Abstract
In the present study, a polyurethane acrylate (PUA) system cured via a thermal–UV (dual-cure process) was developed. The system selected for this work was a two-pack polyurethane acrylate with polyester polyol as the main component and urethane monoacrylate (UMA) as hardener. The polyester polyol was synthesized in a way to provide a final film coating containing both a suitable flexibility and high surface hardness. The thermal and photochemical curing behavior of the resin was studied via the chemorheology technique and the real-time FTIR. The Boltzmann sigmoidal model was implemented and well-fitted to the data obtained from the chemorheology measurements. The comparison between two reactive diluents, butanediol diacrylate (BDDA) and trimethylolpropane triacrylate (TMPTA) showed that BDDA reacts faster than TMPTA in the thermal curing condition. Nevertheless, the network buildup is stronger when TMPTA is used. The photopolymerization is also faster for the case of TMPTA. However, its final double bond conversion is restricted to a lower amount due to steric hindrance and higher viscosity of the system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The photopolymerization is a useful method to produce a polymer network from a liquid resin system. Among several existing techniques, the UV curing is more common because of the reduced energy consumption and lower equipment costs compared to the other routine methods.1 The important limitation for the application of UV-curing systems is the problem with 3D subjects or highly pigmented coatings. In these applications, the whole area of coating is not illuminated and sufficient curing is not achieved. In order to overcome such problems, a second curing system is applied.2,3,4,5 The most usual method is the thermal curing along with the UV irradiation. The final coating properties, however, are obtained by the combination of a UV-curing process and the thermal crosslinking reactions.4,6 The cured films are usually more flexible and adhere better to substrates compared with the 100% UV-curable systems, presumably since shrinkage is reduced.
A UV-curable system is typically composed of three basic components7,8:
-
1.
A resin, i.e., an oligomer or a prepolymer that contains unsaturated double bonds or cyclic structure capable of ring opening characteristic.
-
2.
A reactive diluent, i.e., monomers with different degrees of unsaturation. Monomers play two roles in these systems. They reduce the viscosity to an applicable amount and also take part in the crosslinking reactions via the radiation curing.
-
3.
A photo initiator capable of absorbing the UV radiation and generating free radicals or other reactive species which may, in turn, initiate the polymerization reactions.
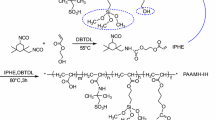
The chemical structure of the main oligomer and the reactive diluents determine the final physical and chemical properties of the cured films.9,10 Due to the high reactivity of acrylate double bonds, the shorter reaction time and a wide range of monomers, the acrylate-based resins are the first choice as oligomer.1,10,11 The most common coating type for the UV-curable systems are the urethane acrylates. Such oligomers possess the potential to combine useful properties of polyurethanes such as high abrasion resistance, appropriate toughness, good tear strength as well as the low-temperature properties with the superior optical and weatherability of polyacrylates.7 Many kinds of the urethane acrylates are formulated as the two-component chemicals, where an acrylic polyol (with polyester or polyether acrylate structure) solution is mixed with a polyisocyanate just before application on the substrate. Another method to synthesize a PUA resin is mixing a polyether or polyester polyol with a urethane monoacrylate (UMA) obtained from an acrylate and an isocyanate component9 (Scheme 1). Such a system behaves similarly to a two-component polyurethane that is thermally cured by the reaction of isocyanate functional groups with the hydroxyl functionalities of polyol and photochemically cured by the radical polymerization of acrylic double bonds in UMA.
Understanding the cure advancement of thermosetting resins has a significant role in describing some important parameters such as the gel point, the induction time before gelation or the working time, and the network architecture all which are related to the bulk chemical formulation and the reactivity.12 These parameters have significant importance to establish the process conditions for thermosets such as the lamination temperatures and the pressure necessary to trigger or block larger scale flow or to control the shrinkage.
The analytical methods used to monitor the cure profile can be classified into two main categories. The first is based on the measurement of the degree of curing before and after curing reactions. The second method continuously monitors the curing reactions and gives very useful information about the reaction kinetics. The infrared spectroscopy and the nuclear magnetic resonance are based on the first method and the real-time FTIR, the photo-DSC, and the dielectric relaxation spectroscopy are based on the second technique.13 The viscoelastic analyses have been also applied to follow the curing kinetics but only for the systems with relatively slow curing rate.
