Abstract
Aflatoxin B1 (AFB1) is the most hazardous mycotoxin for humans. It is mainly produced by Aspergillus flavus and A. parasiticus in a wide range of crops. Alternative strategies to the use of pesticides are more and more studied in order to limit the fungal development as well as the synthesis of aflatoxins in food and feeds. The use of aqueous plants extracts with high an-tioxidant activity has been the issue of different studies because of their impact on AFB1 production. The identification of plant extracts able to block aflatoxinogenesis could represent an ecofriendly and sustainable strategy for small producers to protect their crops from contamination, taking advantage of the local plant ressources. In this study, we characterized the antifungal and anti-aflatoxin B1 activity of 10 Peruvian plants selected for their known biological activities. For some plant extracts, the activity against AFB1 production was correlated with their composition and especially their polyphenols content and subsequent antioxidant activity. This is the case for Aloysia citrodora (AC) that inhibited 78% of the AFB1 synthesis, followed by Annona muricata (AM) (69%), Uncaria tomentosa (UT) (58%), and Myrciaria dubia (MD) (32%). By contrast, extracts of Dysphania ambrosioides (DA) and mainly Minthostachys mollis (MM) were effective against AFB1 synthesis inhibiting 47% and 89% of the production respectively but displayed a very limited antioxidant activity (IC50 DDPH > 400 mg/L), suggesting another route of inhibition. This is the first study demonstrating the anti-aflatoxin B1 effect of aqueous extracts of these plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aflatoxins (AFs) are secondary metabolites naturally produced by foodborne fungi, mostly belonging to the genus Aspergillus and the Flavi section. Aspergillus flavus and Aspergillus parasiticus are the species that have been most frequently reported to produce aflatoxins in a wide range of crops (Bryden, 2012; Taniwaki et al., 2018). The toxins produced by toxigenic fungi are aflatoxins B1, B2, G1, and G2, whereas aflatoxin M1 corresponds to a hydroxylated metabolite produced in liver of exposed animals from AFB1 and possibly excreted in milk of dairy animals. Among these compounds, aflatoxin B1 (AFB1) is the most harmful, classified in the group I of carcinogenic compounds for humans by the International Agency for Research on Cancer (IARC) (IARC, 2012). Indeed, AFB1 is responsible for hepatocarcinoma and is considered to be the natural contaminant leading to the most important number of DALYs (deaths and disability adjusted life years) (Gibb et al., 2015).
Food contamination with AFB1 represents a real public health issue, mainly in tropical and subtropical regions, because of the climatic conditions that favor the development of aflatoxigenic species. However, due to global warming and subsequent fluctuations in climate patterns, AFB1 is an emerging threat in areas such as Europe where it has been recently reported in some crops (Bailly et al., 2018; Gruber-Dorninger et al., 2019; Luo et al., 2021).
Different strategies have been established in order to limit the fungal development as well as the synthesis of aflatoxins in crops, which are applied either in the field or during storage. Mainly, the pre- and post-harvest Good Agricultural Practices (GAP) are fundamental to reduce the development of fungi. However, this cannot avoid totally the contamination as demonstrated by surveys and the frequent presence of aflatoxins in crops. The use of fungicides is also common to control the development of fungi on crops. But their excessive use may have a direct impact on health due to the own toxicity of these compounds. In addition, these chemicals have an impact on the biodiversity and their massive use led to the appearance of resistance mechanisms in target organisms that subsequently reduced their efficacy (Hahn, 2014; Kumar et al., 2021). For these reasons, alternative strategies are required to limit the contamination of crops with aflatoxins. Some atoxigenic strains used as competitor to limit the development of toxigenic strains and aflatoxin contamination of crops have demonstrated their efficiency, but there are still questions on the long-term behavior of these strains, and impact on global biodiversity remains to be elucidated (Ojiambo et al., 2018).
The search for natural resources, mainly plants, that are safe and effective in controlling AFB1 contamination has been the subject of different studies over the years (da Cruz Cabral et al., 2013; Makhuvele et al., 2020). It has been shown that different extracts and essential oils from plants, herbs, and spices have antifungal and anti-AFB1 activity (El Khoury et al., 2017; Hernandez et al., 2021; Rammanee & Hongpattarakere, 2011; Vamvakas et al., 2021). The main active molecules reported from plants are polyphenols, terpenes, and alkaloids (Caceres et al., 2016; Gómez et al., 2018; Marei et al., 2012; Ribeiro et al., 2020). In addition, it has been shown that the anti-AFB1 potential of some plant extracts could be related to their antioxidant activity (Caceres et al., 2017).
Such diversity of anti-aflatoxigenic compounds could represent an eco-friendly strategy for local small producers to protect their crops while valorizing local natural plant resources.
Peru is considered one of the most megadiverse countries in the world (Ministerio del ambiente, 2010); the great variety of climates in the country (28 out of the 32 existing climates in the world) is mainly due to its tropical latitude and the Andes Mountains. All this allows having a wide range of plants, approximately 25,000 species, of which 5000 are highly used by the local population due to their biological and medicinal properties (Bussmann & Sharon, 2006). For example, it has been reported that a great variety of these plants have antibacterial or anti-inflammatory activities and digestive, analgesic, hypotensive properties; and are muscle relaxant, anticonvulsant, immune system enhancers (Berlowski et al., 2013; Bussmann & Sharon, 2006; De Sousa et al., 2010). It has been suggested that some of these bioactive properties, such as antioxidant activity, could be involved in the inhibition of the AFB1 synthesis (Grintzalis et al., 2014). Thus, some of these plants represent good candidates for the inhibition of aflatoxin B1 production. However, the anti-AFB1 activity has not currently been evaluated for these Peruvian medicinal plants.
Within this context, the objective of this work was to screen extracts from different parts of Peruvian plants (leaves, roots, and grains), known for their described various biological activities, for their antifungal and anti-AFB1 potential in Aspergillus flavus. Aqueous extracts of ten plants were analysed after preparation with two different extraction methods. Activity against AFB1 production was correlated with the composition of the extracts and especially their polyphenols content and antioxidant activity.
