Abstract
The bioactive ingredients of green coffee beans were extracted using high pressure-assisted extraction (HPE) and compared against those derived from the traditional heat reflux (HR) extraction method. The effect of extraction conditions on the extraction efficiency of functional components (chlorogenic acids, caffeine, total phenols, and flavonoids); the free radical-scavenging activity of the extract; and the inhibition of α-glucosidase, α-amylase, and food pathogenic bacteria were measured. Using water as the solvent, the high-pressure extraction rates of chlorogenic acid (CGA) and caffeine were 3.20–5.75 mg/100 g and 4.32–7.95 mg/100 g, respectively, which were significantly higher than the rates obtained using the traditional method (2.92 mg/100 g and 3.54 mg/100 g, respectively). Under optimal extraction conditions of 25 °C and 600 MPa for 2.5 min, inhibition of α-glucosidase and α-amylase by the extract reached rates of 27.8% and 26.7%, respectively, with the highest free radical-scavenging activity of 81.5%. HPE exhibited antibacterial activity against both gram-positive (Staphylococcus aureus and Listeria innocua) and gram-negative (Escherichia coli and Salmonella enterica) bacteria, and the lowest minimum inhibitory concentration (4.69 mg/mL) against S. aureus was achieved. Scanning electron microscopic observations evidenced that high pressure partially damaged the intercellular compartments of coffee beans, increased solvent permeability, and improved the extraction rate of bioactive ingredients. The outcomes of the study demonstrated that HPE can be employed as an efficient extraction technique for production of chlorogenic acids and caffeine that might have a potential application in food and related industries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coffee is one of the main globally traded commodities (second only to petroleum), and it contains a wide variety of phytochemicals, many of which are antioxidants. It is well known that the mode used to press coffee strongly influences and determines the quality of green coffee; as such, it establishes the different characteristic components and flavors of wet or dry processed coffee (Farah & Santos, 2015). Chlorogenic acid (CGA) belongs to a major family of phenolic compounds that represent 6–12% of coffee constituents in mass, and it has received considerable research attention due to its potential biological effects, including its antibacterial, antiphlogistic, antimutagenic, and antioxidant activities (Lou et al., 2011). For example, Getachew and Chun (2016) collected five coffee samples and extracted by subcritical water hydrolysis process to recover total phenolics, flavonoids, and CGA, and the extracts showed high antioxidant and antimicrobial activity. It is known that the total CGA content in green coffee beans varies largely in accordance with the species and degree of maturation, and the processing method contributes to changes in the CGA content and its distribution. In this respect, it was shown that processing Brazilian Arabica coffee using a wet method provided higher CGA and trigonelline contents compared to those produced using a semi-dry method (Duarte et al., 2010). In addition, Cho et al. (2014) found that CGA degraded in medium- and dark-roasted coffee beans roasted over a long time, and the beans exhibited a lower antioxidant activity than light-roasted coffee beans. It has also been reported that a 200-mL cup of Arabica coffee contains between 70 and 200 mg CGA, whereas that of Robusta coffee contains between 70 and 350 mg, and Budryn et al. (2009) reported that the CGA concentration in Robusta coffee bean extracts varied between 0.4 g/100 g and 36.0 g/100 g of the dry extract weight, while that of Arabica coffee beans extracts ranged from 0.1 g/100 g to 22.4 g/100 g.
