Abstract
Slow release fertilizers (SRFs) are of vital importance to improve agricultural efficiency. However, their use is still limited due to their relatively high costs. Additionally, most of coating materials used to produce SRFs are nonbiodegradable and toxic to the soil. In this context, we utilized various biopolymers such as tamarind, xanthan, and guar gums together with diatomite to coat urea fertilizer granules. In this study, tamarind–urea-diatomite (TUD), guar–urea-diatomite (GUD), and xanthan–urea-diatomite (XUD) SRF granules were prepared in the presence of epichlorohydrin as crosslinker. The nutrients slow release behavior and the water retention capacity of these SRFs in soil were determined. The water absorbency of the product was 89% TUD, 93% GUD, and 142% XUD of its own weight when it was allowed to swell in tap water at room temperature for 2 h. Poly(methacrylic acid) was applied as the outermost layer of XUD to improve the nitrogen slow release efficiency of XUD SRFs. The results showed that the product had an excellent nutrients slow release property of 79.5% and good water retention capacity of 62.9% after 28 days. This suggested that XUDM could effectively improve the utilization of fertilizer. Furthermore, being biodegradable and low cost could be beneficial in agricultural and horticultural applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, much attention has been paid to fertilizers with slow release and water retention due to their essential roles in agriculture and horticulture applications.1 Compared to the large amount of fertilizers used throughout the world, the total use of slow release fertilizers (SRFs) is still small. Basically, the growth of plants and their quality mainly rely on the quantity of fertilizer and water.2 Regarding the main fertilizers used in agriculture, the global consumption of nitrogen (N), phosphorus (P), and potassium (K) in 2013 was 109.8, 42.2, and 29.4 Mt/year, respectively.3 Therefore, nitrogen is still the most generally applied plant nutrient. To date, urea is a widely used solid nitrogen fertilizer for agricultural production due to its low cost. However, when applied to crops, it is vulnerable to losses from volatilization and leaching which reduce plant nitrogen use efficiency and limit crop yields. Briefly, urea when applied to soil can be rapidly hydrolyzed to NH3 and CO2 by soil urease, followed by NO3 − formation through nitrification.4,5 This could contribute toward environmental pollution in terms of hazardous gaseous emissions and water eutrophication.6 Thus, it is necessary to improve the utilization of water resources and fertilizer nutrients. One possible way to improve nitrogen use efficiency in particular while reducing the environmental hazards is by using SRFs. An ideal slow release fertilizer is defined as a conventional fertilizer coated with natural or semi-natural, environmentally friendly macromolecule material that retards the fertilizers release to such a slow pace that a single application to the soil can meet nutrient requirements for model crop grow.7
Recently, the use of SRFs is a new trend to save fertilizer consumption and to minimize environmental pollution.8,9 Consequently, the technology of coating the urea fertilizer granules for managing the nutrients release has greatly attracted the scientific community.10 Initially, sulfur was the most frequently used as urea coating material. So far, various synthetic polymers such as polyethylene, polystyrene, and polyesters were applied due to their apparent benefit over natural polymers such as set-to-set consistency, foreseeable physic-chemical properties, and tailor-made character. However, the application of sulfur-coated urea may increase the acidity of the soil, because both sulfur and urea contribute to the increased acidity. Moreover, some types of polymers used in the coating of the conventional fertilizers are reported to have poor biodegradability and their use may thus lead to undesirable accumulation of plastic residues.11 In addition, due to their higher costs and process complexity along with issues of environmental pollution caused by those coating materials, research frontiers shifted toward developing low cost, easily fabricable, and environmentally friendly materials. More recently, Wu et al.2,12 claimed that the use of low cost, naturally, and abundantly available resource materials such as polysaccharides and inorganic clay could be a promising approach in the production of coating materials for conventional fertilizers. With this background, here we synthesize low cost and environmentally friendly coated urea fertilizer granules with a slow release of nutrients and water retention properties, based on various natural polysaccharides (tamarind gum, xanthan gum, and guar gum) individually, together with inorganic clay (diatomite).
