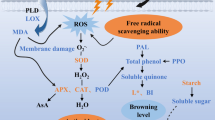
Abstract
In this study, the anti-browning effectiveness of short-term high oxygen pre-stimulation (SHOP), supercooled (SC) storage, or combined (SHOP + SC) treatment for fresh-cut potatoes was explored by evaluations on surface color, texture, phenolic anabolism, membrane integrity, antioxidant capacity, and reactive oxygen species (ROS) balancing. Furthermore, enzyme activity ratios (EA ratios) were further proposed to assess the effect of SHOP on enzymatic browning, which were defined as ratios between defense enzymes, including peroxidase (POD), phenylalanine ammonia-lyase (PAL) or catalase (CAT), and attacked enzymes including polyphenol oxidase (PPO) or lipoxygenase (LOX). As a result, SHOP + SC treatment retarded the browning and delayed the softening of fresh-cut potatoes by inhibiting the activities of related enzymes, restraining the accumulation of malondialdehyde (MDA), and promoting the synthesis of phenolic substances against their oxidation in potatoes. Besides, higher POD activities and lower BI were observed in SHOP + SC compared with SC, but PPO activities were non-statistically significant in both. At the early storage stage, the antioxidant capacities of fresh-cut potatoes treated with SHOP and SHOP + SC were enhanced, and the ROS accumulation was inhibited. Moreover, EA ratios and their changing amplitude of the SC group ranked top, followed by the control, SHOP, and SHOP + SC. Results suggested that the antioxidant capacity was reinforced by SHOP in fresh-cut potatoes due to enhanced resistance against membrane lipid damage resulting from mechanical cutting and persistent SC stimulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There has been a growing demand for fresh-cut vegetables from consumers in recent years due to the convenience and high nutritional value (Kou et al., 2014). The COVID-19 outbreak spurred a boom in the prepared food and dishes market (Zarbà et al., 2022). As reported, the processed fruits and vegetable industry is projected to exceed USD 115 billion by 2026 (Global Market Insights, 2021). The potato (Solanum tuberosum) is one of the most popular vegetables in the world (Zhou et al., 2019). Potatoes that are washed, peeled, cut into pieces or slices, and cooked are suitable and convenient as main and side dishes for consumers (Haverkort et al., 2022). However, fresh-cut processing might cause quality losses such as surface shrinkage, browning, and off-odor, thus shortening the shelf life of potatoes (Shen et al., 2020).
Texture and appearance play a crucial role in food selection preference by affecting taste threshold, pleasantness, and acceptability (Toivonen & Brummell, 2008; Rico et al., 2007; Xylia et al., 2022). The major problem that reduces the commercial value of fresh-cut fruits and vegetables is browning (Fan et al., 2021; Martín-Diana et al., 2008). The leading cause of browning in fresh-cut potatoes was enzymatic browning, which had been widely reported. Once cut, phenols would be released from vacuoles and contacted with polyphenol oxidase (PPO) in the cell membrane to form quinones. Later, the quinones are dehydrated and polymerized to form dark substances (Feumba Dibanda et al., 2020; Ru et al., 2020). Phenylalanine ammonia-lyase (PAL) is also directly associated with enzymatic browning (Liu et al., 2019). PAL (as a defense enzyme) would be activated once the surrounding environment emerges and stimulates fruits and vegetables. In addition, PAL also involves in controlling the synthesis of phenolic substances. The browning substrate content assessed by total phenolic content (TPC) and the browning intermediates determined by soluble quinone content (SQC) is two leading paths for evaluating the enzymatic browning process (Zhang et al., 2022). Moreover, cutting disrupts the dynamic balance between scavenging and producing reactive oxygen species (ROS), leading to excessive accumulation of ROS and malondialdehyde (MDA) to damage plant cell membrane function. Catalase (CAT) and peroxidase (POD) are the primary enzymes in ROS enzymatic scavenging systems (Das & Roychoudhury, 2014; Suman et al., 2021). Lipoxygenase (LOX) catalyzes the generation of reactive oxygen species and oxygen free radicals from unsaturated fatty acids (generated by phospholipase D degradation of plasma membrane phospholipids). These ROS and oxygen free radicals directly act on the unsaturated fatty acids that bind with membrane phospholipids and therefore damage the phospholipid bilayer membrane (Liu et al., 2011; Mao et al., 2007). Hence, modulation of PPO, POD, PAL, CAT, and LOX activities is a fundamental strategy for many anti-browning approaches. Our previous study has revealed that enzyme activity ratios (EA ratios) systematically evaluate the differences in the effects of treatments on attack enzymes (promoting browning) and defense enzymes (preventing browning) in fresh-cut apples (Li et al., 2022).
High oxygen (super-atmospheric oxygen) has recently been widely used in meat, fruit, and vegetable preservation as a green preservation technology (Chen et al., 2015; Garcia-Lomillo et al., 2016; Helena Gomes et al., 2010; Li et al., 2014). At present, it has been confirmed that high oxygen works as abiotic stress, which preserves fruits and vegetables by regulating the PAL metabolic pathway and polyphenol synthesis in the process of fruits and vegetables postharvest. As a result, the fruits and vegetables turn into an induced resistance state (ISR), thereby maintaining the cell membrane integrity and enhancing the resistance to browning (Liu et al., 2019). Generally, most metabolic processes are temperature-dependent, such as microbial growth, respiration, and ethylene production (Koide et al., 2019a). Therefore, decreasing the storage temperature as low as possible without ice crystallization would be the utmost to maintain the texture, flavor, and biologically active substances of fruits and vegetables by reducing metabolic activities (Ahmed et al., 2022; Nadeem et al., 2022). Supercooled (SC) storage is a method without freezing where the storage temperature is below the freezing temperature but above the nucleation temperature (Osuga et al., 2021). SC has been reported to extend the shelf life of garlic, shallots, fresh-cut cabbages, and fresh-cut apples by 1–2.5 folds (James et al., 2009; James & James, 2011; Koide et al., 2019a). Hruschka (1961) suggested that SC storage is possible for fresh-cut potatoes with lower enzyme activities. Adversely, an undercooled environment would promote membrane lipid-related enzyme activities, generate more MDA, and accelerate browning in the storage (Koide et al., 2019b).