Materials and Methods
Chemicals and Reagents
All analytical grade solvents and chemicals were purchased from Sigma-Aldrich (St Quentin- Fallavier, France). Stock solutions of the AFB1 standards were prepared in methanol and stored at 4 °C in the dark. Ethanol 96% and sodium carbonate were purchased from VWR International (Fontenay sous bois, France). Ultrapure water was obtained by using Veolia Purelab Classic (Veolia, Toulouse, France).
Plant Sources and Preparation
Plant Materials
Ten plant species were used in this work (Table 1). The main selection criteria were their commercial availability, their traditional uses in Peru and their known biological properties. The raw materials used in this study were purchased in local markets in the city of Lima. Dried plants were ground by using a mill equipped with a 2 mm grid, and the powder was stored in plastic bottles at 4 °C until use.
Aqueous Extract Preparation
Maceration
Maceration was performed as described by Hernandez et al. (2021) with a liquid/solid ratio of 33. For that, three grams of ground raw material were mixed in 100 mL of distilled water and stirred in a magnetic stirrer at 350 rpm (Thermo Scientific TM RT basic series, Massachusetts, USA) for 15 h at room temperature. The solution was then centrifuged at 15,000 rfc for 15 min (Sigma 6–16 K Centrifuge, Osterode am Harz, Germany) and filtered under vacuum (Buchner filtration, Whatman No. 1 paper). The filtrate volume was completed up to 100 mL with distilled water and sterilized at 121 °C for 20 min (SMI group UNICOM, Montpellier, France). Each extraction was done in triplicate. The final extracts were stored at 4 °C until use.
Accelerated Solvent Extraction (ASE)
Extractions were carried out using a Dionex ASE 300 accelerated solvent extractor (Sunnyvale, CA, USA). Briefly, 3.5 g of the plant samples (as recommended by the apparatus furnisher) were transferred in a 33-mL stainless steel vessel and extracted using 26 mL of distilled water as extracting solvent. To enhance polyphenol extraction, some of the conditions proposed by Repajić et al. (2020) were considered. The extractions were performed at 100 bars, with 5 min equilibration, 5 min static time, and a 120-s purge for three cycles. The extractions were done at 50 °C. About 30 mL of solvent was collected for each extraction. The extract volume was adjusted to 100 mL with distilled water and sterilized at 121 °C for 20 min (SMI group UNICOM, Montpellier, France). Each extraction was done in triplicate. The final extracts were stored at 4 °C until use.
Characterization of the Extracts
Dry Matter Content and Extraction Yield
Dry matter content was obtained by weighting each extract before and after drying in a Memmert oven at 100 °C (Memmert, Schwabach, Germany) until reaching a constant weight. Extraction yield is calculated using the following Formula (1):
Determination of the Total Phenolic Content
Total content of polyphenols in the extracts was determined by using the Folin-Ciocalteu method as described by Hernandez et al. (2021). A 20 µL of the sample was mixed with 10 µL of Folin-Ciocalteu reagent and 170 µL of sodium carbonate at 2.36% in water in a 96-well microplate. Each sample was analyzed in duplicate, and the absorbance was measured at 700 nm using a BMG-Labtech Spectrostar-Nano spectrophotometer (BMG LABTECH SA RL, Champigny s/Marne, France) at 45 °C after 45 min. A range of calibration with Gallic acid was performed at different concentrations (10, 25, 50, 80,100 mg/L) and analyzed in the same way as the samples in order to obtain a calibration curve. The results are expressed in milligrams of Gallic acid equivalents (GAE) per gram of dry extract.
Determination of the Free Radical Scavenging Activity
Antioxidant activity was obtained by using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method adapted from Brand-Williams et al. (1995).
A 150 µL of DPPH reagent was mixed with seven different concentrations of the sample (in a constant volume of 150 µL), and the absorbance was measured at 516 nm after a reaction time of 40 min in a BMG-Labtech Spectrostar Nano spectrophotometer (BMG LABTECH SARL, Champigny s/ Marne, France). The analysis was performed on 5 replicates for each sample concentration. The reaction equation is presented below (2) (Olszowy & Dawidowicz, 2018):
where Ce is the concentration of the extract (in g dry matter/L), A is the absorbance of the extract, and A0 is the absorbance of the blank (DPPH) after 40 min. The radical-scavenging activity is shown as the half-inhibitory concentration (IC50), which represents the concentration of the extract that inhibits 50% of the DPPH radicals. Results are expressed in milligrams of extract per liter (mg/L).
The IC50 is calculated by using the following linear regression (3):
where IC50 is the half-inhibitory concentration, a is the origin at the ordinate, and b is the slope.
Determination of the Condensed Tannins Content
Condensed tannins content of each extract was quantified by an adaptation of Waterman and Mole (1994). Samples were first diluted with distilled water in order to obtain, after hydrolysis, an absorbance lower than 0.200.
For each sample, 2 tubes were used, one as control and one for the test. In each tube, 2 mL of the diluted sample, 1 mL of distilled water, and 3 mL of concentrated hydrochloric acid (12 N) were added. Then, the control tubes were placed on crushed ice, while the sample tubes were incubated at 100 °C for 30 min. The tubes were then cooled on crushed ice.
A total of 0.5 mL of ethanol was added to all tubes (control and test) and vortexed for 10 s. Finally, absorbance was measured at 550 nm using a SHIMADZU UV1800 spectrophotometer (Shimadzu Corp, Kyota, Japan). The tannin concentration is calculated as follows (4):
The test was repeated in triplicate (n = 3), and the results were expressed in mg of condensed tannins per g dry matter (DM) ± standard error of the mean.
Assessment of the Impact of Extracts on Fungal Growth and AFB1 Production in Aspergillus Flavus
Fungal Strain and Culture Conditions
Briefly, 10 µL of a calibrated spore suspension (1000 spores) of Aspergillus flavus strain NRRL 62,477 (El Mahgubi et al., 2013), prepared in Tween 80 from a 7-day culture, was inoculated centrally onto culture medium. The culture medium included 18 mL of Malt Extract Agar (MEA) (Biokar Diagnostics, Allone, France) and 2 mL of autoclaved extract prepared at four different concentrations (0.75, 1.5, 3, and 6 mg DM/mL) by dilution in water. Control cultures were prepared by mixing 2 mL of distilled water with 18 mL of MEA. Cultures were incubated at 27 °C for 7 days. After incubation, the growth was assessed by measuring the colony diameter. Each assay was done in triplicate.