By application of real-time FTIR technique, the conversion of acrylate functional groups at any time can be calculated by monitoring the absorbance peak of the acrylic double bond between two-time steps. At a subsequent time “t,” the amount of absorbance at this peak may be related to the double bond conversion as follows:
where A(0) and A(t) are the normalized amounts of the initial peak and at a time “t,” subsequently. The coating process, as well as the final properties of cured film, is strongly affected by the rheological behavior, so the investigation of the viscoelastic properties during the curing process is important for a reactive system. A link exists between the monomers conversion and the rheological properties. For reactive systems, the determination of the kinetics of conversion as a function of temperature and the thermodynamic activation energy has attracted some interests.12 A number of theoretical and empirical models of viscosity advancement were summarized by Halley and Mackay.14,15 They have shown some specific resin systems that have been evaluated with each model.
The most common and simple model for this purpose is the power-law model:
where η(t) is the time-dependent viscosity and η 0 is the pre-gelled initial viscosity at which crosslinking is initiated. The parameter “n” is the power-law index and shows the rapidity of viscosity rising. This model is so simple and shows a well-defined time depending viscosity in the curing advancement. The power-law model can deviate significantly when network density builds up. At these points, the nonlinear models could describe the dynamic viscosity more accurately in the transition stages during the initial conversion stage and at higher conversions.12,15 One model for defining these changes is the Boltzmann sigmoidal as following:
where η 0 and η ∞ are the viscosities before illumination and after the network formation. They are more related to the torque limit in the measurements. The time t 0, the induction time, is the time that is necessary for changing viscosity from the initial value to (log η 0 + log η ∞)/2. The parameter ∆t is proportional to the slope of the curve at t 0 and is associated with the sigmoidal transition region as viscosity deviates from the initial value.12,16
It is shown that the Boltzmann sigmoidal model represents the viscosity advancement extremely well for the photopolymerized acrylates,12 the thermally cured urethanes,16 the gelation of acrylamide17 and also for the epoxy resins formulated as a chip under fill materials.18,19
In this research, a urethane acrylate resin is synthesized in a way that two curing systems should be applied to achieve desirable properties. The cure advancement of such a system under the UV and thermal curing methods is investigated via the real-time FTIR and chemorheology techniques. Finally, the effect of curing conditions on the pendulum hardness of coating films is studied (Scheme 2).
Experimental
Materials
In this study, terephthalic acid (TPA), adipic acid, trimethylol propane (TMP), neopentyl glycol (NPG) and dibutyltin oxide (DBTO) were used for the synthesis of the polyester polyol. The urethane monoacrylate resulted from the reaction of 2-hydroxyethyl methacrylate (HEMA) and toluene diisocyanate (TDI). Omnirad 73 was used as a photoinitiator. It is a α-hydroxy ketone with a chemical identity of 2-hydroxy-2-methyl-1-phenylpropanone. Butanediol diacrylate (BDDA) and trimethylolpropane triacrylate (TMPTA) were employed as the reactive diluents.
Instruments
The melt viscosity of polyester resin was characterized using CAP2000+ high shear viscometer (Brookfield). The rheological properties were analyzed using a Modular Compact Rheometer (MCR300, Paar Physica, Graz, Austria). The most common geometry to investigate curing analysis is the parallel plates (PP) geometry. Our experiments were performed with standard parallel plates geometry with the diameter of 25 mm (PP25). A humidity chamber installation made in-house was placed to minimize the evaporation during the measurements. The photopolymerization was conducted using a PerkinElmer spectroscope equipped with an LC8 lighting cure UV source accessory (Hamamatsu). The UV light intensity over the sample was 20 mW/cm2 in the wavelength range of 200–440 nm. The specimens were prepared with normal KBr disks and each experiment lasted for 20 min. The absorbance change in wavelength of 810 cm−1 was monitored to check the conversion of acrylic double bonds. The pendulum hardness of the coating films was measured using the instrument called TQC-SP0500 based on ASTM D4366.
