Abstract
An ionic/nonionic polyurethane dispersion with high solid content and low viscosity with a complex hydrophilic chain-extending agent was prepared using isophorone diisocyanate as a hard segment and propylene oxide glycol as a soft segment. The complex hydrophilic chain-extending agent consisted of DPSA and BDSA. The effects of the molar ratio of DPSA/BDSA on the properties of the resultant polyurethane dispersions were studied. The morphologies and properties of the ionic/nonionic PU dispersions were examined using particle-size, TEM, and viscosity analyses. It was found that the ionic/nonionic dispersions possessed wide particle-size distributions due to the addition of the complex hydrophilic chain-extending agent. The ionic/nonionic PU dispersions possessed higher solid content than conventional WPU dispersions because the number and volume percentage of the large particles and small particles of the ionic/nonionic dispersions met the requirements for high solid content. It was observed that the solid content of the ionic/nonionic dispersion increased and then decreased with an increasing molar ratio of DPSA/BDSA. When the ratio ranged from 4:10 to 5:10, the solid content of the ionic/nonionic PU dispersion reached up to 55%. It was also noticed that the apparent viscosity of the ionic/nonionic polyurethane dispersion decreased with an increasing molar ratio of DPSA/BDSA. The complex hydrophilic chain-extending agent consisting of DPSA and BDSA enhanced the solid content and decreased the viscosity of the ionic/nonionic dispersions, which are very important for improving the properties and expanding the applications of PU dispersions. In addition, the ionic/nonionic polyurethane dispersion had good electrolyte-resistance properties, stability at both high and low temperatures, and storage stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyurethane (PU) materials have been generally used in the automobile, paint, furniture, and textile industries and in biomass foam incorporated into renewable raw materials.1,2,3,4 Waterborne polyurethane (WPU), as a nontoxic and nonflammable material, has been abundantly used as environmentally friendly coatings and adhesives that show an excellent range of properties.5,6,7,8,9,10 In the past two decades, extensive investigations have been carried out in this field of study, with numerous papers, patents, and books on syntheses, manufacturing, characterization, and applications of polyurethanes. The wide application of polyurethanes in different fields has caused more demanding requirements of their properties. In particular, WPU is now of great interest due to environmental considerations, especially replacing solvent-based polyurethanes. Developing high-performance WPU, therefore, is of great importance.
However, under unchanged solid content, the drying rate of waterborne polyurethanes (WPU) is slower than that of solvent-based polyurethanes because the latent heat of evaporation of water is higher than that of organic solvents, and therefore, this technology cannot meet fast-paced modern production line requirements. To solve this problem, the focus of international research has mainly been on enhancing the solid content of WPU to reduce the evaporation load of water and to shorten drying time. This is the only way possible to promote environmentally friendly water-based coatings and adhesives to replace the corresponding organic-solvent-based products.11 Moreover, WPU with high solid content has the following advantages relative to WPU with low solid content12: quick drying time, better mechanical properties, higher-capacity utilization, lower transportation costs, and lower energy consumption per unit of product. Therefore, production of high-solid-content products (solid mass fraction of 50% or more) is an important area of development for WPU.
In recent years, early stages of research on WPU with high solid content have been reported. Some of the work has been done on WPU with high solid content of less than 50%, but few studies on WPU with solid content of more than 55% are found in the literature. Methods have been employed to enhance the solid content of WPU, such as mixing polyether diols with sulfonic acid-based and polyester diols as soft segments13 and mixing two kinds of isocyanates as hard segments for blending hybrid-olefin polymers.14
Our group has reported the preparation of a series of novel stable waterborne Si/PU dispersions with high solid content reaching more than 55% and low viscosity less than 300 Pa.s−1, which were successfully prepared through the use of a polyol PES consisting of silicone chains and epoxy acrylate structures and propylene oxide glycol (PPG) as a soft mixing segment.15 However, it was found that the Si/PU had poor electrolyte and storage-stability resistance. Therefore, it is necessary to improve the performance of Si/PU-WPU.