Aflatoxin Extraction and HPLC Quantification
AFB1 analysis was done as described by El Khoury et al. (2017). Culture media were mixed with 30 mL of HPLC-grade absolute chloroform. Samples were shaken for 1 h at room temperature on a horizontal shaking table at 200 rpm; then, the supernatants were filtered through a Whatman 1PS phase separator (GE Healthcare Life Sciences, Vélizy-Villacoublay, France). Filtrates (2 mL) were evaporated to dryness in a STUART SBH200D/3 sample concentrator (Stuart equipment, Paris, France) at 45 °C, dissolved in 2 mL of acetonitrile, filtered using 0.45 µm PTFE disk filters (Thermo Fisher Scientific, Illkirch-Graffenstaden, France) and placed in HPLC vials. HPLC analysis was performed in order to quantify the AFB1 by using an Ultimate 3000 UHPLC (Thermo Fisher Scientific, Illkirch-Graffenstaden, France) with an EvoC18 column (3 µm, 150 × 3.2, Phenomenex, Le Pecq, France) conditioned at 27 °C. The aflatoxin separation was performed by using an elution program consisting in an isocratic mixture composed of acetonitrile and water (25:75 v: v). The mobile phase had a flow rate of 1.2 mL/min. A 10 µL of the sample was injected.
Aflatoxin B1 was detected by using a fluorescent detector at 365 (430) nm excitation (emission) wavelengths, and the UV spectra were confirmed by an additional diode matrix detector (DAD) coupled to the system. AFB1 concentration in each sample was calculated based on a standard calibration curve.
Statistics
Student t-test was used to analyze the differences between control and treated samples. Differences were considered statistically significant when a p value was lower than 0.05. Data analyses were done using STATA MP Software (TX, USA). Graphical values are represented as the mean ± standard error of mean (SEM) of three or four different assays.
Results and Discussion
The aim of this study was to characterize the effect of aqueous extracts from 10 Peruvian plants on the growth of A. flavus and the production of AFB1, in addition to providing some insights into the composition/bioactivity relationship.
Effect of the Plant Aqueous Extracts on Fungal Growth and Aflatoxin B1 Production
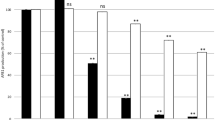
The effect of the plants extracts on the fungal growth and AFB1 production was evaluated by respectively measuring the diameter of the colonies and calculating the percentage of AFB1 production compared to untreated control cultures. Figure 1 presents the effect of the 10 plant aqueous extracts obtained by maceration at a concentration of 6 mg DM/mL after 7 days of incubation at 27 °C.
Effect of Peruvian plant aqueous extracts on AFB1 production (grey bars) and fungal growth (line) in A. flavus NRRL 62477. Results are expressed as percentage of untreated control ± standard error of the mean (n = 4). ns = no statistically significant change, *p value < 0.05; **p value < 0.01; ***p value < 0.001
Six out of the ten tested plant extracts led to a dose-dependent inhibition of AFB1 production. Minthostachys mollis (MM) inhibited AFB1 production by 89% with an IC50afb1 = 0.7 mg DM/mL (concentration reducing AFB1 production by 50% compared to control culture). It was followed in terms of efficacy by Aloysia citrodora (AC) (78% inhibition, IC50afb1 = 2.4 mg DM/mL), Annona muricata (AM) (69% inhibition, IC50afb1 = 3.3 mg DM/mL), and Uncaria tomentosa (UT) (58% inhibition, IC50afb1 = 4.5 mg DM/ml). Dysphania ambrosioides (DA) (47% inhibition, IC50afb1 = 6.5 mg DM/mL) and Myrciaria dubia (MD) (32% inhibition, IC50afb1 = 10.1 mg DM/mL) extracts had a lower effect. These results are consistent with previous studies showing the effectiveness of some aqueous plant extracts in inhibiting the synthesis of aflatoxins (Bluma et al., 2008; Njoki et al., 2017). In particular, aqueous extracts of Micromeria graeca and Mimosa tenuiflora demonstrated a significant anti-AFB1 activity, with IC50afb1 of 0.8 mg DM/mL (El Khoury et al., 2017) and 3 mg DM/mL (Hernandez et al., 2021) respectively.
Regarding the impact on the fungal growth, these 6 extracts presented a significant but slight effect on the growth of A. flavus NRRL 62477 (Fig. 1). The highest percentage of inhibition was observed for MM but it was limited to 3%. A study conducted by Mo et al. (2013) showed that different extracts of black and green teas had an effect on the inhibition of AFB1 but with limited or no impact on the fungal growth. Similar effects have been obtained for the aqueous extracts of Micromeria graeca and Mimosa tenuiflora on A. flavus. The lack of effect on fungal growth suggests that the extracts only interact with secondary metabolism of the fungus. Therefore, the risk of creating a selection pressure that could result in resistance appearance is very weak. Moreover, it is likely that the collateral impact on biodiversity shall also be limited.
Conversely, the extracts of Chamaemelum nobile (CN), Lepidium meyenii (LM), and Arracacia xanthorrhiza (AX) did not have an effect on both the production of AFB1 and the growth of A. flavus compared to control culture, whereas Salvia hispanica (SH) led to a slight stimulation of AFB1 production (p > 0.005), which could result from the presence of certain nutrients in the extract that enhance the synthesis of the mycotoxin.