Synthesis of polyester polyol
The polyester polyol used for the preparation of two-pack urethane acrylate resin should contain hydroxyl functional end-groups to react with the isocyanate functional groups of urethane monoacrylate. Such polyester resin was synthesized from the reaction of TPA and adipic acid with the hydroxyl functionalities of TMP and NPG (Fig. 1). The final hydroxyl value of this resin was 49 mgKOH/gr. The reaction was performed in the presence of DBTO as the catalyst with a distillation column geometry. This geometry was changed to the azeotropic distillation at the last stage because of the low reaction rate. The reaction advancement was monitored with measurement of the acid value and the melt viscosity. The lower the acid value, the larger the melt viscosity and the higher the molecular weight obtained. Figure 2 shows the changes in the melt viscosity versus the acid value for the reaction advancement of polyester polyol resin.
Synthesis of urethane monoacrylate
The urethane monoacrylate was synthesized from HEMA and TDI. For this reason, the equimolar amount of HEMA was added drop-wise to TDI in the presence of hydroquinone at 60°C. After completing the addition of HEMA, the reactor temperature was held at 60°C and mixing was continued until the NCO content reached a specified value. The NCO content was measured according to ASTM D2572 procedure. Theoretically, when the NCO content reached the amount of 13.8 wt%, the half of the isocyanate groups of TDI were reacted with the hydroxyl groups of HEMA. Therefore, one end of the molecule was capped with an acrylic double bond suitable for the UV curing and the free isocyanate groups were used for the thermal curing.
Sample preparation
In order to obtain a proper coating with the dual-curing characteristics, polyester polyol must be mixed with the urethane monoacrylate in the presence of reactive diluents and a photo initiator. The necessary amounts of the two components were calculated from the hydroxyl value of polyester resin and the value of 1.1 for the NCO/OH ratio. This amount is appropriate for achieving some polyurethanes with high chemical resistance and good physical properties.20 For chemorheology experiments, a reactive diluent was added with 90/10 ratio for polyester/diluent. This ratio for the samples used in the UV curing was 80/20 to show the difference between two diluents more distinctively. Photoinitiator was mixed with these specimens with the amount of 5 wt% based on the polyester resin.
Results and discussion
Chemorheology
To investigate the curing behavior of urethane acrylate in the thermal curing, the rheological measurements in the oscillation mode were implemented. Curing was performed at two temperatures, 80 and 100°C, with an angular frequency of 1 Hz. With performing the time test at a constant temperature, the storage (G′) and loss moduli (G′′) were monitored along with time. At the first stages, the resin system has a liquid-like behavior and shows larger G′′. When the reaction between hydroxyl and isocyanate groups starts, some new structures are formed, as proven by showing a rising G′. This experiment was continued to the extent that G′ reached a plateau. The complex viscosity data obtained from this analysis is fitted with the Boltzmann Sigmoidal model using the Microcal Origin (Origin Lab, Northampton, USA) software. The rheometry data and the fitted plot for the Boltzmann model are shown in Figs. 3a and b.
In the thermal curing of urethane system, the reaction occurs between the hydroxyl and isocyanate functional groups. This type of reaction can take place at any temperature and depends on the isocyanate type exhibiting higher reaction rates when temperature rises.21 The experimental data and the fitted Boltzmann model also validate the temperature behavior (Fig. 3 and Table 1). Theoretically, when the polyester-UMA mixture is exposed to thermal curing, the NCO groups of UMA molecules are reacted with the hydroxyl groups of the polyester chains and form a polyester-acrylate molecule with a larger molecular weight. In other words, every OH group of the polyester chain is capped with a UMA molecule. In such a condition no crosslinking reaction takes place and no changes in the rheological behavior, especially in the storage modulus, are envisaged. The rheological experiments showed some differences from the theoretical point of view, especially at higher temperatures. As is mentioned later, the real-time monitoring of storage modulus was continued to a point where G′ reached a plateau and no changes versus time were observed. To investigate the structure of cured specimen, a frequency sweep experiment was performed (Fig. 4). It is understood that the slope of storage modulus versus the angular frequency is two for unstructured liquids. This slope approaches a plateau for the solid-like or completely cross-linked polymer networks.22 Because of the curing condition, the thermal reaction between some of the acrylic double bonds is possible.23 In the studied system, the thermal reactions of acrylic double bonds result in some crosslinking reactions and show a rise in G′. The thermal reaction of acrylic double bonds depends on the double bond reactivity; it is confirmed by the differences between two reactive diluents and less crosslinking at 80°C in comparison with 100°C. The FTIR spectroscopy showed that ca. 6% of acrylic doubles were reacted during thermal curing in the case of the BDDA-diluted samples. For the TMPTA-diluted samples, 1.3% of these functional groups were reacted (Fig. 5).