The objective of this study was to design a novel ionic/nonionic waterborne polyurethane with a complexing hydrophilic chain-extending agent consisting of low-molecular-weight polyether diols (DPSA), which is an ionic/nonionic sulfonate salt, and ionic sulfonate salt diols (BDSA). The influence of the molar ratio of DPSA and BDSA on the properties of the polyurethane (PU) dispersions was considered. The morphology and properties of the PU dispersions with high solid content were examined using particle-size, TEM, and viscosity measurements. This work will provide a new method of preparing high-solid-content WPU, which will expand the application of WPU.
Experimental
Materials
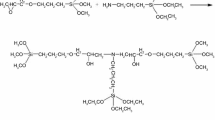
The diisocyanate used was isophorone diisocyanate (IPDI, 98% purity, supplied by Shanghai Chemical Co., China). The propylene oxide glycol (PPG; MW = 1200, supplied by Jilin Chemical Co., China) was used as the soft segment, which was vacuum-dried at 110°C for 2 h. The complex hydrophilic chain-extending agent consisted of DPSA (M = 1100, prepared in the lab) and BDSA (98% purity, prepared in the lab). N-Methyl-2-pyrrolidone (NMP, analytical reaction purity, Shanghai Guanghua Technology Co., China) was used as the solvent, and dibutyltin dilaurate (DBTD; Sn content = 19%, supplied by Beijing Chemical Co., China) was used as the catalyst. The chemical structures of DPSA and BDSA are shown in Fig. 1.
Synthesis of ionic/nonionic polyurethane dispersions
The ionic/nonionic polyurethane dispersions were prepared by the material formulations shown in Table 1. PPG, according to its calculated weight ratio, was placed in a flask, equipped with a thermometer, reflux condenser tube and stirrer, and degassed in vacuum at 110°C for 1 h. IPDI was added to the flask, and the mixture was allowed to cool to 30°C under moderate stirring (175–200 rpm). One drop of DBTDL was added at this temperature. Then, DPSA/BDSA/NMP, according to its calculated molar ratio, was added to the flask. The reaction mixture was heated to 75°C under moderate stirring (175–200 rpm) and was allowed to react at 75°C until the theoretical NCO content was reached. Then, the mixture was allowed to cool to 45°C, and distilled water was added to the reaction mixture with vigorous stirring (1200–1500 rpm). Finally, ionic/nonionic polyurethane dispersions with high solid content were obtained. The compositions of the ionic/nonionic polyurethane prepolymer are listed in Table 1.
Characterization
The mean particle size and particle-size distribution of the ionic–nonionic polyurethane dispersions were measured with a zeta size analyzer. A small amount of aqueous dispersion was added into a deionized water tank, and the pinhole was set at 200 μm. The average particle diameter was measured at 25°C. The polydispersity index (PDI) was calculated in accordance with the following formula.15
where μ − 2 is the second central moment of the distribution, Γ is the line width. Γ is a function of the concentration of the measured system C and scanning angle θ. PDI measures the distribution width of the diffusion coefficient, and PDI is a dimensionless quantity. The PDI value of a monodisperse system is approximately 0.002. With an increase in the PDI value, the distribution becomes wider. A PDI value in a range between 0.2 and 0.25 indicates that the polydispersity of the sample is quite large.
Particle morphology was measured by transmission electron microscopy (TEM), and the dispersion solid content was determined by TGA. The apparent viscosity of the dispersion was measured using an NDJ-8S digital viscometer.
The storage stability of the dispersion was determined by observing the appearance of the dispersion after being placed at room temperature for 1 month. The freeze–thaw stability of the dispersions was determined as follows. A certain amount of dispersion was placed in a −20°C refrigerator to freeze for 18 h and then thawed at room temperature for 6 h. Such operations were repeated approximately 5 times or more. Finally, the number of observed dispersion gels was recorded. The high-temperature stability of the dispersion was determined as follows. The dispersion samples were placed in sealed glass bottles at 80°C under a constant temperature, and then, the appearance of sedimentation or flocculation was viewed and recorded.