Comparison of the Effect on the Fungal Growth and Aflatoxin B1 Production of the Active Plant Extracts Obtained by Maceration and ASE
Plant extracts that showed promising anti-AFB1 activity were further submitted to an accelerated solvent extraction (ASE) in order to evaluate the impact of such extraction method on their activity against AFB1 compared to maceration. The ASE technique is considered a useful process to obtain active compounds from plant biomass since it allows working from a wide range of temperatures (50 − 200 °C), pressure (100 − 150 bars), and different extraction cycles (Richter et al., 1996). In addition, unlike maceration, it implies a great reduction in the amount of solvent to be used as well as a minimum extraction time. In our work, the extraction was carried out at a temperature of 50 °C, for 5 min of static time and using water as the only solvent.
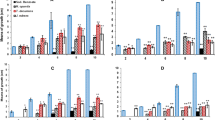
The comparative results of the antifungal and anti-AFB1 activity of the plant extracts obtained by maceration and ASE respectively are shown on Fig. 2. All extracts had an effect on the production of AFB1. This is the first study showing the anti-AFB1 activity of plant aqueous extracts obtained using ASE. Comparing the impact of the extracts obtained using the 2 extraction methods demonstrates that, in most cases, maceration led to the obtention of a more potent extract regarding AFB1 inhibition. This difference is very significant for the MM, UT, and MD extracts (p < 0.001). DA was the only extract for which the extraction method did not modify the anti-AFB1 ability. Differences on the impact on fungal growth were much more limited, similar results being observed for 4 out of the 6 tested extracts. For the 2 remaining (AM and DA), ASE extracts led to a stimulation of fungal growth, that was not observed with those obtained by maceration. Several studies have shown that maceration is a useful technique in obtaining extracts with high antimycotoxin activity. Ponzilacqua et al. (2019) showed that aqueous extracts of the leaves of Rosemary officinalis and Origanum vulgare, obtained by maceration at room temperature, led to the inhibition of AFB1 production by 49% and 38% respectively. In addition, the same extraction conditions were used by Deabes et al. (2022) to prepare an ethanolic-aqueous extract of Ephedra sinica with a high efficacy in the inhibition of AFB1 (98.4%).
Comparison of the effect of plant extracts obtained by Maceration (black) and ASE (grey) on AFB1 production (bars) and fungal growth (lines) in A. flavus NRRL 62477. Results are expressed as percentage of untreated controls ± standard error of the mean (n = 4). ns = no statistically significant change, *p value < 0.05; **p value < 0.01; ***p value < 0.001
Characterization and Composition of the Active Plant Extracts Obtained by Maceration and ASE
Extraction yields and chemical composition of the extracts are presented in Table 2. Extraction yield (mass of extract/mass of dry matter) was used as an indicator of the ability of the experimental conditions to extract possible bioactive compounds from plants. For all the extracts obtained by maceration (15 h, 25 °C), the yield values were higher than 7%. This is consistent with a study carried out on the aqueous extract of Mimosa tenuiflora obtained under the same conditions, reporting an extraction yield of 11% (Hernandez et al., 2021). Furthermore, our yield values were higher than those reported in the literature. Adewole and Ojewole (2009) reported an extraction yield of 3.62% for AM by maceration at 25 °C for 48 h. Almost similar conditions were used for DA obtaining an extraction yield of 14% (Ajaib et al., 2016), while an extraction by maceration at 80 °C in Uncaria tomentosa (UT) showed a yield of 2.65% (Navarro-Hoyos et al., 2018).
Regarding the yields obtained by ASE at 50 °C, the extracts of DA, AC, AM, UT, and MD presented values of 14%, 23%, 13%, 10%, and 12% respectively. These values are not significantly different from those obtained by maceration. By contrast, the extraction yield of MM was deeply modified, increasing from 13% (Maceration) to 22% (ASE 50 °C). A comparative study of the extraction of Plinia cauliflora compounds by ASE at different temperatures (40-60-80-100 °C) and extraction times (3-6-9-12-15 min) suggests that the increase in temperature improves the efficiency of the extraction due to the fact that it favors the rate of diffusion and solubility of the analytes in the solvent (Santos et al., 2012), thus favoring the extraction of compounds such as proteins and sugars which subsequently increases the extraction yield (Herrero et al., 2005). Our results confirm that the extraction conditions such as time and temperature influence on the different yields.
It has been suggested that the synthesis of AFB1 is the result of the fungus response to high levels of oxidative stress (Grintzalis et al., 2014). Currently, plant-derived molecules such as polyphenols have been described able to inhibit AFB1 in relation with their high antioxidant activity that may disrupt the balance of oxidative stress (Caceres et al., 2017; Hernandez et al., 2021). Thus, the evaluation of the antioxidant activity, polyphenol, and condensed tannins content was carried out on the extracts of the 6 active plants. The results are shown in Table 2.
The extracts obtained by maceration and ASE from AC, MD, and DA showed almost similar values regarding their content in polyphenols, condensed tannins, as well as their antioxidant activity. For UT and AM extracts, differences were observed when extracted at 50 °C, leading to a reduction in the antioxidant activity as well as in the content of both polyphenols and condensed tannins. A study conducted by Duraisamy et al. (2020) on the bark of Acacia xanthophloea described that different parameters such as time, temperature, and type of solvent are fundamental for a good tannin isolation. They suggested that the best extraction condition was 60 °C for 4 h, because condensed tannins are molecules of hydrophobic nature and therefore require a longer contact time with water to be extracted. This could explain why a lower content of these molecules was obtained in the extracts by ASE 50 °C as compared with the ones from maceration, as the extraction time was only of 5 min for ASE, compared to 15 h for maceration.
The MM extract, unlike the other plants, showed a large increase in the polyphenol content and antioxidant activity when extracted at 50 °C using ASE. A study by Dent et al. (2013) on the extract of the plant Salvia officinalis that belongs to the same family as MM (Lamiaceae) indicated that the highest polyphenol content was obtained for an ethanolic extraction at 60 °C, the high temperature favoring polyphenols solubility and isolation, and consequently a higher antioxidant activity. In addition, it has been suggested that, occasionally, certain types of terpenoids can be isolated in aqueous extracts (Cowan, 1999; Olech & Łyko, 2020), mainly in relation with an increase in temperatures (Azmir et al., 2013). MM has also been reported as a plant rich in lipophilic molecules among which terpenes. These compounds (such as pulegone) with high antioxidant activity can be extracted using hydro-distillation (Olmedo et al., 2018).