To study the thermal cure advancement more precisely, the Boltzmann sigmoidal model was fitted on the complex viscosity that was obtained from chemorheology experiments. The parameter “t 0” in the Boltzmann sigmoidal model represents the reactivity of the whole system and shows how fast the reactions begin. At 80°C, the effect of two reactive diluents is the same, but with increasing curing temperature, the BDDA-diluted mixture shows a higher reactivity. The parameter “Δt” shows the tendency of the system to build up a network structure. As for t 0, the network is built up at 80°C, exhibiting no significant difference between two diluents used, but TMPTA-diluted samples are crosslinked a little faster. These two behaviors can be explained by the chemical structure of two diluents and physical properties of the mixture. BDDA is a diacrylate with a linear structure that provides a low viscosity in the system, and on the other hand, TMPTA is a triacrylate with higher reactivity. The BDDA-diluted mixture shows lower viscosity, so when thermal curing of acrylic double bonds begins, the diluent molecules have more mobility than the mixture containing TMPTA. The larger mobility results in faster reactions showing a lower t 0 value. The chemical structure of TMPTA leads to a larger crosslinking density with a branching structure; thus, it shows lower Δt values than the mixtures containing BDDA.
The calculation of real gel point is an applicable method to explain the difference between two systems. It is shown that the cross-over point between the low and high viscosity conversion is identified as the gel point.15,24,25 The low conversion regime is generally attributed to the initial stage of polymerization. In such stages, the fluid contains monomers and small-size oligomers. As the polymerization reaction takes place, these oligomers are joined together building a network structure which causes a sudden increase in viscosity.16 The power-law viscosity model can be used to explain viscosity behavior at these two regions (Fig. 6).
To determine the gel point of the system, the complex viscosity obtained from the thermal curing at 100°C was plotted versus time and two power-law models were fitted on each plot for the low and high conversion regimes. The power-law model is well-fitted on the viscosity data at high conversions and a rise in the power-law index occurred after the gel point. The advantage of using the nonlogarithmic scale power-law model is that the determination of small changes especially in the initial stage of the reaction is feasible. The gel point from this method is about 330 s for BDDA and 705 s for the TMPTA-diluted mixture. This result shows the same pattern as the Boltzmann sigmoidal model and the determined gel points are lower than the fitted parameter “t 0.” Increasing the power-law index is more considerable in the samples containing TMPTA which is comparable to the results of “Δt” parameter from the Boltzmann model.
Real-time FTIR
The real-time FTIR (RTIR) is used to study the curing behavior of polymer resins. Monitoring the absorbance peak at a specific wavelength may exhibit the extent of chemical reaction and may be useful to calculate the extent of reaction conversion. The acrylic double bonds have specific absorbance peaks at 810, 986, 1410 and 1636 cm−1. According to the chemical structure of PUA resin, the wavelength of 810 cm−1 is selected to calculate the conversion. The baseline absorbance point at 1730 cm−1 for C=O stretching vibration was used as a baseline to normalize the data. Figure 4 shows the acrylic double bond conversion for the two reactive diluents.