Xanthan gum is a natural polysaccharide commonly used as a food additive and rheology modifier,13 with branched chains and acidic characteristics produced predominantly by Xanthomonas campestris in aerobic conditions from sugar cane, corn, or their derivatives.14 It consists of d-glucosyl, d-mannosyl, and d-glucuronyl acid residues in a 2:2:1 molar ratio and variable proportions of O-acetyl and pyruvyl residues. Trisaccharide side chains are composed of mannose β-(1 → 4) glucuronic acid β-(1 → 2) mannose attached to alternate glucose residues in the backbone by α-(1 → 3) linkages.15,16 Their anionic and hydrophilic surface characteristics facilitate interactions with cations17,18 and other polysaccharides such as glucose, mannose (C6H12O6), potassium gluconate (C6H11KO7), acetate (CH3CO2 −), and pyruvate (CH3COCOOH), inducing stronger gelation.19
Tamarind gum, a crude extract of tamarind seeds, is rich in polysaccharide (~65–72%),20 which contains glucose, xylose, and galactose units, in a molecular ratio of ~3:2:1, respectively.21–23 Its structure is based on a β-(1 → 4)-d-glucan backbone, substituted at position 6 of the glucopyranosyl units mainly by single α-d-xylopyranosyl residues as well as by disaccharide side chains composed of β-d-galactopyranosyl-(1 → 2)-α-d-xylopyranosyl residues.23 In addition, tamarind seed gum is a high molecular weight polysaccharide (720–880 kDa),21,22,24,25 which forms viscous solutions when dissolved in water as many polysaccharide gums extracted from plant materials. Presently, it has the potential for commercial applications, for example in the pharmaceutical industry for controlling drug release.26
Guar gum is a water soluble polysaccharide derived from the seeds of Cyamopsis tetragonolobus, family of Leguminosae. It consists of linear chains of β-(1 → 4)–d-mannopyranosyl units with α-d-galactopyranosyl units attached by (1 → 6) linkages.27 Guar and its derivatives have been used in many applications like food, drug delivery, and health care products because of their natural abundance and their low cost and other desirable functionalities.28
Diatomaceous earth, also known as diatomite, is a fossil material of sedimentary origin, formed over centuries by siliceous skeleton (called “frustule”) of aquatic unicellular microalgae, the diatoms, deposited on bottom of lakes or present in marine environments.29 The main constituent of diatomite is amorphous silica, although it can contain impurities such as organic components and metallic oxides (MgO, Al2O3, Fe2O3) coming from the environment.30 Different strategies, including calcination processes and hot acid treatments, have been developed to remove impurities from frustules.31,32 The silica surface of diatoms is covered by reactive silanol (Si–OH) groups. Due to its hydrophilic property, large availability in many areas of the world, chemical stability, extremely low cost, and nontoxicity, this fossil material is an ideal component for added superabsorbent network to improve the swelling properties. Moreover, the porous structure of diatomite allows a large amount of water to penetrate into the polymer network and may be of benefit to water absorbency of the corresponding superabsorbent.
The ultimate aim of this study was to develop environmentally friendly and low cost urea coating materials to synthesize coated urea fertilizer granule with high absorption and water retention capacity as well as enhanced slow release of crop nutrients when a fertilizer is applied, based on the naturally and abundantly available polysaccharides and inorganic clay. Through this study, we determined properties such as water absorption, water retention capacity, and nutrient release behaviors of the urea fertilizer granules coated with different most common hydrocolloids viz: tamarind gum, guar gum, or xanthan gum together with diatomite clay as the inorganic layer of the granule composite. In this contribution, three different types of coated urea fertilizer granules, i.e., tamarind–urea-diatomite (TUD), guar–urea-diatomite (GUD), and xanthan–urea-diatomite (XUD) were successfully prepared. Each layer was coated onto the urea fertilizer granule through the granulation method in the presence of epichlorohydrin as crosslinker. The schematic diagram of the granulation process and the schematic view of the cross section for the prepared urea fertilizer granule are depicted in Figs. 1a and 1b, respectively. According to different properties determined, urea fertilizer granule coated with xanthan gum and diatomite (XUD) exhibited beneficial water retention and water absorbency capacity as well as nutrients slow release efficiency compared to TUD and GUD SRFs. Therefore, it was selected to be further coated with poly(methacrylic acid) (PMAA), as the outermost layer to improve its properties. Finally, challenges in theory and future prospectives are examined.
Materials and Methods
Materials
Tamarind gum, xanthan gum, and guar gum (Food grade) were supplied by Anrui Biotechnology Co., Ltd (Henan, China). Diatomite was purchased from Guangsenyuan Diatomite Co., Ltd. Urea (AR) and epichlorohydrin (AR) was kindly provided by Shanghai Chemical Reagent Co., Ltd (Shanghai, China). Methacrylic acid (AR) was provided by Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Organic solvents were used without further purification. All other chemicals were commercially available analytical grade reagents unless otherwise stated.
Xanthan gum, tamarind gum, and guar gum viscosity measurement
A 0.5% solution of each polysaccharide (xanthan gum, tamarind gum, or guar gum) was heated at 80°C and stirred gently for a certain time until the solution was completely dissolved in distilled water. NDJ-8S (Shanghai Nirun Intelligent Technology Co., Ltd, China) digital viscometer spindle rotating at 12 rpm at 25°C was used and allowed to rotate for 1 min prior to measurement.