Our results suggest that, although an extraction at a higher temperature (ASE 50 °C) allows obtaining extraction yields slightly higher than those from maceration, this does not necessarily imply a better extraction of polyphenolic compounds and therefore a better bioactivity (antioxidant activity).
Relationship Between the Antioxidant Activity of the Extracts Obtained by Maceration and their Ability to Inhibit AFB1 Production
The studied extracts were prepared from different parts of plants: leaves, fruits, bark, etc. This directly influenced the composition of aqueous extracts, their richness in polyphenols, condensed tannins, and subsequent antioxidant activity.
It is known that AFB1 and some of its precursors such as norsoloric acid and versicolorin are highly oxygenated molecules and that is why it was hypothesized that AFB1 production could be, by itself, a way for the fungus to resist to oxidative stress (Grintzalis et al., 2014). Currently, the effect of some plant-derived molecules such as polyphenols, alkaloids, and essential oils on the inhibition of AFB1 has been very often related to their antioxidant activity that may decrease the oxidative stress surrounding the fungus (Hernandez et al., 2021; Marei et al., 2012).
Of the 6 extracts that inhibited AFB1, 4 of them (UT, AM, AC, MD) showed a significant antioxidant activity, likely linked to their polyphenol content.
UT extract was prepared from the bark of the plant. As expected, it was the richest in polyphenols, mostly represented by condensed tannins. These molecules are known for their antioxidant activity and UT extract displayed the highest antioxidant activity (13 mg/L). By comparison, Trolox®, a common antioxidant reference, has an IC50 of 3.5 mg/L (Muñoz-Acevedo et al., 2011). Our results are in agreement with some reported before by Navarro-Hoyos et al. (2018). In the same way, Hernandez et al. (2021) showed that the condensed tannins of the aqueous extract of Mimosa tenuiflora are one of the main molecules involved in the inhibition of AFB1. This extract had a tannin content equivalent to 171 mg/g DM, while our UT extract contained two times more tannins (374 mg/g DM).
AM extract had a concentration in condensed tannins (145 mg/g DM) almost similar to that reported for Mimosa tenuiflora (Hernandez et al., 2021). Therefore, once again these molecules could be involved in the anti-AFB1 activity of this plant extract.
MD and AC also presented a high content of polyphenols and an important antioxidant activity. However, unlike UT and AM, condensed tannins were not the most representative molecules. MD extract was obtained from fruits. It was demonstrated that these fruits were rich in other polyphenols than tannins such as flavonols, flavanones, anthocyanins, and catechin and that these compounds correlate with the antioxidant activity, together with other components such as vitamin C or β-carotene (Azmir et al., 2013; Olmedo et al., 2018). For AC extract, made from leaves, it has been proved that aqueous extracts are rich in hydroxycinnamic acids, flavones, and flavonols, being the main responsible for their antioxidant activity (Muñoz-Acevedo et al., 2011). So, these antioxidant polyphenols could be responsible for the anti-AFB1 activity of these two extracts. Indeed, it has been reported that various types of polyphenols such as flavonoids (Quercetin, hyperoside, vitexin, apigenin), phenolic acids, and epicatechins may inhibit AFB1 synthesis (Abdel-Razek et al., 2017; Nobili et al., 2019; Vamvakas et al., 2021; Zhou et al., 2015).
Finally, our results demonstrated that MM and DA extracts had a different composition. These two extracts were obtained from leaves. They showed only a slight polyphenol content with 94 and 32 mg GAE/g DM respectively. Condensed tannins concentration was limited as observed for AC, but for MM and DA, this also went with a very limited antioxidant capacity with IC50 of 417 mg/L and > 600 mg/L, respectively. However, an important effect was observed on the inhibition of AFB1, mainly for MM, which was the most effective plant (IC50afb1 = 0.7 mg DM/mL). Thus, the efficacy of MM and DA in the inhibition of AFB1 was not directly related to their antioxidant activity, and other phytoconstituents must probably be involved. The information about the phytochemical composition of DA and MM aqueous extracts is limited. However, the anti-AFB1 and/or antifungal activity of these plants has been reported using other types of extracts. For instance, a study carried out on the essential oil of DA leaves showed that a concentration of 10 µg/mL completely inhibited the production of the AFB1 as well as the growth of A. flavus (Kumar et al., 2007). Bustamante Gonzales (2018) showed that the alkaloids and flavonoids extracted from an ethanolic extract of MM had an antifungal activity against Botrytis cinerea fungus. Cano et al. (2008) reported the presence of volatiles compounds such as pulegone (36.6%) and menthone (24.2%) as the main components of essential oils of MM collected from the central Andes of Peru and that these compounds had an antifungal activity in Trichophyton mentagrophytes. Nevertheless, further studies are needed in order to identify the main phytoconstituents of aqueous extracts of MM that could be responsible for its high anti-AFB1 effect as well as the characterization of its mechanism of action.
Conclusions
The present study characterized the antifungal and anti-aflatoxin B1 activity of aqueous extracts of 10 Peruvian plants obtained by two different extraction methods. To prepare the extracts, various parts of the plants were used. Roots (AX, LM) and seeds (SH) were not active, whereas extracts obtained from leaves (MM, AC, AM) and bark (UT) were the most effective against AFB1. Moreover, the extracts obtained by maceration showed the best inhibition of aflatoxin B1. The anti-AFB1 activity of most of them could be related to their high content in polyphenols and subsequent antioxidant activity. This composition/property relationship between polyphenol content and anti-AFB1 activity may allow identifying agricultural by-products as new candidates to preserve crops from AFB1 contamination.
Conversely, the most effective plant, MM, has a low antioxidant activity suggesting that other types of bioactive substances than polyphenols could be involved in its effectiveness. This is the first study showing the anti-AFB1 activity of these plants. Moreover, the obtained results allow considering the use of aqueous extracts as a sustainable and eco-friendly source in the protection of crops against AFB1.
As the active plants are very abundant in South America, they could represent an easily available resource for local producers to limit AFB1 contamination of crops before harvest. The use of aqueous extracts could also allow carrying the active molecules via the irrigation systems.