As is shown in Fig. 7, the TMTPA-diluted samples react more rapidly via illumination than the BDDA-diluted ones. This behavior is related to the chemical nature of TMPTA and its higher reactivity compared with other diluents.26 The higher reactivity of three double bonds in TMPTA results in a shorter reaction time, but the final conversion with this diluent was much lower than the conversion of the mixture containing BDDA. The conversion limitation is due to the lower viscosity of BDDA and the tendency of TMPTA to make the branching structure and steric hindrance in the presence of aromatic components.27 Such behavior is already reported for hexanediol diacrylate (HDDA) and TMPTA.28 Thus, when a mixture contains TMPTA, the double bond conversion starts rapidly; however, as the reaction continues, the molecular mobility becomes dominant and the conversion is limited to lower values.
Mechanical properties
Table 2 shows the results for pendulum hardness, adhesion by the cross-cut experiment and the pencil hardness of films that cured with the UV irradiation and temperature (dual-cure). The hardness measurement using the pendulum hardness is a useful way to study the extent of cure in the coating films.2,5,29 Once the coating films are exposed to the curing condition, the reaction of functional groups results in crosslinking between polymer chains, so the pendulum hardness of film increases. The comparison of these data after each step of curing can be useful to explain the effectiveness of curing method.
The results (Table 2) show that the thermal curing as expected is the main curing method and use of the UV irradiation completes the extent of curing leading to a much higher hardness. The TMPTA-diluted samples reach a smaller hardness value after the dual curing in comparison with the BDDA-diluted samples; it is because of the lower conversion rate of the samples containing TMPTA in both curing systems. This is shown both by the chemorheology and real-time FTIR experiments.
Conclusions
The UV–thermal dual-cure polyurethane acrylate was synthesized as a two-component coating using polyester polyol and urethane monoacrylate structure. The curing of such mixtures with two reactive diluents, BDDA and TMPTA, was studied implementing chemorheology and real-time FTIR spectroscopy. The Boltzmann sigmoidal model described the PUA thermal curing, effectively. The result showed that the BDDA-containing samples react faster than the TMPTA-containing samples; nevertheless growing a stronger network was more considerable when TMPTA was used as the reactive diluent. The absorbance change in the wavelength of 810 cm−1 in the real-time FTIR experiment was used to calculate the amount of acrylic double bond conversion versus time. By using TMPTA, the radical polymerization reactions begin faster, but the final conversion is restricted. The pendulum hardness was implemented to investigate the effect of each curing method on the cure extent of coating films. The result showed that the thermal curing had a main effect on the reaction advancement and the UV curing. This helps to achieve the desired properties. The TMPTA-diluted samples were softer in comparison with the BDDA-containing samples because of slower reaction times and restricted double bond conversion.
References
Endruweit, A, Johnson, MS, Long, AC, “Curing of Composite Components by Ultraviolet Radiation: A Review.” Polym. Compos., 27 (2) 119–128 (2006)
Jian, Z, Yong, H, Ming, X, Jun, N, “Preparation and Properties of Dual-Cure Polyurethane Acrylate.” Prog. Org. Coat., 66 (1) 35–39 (2009)
Decker, C, Masson, F, Schwalm, R, “Dual-Curing of Waterborne Urethane-Acrylate Coatings by UV and Thermal Processing.” Macromol. Mater. Eng., 288 (1) 17–28 (2003)
Studer, K, Decker, C, Beck, E, Schwalm, R, “Kinetic Study of the Dual-Curing of Isocyanate Acrylate Hydrogen Donor Combinations.” (2003).
Studer, K, Decker, C, Beck, E, Schwalm, R, “Thermal and Photochemical Curing of Isocyanate and Acrylate Functionalized Oligomers.” Eur. Polym. J., 41 (1) 157–167 (2005)
Dean, RE, Kania, CM, Kutchko, C, Rearick, BK, Retsch JR, WH, Schwendeman, JE, Hu S, “Patent US7794844—Dual Cure Coating Compositions, Multi-component Composite Coatings, and Related Coated Substrates.”