Preparation of TUD, GUD, and XUD SRF granules
Urea fertilizer core granules ranging from 1.8 to 2 mm in diameter were sieved and used for further experiments. During the sieving, we tried to remove granules that had defects on the surface. TUD SRF granule as an example has been prepared as follows: the sieved urea fertilizer granule was placed into homemade pan granulator (PZL-0.4 Pan Granulator, Central China Normal University, China), the pan of 45 cm diameter and 25 cm depth was positioned at an angle of 20° to the horizontal. At this step, a small amount of distilled water (1 wt% of the core weight) was sprayed onto the urea fertilizer granule (the core) in the pan granulator under rotation and then granulated for 10 min at 35 rpm. Then the tamarind powder as the inner coating layer was fed to the surface of the core granule under rotation. Still in the granulator rotating at the predicted speed, epichlorohydrin (2–3 wt% of the granules total weight) as crosslinker was sprayed at regular time intervals of 20 min for 2 h. In the coating process, it was assured that the used material equally covered the whole granule’s surface with sufficient thickness. Thereafter, the granule was taken out and dried at room temperature (RT) for 8 h. Finally, the granule was placed into the pan granulator rotating at 35 rpm and diatomite powder as the outer coating was coated onto the surface of the granule under rotation. During this step, the predicted amount of crosslinker was sprayed periodically. The process was finished until a compact and homogeneous coating formed on the fertilizer granule. The granulated product was fed into a dryer at 50°C to remove excess water and to harden the granule. The dried product was passed over a screen where the particles smaller or larger than the desired size were removed. The preparation process for GUD and XUD SRF granules was the same as above.
Characterization of the prepared coated urea SRFs
The average diameter of coated fertilizer was determined using a micrometer for 10 granules. The prepared granules were split into halves and the fractions obtained were put on slides and examined through dissecting microscope.
Measurement of water absorbency of TUD, GUD, and XUD SRFs
Prepared XUD, TUD, and GUD SRF granules were separately immersed into different containers filled with 200 mL of tap water and allowed to swell at RT for 2 h. The swollen XUD, TUD, and GUD SRF granules were filtrated through a 100-mesh sieve for 5 min to remove nonabsorbed water and weighed. The water absorbency was calculated using the following equation:
where m and m 0 denote the weight of swollen TUD, GUD, or XUD and the weight of the dry TUD, GUD, or XUD, respectively, and W% is the water absorbency percentage.
Slow release behavior of TUD, GUD, and XUD SRFs in soil
The soil used in this study was taken from the campus of Central China Normal University (CCNU) located in central China. The soil was put into a desiccator at RT for 2 weeks and then passed through a 26-mesh sieve. Then 1 g of each prepared compound (TUD, GUD or XUD) was well mixed with 200 g of desiccated soil and kept in a 200 mL beaker, and then 25 wt% of deionized water was added to each beaker. Thereafter, each beaker was properly covered and incubated for different periods at RT. Through the experiment, the soil moisture was maintained at 25% of the total weight (soil and granule) by weighing and adding distilled water if necessary. For each granule, six beakers were prepared in parallel to facilitate the determination of released nitrogen at different settled incubation periods. After 1, 3, 5, 7, 14, and 28 days incubation periods, the leftover TUD, GUD, or XUD granule SRFs in the beakers were removed and washed with distilled water, dried at RT overnight, weighed, and then placed into a nitrification tube. 10 mL of 98% concentrated H2SO4 and 1 g of mixed catalyst (copper sulfate pentahydrate: 1:20) were added and the whole mixture was subjected to heating in digestion chamber until the transparent residue contents were obtained (at 400°C for 1.5 h). The solution was cooled down and 5 mL of 50% (w/v) NaOH solution were added to the mixture. Thereafter, 1–2 drops of combined indicator (methyl red and methylene blue) were added to the mixture and then titrated with 0.05 N hydrochloric acid until the process reached its end point. Also a blank was run through along with the sample. After titration, the released nitrogen amount percentage was calculated, as shown in equation 2:
where ∆V represents the difference between hydrochloric acid volume used for the sample and blank solution titration in mL; C represents the concentration of hydrochloric acid in normality; 0.01401 value stands for the molecular weight of nitrogen, m represents the weight of the sample in g, and W N% represents the released nitrogen amount percentage.
Largest water-holding ratio of soil with TUD, GUD, or XUD SRFs
The soil used in this study was taken from the campus of CCNU located in central China. A 2 g sample fertilizer was mixed with 200 g of dry soil and loaded into a PVC pipe of 4.5 cm diameter and 20 cm length and the bottom of the tube was sealed with a 200-mesh nylon cloth and weighed (M 1). Tap water was added slowly to the tube from the top until the water seeped out of the bottom. After there was no leaking water at the bottom, the tube was weighed again (M 2). The largest water-holding ratio (M%) of the soil was calculated using the following equation:
Measurement of the water retention of TUD, GUD, and XUD SRFs in soil
The soil used in this study was taken from the campus of CCNU located in central China. 2 g of slow release fertilizer was mixed with 200 g of dry soil (below 2 mm in diameter) and kept in a plastic beaker and then 100 g of tap water was slowly added into the beaker and weighed (m 1). The beakers were maintained at RT and weighed every 4 days to determine the weight of the evaporated water (m i ) over a period of 28 days. A blank experiment was prepared as a reference. The retained water ration (M%) was determined by using the following equation:
Preparation of enhanced properties efficiency of XUD SRFs
XUD SRF was coated with the outermost layer using a PMAA wrap. The main purpose of this experiment was to determine whether the addition of the PMAA outermost layer could enhance XUD urea-coated fertilizer properties such as nutrients slow release efficiency and water retention capacity.
