.2 and 0.4. In addition, the phenomena of dilation, dissolution of solutes, and filling of large pores occur when aw is between 0.6 and 0.7. This is a common behavior for food.29
The Xm obtained by the GAB model varied from 0.16 to 0.22 (microbiologically adequate values), which are close to those reported by Telis et al.30 and Sousa31 when they studied isotherms in persimmons and figs, respectively, with similar coating.
The reverse behavior is observed in Fig. 1 as aw is over 0.80, which usually happens in fruits when their sugars are dissolved. This was not observed in the desorption isotherms of kiwi (Type II isotherms), since it has a low sugar content (15%) (Fig. 1).
Drying process
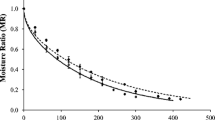
The drying process was divided into two periods. The first one was at a constant rate (the product surface behaves as if it is only composed of water, and the moisture content removed strictly depends on the nature of the product). The second period had a decreasing drying rate in which the migration rate of water decreases with the removal of surface water. This is dependent on the diffusion of the internal water from the product to the surface (Fig. 2). The duration of each drying period mainly depends on the initial moisture content of the food and the humidity required for storage. The drying periods for the 60°C processes were more evident because the transition between the periods is closely related to the process temperature.
The constant drying rate period for the formulations studied was significant at the beginning of the drying process, which were 30 and 15 min at 50 and 60°C, respectively. Even though the constant rate period for fruit drying is considered non-existent, this period may be significant in the first minutes of the process when the water content is very high. Afterward, the drying rate decreased more rapidly at the 60°C temperature (Fig. 2) at the beginning of the decreasing rate period, and remained until the end of the drying process, thus requiring a shorter time to obtain a microbiologically adequate equilibrium moisture.
The drying process of the kiwi was adjusted to the experimental data (R2 ≈ 1) for the Page in empirical model which is commonly used to describe kinetic curves of fruit drying.32,33,34 For parameter n, which is related to the process variables (temperature, velocity, and air humidity), there was no correlation with the process temperatures studied, with its average value being 1.18 ± 0.07. On the other hand, k was strongly influenced by the temperature for all conditions studied, and the order of magnitude of this equation parameter was 10−3 for all coated samples (Fig. 2).
In a similar study, Canizares and Mauro32 obtained lower values of k (of the order of 10−5) using pectin coating on papaya, which is justified by the influence of the geometry used and the fruit composition. There was a significant difference among the formulations between the k value obtained for the F1 formulation, and the other which may be associated with the initial moisture differences of the samples inherent to the coating application. Mean values of the effective diffusivity were 1.20 × 10−10 and 1.52 × 10−10 m2/s for the temperatures of 50 and 60°C, respectively (R2 ≥ 95%). This suggests that the temperature variable has a positive effect on the effective diffusivity value, so that an increase of 10°C resulted in higher values (Table 4).
Coating characterization
The F3 formulation (ascorbic acid + citric acid coating) showed the highest TAA among the tested coatings, possibly because the antioxidant compound that was added was of standard chemical quality with high purity (≥ 95%) (Table 2). The F6 formulation (acerola extract) was noted for its high TAA among the added fruit extract formulations (F4, F5, F6, and F7), significantly differing from the other formulations. This may be due to the high content of total phenolic compounds (p < 0.05), which were seven times higher than the values presented by the F4 and F5 formulations, and two times higher than the F7 formulation (mixture of extracts), which also presented a relevant TAA attributed to the TPC content (Table 5).
On the other hand, compounds with and without the antioxidant capacity for formulations containing fruit extracts form the additive content added to the coatings (1 g total solids in the pectin extract). Thus, TAA is lower than that obtained by the same 1 g of pure compounds (F3), even when considering the synergistic effect inherent in natural compounds.35
Evaluation of kiwi slices after the drying process
Color analysis
Figure 3 shows the changes in the surface color for kiwifruit samples before and after the drying processes at 50 and 60°C according to the L*, a*, and b* color parameters. After drying, the lightness (L*) of the kiwifruit samples increased in the F6 and F7 formulations for both temperatures. This may be associated with the presence of the acerola extract because its high acidity increases the gel forming capacity of the pectin used. A decrease in this parameter was observed in the drying process at 50°C for the uncoated slices, as expected.