Şabani, S, Önen, AH, Güngör, A, “Preparation of Hyperbranched Polyester Polyol-Based Urethane Acrylates and Applications on UV-Curable Wood Coatings.” J. Coat. Technol. Res., 9 (6) 703–716 (2012)
Cahn, RW, Materials Science and Technology, Processing of Polymers, Vol. 18. John Wiley & Sons, Hoboken, 1997
Srivastava, A, Agarwal, D, Mistry, S, Singh, J, “UV Curable Polyurethane Acrylate Coatings for Metal Surfaces.” Pigment Resin Technol., 37 (4) 217–223 (2008)
Schwalm, R, UV Coatings: Basics, Recent Developments, and New Applications. Elsevier, Amsterdam, 2006
Decker, C, “UV-Radiation Curing Chemistry.” Pigment Resin Technol., 30 (5) 278–286 (2001)
Love, BJ, Ruinet, FP, Teyssandier, F, “Chemorheology of Photopolymerizable Acrylates Using a Modified Boltzmann Sigmoidal Model.” J. Polym. Sci. Part B Polym. Phys., 46 (21) 2319–2325 (2008)
Lee, SS, Luciani, A, Månson, J-AE, “A Rheological Characterisation Technique for Fast UV-Curable Systems.” Prog. Org. Coat., 38 (3–4) 193–197 (2000)
Halley, PJ, Mackay, ME, “Chemorheology of Thermosets—An Overview.” Polym. Eng. Sci., 36 (5) 593–609 (1996)
Halley, PJ, George, GA, Chemorheology of Polymers: From Fundamental Principles to Reactive Processing. Cambridge University Press, Cambridge, 2009
Teyssandier, F, Love, BJ, “Cure Advancement of Urethane Networks Using a Sigmoidal Chemorheological Model.” Polym. Eng. Sci., 50 (3) 499–503 (2010)
Love, BJ, “Revisiting Boltzmann Kinetics in Applied Rheology,” SPE Plast. Res. Online, 2009.
Teyssandier, F, Sun, YY, Wong, CP, Love, BJ, “Cure Versus Flow in Dispersed Chip-Underfill Materials.” Macromol. Mater. Eng., 293 (10) 828–831 (2008)
Love, BJ, Teyssandier, F, Sun, YY, Wong, CP, “Sigmoidal Chemorheological Models of Chip-Underfill Materials Offer Alternative Predictions of Combined Cure and Flow.” Macromol. Mater. Eng., 293 (10) 832–835 (2008)
Oldring, PKT, Tuck, N, Resins for Surface Coatings, Alkyds & Polyesters. Wiley, Hoboken, 2000
Oldring, PKT, Limited, ST, Tuck, N, Resins for Surface Coatings: Polyurethanes, Polyamides, Phenoplasts, Aminoplasts, Maleic Resins. Wiley, Hoboken, 2001
Kotsilkova, R, Pissis, P, Thermoset Nanocomposites for Engineering Applications. Smithers Rapra Publishing, 2007
Studer, K, Decker, C, Beek, E, Schwalm, R, Gruber, N, “Redox and Photoinitiated Crosslinking Polymerization I. Dual-Cure Isocyanate-Acrylate System.” Prog. Org. Coat., 53 (2) 126–133 (2005)
Lipshitz, SD, Macosko, CW, “Rheological Changes During a Urethane Network Polymerization.” Polym. Eng. Sci., 16 (12) 803–810 (1976)
Lipshitz, SD, Macosko, CW, “Kinetics and Energetics of a Fast Polyurethane Cure.” J. Appl. Polym. Sci., 21 (8) 2029–2039 (1977)
Arceneaux, JA, Willard, K, “UV & EB Chemistry and Technology,” RadTech Printer’s Guide
Pappas, SP, Radiation Curing: Science and Technology. Springer, US, 1992
Kim, DS, Seo, WH, “Ultraviolet-Curing Behavior and Mechanical Properties of a Polyester Acrylate Resin.” J. Appl. Polym. Sci., 92 (6) 3921–3928 (2004)
Maag, K, Lenhard, W, Löffles, H, “New UV Curing Systems for Automotive Applications.” Prog. Org. Coat., 40 (1–4) 93–97 (2000)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarrafi, M., Kaffashi, B. & Bastani, S. Investigation of cure advancement in dual-cure polyurethane-acrylate coatings over metal substrates. J Coat Technol Res 15, 527–534 (2018). https://doi.org/10.1007/s11998-017-0008-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-017-0008-5