The XUD granule slow release fertilizer used for this experiment was prepared as previously described in the “Preparation of TUD, GUD, and XUD SRF granules” section. XUD SRF granule was put into the granulator rotating at 35 rpm and 3.5% w/v of PMAA solvent was poured to the XUD SRF granules under rotation. Meanwhile, epichlorohydrin (2–3 wt% of the granule weight) was sprayed at regular time intervals of 20 min for 2 h as crosslinker. The obtained coated granule was named Xanthan-Urea-Diatomite and PMAA SRFs (XUDM SRFs). The granulated product was dried to a constant mass at 50°C.
Results and discussion
Morphology and characteristics of the coated fertilizers
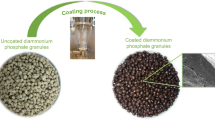
Figures 2a and 2b show the morphology of the real image of the uncoated urea fertilizer granule (the core) and the final product (urea coated with inner layer (xanthan, guar, or tamarind) and outer layer (diatomite)), respectively. The coating thickness of the prepared coated urea fertilizer was 1.9 mm urea, 0.495 mm polysaccharide, and 0.798 mm diatomite, and the granules selected for further analysis were composed of 56% urea, 13% polysaccharide, and 21% diatomite, calculated as the average for 10 coated urea fertilizer granules. From Fig. 2e, the dissecting microscope image at magnification of ×10 showed the three-layer structure of the prepared SRFs. The porous outer layer was composed of diatomite which could absorb a large amount of water. The middle layer was polysaccharide (xanthan, guar, or tamarind) (Fig. 2d), which could reduce the rate of water diffusion into the core and the nutrient diffusion outside the core. This could enhance the nutrient slow release efficiency of XUD, TUD, or GUD. The inner core (Fig. 2c) was a water soluble urea fertilizer granule. Thus, after the fertilizer core was dissolved by water, it had to pass through the two layers i.e., polysaccharide layer (inner layer) and diatomite layer (outer layer).
Images for uncoated (a) and coated urea fertilizer granule (b). Dissecting microscope images for (c) urea fertilizer granule (the core), (d) cross section view of urea coated with polysaccharide, and (e) cross section view of urea coated with middle layer (polysaccharide) and outer layer (diatomite)
Polysaccharides viscosity test result
The determined viscosity for tamarind gum, guar gum, and xanthan gum was 185, 395, and 449 mPa s, respectively. It was observed that the viscosity of xanthan gum was stronger than other polysaccharides used in this study. The researchers reported that in static conditions, a small amount of xanthan gum (in most foods, 0.5%) induces a large increase in the viscosity of a liquid. Moreover, unlike other gums, xanthan gum shows high stability under a wide range of temperatures and pH.33 The high viscosity of coating material can play a great role in polymer bonding networks, and its strong adhesion in the matrix of the coated granule could be more advantageous to the hardness of the prepared SRFs. Hence, we used biopolymers (tamarind, xanthan, or guar gum) as the middle layer of the prepared three-layered SRFs in order to obtain a product with enhanced mechanical strength of the coating layers.
Water absorbency of TUD, GUD, and XUD SRFs test results
Water absorption is one of key properties of a better slow release fertilizer. TUD, GUD, and XUD granule SRFs were immersed in a container filled with tap water at RT for 2 h; the water absorption was found to be 89, 93, and 142%, respectively, as demonstrated in Fig. 3. XUD indicated a higher water absorption than TUD, and GUD granule SRFs. During this study, the coating materials used to prepare XUD, TUD, and GUD granule SRFs were the same except for the polysaccharide layer (middle layer). It was noticed that the type of polysaccharides used as coating materials (tamarind, xanthan, or guar gum) had a different influence on the water absorbency of the prepared granules. The highest water absorbency of XUD SRFs could be explained as follows: first, the presence of carboxyl groups on the side chains renders xanthan gum molecules anionic.34 These anionic chains on the xanthan gum molecules enhance hydration and make xanthan gum more soluble in cold water as well as hot water compared to other hydrocolloids such as guar gum, tamarind gum, etc. Second, xanthan gum is a high molecular weight polysaccharide with high viscosity even at low polymer concentrations. Therefore, the high viscosity of xanthan could enhance the interaction between the materials used to coat XUD as it has been used as the middle layer. Hence, the high hydrodynamic size, molecular weight, and water solubility of xanthan gum as well as its anionic chains are believed to be the most important determinants of the highest water absorbency capacity of XUD compared to TUD and GUD granule SRFs.