Results and discussion
Average particle diameter and distribution of the dispersions
Figure 2 shows the particle-size distributions of the ionic–nonionic PU dispersions with different weight ratios of DPSA and BDSA. It is noticed that the ionic–nonionic PU dispersions possess multivariate particle-size distributions. According to the literature,16 multivariate particle-size distributions have important contributions of high solid content and low viscosity. For polymer dispersions, if the large gap among large particles can be filled with the appropriate number of small particles and the shape of the particles is deformed, the volume fraction of the polymer in the dispersions will be further enhanced; i.e., the solid content of dispersions will be further enhanced. For the ionic–nonionic PU dispersion prepared using the mixed hydrophilic chain extender composed of DPSA and BDSA, there are two kinds of hydrophilic groups that are nonionic in the structure of DPSA: the (–CH2–CH2–O–)n unit and –SO3Na hydrophilic groups. We also know that the hydrophilic properties of the nonionic (–CH2–CH2–O–)n unit are much weaker than those of the –SO3Na hydrophilic groups, and its volume is greater than that of the –SO3Na group. In the emulsification process, part of the –SO3Na groups wrapped inside the segment by the nonionic (–CH2–CH2–O–)n unit cannot contact water molecules due to the steric effects of the macromolecular chains of (–CH2–CH2–O–)n on –SO3Na. The (–CH2–CH2–O–)n unit with weak hydrophilicity plays a major role in the emulsion; thus, making the emulsified aqueous polyurethane dispersion particle size become larger. On the other hand, 1-4-butenediol-2-sodium (BDSA) not only has a small-molecule chemical structure and strong hydrophilic properties, but there are also two symmetrical primary –OH groups on the molecular ends of BDSA. The two primary –OH groups have the same reactivity, which can quickly react with –NCO on the end of the linear PU prepolymer, thus making the emulsified aqueous polyurethane dispersion particle size smaller. To a certain extent, the mixed hydrophilic chain extender composed of DPSA and BDSA broadens the particle-size distribution and changes the particle accumulation mode. As long as the molar ratio of DPSA and BDSA is controlled in a certain range, the requirements of low viscosity and high solid content can be met. It is also found in Fig. 2 that, with an increase in the weight ratio of the DPSA and BDSA, the volume fraction of the large particles increased gradually and the volume fraction of small particles decreased. When the ratio was controlled in a certain range, the gap between the large particles could be filled with small particles, resulting in an increase in the solid content of the system. If the weight ratio is too large or too small, the number and the volume fraction of particles will change, resulting in not enough small particles filling the gap between large particles and resulting in a decrease in the solid content. Therefore, as long as an adjustment of the weight ratio of DPSA and BDSA can be made, a high solid content for the ionic–nonionic PU dispersion can be obtained. As shown in Fig. 3, when the ratio of DPSA and BDSA ranges from 4:10 to 5:10, the solid content of the dispersion reaches 55%.
Figure 4 shows the average particle diameters of the dispersions with different molar ratios of DPSA and BDSA. With all other experimental conditions unchanged, the ionic–nonionic PU dispersions with higher DPSA content possess higher average particle diameters, as shown in Fig. 5. The reason for this is analyzed as follows. According to the laws of thermodynamics,17 for polymers dispersed in water at a constant temperature and pressure, the process of free enthalpy is expressed as ΔG = σΔA. When ΔG is negative, the process is spontaneous. Here, σ is the interface between the polymer and water, and ΔA is the change in the value of the two-phase interfacial area after the polymer is dispersed into water. If the dispersion process is achieved, in this case, ΔA > 0, and σ must be positive, which leads to ΔG > 0. However, ΔG > 0 shows that the dispersing process is nonspontaneous; that is, only by means of external forces can the dispersion process occur. When the applied force is constant (stirring speed, r/min), to make ΔA increase and the particle radius decrease, the interfacial tension must be low. For polymers with good hydrophilicity, such as pure water-based polyurethane, σ is smaller and ΔA can be larger, and accordingly, the particle size is small. In contrast, along with an increase in the molar ratio of DPSA and BDSA, the relative content of the hydrophobic component in the PU chains is enhanced. Therefore, the prepolymer containing higher DPSA content is more difficult to be dispersed in water during the dispersion step and subsequently results in the dispersion with a larger particle size.
Figure 5 shows the particle polydispersity index of the ionic–nonionic PU dispersions with different weight ratios of DPSA and BDSA. As shown, the ionic–nonionic PU dispersions with higher DPSA content possess higher PDI and wider particle-size distributions. However, the PDI values remain between 0.1 and 0.3, which further illustrates that ionic–nonionic PU dispersions with wide particle-size distributions were obtained.