High pressure-assisted extraction (HPE) is a novel technique used to extract the active ingredients from various matrices. The HPE operation usually applies a hydrostatic pressure of 100–600 MPa to the raw material at room temperature. Compared to conventional extraction techniques, such as heat reflux extraction, HPE can be effectively used to improve the extraction rate by increasing the mass transfer rates and the possible rupture of the cellular wall, membrane, and organelles, which results in higher product yields at a reduced processing time and lower solvent consumption (Huang et al., 2013). HPE has been used to extract various interesting components from a wide variety of sample matrices, and it is also a promising alternative sample preparation technique employed in a number of applications, such as extracting lycopene from ketchup, anthocyanins from grape skin, flavonoids from litchi peel, ginsenosides from ginseng root and catechins, and caffeine and polyphenols from tea. The extraction of CGA from various plant samples using infrared, microwave, and ultrasonic extraction techniques has also been reported. For example, Upadhyay et al. (2012) reported that the yield of CGA rapidly reached 31–62% within 5 min under optimal microwave-assisted extraction conditions, where water was used as the extraction solvent at an extraction condition of 50 °C and 800 W, respectively. In addition, Li et al. (2005) reported that ultrasonic extraction technique was highly efficient for extracting CGA from Eucommia ulmoides, and Tang et al. (2012) showed that GGA could be extracted from honeysuckle at a high extraction efficiency via infrared-assisted extraction. However, no studies have reported the use of HPE for extracting CGA from plant samples. Therefore, this study aimed to compare the use of HPE to extract CGA with a conventional extraction method. The amounts of caffeine, total polyphenols, and total flavonoids obtained from green coffee beans were measured, and the radical-scavenging activity, inhibition of α-glucosidase and α-amylase, and inhibition of food pathogenic bacteria were evaluated. Furthermore, the morphological changes of HPE coffee beans were observed using scanning electron microscopy (SEM).
Materials and Methods
Plant Material
Arabica green coffee beans were obtained from Nantou Coffee Farm in Shuili Township, Nantou County, Taiwan. The freeze-dried coffee beans were ground into powder with a grinder (SG-10TW, Cuisinart, USA), and the ground coffee was packed in low-density polyethylene bags and stored until used in high pressure processing and subsequent further analysis.
HPE
The coffee powder was mixed with water, methanol, and ethanol (1:4; w/v) and then vacuum-sealed in sterile polyethylene bags for HPE. The high-pressure machine (Foodlab Plunger Press model S-FL-850–9 W; Stansted Fluid Power Ltd., Stansted, UK) used in this study had a chamber volume of 0.3 L, and water was used as the pressure transmission medium. The filled mixture was placed in a high-pressure chamber and subjected to a pressure of 200–600 MPa at 5–50 °C (initial temperature). Immediately following high-pressure treatment, the extraction mixture was centrifuged at 4,000 rpm for 10 min, and the supernatant was collected and stored at 4 °C for subsequent component analysis.
Heat Reflux Extraction
The method described by Upadhyay et al. (2012) was used as reference for heat reflux extraction. Ground coffee was degreased and extracted in a hot water bath at 50 °C for 5 min. The slurry was then filtered to obtain a clear extract for conducting a quantitative analysis.
Analytical Methodologies
Extraction Rate
The yield of the extract was calculated by evaporating all water from a known amount of the extract at 50 °C with a rotary evaporator.
CGA
The CGA content of the coffee bean extract was determined using the method described by Navarra et al. (2017) with some modifications. In this respect, the diluted extracts were measurement of absorption at a 325 nm using a spectrophotometer (SPECTROstar Nano, BMG). The CGA content was calculated based on the standard curve.
Caffeine
The caffeine content of the coffee extract was determined using the method described by Belay et al. (2008) with some modifications. The extracts were dried, and the absorbance was measured at 275 nm using a spectrophotometer (SPECTROstar Nano, BMG). The amount of caffeine was calculated according to a standard curve prepared using a reference standard.
Total Polyphenols and Flavonoid
The total polyphenols and flavonoid contents of the coffee extracts were determined according to the method described by Alyahya et al. (2021). The total polyphenol content (TPC) was expressed in gallic acid (GC) equivalents, and the total flavonoid content (TFC) was expressed as a catechin equivalent per gram dry weight of the sample (mg CE/g DW).