Water retention behavior of TUD, GUD, and XUD SRFs in soil
The water retention capacity of swollen TUD, GUD, and XUD SRFs in soil was determined and compared to the water-holding capacity of the swollen soil without the coated fertilizers. In Fig. 4, it is clear that the water moisture was gradually decreasing over a period of 28 days at RT. The soil without coated fertilizers showed the fastest water evaporation—where only 50% of water moisture was remaining at the 28th day. With TUD and GUD SRFs, both types showed approximately the same water retention ability in soil after 28 days as their water-holding capacity was 62% and 61%, respectively. In addition, swollen TUD and GUD SRFs in soil showed rapid water evaporation at the beginning of the incubation time. However, from the 16th day, a decelerated water evaporation rate was noted. Swollen XUD granule SRFs in soil showed the powerful water retention capacity compared to TUD as well as the GUD granule SRFs. After 28 days, 71% of water still remained, which indicated that only 29% of the water had evaporated. This shows that the water evaporation rate for swollen XUD in soil was slightly slower and the granule has an excellent property for water retention in soil. In a relatively dry season, it can keep a certain amount of water to be supplied for plant growth.
Largest water-holding ratio of soil with TUD, GUD, or XUD SRFs test results
The largest water-holding ratio for soil without coated fertilizer, soil with TUD, GUD, or XUD SRFs was calculated by using equation 3. As shown in Fig. 5, we found that the maximum water-holding ratio for soil without coated fertilizer was 17.2% and the one for soil with TUD, GUD, or XUD SRFs was 20.3%, 19.8%, and 22%, respectively, (the mass ration of SRFs to soil was 1:100). It was found that the largest water-holding ratio of soil with XUD, TUD, or GUD SRFs was respectively 4.8%, 3.1%, and 2.6% higher than the one of soil without any coated fertilizer. This showed that all prepared SRFs had better absorbency in soil. However, XUD SRFs indicated excellent water absorbency in soil compared to TUD and GUD SRFs, which could obviously improve the water-holding capacity of soil. Moreover, this means that during rainy season and irrigation, XUD SRFs have adequate capacity to store a certain amount of water which could be used for crop growth during dry seasons for a prolonged time.
Slow release behavior of TUD, GUD, and XUD SRFs in soil test results
One of the key properties of a slow release fertilizer is to provide the plant nutrients by releasing nitrogen in delayed manner. As shown in Fig. 6, for the urea fertilizer granule without any treatment nitrogen, release reached 99% after 5 days and 2 days later, it was found that all nutrients were completely released. TUD SRFs nitrogen release reached 78% after 24 h, at the 14th day the nitrogen release was 96% and again it was found to be 98% after 28 days. This indicated that almost all nitrogen amounts appeared to be completely released after 14 days. For GUD SRFs, the nitrogen release was 74% after 24 h and it reached 98% after 28 days. XUD SRFs presented slightly lower nitrogen release capacity compared to TUD and GUD SRFs as 68% of nitrogen was released after 24 h and after 28 days the nitrogen release reached 92%. This indicates that the slowest nitrogen release ability of XUD SRFs is slightly more beneficial than TUD and GUD SRFs. This may be because xanthan gum showed a higher adhesion behavior compared to tamarind gum and guar gum, which places it more closely to the core and outer layer with enhanced polymer bonding network. Therefore, as XUD SRF showed the best water retention and nitrogen slow release properties compared to TUD and GUD SRFs, it was selected to be further coated with an additional layer by overwrapping a poly(methacrylic acid) (PMAA) layer to XUD granule SRFs as the outermost shell in order to enhance its properties. The obtained product (urea fertilizer granule coated with xanthan, diatomite, and PMAA) was named XUDM SRF. The XUDM SRFs results for water retention capacity and nutrients slow release behavior in soil are presented and discussed in the following sections, as well as shown in Figs. 7 and 8, respectively.
XUD SRFs enhanced properties by applying PMAA as outermost layer
Water retention behavior of XUDM SRFs in soil test results
The presence of water in soil is essential for vegetation growth. Liquid water ensures the feeding of plants with nutritional elements, which increases the growth quality of plants.35 In this study, the water retention capacity of XUDM SRFs in soil was studied and determined as shown in Fig. 7. However, in comparison to the water retained by the XUD SRFs, it can be found that XUDM SRFs in soil retained slightly less water. This could be explained simply as follows: first, in the mixtures, each granule is surrounded by soil particles and subjected to a confining pressure by these particles.36 Second, the presence of ions in soil solution makes the osmotic pressure difference between the polymeric network of XUDM and the external solution decrease, resulting in the water absorbency of the XUDM. Meanwhile, the penetration of cations (such as Na+, K+, Ca2+, and Mg2+) into the polymer network of XUDM makes the screening effect of them on the anionic groups (−COO−) in the polymeric network more evident,37,38 which also decreases the water absorbency of the XUDM SRFs.