Data Availability
Data will be available on request to corresponding author.
References
Abdel-Razek, A., Badr, A., & Shehata, M. (2017). Characterization of Olive oil by-products: Antioxidant activity, its ability to reduce aflatoxigenic fungi hazard and its aflatoxins. Annual Research & Review in Biology, 14, 1–14. https://doi.org/10.9734/ARRB/2017/35065
Adewole, S., & Ojewole, J. (2009). Protective effects of Annona muricata linn. (Annonaceae) leaf aqueous extract on serum lipid profiles and oxidative stress in hepatocytes of streptozotocin-treated diabetic rats. African Journal of Traditional, Complementary and Alternative Medicines, 6(1), 30–41. https://doi.org/10.4314/ajtcam.v6i1.57071
Ajaib, M., Hussain, T., Farooq, S., & Ashiq, M. (2016). Analysis of antimicrobial and antioxidant activities of chenopodium ambrosioides: an ethnomedicinal plant. 2016, 1–11. https://doi.org/10.1155/2016/4827157
Azmir, J., Zaidul, I. S. M., Rahman, M. M., Sharif, K. M., Mohamed, A., Sahena, F., Jahurul, M. H. A., Ghafoor, K., Norulaini, N. A. N., & Omar, A. K. M. (2013). Techniques for extraction of bioactive compounds from plant materials: a review. Journal of Food Engineering, 117(4), 426–436. https://doi.org/10.1016/j.jfoodeng.2013.01.014
Bailly, S., Mahgubi, A. E., Carvajal-Campos, A., Lorber, S., Puel, O., Oswald, I. P., Bailly, J.-D., & Orlando, B. (2018). Occurrence and identification of Aspergillus section flavi in the context of the emergence of aflatoxins in French maize. Toxins, 10(12), 525. https://doi.org/10.3390/toxins10120525
Berlowski, A., Zawada, K., Wawer, I., & Paradowska, K. (2013). Antioxidant properties of medicinal plants from Peru. 4, 71–77. https://doi.org/10.4236/fns.2013.48A009
Bluma, R., Amaiden, M. R., & Etcheverry, M. (2008). Screening of Argentine plant extracts: impact on growth parameters and aflatoxin B1 accumulation by Aspergillus section Flavi. International Journal of Food Microbiology, 122(1–2), 114–125. https://doi.org/10.1016/j.ijfoodmicro.2007.11.050
Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology, 28(1), 25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Bryden, W. L. (2012). Mycotoxin contamination of the feed supply chain: implications for animal productivity and feed security. Animal Feed Science and Technology, 173(1), 134–158. https://doi.org/10.1016/j.anifeedsci.2011.12.014
Bussmann, R. W., & Sharon, D. (2006). Traditional medicinal plant use in Northern Peru: tracking two thousand years of healing culture. Journal of Ethnobiology and Ethnomedicine, 2(1), 47–64. https://doi.org/10.1186/1746-4269-2-47
Bustamante Gonzales, S. R. (2018). Evaluación del potencial antifúngico de los extractos etanólicos de Phyllanthus niruri y Minthostachys mollis frente al hongo Botrytis cinerea. Universidad Nacional Mayor de San Marcos. https://cybertesis.unmsm.edu.pe/handle/20.500.12672/9058
Caceres, I., El Khoury, R., Bailly, S., Oswald, I. P., Puel, O., & Bailly, J.-D. (2017). Piperine inhibits aflatoxin B1 production in Aspergillus flavus by modulating fungal oxidative stress response. Fungal Genetics and Biology, 107, 77–85. https://doi.org/10.1016/j.fgb.2017.08.005
Caceres, I., El Khoury, R., Medina, Á., Lippi, Y., Naylies, C., Atoui, A., El Khoury, A., Oswald, I. P., Bailly, J.-D., & Puel, O. (2016). Deciphering the anti-aflatoxinogenic properties of eugenol using a large-scale q-PCR approach. Toxins, 8(5), 123. https://doi.org/10.3390/toxins8050123
Cano, C., Bonilla, P., Roque, M., & Ruiz, J. (2008). Actividad antimicótica in vitro y metabolitos del aceite esencial de las hojas de Minthostachys Mollis (muña). Revista Peruana De Medicina Experimental y Salud Publica, 25(3), 298–301.
Cowan, M. M. (1999). Plant products as antimicrobial agents. Clinical Microbiology Reviews, 12(4), 564–582. https://doi.org/10.1128/CMR.12.4.564
Da Cruz Cabral, L., Fernández Pinto, V., & Patriarca, A. (2013). Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. International Journal of Food Microbiology, 166(1), 1–14. https://doi.org/10.1016/j.ijfoodmicro.2013.05.026
De Sousa, O. V., Vieira, G. D.-V., De Pinho, J. de J. R. G., Yamamoto, C. H., & Alves, M. S. (2010). Antinociceptive and anti-inflammatory activities of the ethanol extract of Annona muricata L. leaves in animal models. International Journal of Molecular Sciences, 11(5), 2067–2078. https://doi.org/10.3390/ijms11052067
Deabes, M., Khalil, W., Kh, A.-E.-A., Ibrahim, S., & Naguib, K. (2022). The inhibitory effect of different ephedra plant extracts on the Aspergillus flavus growth and aflatoxin B 1 gene expression. Jordan Journal of Biological Sciences, 15, 59–66. https://doi.org/10.54319/jjbs/150108
Dent, M., Dragović-Uzelac, V., Penić, M., Bosiljkov, T., & Levaj, B. (2013). The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in dalmatian wild sage (Salvia officinalis L.) extracts. Food Technology and Biotechnology, 51(1), 84–91.