Opportunely, SHOP treatment has been proven to control browning by maintaining membrane integrity (Li et al., 2022). Therefore, the combination of SHOP and SC storage is expected to have an enhanced anti-browning effect. In this study, physicochemical changes and quality evaluations of SHOP-treated fresh-cut potatoes were observed and analyzed during 9 days of SC storage. Specifically, the anti-browning effects and cold injury resistance of fresh-cut potatoes after pre-stimulation with high oxygen (80% O2 for 30 min) before SC storage was further explored. In addition, the EA ratios were used to study and confirm that SHOP could counteract the SC stress to fresh-cut potatoes, improve the resistance to quality deterioration, and prolong the shelf life of fresh-cut potatoes.
Materials and Methods
Vegetable Materials
The yellow-fleshed potatoes were obtained from Jiaohe City (Shandong province, China) and then transported through the cold chain to the laboratory. Potatoes without disease, blemishes, or mechanical damage were selected for pre-cold treatment at 4 °C for 18 h.
Determination of Freezing and Supercooling Points
The supercooling point of potatoes was assessed by the method by Koide et al. (2019b) with minor modifications. A T-type thermocouple was positioned at the geometric center of each potato sample (2 × 2 × 2 cm), which was then placed in a polystyrene box in a freezer (BC-142FQD, TCL Technology Group Co. LTD). Temperatures were recorded every 3 s by a datalogger (FLUKE 2638A, Fluke Electronic Instruments Co, USA) until the temperature of samples cooled down to –20 °C (Fig. S1). The time–temperature distribution of the fresh-cut potatoes (n = 10) was used to ascertain the initial freezing point and the supercooling point as the description by Kaale et al. (2011).
Experimental Design
The experimental design of this study is demonstrated in Fig. 1. In sealed bottles, half of the 8 kg selected potatoes were treated with 80% O2 + 20% N2 for 30 min, while the other half was immersed in 21% O2 + % N2 for 30 min. An O2/CO2 analyzer was used to measure the oxygen concentration (Checkpoint II, PBI Dansensor Denmark). After that, potato tubers were peeled and cut into slices (4 mm thick) by a slicer with adjustable thickness. All potato slices were rinsed by distilled water, then wiped the distilled water on the surface and packaged in food ziplock bags (Polyethylene, 140 × 200 mm, 0.7 mm thick, oxygen transmission rate is 1500–2700 mL m−2 day−1, Heyuan Huafeng Plastic Co., Ltd.) (Heo et al., 2019; Norrrahim et al., 2013; Yaptenco et al., 2007). Subsequently, potato slices were stored at –2 ± 0.5 °C (SC storage) or 4 ± 0.5 °C for 9 days. In summary, a total of 4 groups of samples were evaluated and analyzed on days 0, 2, 4, 6, and 9, namely the control (21% O2, 4 °C), SHOP (80% O2, 4 °C), SC (21% O2, –2 °C), and SHOP + SC (80% O2, –2 °C) (Fig. 1).
Browning Index (BI) and ΔE
A chromameter (HP-200, Hanpu Photoelectric Technology Co., Ltd, Shanghai, China) was used to measure the surface color in values of L* (light), a* (reddish-greenish), b* (yellowish-bluish), and ΔE (CIE, 1976). Browning index (BI) was calculated to assess browning degrees (Buera et al., 1986).
Firmness and Fracturability
The firmness and fracturability were evaluated by a texture Analyzer (TA-XT plus, Stable Micro Systems, Surrey, UK) equipped with a 100-mm-diameter-cylindrical probe for texture profile analysis (TPA) (Phinney et al., 2017). Potato slices were cut into round flakes (10 mm in diameter, 4 mm in thickness). The trigger type was “auto force” with a test speed of 15 mm s−1, and a trigger force of 1.5 N until 75% strain.
MDA Content
MDA was measured according to the method by Hu et al. (2021) and expressed as μmol kg−1 on a fresh weight basis. Briefly, trichloroacetic acid (w/v, 10%, 5 mL) was homogenized with frozen potato samples (5 g) at 0 °C. The mixture was centrifuged at 6000 × g at 4 ℃ for 20 min. Supernatant or trichloroacetic acid solution (2 mL) was mixed with 0.6% (w/v) thiobarbituric acid solution (2 mL) and then incubated in a boiling water bath for 20 min. After cooling to room temperature, absorbances were measured at 532 nm, 600 nm, and 450 nm.
Antioxidant Capacity
According to the method reported by Tang et al. (2015), the phenols extraction method in fresh-cut potatoes was slightly modified. The frozen potato samples were lyophilized in a vacuum freeze dryer for 24 h and then ground to powder for further use. Potato freeze-dried powder (1 g) and 70% methanol containing 0% glacial acetic acid (v/v, 5 mL) were mixed evenly, extracted at room temperature with a shaker (HY 60, Wuhan Huicheng Biotechnology Co., LTD, Wuhan, China) in the dark for 2 h, then sonicated for 30 min. The mixture was centrifuged at 6000 × g for 20 min. Three extractions were performed, and the supernatant was pooled to 15 mL. The supernatant was used as a crude extraction for further use.
The Folin-Ciocalteu method was used to assay TPC with minor modifications (Tang et al., 2015). Each sample or standard (25 µL) was transferred to a 96-well plate and mixed with freshly diluted Folin-Ciocalten reagent (1 mol L−1 Folin-Ciocalten:water = 1:4, 125 µL). After 10 min reaction, 125 µL of 7.5% Na2CO3 was added. After 30 min, the absorbance was read at 765 nm and methanol was the blank (Spectra Max190, Molecular Devices Corporation, USA). TPC was expressed as grams of gallic acid equivalents per kilogram on a dry weight basis (g GAE kg−1) (y = 0.0049 x + 0.0736, r2 = 0.9999). A previously reported method was used to determine SQC (Qiao et al., 2021).
1,1-Diphenyl-2-trinitrophenylhydrazine (DPPH) free radical scavenging activity and Ferric reducing antioxidant power (FRAP) assay were evaluated by the method by Tang et al. (2015). DPPH free radical scavenging activity was expressed on a fresh weight basis as mmol Trolox equivalents per kilogram (mmol TE kg−1) (y = −0.0009 x + 0.0555, r2 = 0.9999). The FRAP antioxidant activity was expressed on a dry weight basis as mmol ascorbic acid equivalent per kilogram (mmol AAE kg−1) (y = 0.0017 x + 0.0032, r2 = 0.9996).