In Fig. 7, we can find that the water retention ability of XUDM SRFs in soil is 12.3% higher than that of soil without any slow release fertilizer after 28 days. The soil water loss reached 49.4%, while XUDM water loss reached 37.1% over a period of 28 days at RT. It was noted that soil with coated fertilizer mixtures retained more water than the control soil without coated fertilizer. This showed that XUDM slow release fertilizer had a beneficial water retention capacity in soil which could effectively store rain water or irrigation water resources to be supplied to a plant over a long period of time during dry seasons.
Slow release behavior results for XUDM SRFs in soil test results
In Fig. 8, it was found that the XUD SRFs without the outermost layer of PMAA nitrogen release reached 68% after the first day of incubation, and 92% after 28 days. On the other hand, it was reported that the plant needs only a small quantity of food during its early stage of growth.6 To overcome this problem, PMAA was used to coat XUD SRFs as the outermost layer by readying XUDM SRFs. As shown in Fig. 8, XUDM SRFs exhibited a significant improved nutrients slow release efficiency compared to XUD SRFs, where at the first day only 43% of the nitrogen was released, and after 28 days the nitrogen release was 79.5%. This indicated that XUDM SRFs had enough ability to supply the plant nutrients gradually for a prolonged period of time. The possible way to explain the slow release behavior of XUDM SRFs in this study, is that diatomite was used as a matrix which has a special surface area with many porous areas through the lattice. When the water diffused into fertilizer cores, part of nutrients would be adsorbed by diatomite, which consequently slowed the release rate of nutrients. The silica surface of diatoms is covered by negatively charged reactive silanol (Si–OH) groups. The silanol group is an active one which tends to react with many polar organic compounds and various functional groups,39 causing minerals to absorb cation molecules (NH4 + from urea) to balance the charge deficit, which consequently slowed the release rate of nitrogen.11 This suggested that the application of PMAA as the outermost layer enhanced the nutrients slow release efficiency of XUD SRFs, which could be beneficial to the plant growth and yield.
Conclusion
In summary, the new low cost and environmentally friendly SRFs were developed by coating urea fertilizer granules with various different biodegradable polysaccharides such as tamarind gum, guar gum, and xanthan gum, together with diatomite in the presence of epichlorohydrin as crosslinker. The synthesized granule SRFs were composed of a three-layer structure. Their core was water soluble urea fertilizer granule, the inner layer was polysaccharide, and the outer layer was diatomite. The water absorbency result of the product was 89% TUD, 93% GUD, and 142% XUD of its own weight when it was allowed to swell in tap water at RT for 2 h. Considering all experimentally obtained results, it was observed that XUD SRFs presented more beneficial water retention capacity and nitrogen slow release efficiency compared to TUD and GUD SRFs. Therefore, XUD SRFs nitrogen slow release efficiency was improved by coating PMAA as the outermost layer to prepare XUDM SRFs. The results showed that the addition of XUDM SRFs in soil can contribute to the improved nitrogen use efficiency along with enhanced yield. Another important attribute of XUDM SRF is that it has a better water retention capacity in, 12.3% higher than the one of the soil without any slow release fertilizer after a period of 28 days, which could obviously improve the water retention capacity of the soil. Therefore, this approach showed a promise in the utilization of abundant, low cost, and natural resource such as diatomite and polysaccharides in the production of the coating material, which could significantly reduce the production cost and make the technique quite environmentally friendly.
However, the rigorous literature revealed that when urea is applied to the soil, it undergoes a series of chemical, physical, and biological transformations to produce nutrients available to plants;6,40 moreover, its nutrients release is affected by several factors such as temperature, soil pH, cation exchange capacity (CEC), organic matter, dose coverage, and fertilization location,41,42 which could result in the nutrients loss of fertilizers. Thus, the authors would like to suggest taking into consideration the above discussed factors, while preparing coated urea SRFs for further studies, in order to enhance its nutrients slow release efficiency and water retention capacity.
References
Zhang, Y, Liang, X, Yang, X, Liu, H, Yao, J, “An Eco-Friendly Slow-Release Urea Fertilizer Based on Waste Mulberry Branches for Potential Agriculture and Horticulture Applications.” ACS Sustain. Chem. Eng., 2 1871–1878 (2014)
Wu, L, Liu, M, Rui, L, “Preparation and Properties of a Double-Coated Slow-Release NPK Compound Fertilizer with Superabsorbent and Water-Retention.” Bioresour. Technol. , 99 547–554 (2008)
Heffer, P, Prudhomme, M, “Fertilizer Outlook 2012–2016”. International Fertilizer Industry Association (IFA) Annual Conference. Doha, Qatar, 2012
Gioacchini, P, Nastri, A, Marzadori, C, Giovannini, C, Antisari, LV, Gessa, C, “Influence of Urease and Nitrification Inhibitors on N Losses from Soils Fertilized with Urea.” Biol. Fert. Soils, 36 129–135 (2002)
Zhou, L, Chen, L, Li, R, Wu, Z, “Behavior of Soil Urea N and Its Regulation Through Incorporating with Inhibitors Hydroquinone and Dicyandiamide”. Fertilization in the Third Millenium—Fertilizer, Food Security and Environmental Protection, Proceedings, pp. 1175-1192. Liaoning Science and Technology Publishing House, China (2003).