Particle morphology of the dispersions
Figure 6 shows TEM photographs of dispersion particles with different weight ratios of DPSA and BDSA. From the three photographs, it is shown that the ionic–nonionic PU dispersions are generally spherical. In image (a), the particle diameter of the dispersion is larger and uneven, the gap between the large particles is very large, and the number of small particles is low and not high enough to fill the large gap between the particles. Therefore, the solid content is not high. In images (b) and (c), the particle sizes are significantly reduced, the densities of the particles become more intensive, and the numbers of particles per unit volume are significantly increased. Therefore, the solid content of the emulsions is increased, which further validates the previous conclusions. In addition, in image (c), the small particles stick to each other. The density of particles is too large, resulting in difficulty achieving active radical diffusion.
Rheology of the dispersions
A water-based polyurethane can be thought of as a PU ionomer and water binary dispersion, and the apparent viscosity of this dispersion shows the characteristic of shear thinning. In general, the power-law model can reflect the rheological properties. According to the Ostwald–dewael power-law equation, the rheological behavior is described by the following equation.18
where σ is the shear stress, \(\mathop \gamma \limits^{ \bullet }\) is the shear rate, n is the flow index (n = 1 indicates a Newtonian fluid; n < 1 indicates a non-Newtonian fluid), k is the consistency coefficient (larger k values indicate a greater apparent viscosity; k values can reflect the impact of structure on the viscosity), and η a is the apparent viscosity. The values of n and k can be obtained using linear regression. Experimental data are shown in Table 2.
Table 2 shows that with an increase in the molar ratio of DPSA and BDSA the n value increases and approaches 1. This result shows that the ionic–nonionic PU dispersions change from a non-Newtonian fluid to a Newtonian fluid, but in fact, they are still non-Newtonian fluids. With an increase in the molar ratio of DPSA and BDSA, the consistency coefficient k decreases from 0.892 to 0.633. This shows that the dispersion gains better mobility. Figure 7 shows the viscosity and rheological curves of the ionic–nonionic PU dispersions. Figure 7 shows that, with an increase in the shear rate, the apparent viscosity of the dispersion decreases, and when the shear rate is not too large (less than 100 s−1), the apparent viscosity decreases obviously. Upon further increase in the shear rate, the apparent viscosity decreases gradually. Overall, in the entire range of the shear rate, the apparent viscosity of the dispersion is not more than 420 mPa.s. This shows that the dispersions with high solid content (greater than 45%) also have good mobility.
In addition, it can be seen from Fig. 7 that, with a reduction of the molar ratio of DPSA and BDSA, the apparent viscosity of the PU dispersions increases significantly. This is because, with a decrease in the molar ratio of DPSA and BDSA, the content of strongly hydrophilic –SO3Na groups increases and the content of weakly hydrophilic –CH2–CH2–O– groups decreases in the PU molecular chain segment. At a fixed total content of hydrophilic groups, –SO3Na plays a leading role in the emulsification process. Therefore, when there are more –SO3Na groups in the complex of the hydrophilic chain extender, the hydrophilic properties of PU segments will be strengthened, resulting in a smaller size of the PU particles and a greater number of PU particles. Then, this will further lead to particles with larger surface areas, a smaller average distance between particles, and stronger interactions between particles. Therefore, the displacement of particles will be difficult, resulting in an increase in viscosity.
The stability of the dispersions
In the experiment, the dispersions were diluted to a solid content of 5 wt.%. Under electromagnetic stirring, solutions of 10 wt.% NaCl, CaCl2, and AlCl3 were added to the PU dispersions and diluted until flocculation or demulsification occurred. The volume of the three solutions was recorded when flocculation or demulsification occurred. If the volume of solution recorded is lower, then the electrolyte-resistance stability of the system is worse, and vice versa. The results are shown in Table 3.
The data in Table 3 show that adding a small amount of NaCl, CaCl2, and AlCl3 electrolyte solution to the WPU dispersion will not cause precipitation to occur. This shows that the dispersion systems have a certain electrolyte resistance. However, when the volume of the added strong electrolyte exceeds a certain value, the dispersions will appear flocculated.