The drying process also promoted a significant increase in the a* and b* parameter values for both analyzed temperatures (Fig. 3). This suggests that the colors of the slices are more distant from green and blue and approach red and yellow. This effect was more strongly noted for the a* parameter in the samples with red colored fruit extract coatings, which were more evident after drying due to the decrease in the water content of the product.
The changes for the F1, F2, F3, and F4 formulations were more discreet, with only kiwi characteristics being attributed either due to lack of coating (F1) or lack of color (F2, F3, and F4).
Effect of drying on the bioactive content and total antioxidant activity (TAA)
The drying process in kiwifruit promoted a significant reduction in the content of the evaluated bioactive compounds, vitamin C and total phenolic compounds (TPC), consequently leading to a decrease in total antioxidant activity (Fig. 4). However, it was observed that the application of the F3, F6, and F7 coating formulations in drying performed at 50°C contributed to maintaining the bioactive contents in kiwis, although this effect was observed only for the F3 and F6 formulations when they were dried at 60°C. This effect could be associated with the ascorbic acid antioxidant capacity since both formulations have a high concentration of this compound. Sakooei-Vayghan et al.36 used pectin coating incorporated with ascorbic acid and observed an increase in TPC, vitamin C retention, and total antioxidant activity of dried apricot cubes compared to pectin or pectin + citric acid coatings (Table 5).
Figure 4 shows that the retention percentages of the bioactive compounds (vitamin C and TPC) for kiwifruit enriched with fruit extracts were higher than the ones observed in the fruits with F1 and F2 formulations. This implies that the added coating of compounds with antioxidant properties promoted improved kiwifruit functional quality associated with vitamin C and TPC content, and consequently of its antioxidant activity.
The effect of F3, F6, and F7 coatings on kiwifruit slices during the drying processes is attributed to the high vitamin C and TPC contents, which are related to the antioxidant compounds added (Table 3) and associated with a high antioxidant protection against the degradation effects of oxidation, even after prolonged exposure to convective flows of heated air.
A similar behavior was observed for the TAA (Fig. 4), reinforcing the idea of the correlation between the bioactive compounds and their antioxidant capacity, which was also approached by Bennett et al.33 in a study with different dried fruits. On the other hand, the F4 and F5 formulations were able to preserve the phytochemical compound and also the fruit antioxidant capacity (retention of 50% and 60%, respectively), in addition to being the coating with the lower antioxidant capacity.
Stability study of coated kiwis throughout storage
The added coatings of antioxidant compounds contributed to reduce the degradation of the bioactive content (vitamin C and TPC) in kiwifruit throughout the storage period. This behavior is more evident in the fruits with the F6 (acerola extract) coating, which consequently influenced its TAA (Fig. 5). Although F6 fruits initially showed lower vitamin C content than F3 fruits, the content of this vitamin was similar for both formulations at the end of storage, as well as for TPC content, suggesting that the synergism of antioxidants in the extract may act more effectively against oxidative processes. The nutrient losses in fresh fruits can occur during processing,37 as in the case of drying in this study, or during storage due to prolonged exposure to adverse conditions.38
A decrease in the TPC content up to the 14th day of storage showed a stable and statistically similar behavior in the F5, F6, and F7 formulations. This suggests that the added antioxidant in the coating is no longer the dominant factor to prevent oxidation of the fruit, but the physical covering itself has become the dominant factor. It is possible that the effect of pore decrease promoted by the coating reduced the compound migration (oxygen and volatile), maintaining these phytochemical compounds. The pore reduction effect was observed in the dried banana cross-sectional scanning electron microscopy images which was pretreated by the use of carboxymethyl cellulose (CMC) coating.39
In comparing the F1 and F2 formulations, the protective effect of the pectin coating during the drying process was seen to be much more representative than during the storage.
Although the formulations had similar antioxidant capacities, the retention percentages also followed the trend presented for most of the samples corresponding to the measurements of phenolic compounds and vitamin C (Fig. 4).