Azeem, B, KuShaari, K, Man, ZB, Basit, A, Thanh, TH, “Review on Materials & Methods to Produce Controlled Release Coated Urea Fertilizer.” J. Controll. Release, 181 11–21 (2014)
Blouin, GM, “Method of Making Sulfur-Coated Fertilizer Pallet Having a Controlled Dissolution Rate.” Google Patents US 3295950 A, 1967.
Akelah, A, “Novel Utilizations of Conventional Agrochemicals by Controlled Release Formulations.” Mater. Sci. Eng. C, 4 83–98 (1996)
Jarosiewicz, A, Tomaszewska, M, “Controlled-Release NPK Fertilizer Encapsulated by Polymeric Membranes.” J. Agric. Food Chem., 51 413–417 (2003)
Naz, MY, Sulaiman, SA, “Testing of Starch-Based Carbohydrate Polymer Coatings for Enhanced Urea Performance.” J. Coat. Technol. Res., 11 747–756 (2014)
Ni, B, Liu, M, Lü, S, Xie, L, Wang, Y, “Environmentally Friendly Slow-Release Nitrogen Fertilizer.” J. Agric. Food Chem., 59 10169–10175 (2011)
Wu, L, Liu, M, “Preparation and Properties of Chitosan-Coated NPK Compound Fertilizer with Controlled-Release and Water-Retention.” Carbohydr. Polym., 72 240–247 (2008)
Chang, I, Im, J, Prasidhi, AK, Cho, GC, “Effects of Xanthan Gum Biopolymer on Soil Strengthening.” Constr. Build. Mater., 74 65–72 (2015)
Dumitriu, S (ed.) Polysaccharides: Structural Diversity and Functional Versatility, pp. 459–475. Marcel Dekker, New York (2005)
Bueno, VB, Bentini, R, Catalani, LH, Petri, DFS, “Synthesis and Swelling Behavior of Xanthan-Based Hydrogels.” Carbohydr. Polym., 92 1091–1099 (2013)
Feng, E, Ma, G, Wu, Y, Wang, H, Lei, Z, “Preparation and Properties of Organic–Inorganic Composite Superabsorbent Based on Xanthan Gum and Loess.” Carbohydr. Polym., 111 463–468 (2014)
Bergmann, D, Furth, G, Mayer, C, “Binding of Bivalent Cations by Xanthan in Aqueous Solution.” Int. J. Biol. Macromol., 43 245–251 (2008)
Nolte, H, John, S, Smidsrød, O, Stokke, BT, “Gelation of Xanthan with Trivalent Metal Ions.” Carbohydr. Polym., 18 243–251 (1992)
Laneuville, SI, Turgeon, SL, Sanchez, C, Paquin, P, “Gelation of Native β-Lactoglobulin Induced by Electrostatic Attractive Interaction with xanthan Gum.” Langmuir, 22 7351–7357 (2006)
Kumar, CS, Bhattacharya, S, “Tamarind Seed: Properties, Processing and Utilization.” Crit. Rev. Food Sci., 48 1–20 (2008)
Wang, Q, Ellis, PR, Ross-Murphy, SB, Burchard, W, “Solution Characteristics of the Xyloglucan Extracted from Detarium senegalense Gmelin.” Carbohydr. Polym., 33 115–124 (1997)
Freitas, RA, Martin, S, Santos, GL, Valenga, F, Buckeridge, MS, Reicher, F, Sierakowski, MR, “Physico-Chemical Properties of Seed Xyloglucans from Different Sources.” Carbohydr. Polym., 60 507–514 (2005)
Patel, TR, Morris, GA, Ebringerová, A, Vodeničarová, M, Velebný, V, Ortega, A, Garcia de la Torre, J, Harding, SE, “Global Conformation Analysis of Irradiated Xyloglucans.” Carbohydr. Polym., 74 845–851 (2008)
Sims, IM, Gane, AM, Dunstan, D, Allan, GC, Boger, DV, Melton, LD, Bacic, A, “Rheological Properties of Xyloglucans from Different Plant Species.” Carbohydr. Polym., 37 61–69 (1998)
Khounvilay, K, Sittikijyothin, W, “Rheological Behaviour of Tamarind Seed Gum in Aqueous Solutions.” Food Hydrocoll., 26 334–338 (2012)
Sumathi, S, Alok, R, “Release Behavior of Drugs from Tamarind Seed Polysaccharide Tablets.” J. Pharm. Pharm. Sci., 5 12–18 (2002)
Soumya, RS, Vineetha, VP, Reshma, PL, Raghu, KG, “Preparation and Characterization of Selenium Incorporated Guar Gum Nanoparticle and Its Interaction with H9c2 Cells.” PLoS ONE, 8 74411 (2013)