This is because counterions (Na+, Ca2+ and Al3+) in the electrolyte will spread to the external and internal PU polymer chains when the electrolyte solution is added constantly. Part of the counterions will penetrate into the ionic group on the PU chain, thereby shielding the effective charge and making this part of the ion electrostatic field balanced, which causes the repulsion between the PU ionic segments to become weakened, the molecular chains to become curly, and the particles to agglomerate. As seen in Table 3, with the same content of hydrophilic groups and an increase in the DPSA/BDSA molar ratio, an increase in the amount of electrolyte solution dropped into the system results in significant flocculation. This indicates that the molar ratio of DPSA/BDSA has a great effect on the electrolyte-resistance stability of PU dispersions. In addition, with an increasing ratio, the electrolyte-resistance stability of the emulsion is enhanced. This is because, with an increase in the molar ratio of DPSA/BDSA, the proportion of the nonionic segment in the PU dispersion, and the stability of the dispersion, sterics play a dominant role, and therefore, the electrolyte-resistance properties of the PU dispersions are enhanced.
Table 4 presents the stability data of the ionic–nonionic PU dispersions at low temperature, high temperature, and room temperature. The table shows that, with an increase in the weight ratio of DPSA/BDSA, the stability of dispersion decreases. There are two main reasons for this. When the molar ratio of DPSA/BDSA is greater than 6/10, after four freeze–thaw cycles, the emulsion turns into a gel, and when the molar ratio of DPSA/BDSA is less than 6/10, after five freeze–thaw cycles, the emulsion turns into a gel. This indicates that the frost resistance of the ionic/nonionic PU dispersion is better than a simple ionic dispersion. The reason for this is that the stability of the ionic–nonionic dispersion is not only due to the electric-double-layer structure of the latex particles but also attributed to the “hair-like” structure on the surface of the particles, which results in enhanced frost resistance. Furthermore, data on the temperature and storage performance of the dispersions show that the ionic–nonionic PU dispersions have good high-temperature and storage stability.
Conclusion
A series of the ionic/nonionic PU dispersions with high solid content were prepared by self-emulsification, which involved modification of a homemade complex hydrophilic chain-extending agent of DPSA and BDSA, isophorone diisocyanate (IPDI) as a hard segment, and propylene oxide glycol (PPG) as a soft segment. The effects of the weight ratio of DPSA/BDSA on the properties of the resultant polyurethane dispersions were studied. The morphologies and properties of the ionic/nonionic PU dispersions were examined by particle-size, TEM, and viscosity analyses. The study of particle morphology and solid content showed that the ionic/nonionic PU dispersions possessed wide particle-size distributions. The ionic/nonionic PU dispersions possessed higher solid content than conventional WPU because the number and volume percentage of larger particles and smaller particles in the ionic/nonionic PU dispersions met the requirements for high solid content. It was observed that the solid content of the ionic/nonionic PU dispersion increased and then decreased with an increasing weight ratio of DPSA/BDSA. When the ratio ranged from 4:10 to 5:10, the solid content of the ionic/nonionic PU dispersions reached up to 55%. The study of the rheology showed that the apparent viscosity of the ionic/nonionic PU dispersions decreased with an increasing weight ratio of DPSA/BDSA, and the dispersions changed from the non-Newtonian fluids to Newtonian fluids. The complex hydrophilic chain-extending agent enhanced the solid content and decreased the viscosity of the ionic/nonionic PU dispersions, which are very important to improve the properties and expand the applications of PU dispersions. In addition, the ionic/nonionic PU dispersions had better electrolyte-resistance properties than conventional WPU as well as storage stability at low temperature, high temperature, and room temperature.