Duraisamy, R., Shuge, T., Worku, B., Kerebo Berekete, A., & M Ramasamy, K. (2020). Extraction, screening and spectral characterization of Tanninsfrom Acacia Xanthophloea (fever tree) bark. Research Journal of Textile and Leather, 1(1), 1–10. https://doi.org/10.46590/rjtl.2020.010101
El Khoury, R., Caceres, I., Puel, O., Bailly, S., Atoui, A., Oswald, I. P., El Khoury, A., & Bailly, J.-D. (2017). Identification of the anti-aflatoxinogenic activity of Micromeria graeca and elucidation of its molecular mechanism in Aspergillus flavus. Toxins, 9(3), 87. https://doi.org/10.3390/toxins9030087
El Mahgubi, A., Puel, O., Bally, S., Tadrist, S., Querin, A., Ouadia, A., Oswald, I. P., & Bailly, J.-D. (2013). Distribution and toxigenicity of Aspergillus section flavi in spices marketed in Morocco. Food Control, 32, 143–148. https://doi.org/10.1016/j.foodcont.2012.11.013
Gibb, H., Devleesschauwer, B., Bolger, P. M., Wu, F., Ezendam, J., Cliff, J., Zeilmaker, M., Verger, P., Pitt, J., Baines, J., Adegoke, G., Afshari, R., Liu, Y., Bokkers, B., van Loveren, H., Mengelers, M., Brandon, E., Havelaar, A. H., & Bellinger, D. (2015). World Health Organization estimates of the global and regional disease burden of four foodborne chemical toxins, 2010: a data synthesis. F1000Research, 4, 1393. https://doi.org/10.12688/f1000research.7340.1
Gómez, J. V., Tarazona, A., Mateo-Castro, R., Gimeno-Adelantado, J. V., Jiménez, M., & Mateo, E. M. (2018). Selected plant essential oils and their main active components, a promising approach to inhibit aflatoxigenic fungi and aflatoxin production in food. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 35(8), 1581–1595. https://doi.org/10.1080/19440049.2017.1419287
Grintzalis, K., Vernardis, S. I., Klapa, M. I., & Georgiou, C. D. (2014). Role of oxidative stress in sclerotial differentiation and aflatoxin B1 biosynthesis in Aspergillus flavus. Applied and Environmental Microbiology, 80(18), 5561–5571. https://doi.org/10.1128/AEM.01282-14
Gruber-Dorninger, C., Jenkins, T., & Schatzmayr, G. (2019). Global mycotoxin occurrence in feed: A ten-year survey. Toxins, 11(7), 375. https://doi.org/10.3390/toxins11070375
Hahn, M. (2014). The rising threat of fungicide resistance in plant pathogenic fungi: botrytis as a case study. Journal of Chemical Biology, 7(4), 133–141. https://doi.org/10.1007/s12154-014-0113-1
Hernandez, C., Cadenillas, L., Maghubi, A. E., Caceres, I., Durrieu, V., Mathieu, C., & Bailly, J.-D. (2021). Mimosa tenuiflora aqueous extract: role of condensed tannins in anti-aflatoxin B1 activity in Aspergillus flavus. Toxins, 13(6), 391. https://doi.org/10.3390/toxins13060391
Herrero, M., Martín-Álvarez, P. J., Señoráns, F. J., Cifuentes, A., & Ibáñez, E. (2005). Optimization of accelerated solvent extraction of antioxidants from Spirulina platensis microalga. Food Chemistry, 93(3), 417–423. https://doi.org/10.1016/j.foodchem.2004.09.037
IARC. (2012). Fungi producing significant mycotoxins. IARC Scientific Publications, 158, 1–30.
Kumar, J. K., Monica, S. S., Bojan, V., Suganthi, A., & Paramasivam, M. (2021). Impact of pesticide exposure on environment and biodiversity: a review. Agricultural Reviews, 1, 1–12. https://doi.org/10.18805/ag.R-2325
Kumar, R., Mishra, A. K., Dubey, N. K., & Tripathi, Y. B. (2007). Evaluation of Chenopodium ambrosioides oil as a potential source of antifungal, antiaflatoxigenic and antioxidant activity. International Journal of Food Microbiology, 115(2), 159–164. https://doi.org/10.1016/j.ijfoodmicro.2006.10.017
Luo, S., Du, H., Kebede, H., Liu, Y., & Xing, F. (2021). Contamination status of major mycotoxins in agricultural product and food stuff in Europe. Food Control, 127, 108–120. https://doi.org/10.1016/j.foodcont.2021.108120
Makhuvele, R., Naidu, K., Gbashi, S., Thipe, V. C., Adebo, O. A., & Njobeh, P. B. (2020). The use of plant extracts and their phytochemicals for control of toxigenic fungi and mycotoxins. Heliyon, 6(10), e05291. https://doi.org/10.1016/j.heliyon.2020.e05291
Marei, G. I. K., Abdel Rasoul, M. A., & Abdelgaleil, S. A. M. (2012). Comparative antifungal activities and biochemical effects of monoterpenes on plant pathogenic fungi. Pesticide Biochemistry and Physiology, 103(1), 56–61. https://doi.org/10.1016/j.pestbp.2012.03.004
Ministerio del ambiente. (2010). Peru: Economia y diversidad biologica. Zona de comunicaciones SAC.