The superficial color change is a determining factor for evaluating the dried product quality, since they are the results of oxidative processes. The changes in the a* parameter were intense, presenting a linear growth along the storage, unlike the behavior for the b* parameter, whose effects were more evident for the fruits with the F1 formulation (Fig. 6).
The F2 formulation presented the lowest changes in the color parameters at the end of the study considering the coated slices, as in addition to protecting the fruit, it did not interfere in the color of the slices. The F6 formulation presented the highest parameter a* values at the end of storage, which can be attributed to the characteristic red color of the acerola used to make the extract. In contrast, the F2 formulation presented the lowest value, suggesting that the pectin coating prevented the darkening processes, in addition to not significantly altering the fruit color. Moreover, there were small changes for the coated samples during storage regarding the L* parameter. There was also a significant difference on the 7th day for F1 compared to the measurement after drying, a fact which was not observed for F2 and which also highlights the effect of the coating on the lightness parameter.
Conclusions
Application of antioxidant compounds (fruit extracts and synthetic antioxidants) significantly contributed to increase the antioxidant capacity of the pectin coating with the drying temperature promoting a positive effect on diffusivity of the antioxidant compounds. The antioxidant pectin coating reduced undesirable effects of nutrient oxidation, and it maintained the quality characteristics of the kiwifruit throughout the storage for 31 days at 40°C. The F3 (pure compound coating) and F6 (covering with acerola extract) obtained the best results among the coating formulations studied in terms of maintaining the nutritional quality of the fruit, which was probably due to their higher antioxidant capacity.
References
Presti, G, Guarrasi, V, Gulotta, E, Provenzano, F, Provenzano, A, Giuliano, S, Monfreda, M, Mangione, MR, Passantino, R, Biagio, PLS, Costa, MA, Giacomazza, D, “Bioactive Compounds from Extra Virgin Olive Oils: Correlation Between Phenolic Content and Oxidative Stress Cell Protection.” Biophys. Chem., 230 109–116 (2017)
Gonzáles-Vallinas, M, González-Castejón, M, Rodríguez-Casado, A, De Molina, AR, “Dietary Phytochemicals in Cancer Prevention and Therapy: A Complementary Approach with Promising Perspectives.” Nutr. Rev., 71 (9) 585–599 (2013)
Saenglee, S, Jogloy, S, Patanothai, A, Leid, M, Senawong, T, “Cytotoxic Effects of Peanut Phenolics Possessing Histone Deacetylase Inhibitory Activity in Breast and Cervical Cancer Cell Lines.” Pharmacol. Rep., 68 1102–1110 (2016)
Du, H, Li, L, Bennett, D, Guo, Y, Key, TJ, Bian, Z, Sherliker, P, Gao, H, Yiping, C, Yang, L, Chen, J, Wang, S, Du, R, Su, H, Collins, R, Peto, R, Chen, Z, “Fresh Fruit Consumption and Major Cardiovascular Disease in China.” N. Engl. J. Med., 374 (14) 1332–1343 (2016)
Kim, J, Lee, HJ, Lee, KJ, “Naturally Occurring Phytochemical for the Prevention of Alzheimer’s Disease.” J. Neurochem., 112 1415–1430 (2010)