References
Guyot, A, Chu, F, Schneider, M, et al., “High Solid Content Latexes.” J. Appl. Polym. Sci., 10 1573–1615 (2002)
Li, Q, Sun, D, Wang, Y, “Synthesis of HDI–IPDI Based High Solid Content Aqueous Polyurethane Dispersion and Study on the Micromorphology of the Dispersion.” Polym. Mater. Sci. Eng., 23 59–62 (2007)
Liu, JP, Wang H, Shen LI, Wang XP, “Advances in Research on High-Solid Content Polymer Emulsion.” China Adhes., 27 32–35 (2006)
Mequanint, K, Sanderson, R, “Nano-Structure Phosphorus-Containing Polyurethane Dispersions: Synthesis and Crosslinking with Melamine Formaldehyde Resin.” Orig. Polym., 44 2631–2639 (2003)
Cakic, SM, Stamenkovic, JV, Djordjevic, DM, et al., “Synthesis and Degradation Profile of Cast Films of PPG–DMPA–IPDI Aqueous Polyurethane Dispersions Based on Selective Catalysts.” Polym. Degrad. Stab., 94 2015–2022 (2009)
Sun, DC, Huang, ZY, “Synthesis and Characterization of Anionic/Non-Ionic Type High Solid Content Polyurethane Dispersion Based on Sulfonate and Monofunctional Polyethylene Oxide Polyether.” J. Chem. Ind. Eng., 60 497–501 (2009)
García-Pacios, V, Costa, V, Colera, M, et al., “Effect of Polydispersity on the Properties of Waterborne Polyurethane Dispersions Based on Polycarbonate Polyol.” Int. J. Adhes. Adhes., 30 456–465 (2010)
García-Pacios, V, Iwata, Y, Colera, M, et al., “Influence of the Solids Content on the Properties of Waterborne Polyurethane Dispersions Obtained with Polycarbonate of Hexanediol.” Int. J. Adhes. Adhes., 31 787–794 (2011)
Wei, XL, Zhang, FX, Xiao, ZL, “Preparation and Property Study of High-Solid Content and Low-viscosity Polyurethane Microemulsions.” Acta Polym. Sin., 1 28–34 (2009)
Kim, KB, Shin, HJ, “Modification of Waterborne Polyurethane by Forming Latex Interpenetrating Polymer Networks with Acrylic Rubber.” Colloid Polym., 280 716–724 (2002)
Jofre-Reche, JA, García-Pacios, V, et al., “Role of the Interactions Between Carbonate Groups on the Phase Separation and Properties of Waterborne Polyurethane Dispersions Prepared with Copolymers of Polycarbonate Diol.” Prog. Org. Coat., 12 199–211 (2015)
Li, QA, Dong, CS, Yu, XW, “Study on the Synthesis for Poly(Lactic Acid) by Direct Condensation Polymerization.” China Adhes., 8 59–64 (2007)
Kang, YS, Kim, BK, “High Solid and High Performance UV Cured Waterborne Polyurethanes.” Colloids Surf. A, 10 58–63 (2010)
Lee, HT, Wu, SY, “Effect of Sulfonated Polyol on the Properties of the Resultant Aqueous Polyurethane Dispersions.” Colloids Surf. A, 276 176–185 (2006)
Wei, XL, Zhang, FX, Xiao, ZL, “Study on High-Solid Content Si/PU Polyurethane Dispersion with PES/PPG Composite Soft Segment.” J. Appl. Polym. Sci., 5 1–5 (2012)
Rahman, MM, Yoo, HJ, Jung, C, “Synthesis and Characterization of a Clay/Waterborne Polyurethane/Clay Nanocomposite—Effect on Adhesive Strength.” Macromol. Symp., 250 252–258 (2007)
Kuan, H-C, Chuang, W-P, Ma, C-C, “Synthesis and Characterization of a Clay/Waterborne Polyurethane Nano-Composite.” J. Mater. Sci., 40 180–186 (2005)
Lee, D, Lee, S-H, Kim, S, “Micro-Phase-Separation Behavior of Amphiphilic Polyurethanes Involving Poly(Ethylene Oxide) and Poly(Tetramethylene Oxide).” J. Polym. Sci., 41 20–25 (2003)
Acknowledgments
This work was partially supported by the Chemical Additives and Surfactants Key Laboratory of Sichuan Universities (Projects 2015JXY02 and 2014JXY04), the Sichuan University Talents Project (Projects 2014RC14 and 2015RC55), and the Sichuan Province Department of Education (Project 16ZB0254). The authors also thank all project team members for providing performance testing of the products in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, F., Wei, X. Study of ionic/nonionic polyurethane dispersions with high solid content and low viscosity using a complex hydrophilic chain-extending agent. J Coat Technol Res 15, 141–148 (2018). https://doi.org/10.1007/s11998-017-9965-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-017-9965-y