Production of H2O2 was measured by the hydrogen peroxide assay kit (Beijing Solarbio Science & Technology Co. Ltd., Beijing, China). The superoxide anion (O2·−) scavenging activity was assessed by a superoxide anion assay kit (Shanghai Yuanye Bio-Technology Co., Ltd., Shanghai, China).
Enzyme Activity Assay
PPO, POD, PAL, and CAT activities were evaluated referring to the method by our lab (Du et al., 2021). The 1 absorbance change per minute at 420 and 470 nm was denoted as one unit for PPO and POD activity, respectively. One unit of CAT and PAL activity was defined as 0.01 absorbance changes per minute at 240 and 290 nm, respectively. The results were expressed as U kg−1 on a fresh weight basis.
LOX activity was determined according to the method by Qiao et al. (2021) with minor modifications. A total of 5.0 g potato tissues and 5.0 mL extraction buffer were ground and homogenized in ice bath condition. Then the mixture was centrifuged at 12,000 × g at 4 °C for 30 min. Subsequently, 2.7 mL phosphoric acid buffer (0.1 mol L−1, pH 6.8) and 100 μL 0.5% linoleic acid solution were incubated at 30 °C for 10 min. Then 200 µL crude enzyme extract was added, mixed evenly, and immediately measured for absorbance. LOX activity was defined as 0.01 absorbance changes per minute at 234 nm. The results were expressed as U kg−1 on a fresh weight basis.
Enzyme Activity Ratio
The definition of EA ratio refers to our previous work (Li et al., 2022), while the scope of its definition was further extended as follows formulas in this study, which was used to reflect the resisting capacity against the cutting and chilling injury.
0—values on day 0; n — values on days 2, 4, 6, and 9.
Statistical Analysis
Data were expressed as mean ± SD. Origin 2018 (OriginLab Corp., USA) and SPSS 19 (IBM, USA) were used for plotting figures and experimental data analysis. The initial freezing and supercooling points were 10 replicates, and BI, ΔE, firmness, and fracturability were 5 replicates, which were analyzed by Tukey’s test with P ≤ 0.05. The other indicators were 3 replicates and analyzed by the least significant difference (LSD) method with P ≤ 0.05.
Results and Discussion
Freezing and Supercooling Points
As shown in Table 1 and Fig. S1, the average initial freezing point was –1.6 ± 0.4 °C, and the minimal supercooling point was –2.5 °C in fresh-cut potatoes, which suggests that fresh-cut potatoes can be stored at −2 °C. This result was in line with the previously reported initial freezing point in whole potatoes (–2 °C) (Comandini et al., 2013; Hruschka, 1961), leaf lettuces (–0.2 °C), carrot (–1.6 °C), parsnip (–2.2 °C), and broccoli (–2.1 °C) (James & James , 2011; Nguyen Quang et al., 2017). Therefore, the SC storage temperature in this study was set at −2.0 ± 0.5 °C.
Overall Visual and Textural Quality
Surface color is a crucial sensory parameter of fresh-cut potatoes. Browning occurrence lowers the purchase desirability of consumers directly (Osuga et al., 2021). The BI and ΔE usually evaluate the change of surface color in fresh-cut fruits and vegetables. The higher BI and ΔE are generally considered more severe browning (Toivonen & Brummell, 2008). It has been reported that the surface color of fresh-cut potatoes without any treatment changed drastically within 4 days at 4 °C (Liu et al., 2019; Zhou et al., 2019). In this study, SHOP, SC, and SHOP + SC postponed the increase of ΔE (Fig. 2b) and BI (Fig. 2c) and delayed the decline of firmness (Fig. 2d) and fracturability (Fig. 2e) of fresh-cut potatoes. From Fig. 2b, c, the surface color of potato samples from all groups gradually got darker (higher BI and ΔE) throughout the storage duration. In this study, the serene browning of the control and SHOP (6 days) occurred earlier than SC (9 days), while SHOP + SC maintained the lowest BI (113.10) and ΔE (10.26) with a light bright appearance after 9-day storage. The trends above were consistent with the study by Liu et al. (2019), where SHOP inhibited browning by activating PAL to enhance the antioxidant ability in fresh-cut potatoes. In addition, the SC and SHOP + SC maintained higher firmness (53.2 N, 55.3 N) and fracturability (56.8 N, 53.6 N) compared with the control and SHOP on day 9 (Fig. 2d, e). Similar effect as supercooled preservation controlled the microbial population and maintained color parameters and firmness has been previously reported in fresh-cut apples (Osuga et al., 2021). Therefore, the combination of SHOP and SC treatment is believed to be more effective than SHOP or SC alone in maintaining the storage quality and regulating the browning process.
SHOP combined with SC storage maintained the surface color and texture of fresh-cut potatoes at –2 °C for 9 days. Photographs of fresh-cut potatoes (a), BI (b), ΔE (c), firmness (d), and fracturability (e). Vertical bars indicate standard errors (n = 5). Different letters denote significant differences between treatments within each time interval (P ≤ 0.05)
Phenolic Compound Metabolism and Lipid Peroxidation
PPO is a critical enzyme for controlling the enzymatic browning in plants. After exposure to oxygen, phenols were catalyzed by PPO to form quinones, which polymerize themselves in plants or interact with amino acids and proteins in cells and finally produce black or brown deposits (Shen et al., 2020). As a wound-inducing enzyme, PAL is easily activated after cleavage (Qiao et al., 2022). The TPC (Fig. 3a), SQC (Fig. 3b), and PAL activity (Fig. 3c) of fresh-cut potatoes were increased, but the PPO activity (Fig. 3d) was inhibited by SHOP on day 0. On day 2, the PAL and POD activities (Figs. 3c and 4a) of SC were 35.6–49.2% lower than SHOP + SC. Moreover, the increment of TPC in SHOP + SC was 0.85–0.90 times that of the control and SC during the storage. Those results pointed out that SHOP inhibited enzymatic browning by activating the phenylpropane metabolic pathway, synthesizing secondary metabolites, and inhibiting the activity of PPO at conventional refrigeration temperature (4 °C). High oxygen (O2 ≥ 75%) pre-stimulation and high oxygen packaging were used to block the browning of fresh-cut watercored apples, fresh-cut eggplants, and mushrooms, which were consistent with this study (Li et al., 2014, 2017, 2022). In addition, the TPC of SHOP and SHOP + SC increased sharply from day 4 to day 6 and from day 6 to day 9, respectively. This result was consistent with the changes in photos (Fig. 1a), BI (Fig. 1b), and ΔE (Fig. 1c). From day 4 to day 9, non-statistically significance was observed in PPO activities between SC and SHOP + SC, while the highest POD activity and the lowest BI were obtained in SHOP + SC. Sun et al. (2015) reported that in the presence of H2O2, POD catalyzed the oxidation of phenolic substances, leading to tissue browning. This suggested that the main pathway of browning in SHOP + SC might be mediated by POD. In addition, the lower PAL activity and TPC in SHOP + SC in this study indicated that SHOP alleviated the stress of the SC environment on fresh-cut potatoes, maintained a lower biochemical reaction activity, and achieved the purpose of inhibiting browning.