Tresserra-Rimbau, A, Guasch-Ferré, M, Salas-Salvadó, J, Toledo, E, Corella, D, Castaner, O, Guo, X, Gómez-Gracia, E, Lapetra, J, Arós, F, Fiol, M, Ros, E, Serra-Majem, L, Pintó, X, Fitó, M, Babio, N, Martínez-González, MA, Sorli, JV, López-Sabater, MC, Estruch, R, Lamuela-Raventós, RM, “Intake of Total Polyphenols and Some Classes of Polyphenols is Inversely Associated with Diabetes in Elderly People at High Cardiovascular Disease Risk.” J. Nutr., 146 767–777 (2016)
Soquetta, MB, Stefanello, FS, Da Mota Huerta, K, Monteiro, SS, Da Rosa, CS, Terra, NN, “Characterization of Physiochemical and Microbiological Properties, and Bioactive Compounds, of Flour Made from the Skin and Bagasse of Kiwi Fruit (Actinidia deliciosa).” Food Chem., 199 471–478 (2016)
Park, YS, Im, MH, Choi, JH, Yim, SH, Leontowicz, H, Leontowicz, M, Suhaj, M, Gorinstein, S, “The Effects of Ethylene Treatment on the Bioactivity of Conventional and Organic Growing ‘Hayward’ Kiwi Fruit.” Sci. Hortic., 164 589–595 (2013)
Kaya, A, Aydın, O, Dincer, I, “Experimental and Numerical Investigation of Heat and Mass Transfer During Drying of Hayward Kiwi Fruits (Actinidia deliciosa Planch).” J. Food Eng., 88 323–330 (2008)
Darvishi, H, Zarein, M, Farhudi, Z, “Energetic and Exergetic Performance Analysis and Modeling of Drying Kinetics of Kiwi Slices.” J. Food Sci. Technol., 53 2317–2333 (2016)
Laurienzo, P, Stasio, M, Di Malinconico, M, Volpe, MG, “De-Hydration of Apples by Innovative Bio-Films Drying.” J. Food Eng., 97 491–496 (2010)
Lago-Vanzela, ES, Do Nascimento, P, Fontes, EAF, Mauro, MA, Kimura, M, “Edible Coatings from Native and Modified Starches Retain Carotenoids in Pumpkin During Drying.” LWT Food Sci. Technol., 50 420–425 (2013)
Santagata, G, Mallardo, S, Fasulo, G, Lavermicocca, P, Valerio, F, Biase, MD, Stasio, MD, Malinconico, M, Volpe, MG, “Pectin-Honey Coating as Novel Dehydrating Bioactive Agent for Cut Fruit: Enhancement of the Functional Properties of Coated Dried Fruits.” Food Chem., 258 104–110 (2018)
Tesfay, SZ, Magwaza, LS, “Evaluating the Efficacy of Moringa Leaf Extract, Chitosan and Carboxymethyl Cellulose as Edible Coatings for Enhancing Quality and Extending Postharvest Life of Avocado (Persea americana Mill.) Fruit.” Food Packag. Shelf Life, 11 40–48 (2017)
Tesfay, SZ, Magwaza, LS, Mbili, N, Mditshwa, A, “Carboxyl Methylcellulose (CMC) Containing Moringa Plant Extracts as New Postharvest Organic Edible Coating for Avocado (Persea americana Mill.) Fruit.” Sci. Hortic., 226 201–207 (2017)
Saba, MK, Sogvar, OB, “Combination of Carboxymethyl Cellulose-Based Coatings with Calcium and Ascorbic Acid Impacts in Browning and Quality of Fresh-Cut Apples.” LWT Food Sci. Technol., 66 165–171 (2016)
Rojas-Graü, MA, Tapia, MS, Rodríguez, FJ, Carmona, AJ, Martin-Belloso, O, “Alginate and Gellan-Based Edible Coatings as Carriers of Antibrowning Agents Applied on Fresh-Cut Fuji Apples.” Food Hydrocoll., 21 118–127 (2007)
Eça, KS, Machado, MT, Hubinger, MD, Menegalli, FC, “Development of Active Films from Pectin and Fruit Extracts: Light Protection, Antioxidant Capacity, and Compounds Stability.” J. Food Sci., 80 (11) 2389–2896 (2015)
Jowitt, R, Escher, F, Kent, M, McKenna, B, Roques, M, Physical Properties Foods II. Elsevier Applied Science Publishers, London, 1987
Page, C, Factors Influencing the Maximum Rate of Drying Shelled Corn in Layers. M.Sc. Dissertation. Purdue University, Indiana, USA, 1949
Crank, J, The Mathematics of Diffusion. Oxford University Press, Oxford, 1975
Waterhouse, AL, “Determination of Total Phenolics.” In: Current Protocols in Food Analytical Chemistry, vol. 6, chap. 1, pp. I1.1.1–I1.1.8. Wiley (2002)