Mo, H. Z., Zhang, H., Wu, Q. H., & Hu, L. B. (2013). Inhibitory effects of tea extract on aflatoxin production by Aspergillus flavus. Letters in Applied Microbiology, 56(6), 462–466. https://doi.org/10.1111/lam.12073
Muñoz-Acevedo, A., Méndez, L. Y. V., Stashenko, E. E., & Kouznetsov, V. (2011). Improved Trolox® equivalent antioxidant capacity assay for efficient and fast search of new antioxidant agents. 1, 86–102. https://doi.org/10.1080/22297928.2011.10648207
Navarro-Hoyos, M., Alvarado-Corella, D., Moreira-Gonzalez, I., Arnaez-Serrano, E., & Monagas-Juan, M. (2018). Polyphenolic composition and antioxidant activity of aqueous and ethanolic extracts from Uncaria tomentosa bark and leaves. Antioxidants (Basel, Switzerland), 7(5), 65. https://doi.org/10.3390/antiox7050065
Njoki, L. M., Okoth, S. A., & Wachira, P. M. (2017). Effects of medicinal plant extracts and photosensitization on aflatoxin producing Aspergillus flavus (Raper and Fennell). International Journal of Microbiology, 2017, 1–9. https://doi.org/10.1155/2017/5273893
Nobili, C., De Acutis, A., Reverberi, M., Bello, C., Leone, G. P., Palumbo, D., Natella, F., Procacci, S., Zjalic, S., & Brunori, A. (2019). Buckwheat hull extracts inhibit Aspergillus flavus growth and AFB1 biosynthesis. Frontiers in Microbiology, 10(1997). https://doi.org/10.3389/fmicb.2019.01997
Ojiambo, S., Battilani, P., Cary, J. W., Blum, B. H., & Carbone, I. (2018). Cultural and genetic approaches to manage aflatoxin contamination: recent insights provide opportunities for improved control. Phytopathology, 108(9), 1024–1037. https://doi.org/10.1094/PHYTO-04-18-0134-RVW
Olech, M., & Łyko, L. (2020). Influence of accelerated solvent extraction conditions on the LC-ESI-MS/MS polyphenolic profile, triterpenoid content, and antioxidant and anti-lipoxygenase activity of Rhododendron luteum sweet leaves. Antioxidants, 9, 822. https://doi.org/10.3390/antiox9090822
Olmedo, R., Ribotta, P., & Grosso, N. R. (2018). Antioxidant activity of essential oils extracted from Aloysia triphylla and Minthostachys mollis that improve the oxidative stability of sunflower oil under accelerated storage conditions. European Journal of Lipid Science and Technology, 120(8), 1–10. https://doi.org/10.1002/ejlt.201700374
Olszowy, M., & Dawidowicz, A. L. (2018). Is it possible to use the DPPH and ABTS methods for reliable estimation of antioxidant power of colored compounds? Chemical Papers, 72(2), 393–400. https://doi.org/10.1007/s11696-017-0288-3
Ponzilacqua, B., Rottinghaus, G. E., Landers, B. R., & Oliveira, C. A. F. (2019). Effects of medicinal herb and Brazilian traditional plant extracts on in vitro mycotoxin decontamination. Food Control, 100, 24–27. https://doi.org/10.1016/j.foodcont.2019.01.009
Rammanee, K., & Hongpattarakere, T. (2011). Effects of tropical citrus essential oils on growth, aflatoxin production, and ultrastructure alterations of Aspergillus flavus and Aspergillus parasiticus. Food and Bioprocess Technology, 4(6), 1050–1059. https://doi.org/10.1007/s11947-010-0507-1
Repajić, M., Cegledi, E., Kruk, V., Pedisić, S., Çınar, F., Kovačević, D. B., Žutić, I., & Dragović-Uzelac, V. (2020). Accelerated solvent extraction as a green tool for the recovery of polyphenols and pigments from wild nettle leaves. Processes, 8(7), 803. https://doi.org/10.3390/pr8070803
Ribeiro, L. P., Domingues, V. C., Gonçalves, G. L. P., Fernandes, J. B., Glória, E. M., & Vendramim, J. D. (2020). Essential oil from Duguetia lanceolata St.-Hil. (Annonaceae): suppression of spoilers of stored-grain. Food Bioscience, 36. https://doi.org/10.1016/j.fbio.2020.100653
Richter, B. E., Jones, B. A., Ezzell, J. L., Porter, N. L., Avdalovic, N., & Pohl, C. (1996). Accelerated solvent extraction: A technique for sample preparation. Analytical Chemistry, 68(6), 1033–1039. https://doi.org/10.1021/ac9508199
Santos, D. T., Veggi, P. C., & Meireles, M. A. A. (2012). Optimization and economic evaluation of pressurized liquid extraction of phenolic compounds from jabuticaba skins. Journal of Food Engineering, 108(3), 444–452. https://doi.org/10.1016/j.jfoodeng.2011.08.022
Taniwaki, M. H., Pitt, J. I., & Magan, N. (2018). Aspergillus species and mycotoxins: Occurrence and importance in major food commodities. Current Opinion in Food Science, 23, 38–43. https://doi.org/10.1016/j.cofs.2018.05.008
Vamvakas, S. -S., Chroni, M., Genneos, F., & Gizeli, S. (2021). Vaccinium myrtillus L. dry leaf aqueous extracts suppress aflatoxins biosynthesis by Aspergillus flavus. Food Bioscience, 39. https://doi.org/10.1016/j.fbio.2020.100790
Waterman, P. G., & Mole, S. (1994). Analysis of phenolic plant metabolites. Balckwell Scientific Oxford, UK. Boston, MA, USA. ISBN 978–0–632–02969–3.
Zhou, W., Hu, L. B., Zhao, Y., Wang, M. Y., Zhang, H., & Mo, H. Z. (2015). Inhibition of fungal aflatoxin B1 biosynthesis by diverse botanically-derived polyphenols. Tropical Journal of Pharmaceutical Research, 14(4), 605–609. https://doi.org/10.4314/tjpr.v14i4.7
Funding
This Ministère de l’Enseignement Supérieur et de la Recherche (MEST) funded the Ph-D grant of Laura F Cadenillas. This work has also benefited from a State grant managed by the National Research Agency under the “Investissements d'Avenir” programme with the reference ANR-18-EURE-0021.
Author information
Authors and Affiliations
Contributions
Conceptualization: Jean-Denis Bailly and Vanessa Durrieu. Methodology: Céline Mathieu, Jean-Denis Bailly, and Vanessa Durrieu. Formal analysis: Laura F Cadenillas and Christopher Hernandez. Writing-original draft preparation: Laura F Cadenillas, Jean-Denis Bailly, and Vanessa Durrieu. Writing-review and editing: Laura F Cadenillas, Jean-Denis Bailly, and Vanessa Durrieu. Supervizion: Jean-Denis Bailly and Vanessa Durrieu.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cadenillas, L.F., Hernandez, C., Mathieu, C. et al. Screening of the Anti-Aflatoxin B1 Activity of Peruvian Plant Extracts: Relation with their Composition. Food Bioprocess Technol 16, 1324–1334 (2023). https://doi.org/10.1007/s11947-023-03002-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-023-03002-7