SHOP combined with SC delayed the synthesis of phenolic substances, reduced phenolic oxidation, and delayed the lipid peroxidation of fresh-cut potatoes at –2 °C for 9 days. TPC (a), SQC (b), PAL activity (c), PPO activity (d), MDA (e), and LOX activity (f). Vertical bars indicate standard errors (n = 3). Different letters denote significant differences between treatments within each time interval (P ≤ 0.05)
SHOP combined with SC improved the antioxidant capacity and inhibited the accumulation of ROS of fresh-cut potatoes at –2 °C for 9 days. POD activity (a), CAT activity (b), O2·− content (c), H2O2 content (d), DPPH scavenging effect (e), and FRAP (f). Vertical bars indicate standard errors (n = 3). Different letters denote significant differences between treatments within each time interval (P ≤ 0.05)
Cell membrane integrity plays an essential part in browning development. MDA is a reference index to evaluate the degree of membrane lipid peroxidation, and higher MDA content generally means more severe damage to the cell membrane (Aghdam & Bodbodak, 2014; Zheng et al., 2019). LOX catalyzes the oxidation of polyunsaturated fatty acids, commonly observed in browning and chilling injury phenomena (Liu et al., 2011). In this study, the MDA content (Fig. 3e) of SC and SHOP + SC rose and then decreased throughout the storage process, while the MDA content of the control and SHOP continued to increase. The samples of SC group had the highest MDA content (Fig. 3e) and LOX activities (Fig. 3f) compared with the other three groups on day 2 and day 4, followed by SHOP + SC. Ma et al. (2022) reported that cold shock treatment regulated the membrane lipid metabolism of peach fruits to alleviate the chilling injury. Similarly, heat treatment protects against cold injury by improving the unsaturated/saturated fatty acid ratio (unSFA/SFA) and maintaining the membrane integrity of cold-sensitive fruits and vegetables (Aghdam & Bodbodak, 2014). Besides, severe browning was associated with higher MDA content and LOX activity in fresh-cut pears, potatoes, lettuce, and other fruits and vegetables (Li et al., 2014; Martín-Diana et al., 2008; Qiao et al., 2021; Zheng et al., 2019). In this study, the lowest MDA content (Fig. 3e) and LOX activity (Fig. 3f) and a relatively steady changing trend were found in SHOP + SC, which indicated the synergetic role of SHOP and SC in maintaining membrane integrity.
Antioxidant Capacity
The homeostasis between ROS production and elimination will be disrupted and induce severe accumulation of ROS under the stress conditions, such as drought, mechanical damage, and cold or heat shock (Aghdam & Bodbodak, 2014; Imahori et al., 2008). Excessive ROS (O2·− and H2O2) accelerates membrane lipid peroxidation, ultimately aggravating tissue chilling injury and browning (Imahori et al., 2008). O2·− leads to high local free radical concentration. However, SOD catalyzes O2·− into H2O2, which passes through the cell membrane to all parts of the cell to reduce the oxidative damage caused by excessive local ROS (Das & Roychoudhury, 2014). Due to the vigorous SOD activity and widespread presence in various parts of cells, H2O2 is the true representative of reactive oxygen species in tissue cells (Sies et al., 2022). H2O2 induced a plant defense system and activated the activity of defense enzymes (POD, PAL, and CAT) (Jaafar et al., 2012). H2O2 catalyzed by CAT to H2O and O2, which would reduce the oxidative damage caused by free radicals as well. Within the early 4 days of storage, the O2·− and H2O2 contents (Fig. 4c, d) of SHOP, SC, and SHOP + SC increased sharply, and then the H2O2 content gradually decreased on day 6 and was lower than that of the control. However, after 4 days of storage, the O2·− content in each treatment group was generally inhibited compared with the control (Fig. 4d). Those changes were similar to MDA content and LOX activity (Fig. 3e, f). This indicated that O2·− and H2O2 were induced by SHOP treatment to cause transient stress increase, and then it acted as a signaling molecule to regulate downstream related metabolic systems and other physiological and biochemical reaction processes, which enhanced the cold resistance and browning resistance of fresh-cut potatoes. Zheng et al. (2019) and Bai et al. (2022) found that melatonin and eugenol treatment reduced the ROS content of fresh-cut pears and fresh-cut yams to inhibit their browning, a pathway similar to this study.
Many studies have found that POD manifests itself as two mechanisms of action in fruits refrigeration. One mechanism is expressed in large quantities in the early stages of cold adversity, manifesting itself as a protective effect (Magri et al., 2022). Another mechanism is activated late in refrigeration adversity and manifests itself as a harmful effect, thereby reducing the storability of fruits (Li et al., 2019). Figure 4a, b shows that POD and CAT activities were increased by SHOP in fresh-cut potatoes from day 0 to day 2, while these enzyme activities decreased to 22.4 − 50.7% of the control on day 4. The POD and CAT activities of SHOP + SC gradually decreased lower than SC with the passage of storage time, which can be inferred that POD and CAT are non-key factors of the antioxidant system in the late refrigeration stage. Those results may be caused by POD and CAT metabolism disorders in long-term low-temperature storage (Boonyaritthongchai & Supapvanich, 2017). Overall, SHOP increased the activity of potato tubers and fresh-cut potato defense enzymes at the early stage of storage and inhibited the metabolic disorders of POD and CAT caused by supercooling.