AOAC, Official Methods of Analysis of A.O.A.C International. William Horwitz, Arlington, 2006
Brand-Williams, W, Cuvelier, ME, Berset, C, “Use of a Free Radical Method to Evaluate Antioxidant Activity.” LWT Food Sci. Technol., 28 25–30 (1995)
Shivhare, US, Arora, S, Ahmed, J, Raghavan, GSV, “Moisture Adsorption Isotherms for Mushroom.” LWT Food Sci. Technol., 37 133–137 (2004)
Lahsasni, S, Kouhila, M, Mahrouz, M, “Adsorption–Desorption Isotherms and Heat of Sorption of Prickly Pear Fruit (Opuntia ficus indica).” Energy Convers. Manag., 45 249–261 (2004)
Moreira, R, Chenlo, F, Torres, MD, Vallejo, N, “Thermodynamic Analysis of Experimental Sorption Isotherms of Loquat and Quince Fruits.” J. Food Eng., 88 514–521 (2008)
Van den Berg, C, “Description of Water Activity of Foods for Engineering Purposes by Means of the GAB Model of Sorption.” In: McKenna, BM (ed.) Engineering and Foods, vol. 1, pp. 311–321. Elsevier Applied Science Publishers, New York, US (1984)
Lewicki, PP, “The Applicability of the GAB Model to Food Water Sorption Isotherms.” Int. J. Food Sci. Technol., 32 533–557 (1997)
Telis, VRN, Gabas, AL, Menegalli, FC, Telis-Romero, J, “Water Sorption Thermodynamic Properties Applied to Persimmon Skin and Pulp.” Thermochim. Acta, 343 49–56 (2000)
Sousa, SD, Obtenção de figos secos por desidratação osmótica e secagem convectiva. University of Campinas, Campinas, BR, 2008
Canizares, D, Mauro, MA, “Enhancement of Quality and Stability of Dried Papaya by Pectin-Based Coatings as Air-Drying Pretreatment.” Food Bioprocess. Technol., 8 1187–1197 (2015)
Bennett, LE, Jegasothy, H, Konczak, I, Frank, D, Sudharmarajan, S, Clingeleffer, PR, “Total Polyphenolics and Antioxidant Properties of Selected Dried Fruits and Relationships to Drying Conditions.” J. Funct. Foods, 3 115–124 (2011)
Maskan, M, “Kinetics of Colour Change of Kiwifruits During Hot Air and Microwave Drying.” J. Food Eng., 48 169–175 (2001)
Meletis, CD, Barker, JE, “Synergy in Nutrients.” J. Altern. Complement. Med., 10 326–329 (2004)
Sakooei-Vayghan, R, Peighambardoust, SH, Hesari, J, Peressini, D, “Effects of Osmotic Dehydration (With and Without Sonication) and Pectin-Based Coating Pretreatments on Functional Properties and Color of Hot-Air Dried Apricot Cubes.” Food Chem., 311 125978 (2020)
Zhao, YP, Chang, KC, “Sulfite and Starch Affect Color and Carotenoids of Dehydrated Carrots (Daucus carota) During Storage.” J. Food Sci., 60 324–326 (1995)
Lin, D, Zhao, Y, “Innovations in the Development and Application of Edible Coatings for Fresh and Minimally Processed Fruits and Vegetables.” Compr. Rev. Food Sci. Food Saf., 6 60–75 (2007)
Dehsheikh, FN, Dinani, ST, “Coating Pretreatment of Banana Slices Using Carboxymethyl Cellulose in an Ultrasonic System Before Convective Drying.” Ultrason. Sonochem., 52 401–413 (2019)
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by KSE. The first draft of the manuscript was written by ECMP and LdSO, and all authors commented on previous versions of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Sitônio Eça, K., Maciel Porto, E.C., de Siqueira Oliveira, L. et al. Applying pectin coatings enriched with antioxidant compounds as pre-treatment for kiwi (Actinidia deliciosa) drying process. J Coat Technol Res 18, 1131–1142 (2021). https://doi.org/10.1007/s11998-021-00469-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-021-00469-x