Gupta, AP, Verma, DK, “Preparation and Characterization of Carboxymethyl Guar Gum Nanoparticles.” Int. J. Biol. Macromol., 68 247–250 (2014)
Rea, I, Martucci, NM, De Stefano, L, Ruggiero, I, Terracciano, M, Dardano, P, Migliaccio, N, Arcari, P, Taté, R, Rendina, I, Lamberti, A, “Diatomite Biosilica Nanocarriers for siRNA Transport Inside Cancer Cells.” BBA-Gen. Subj., 1840 3393–3403 (2014)
Şan, O, Gören, R, Özgür, C, “Purification of Diatomite Powder by Acid Leaching for Use in Fabrication of Porous Ceramics.” Int. J. Miner. Process., 93 6–10 (2009)
Goren, R, Baykara, T, Marsoglu, M, “A Study on the Purification of Diatomite in Hydrochloric Acid.” Scand. J. Metall., 31 115–119 (2002)
Goren, R, Baykara, T, Marsoglu, M, “Effects of Purification and Heat Treatment on Pore Structure and Composition of Diatomite.” Brit. Ceram. T, 101 177–180 (2002)
Zohuriaan, MJ, Shokrolahi, F, “Thermal studies on natural and modified gums.” Polym Test, 23 575–579 (2004)
Milani, J, Maleki, G, “Hydrocolloids in Food Industry”. INTECH Open Access Publisher, 2012.
Bakass, M, Mokhlisse, A, Lallemant, M, “Absorption and Desorption of Liquid Water by a Superabsorbent Polymer: Effect of Polymer in the Drying of the Soil and the Quality of Certain Plants.” J. Appl. Polym. Sci., 83 234–243 (2002)
Bhardwaj, A, Shainberg, I, Goldstein, D, Warrington, D, Levy, JG, “Water Retention and Hydraulic Conductivity of Cross-Linked Polyacrylamides in Sandy Soils.” Soil Sci. Soc. Am. J., 71 406–412 (2007)
Ni, B, Liu, M, Lü, S, Xie, L, Zhang, X, Wang, Y, “Novel Slow-Release Multielement Compound Fertilizer with Hydroscopicity and Moisture Preservation.” Ind. Eng. Chem. Res., 49 4546–4552 (2010)
Zheng, Y, Li, P, Zhang, J, Wang, A, “Study on Superabsorbent Composite XVI. Synthesis, Characterization and Swelling Behaviors of Poly (Sodium Acrylate)/Vermiculite Superabsorbent Composites.” Eur. Polym. J., 43 1691–1698 (2007)
Khraisheh, MAM, Al-Ghouti, MA, Allen, SJ, Ahmad, MN, “Effect of OH and Silanol Groups in the Removal of Dyes from Aqueous Solution Using Diatomite.” Water Res., 39 922–932 (2005)
Trenkel, ME, Slow- and Controlled-Release and Stabilized Fertilizers: An Option for Enhancing Nutrient Use Efficiency in Agriculture.. IFA, International Fertilizer Industry Association, Paris (2010)
Navia, DRJ, Betancourt, ARE, Diez, JMC, Sepúlveda, RNZ, Curaqueo, FGA, Cea, LMX, Toro, ACA, González, RA, González, QME, “Controlled-Release Nitrogen Fertilizer Using Biochar as a Renewable Support Matrix.” Google Patents WO2014091279 A1, 2014.
Quijón, MEG, “Development of a Urea-Based Controlled-Release Nitrogen Fertilizer Using Biochar as Support Material.” Doctoral Thesis In Fulfillment of the Requirements for the Doctor Degree in Sciences of Natural Resources at Universidad de La Frontera, Temuco, Chile, pp. 72–74, 2003.
Acknowledgments
This work was supported by the Scientific Research Foundation for the Returned Overseas Chinese Scholars (State Education Ministry, 2010-1174), and China Spark Program (Ministry of Science and Technology, 2013GA740073).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jean Felix Mukerabigwi and Qing Wang contributed equally to the work.
Rights and permissions
About this article
Cite this article
Mukerabigwi, J.F., Wang, Q., Ma, X. et al. Urea fertilizer coated with biodegradable polymers and diatomite for slow release and water retention. J Coat Technol Res 12, 1085–1094 (2015). https://doi.org/10.1007/s11998-015-9703-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-015-9703-2