Two chemical model systems, the DPPH scavenging effect and FRAP were used to evaluate the antioxidant activities of fresh-cut potatoes (Yu et al., 2019). SHOP had higher DPPH free radical scavenging and iron ion reducing ability, consistent with that of Liu et al. (2019), who tested that the DPPH free radical scavenging rate of fresh-cut potatoes was 53% after 80% O2 pretreatment, which was higher than 49% in the control (21% O2). However, the combined treatment did not enhance the antioxidant capacity of fresh-cut potatoes. In addition, the fluctuations of the DPPH scavenging effect (Fig. 4e) of the control, SHOP, SC, and SHOP + SC within 9 days of storage were 20.48, 18.37, 6.57, and 4.28 mmol TE kg−1, respectively. Moreover, the fluctuations in FRAP (Fig. 4f) of the control, SHOP, SC, and SHOP + SC were 9.01, 4.32, 3.77, and 4.25 mmol AAE kg−1, respectively. The above results may be due to persistent damage to fresh-cut potatoes caused by SC, but SHOP provided potato tubers with excellent resistance against minimal processing and supercooled storage, resulting in less damage in SHOP + SC than in SC after day 4, which failed to induce higher defense enzyme activities and antioxidant capacity.
Enzyme Activity Ratios
It has been reported that the enzyme activity (EA) ratio was applied as an overall and direct indicator to express the effectiveness of fresh-cut watercored apples in anti-browning ability (Li et al., 2022). The EA ratio refers to the proportion of the increments of the attack enzyme and the defense enzyme per unit of time, which can express the intensity of the life activity of the samples during storage. The trends of changes in the activities of various enzymes measured in this experiment were fluctuant within 9 days of storage. Still, after reprocessing the enzyme activity data by EA ratio, the differences and trends of changes in each treatment group gradually became clear. The Ratio1 (Fig. 5a), Ratio2 (Fig. 5b), and Ratio3 (Fig. 5c) of SHOP and SHOP + SC had minor variations than the control and SC. Moreover, the Ratio4 (Fig. 5d), Ratio5 (Fig. 5e), and Ratio6 (Fig. 5f) of the control and SC, and SHOP and SHOP + SC had the same trends. In Fig. 5a, Ratio1 was higher in SHOP and SHOP + SC than in the control and SC. Among them, the fluctuation ranges of Ratio1 values of SC and SHOP + SC within 9 days of storage were 3.9 and 1.7, respectively. In contrast, Ratio2 and Ratio3 (Fig. 5b, c) in SC and SHOP + SC showed a smaller variation range than the control and SC. The values range of SC in Ratio4, Ratio5, and Ratio6 was 12.6–47.5, 19.1–72.17, and 14.0–29.2 times higher than the other three treatment groups, respectively. Those results have shown that SHOP inhibited the persistent damage to fresh-cut potatoes caused by the storage environment and maintained the fresh-cut potatoes in a stable state of lower biological activity. In addition, except for Ratio1, the ratios of other enzyme activities in the control reached the maximum on day 6, while Ratio1 and Ratio2, Ratio3, Ratio4, and Ratio5 (Fig. 5a–e) of SC were on day 9. Overall, SHOP induced a more extraordinary ability to resist supercooling and cutting injury for the cell membrane within 4 days of storage. We also found that the change point of EA ratios was consistent with the color mutation point of fresh-cut potatoes, supporting those ratios as comprehensive and direct indicators of when the quality of fresh-cut potatoes reaches the plateau.
SHOP combined with SC storage showed a slight fluctuation on Ratio1, Ratio2, Ratio3, Ratio4, Ratio5, and Ratio6 of fresh-cut potatoes at –2 °C for 9 days. Ratio1 ((PODn-POD0)/(PPOn-PPO0), (a)), Ratio2 ((PALn-PAL0)/(PPOn-PPO0), (b)), Ratio3 ((CATn-CAT0)/(PPOn-PPO0), (c)), Ratio4 ((PODn-POD0)/(LOXn-LOX0), (d)), Ratio5 ((PALn-PAL0)/(LOXn-LOX0), (e)), and Ratio6 ((CATn-CAT0)/(LOXn-LOX0), (f)). Data are represented by means
According to our results and previous studies, the mechanism of SHOP treatment to inhibit browning and chilling injury of fresh-cut potatoes was summarized in Fig. 6 (Li et al., 2014, 2022; Mao et al., 2007; Sies et al., 2022). The surface color, texture, phenolic compounds anabolism, membrane integrity, antioxidant capacity, ROS balancing, and EA ratios in fresh-cut potatoes were comprehensively considered in this study. The results demonstrated that SHOP treatment enhanced the antioxidant capacity in the early stage of storage, promoted the synthesis of phenolic compounds, and inhibited PPO activity and phenolic oxidation, thereby maintaining the stability of the cell membrane system under conventional refrigerated and supercooled storage temperatures. Therefore, SHOP inhibits the browning and texture softening of fresh-cut potatoes by enhancing resistance to membrane lipid damage caused by mechanical cutting and continuous SC stimulation.
Schematic overview of the proposed model for SHOP-induced cold tolerance and anti-browning. ↓: Stimulatory effect; ⊥: Inhibitory effect; enzyme abbreviations in blue and orange: attack enzymes and defend enzymes, respectively. The photographs at the bottom exhibit the appearance of fresh-cut potatoes at –2 °C for 9 days after treatment with SHOP combined with SC storage
Conclusions
This study indicated that the combination of SHOP and SC was more effective in controlling browning and delaying texture decline of fresh-cut potatoes than either treatment alone. The activities of PPO and POD were reduced and the SQC was increased by these three treatments compared with the control. There was no statistical difference in PPO activity between SC and SHOP + SC, but the POD activity and BI of SC were higher than that of SHOP + SC. Moreover, phenolic and ROS metabolism, including PAL and CAT activities, TPC, DPPH, and H2O2 content, was affected by SHOP, SC, and SHOP + SC. Moreover, the SHOP + SC had the highest membrane integrity with the lowest LOX activity and MDA content among all the groups. Furthermore, minor changes in EA ratios in the SHOP and SHOP + SC indicated reduced and alleviated biological activities. Overall, SHOP combined with SC has the potential to be an effective and promising method in maintaining texture and controlling the browning of fresh-cut potatoes. Compared with the use of food additives, SHOP + SC treatment as a combined physical preservation method has the advantages of effective, environmentally friendly, ease of operation, which is promising for application in fresh-cut industry.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Aghdam, M. S., & Bodbodak, S. (2014). Postharvest heat treatment for mitigation of chilling injury in fruits and vegetables. Food Bioprocess Technology, 7, 37–53. https://doi.org/10.1007/s11947-013-1207-4
Ahmed, Z. F., Kaur, N., Maqsood, S., & Schmeda-Hirschmann, G. (2022). Preharvest applications of chitosan, salicylic acid, and calcium chloride have a synergistic effect on quality and storability of date palm fruit (Phoenix dactylifera L.). HortScience, 57(3), 422–430. https://doi.org/10.21273/HORTSCI16416-21
Bai, T., Li, J., Murtaza, A., Lqbal, A., Zhu, L., Zhang, J., Zhang, B., Xu, X., Pan, S., & Hu, W. (2022). Scavenging of ROS after eugenol treatment as mechanism of slowing down membrane lipid metabolism to maintain the surface color of fresh-cut yam. Food Bioprocess Technology, 15, 1821–1835. https://doi.org/10.1007/s11947-022-02833-0
Boonyaritthongchai, P., & Supapvanich, S. (2017). Effects of methyl jasmonate on physicochemical qualities and internal browning of ‘queen’ pineapple fruit during cold storage. Horticulture, Environment, and Biotechnology, 58(5), 479–487. https://doi.org/10.1007/s13580-017-0362-3
Buera, M. P., Lozano, R. D., & Petriella, C. (1986). Definition of colour in the non enzymatic browning process. Die Farbe, 32(33), 318–322.
Chen, L., Zhou, G., & Zhang, W. (2015). Effects of high oxygen packaging on tenderness and water holding capacity of pork through protein oxidation. Food Bioprocess Technology, 8, 2287–2297. https://doi.org/10.1007/s11947-015-1566-0
CIE, O. (1976). Official recommendations on uniform color space, color difference equations and metric color terms. CIE Publ, 15, 1976.
Comandini, P., Blanda, G., Soto-Caballero, M. C., Sala, V., Tylewicz, U., Mujica-Paz, H., Valdez Fragoso, A., & Gallina Toschi, T. (2013). Effects of power ultrasound on immersion freezing parameters of potatoes. Innovative Food Science & Emerging Technologies, 18, 120–125. https://doi.org/10.1016/j.ifset.2013.01.009
Das, K., & Roychoudhury, A. (2014). Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Frontiers in Environmental Science, 2(53). https://doi.org/10.3389/fenvs.2014.00053
Du, M., Liu, Z., Zhang, X., Li, H., Liu, Z., Li, X., Song, J., Jia, X., & Wang, L. (2021). Effect of pulsed controlled atmosphere with CO2 on the quality of watercored apple during storage. Scientia Horticulturae, 278, 109854. https://doi.org/10.1016/j.scienta.2020.109854
Fan, K., Zhang, M., Guo, C., Dan, W., & Devahastin, S. (2021). Laser-induced microporous modified atmosphere packaging and chitosan carbon-dot coating as a novel combined preservation method for fresh-cut cucumber. Food and Bioprocess Technology, 14(5), 968–983. https://doi.org/10.1007/s11947-021-02617-y
Feumba Dibanda, R., Panyoo Akdowa, E., Rani, P. A., Metsatedem Tongwa, Q., & Mbofung, F. C. M. (2020). Effect of microwave blanching on antioxidant activity, phenolic compounds and browning behaviour of some fruit peelings. Food Chemistry, 302, 125308. https://doi.org/10.1016/j.foodchem.2019.125308
Garcia-Lomillo, J., González-SanJosé, M. L., Skibsted, L. H., & Jongberg, S. (2016). Effect of skin wine pomace and sulfite on protein oxidation in beef patties during high oxygen atmosphere storage. Food Bioprocess Technology, 9, 532–542. https://doi.org/10.1007/s11947-015-1649-y
Global Market Insights, I. (2021). Processed fruits and vegetables market to hit $425 billion by 2026. Retrieved January 10, 2022, from https://www.prnewswire.com/news-releases/processed-fruits-and-vegetables-market-to-hit-425-billion-by-2026-says-global-market-insights-inc-301207816.html
Haverkort, A. J., Linnemann, A. R., Struik, P. C., & Wiskerke, J. S. C. (2022). On processing potato: 1. Survey of the ontology, history and participating actors. Potato Research. https://doi.org/10.1007/s11540-022-09562-z
Helena Gomes, M., Beaudry, R. M., Almeida, D. P. F., & Xavier Malcata, F. (2010). Modelling respiration of packaged fresh-cut ‘Rocha’ pear as affected by oxygen concentration and temperature. Journal of Food Engineering, 96, 74–79. https://doi.org/10.1016/j.jfoodeng.2009.06.043
Heo, J., Choi, M., & Hong, J. (2019). Facile surface modification of polyethylene film via spray-assisted layer-by-layer self-assembly of graphene oxide for oxygen barrier properties. Scientific Reports, 9, 2754. https://doi.org/10.1038/s41598-019-39285-0
Hruschka, H. W. (1961). Seed potato productivity after cooling, supercooling, or freezing (No. 507). USDA Marketing Research Report. https://doi.org/10.22004/ag.econ.313071
Hu, W., Guan, Y., Ji, Y., & Yang, X. (2021). Effect of cutting styles on quality, antioxidant activity, membrane lipid peroxidation, and browning in fresh-cut potatoes. Food Bioscience, 44, 101435. https://doi.org/10.1016/j.fbio.2021.101435
Imahori, Y., Takemura, M., & Bai, J. (2008). Chilling-induced oxidative stress and antioxidant responses in mume (Prunus mume) fruit during low temperature storage. Postharvest Biology and Technology, 49(1), 54–60. https://doi.org/10.1016/j.postharvbio.2007.10.017
Jaafar, H. Z. E., Ibrahim, M. H., & Mohamad Fakri, N. F. (2012). Impact of soil field water capacity on secondary metabolites, phenylalanine ammonia-lyase (PAL), maliondialdehyde (MDA) and photosynthetic responses of malaysian kacip fatimah (Labisia pumila Benth). Molecules, 17, 7305–7322. https://doi.org/10.3390/molecules17067305
James, C., Seignemartin, V., & James, S. J. (2009). The freezing and supercooling of garlic (Allium sativum L.). International Journal of Refrigeration, 32, 253–260. https://doi.org/10.1016/j.ijrefrig.2008.05.012
James, C. H. P., & James, S. J. (2011). Super-cooling phenomena in fruits, vegetables and seafoods. International congress on engineering and food (11th ed., pp. 22–26). Athens, Greece
Kaale, L. D., Eikevik, T. M., Rustad, T., & Kolsaker, K. (2011). Superchilling of food: A review. Journal of Food Engineering, 107, 141–146. https://doi.org/10.1016/j.jfoodeng.2011.06.004
Koide, S., Kumada, R., Hayakawa, K., Kawakami, I., & Uemura, M. (2019a). Survival of cut cabbage subjected to subzero temperatures. Acta Horticulturae, 1256, 329–334. https://doi.org/10.17660/ActaHortic.2019.1256.46
Koide, S., Ohsuga, R., Orikasa, T., & Uemura, M. (2019b). Evaluation of electrical and physiological properties of supercooled fresh cut spinach. Japanese Society of Food Science and Technology, 66(9), 335–340. https://doi.org/10.3136/nskkk.66.335
Kou, X., Guo, W., Guo, R., & Li, X. (2014). Effects of chitosan, calcium chloride, and pullulan coating treatments on antioxidant activity in pear cv. “Huang guan” during storage. Food & Bioprocess Technology, 7(3), 671–681. https://doi.org/10.1007/s11947-013-1085-9
Li, J., Zhou, X., Wei, B., Cheng, S., Zhou, Q., & Ji, S. (2019). GABA application improves the mitochondrial antioxidant system and reduces peel browning in ‘Nanguo’ pears after removal from cold storage. Food Chemistry, 297, 124903. https://doi.org/10.1016/j.foodchem.2019.05.177
Li, L., Sun, H., Kitazawa, H., & Wang, X. (2017). Effects of a high O2 dynamic-controlled atmosphere technology on the browning of postharvest white mushroom (Agaricus bisporus) in relation to energy metabolism. Food Science and Technology International, 23(5), 385–395. https://doi.org/10.1177/1082013217695146
Li, X., Jiang, Y., Li, W., Tang, Y., & Yun, J. (2014). Effects of ascorbic acid and high oxygen modified atmosphere packaging during storage of fresh-cut eggplants. Food Science and Technology International, 20, 99–108. https://doi.org/10.1177/1082013212472351
Li, X., Liu, Z., Ran, Y., Li, L., Chen, L., Lin, Q., Liang, F., Li, J., Li, X., & Tang, Y. (2022). Short-term high oxygen pre-stimulation inhibits browning of fresh-cut watercored Fuji apples. Postharvest Biology and Technology, 191, 111959. https://doi.org/10.1016/j.postharvbio.2022.111959
Liu, H., Song, L., You, Y., Li, Y., Duan, X., Jiang, Y., Joyce, D. C., Ashraf, M., & Lu, W. (2011). Cold storage duration affects litchi fruit quality, membrane permeability, enzyme activities and energy charge during shelf time at ambient temperature. Postharvest Biology and Technology, 60, 24–30. https://doi.org/10.1016/j.postharvbio.2010.11.008
Liu, X., Wang, T., Lu, Y., Yang, Q., Li, Y., Deng, X., Liu, Y., Du, X., Qiao, L., & Zheng, J. (2019). Effect of high oxygen pretreatment of whole tuber on anti-browning of fresh-cut potato slices during storage. Food Chemistry, 301, 125287. https://doi.org/10.1016/j.foodchem.2019.125287
Ma, Y., Hu, S., Chen, G., Zheng, Y., & Jin, P. (2022). Cold shock treatment alleviates chilling injury in peach fruit by regulating antioxidant capacity and membrane lipid metabolism. Food Quality and Safety, 6,. https://doi.org/10.1093/fqsafe/fyab026
Magri, A., Cice, D., Capriolo, G., & Petriccione, M. (2022). Effects of ascorbic acid and melatonin treatments on antioxidant system in fresh-cut avocado fruits during cold storage. Food Bioprocess Technology, 15, 2468–2482. https://doi.org/10.1007/s11947-022-02892-3
Mao, L., Pang, H., Wang, G., & Zhu, C. (2007). Phospholipase D and lipoxygenase activity of cucumber fruit in response to chilling stress. Postharvest Biology and Technology, 44, 42–47. https://doi.org/10.1016/j.postharvbio.2006.11.009
Martín-Diana, A. B., Rico, D., & Barry-Ryan, C. (2008). Green tea extract as a natural antioxidant to extend the shelf-life of fresh-cut lettuce. Innovative Food Science & Emerging Technologies, 9, 593–603. https://doi.org/10.1016/j.ifset.2008.04.001
Nadeem, A., Ahmed, Z. F. R., Hussain, S. B., Omar, A. E. D. K., Amin, M., Javed, S., Ali, A., Ullah, S., Razzaq, K., Rajwana, I. A., Nayab, S., Ziogas, V., Alam-Eldein, S. M., & Mira, A. M. (2022). On-tree fruit bagging and cold storage maintain the postharvest quality of mango fruit. Horticulturae, 8, 814. https://doi.org/10.3390/horticulturae8090814
Nguyen Quang, T., 岩村, 幸., Shrestha, R., & 杉村, 延. (2017). 植物工場におけるレタスの過冷却保存に関する基礎的研究. 日本食品工学会誌, 18, 25–32. https://doi.org/10.11301/jsfe.16473. A study on supercooled storage of leaf lettuces produced in plant factory. Retrieved August 10, 2022, from https://www.jstage.jst.go.jp/AF06S010ShoshJkuDld?sryCd=jsfe&noVol=18&noIssue=1&kijiCd=18_16473&kijiLangKrke=en&kijiToolIdHkwtsh=AT0072&request_locale=EN
Norrrahim, M. N. F., Ariffin, H., Hassan, M. A., Ibrahim, N. A., & Nishida, H. (2013). Performance evaluation and chemical recyclability of a polyethylene/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) blend for sustainable packaging. RSC Advances, 3, 24378–24388. https://doi.org/10.1039/c3ra43632b
Osuga, R., Koide, S., Sakurai, M., Orikasa, T., & Uemura, M. (2021). Quality and microbial evaluation of fresh-cut apples during 10 days of supercooled storage. Food Control, 126, 108014. https://doi.org/10.1016/j.foodcont.2021.108014
Phinney, D. M., Frelka, J. C., Wickramasinghe, A., & Heldman, D. R. (2017). Effect of freezing rate and microwave thawing on texture and microstructural properties of potato (Solanum tuberosum). Journal of Food Science, 82, 933. https://doi.org/10.1111/1750-3841.13690
Qiao, L., Gao, M., Wang, Y., Tian, X., Lu, L., & Liu, X. (2022). Integrated transcriptomic and metabolomic analysis of cultivar differences provides insights into the browning mechanism of fresh-cut potato tubers. Postharvest Biology and Technology, 188, 111905. https://doi.org/10.1016/j.postharvbio.2022.111905
Qiao, L., Gao, M., Zheng, J., Zhang, J., Lu, L., & Liu, X. (2021). Novel browning alleviation technology for fresh-cut products: Preservation effect of the combination of Sonchus oleraceus L. extract and ultrasound in fresh-cut potatoes. Food Chemistry, 348, 129132. https://doi.org/10.1016/j.foodchem.2021.129132
Rico, D., Martín-Diana, A. B., Barat, J. M., & Barry-Ryan, C. (2007). Extending and measuring the quality of fresh-cut fruit and vegetables: A review. Trends in Food Science & Technology, 18, 373–386. https://doi.org/10.1016/j.tifs.2007.03.011
Ru, X., Tao, N., Feng, Y., Li, Q., & Wang, Q. (2020). A novel anti-browning agent 3-mercapto-2-butanol for inhibition of fresh-cut potato browning. Postharvest Biology and Technology, 170, 111324. https://doi.org/10.1016/j.postharvbio.2020.111324
Shen, X., Zhang, M., Fan, K., & Gao, Z. (2020). Effects of ε-polylysine/chitosan composite coating and pressurized argon in combination with MAP on quality and microorganisms of fresh-cut potatoes. Food Bioprocess Technology, 13, 145–158. https://doi.org/10.1007/s11947-019-02388-7
Sies, H., Belousov, V. V., Chandel, N. S., Davies, M. J., Jones, D. P., Mann, G. E., Murphy, M. P., Yamamoto, M., & Winterbourn, C. (2022). Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nature Reviews Molecular Cell Biology, 23, 499–515. https://doi.org/10.1038/s41580-022-00456-z
Suman, S., Bagal, D., Jain, D., Ragini, S., Singh, I. K., & Singh, A. (2021). Biotic stresses on plants: Reactive oxygen species generation and antioxidant mechanism. In T. Aftab & K. R. Hakeem (Eds.), Frontiers in plant-soil interaction (14th ed., pp. 381–411). Academic Press.
Sun, Y., Zhang, W., Zeng, T., Nie, Q., Zhang, F., & Zhu, L. (2015). Hydrogen sulfide inhibits enzymatic browning of fresh-cut lotus root slices by regulating phenolic metabolism. Food Chemistry, 177, 376–381. https://doi.org/10.1016/j.foodchem.2015.01.065
Tang, Y., Li, X., Zhang, B., Chen, P. X., Liu, R., & Tsao, R. (2015). Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chemistry, 166, 380–388. https://doi.org/10.1016/j.foodchem.2014.06.018
Toivonen, P. M. A., & Brummell, D. A. (2008). Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biology and Technology, 48, 1–14. https://doi.org/10.1016/j.postharvbio.2007.09.004
Xylia, P., Chrysargyris, A., Shahwar, D., Ahmed, Z. F. R., & Tzortzakis, N. (2022). Application of rosemary and eucalyptus essential oils on the preservation of cucumber fruit. Horticulturae, 8, 774. https://doi.org/10.3390/horticulturae8090774
Yaptenco, K. F., Kim, J. G., & Lim, B. S. J. P. A. S. (2007). Gas transmission rates of commercially available polyethylene and polypropylene films for modified atmosphere packaging. Philippine Agriculturist, 90(1), 22–27.
Yu, X., Jing, Y., & Yan, F. (2019). Chitooligosaccharide–lysine maillard reaction products: Preparation and potential application on fresh-cut kiwifruit. Food Bioprocess Technology, 12, 1133–1143. https://doi.org/10.1007/s11947-019-02284-0
Zarbà, C., Chinnici, G., Pecorino, B., Pappalardo, G., & D’Amico, M. (2022). Recent scenarios in Italy on fresh-cut products in the Covid-19 context. AIMS Agriculture and Food, 7, 403. https://doi.org/10.3934/agrfood.2022026
Zhang, L., Deng, N., Yagoub, A. E. A., Chen, L., Mustapha, A. T., Yu, X., & Zhou, C. (2022). Ultrasound-assisted probiotics fermentation suspension treatment under mild heat to improve the storage quality of freshly cut lotus root. Food Chemistry, 397, 133823. https://doi.org/10.1016/j.foodchem.2022.133823
Zheng, H., Liu, W., Liu, S., Liu, C., & Zheng, L. (2019). Effects of melatonin treatment on the enzymatic browning and nutritional quality of fresh-cut pear fruit. Food Chemistry, 299, 125116. https://doi.org/10.1016/j.foodchem.2019.125116
Zhou, F., Jiang, A., Feng, K., Gu, S., Xu, D., & Hu, W. (2019). Effect of methyl jasmonate on wound healing and resistance in fresh-cut potato cubes. Postharvest Biology and Technology, 157, 110958. https://doi.org/10.1016/j.postharvbio.2019.110958
Funding
This work was supported by the Key Research and Development Program of Shandong Province (2021CXGC010809), the Key Science and Technology Planning program of Tianjin (22ZYJDSS00090), Haihe Scholar Program (1185/10348) from Tianjin University of Science and Technology and Construction Project of “Quancheng Scholars.”
Author information
Authors and Affiliations
Contributions
Xuejin Li (First Author) and Yuqian Jiang (First Author) wrote the main manuscript text and prepared all figures and table. Yue Liu and Lu Li were responsible for data curation. Fuhao Liang, Xiaodong Wang, Dandan Li, and Na Pan participated in experiment preparation and data determination. Xihong Li, Xiangzheng Yang (Corresponding Author), and Yao Tang (Corresponding Author) provided funders and writing–review and editing. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Jiang, Y., Liu, Y. et al. Effects of Short-Term High Oxygen Pre-Stimulation on Browning Resistance and Low-Temperature Tolerance of Fresh-Cut Potatoes in Supercooled Storage. Food Bioprocess Technol 17, 709–721 (2024). https://doi.org/10.1007/s11947-023-03157-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-023-03157-3