Functional Capacity
Radical-Scavenging Activity
The capacity of the coffee extracts to remove 1,1-diphenyl-2-picryl-hydrazyl radical (DPPH; Aldrich, USA) was determined using the method described by Shimada et al. (1992) with slight modifications. Briefly, 1 mL of sample solution and 5 mL of freshly prepared 0.1 mM DPPH solution were thoroughly mixed and kept in the dark for 60 min. The absorbance of the reaction mixture at 517 nm was read by a spectrophotometer (U-2000, Hitachi, Japan). The blank was prepared by replacing the extract with water (1 mL). The percentage of free radical-scavenging activity was calculated as
Inhibition of α-Glucosidase
The method described by Oboh et al. (2016) was used as a reference with minor modifications. First, 20 μL of α-glucosidase (0.2 U/mL) and 10 μL of the extract (0–100 mg/mL) were mixed as the inhibitor in 0.067 mol/L of phosphate buffer (pH = 6.8) and maintained at 37 °C for 15 min. Subsequently, 20 μL of 2.5 mmol/L p-nitrophenyl-α-D-glucopyranoside (PNPG) solution was added to the mixture, and it was then maintained at 37 °C for 20 min. Finally, 80 μL of 0.2 mol/L sodium carbonate (Na2CO3) was added to terminate the reaction, and the group without the inhibitor was used as the control group. The absorbance of each sample at 405 nm was recorded, and the inhibition rate of α-glucosidase was calculated using the following formula,
where Ati is absorbance of samples containing α-glucosidase, Abi is absorbance of samples without α-glucosidase, At is absorbance of control samples containing α-glucosidase, and Ab is absorbance of control samples without α-glucosidase.
Inhibition of α-Amylase
The method described by Oboh et al. (2016) was used as reference to prepare the α-amylase solution (100 μL, > 6.67 U/mL), and different concentrations of extracts (100 μL; 0–50 μmol/mL) or acarbose solution (as a control) were placed in a centrifuge tube at 37 °C for 10 min. A corn starch solution (300 μL; 5 mg/mL) was then heated at 90 °C for 20 min, added to the centrifuge tube, and mixed at 37 °C for 15 min. DNS chromogenic reagent (100 μL prepared from DNS, NaOH, potassium sodium tartrate tetrahydrate, phenol, and sodium sulfite) was added to terminate the reaction. The centrifuge tube containing the reaction mixture was subsequently heated at 100 °C for 10 min. After rapid cooling, the mixture was diluted to 2 mL with phosphate-buffered saline (PBS; 20 mmol/L, pH 6.9) to obtain the test solution. Finally, the absorbance of the test solution at 540 nm was measured using a spectrophotometer. The inhibition rate of the extract on alpha-amylase was calculated using the following equation,
where A0 is the absorbance of the mixture of α-amylase, corn starch, and the DNS chromogenic reagent; A1 is the absorbance of the mixture of α-amylase, extract, corn starch, and the DNS chromogenic reagent; and A2 is the absorbance of the mixture of α-amylase, extract, and the corn starch solution.
Antibacterial Capacity
The minimum inhibitory concentrations (MICs) of coffee extracts against food pathogenic bacteria were described by Duangjai et al. (2016). E. coli BCRC 10,675, S. enterica BCRC 10,242, S. aureus BCRC 10,451, and L. monocytogenes BCRC 15,354 were purchased from the Bioresources Collection and Research Center (BCRC), ROC. The stock cultures were maintained on nutrient agar (NA) or potato dextrose agar (PDA), according to culture manual. A bacterial suspension was subsequently added to obtain a final concentration at 5 × 105 CFU/mL. All plates were incubated at 37 °C for 24 h, and the MIC was defined as the lowest concentration of the extract that inhibited the growth of bacteria. The suspension in the well of the MIC (and higher concentrations) was spotted on a plate and incubated. The lowest concentration that inhibited growth of bacteria was defined as the minimal bactericidal concentration.
SEM Observation
The ground coffee was fixed using 10 mL of 1.0% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) on a specimen holder with aluminum tape. It was then subjected to three 10-min washes in buffer and then post-fixed for 1 h in 1% osmium tetroxide. The samples were then dehydrated using a series of 10-mL ethanol solutions (10–90% ethanol in 10%-steps) for 15 min. The samples were subsequently soaked in isopentyl acetate prior to critical point drying in a CO2 medium using a critical point dryer (Hitachi HCP-2), and they were then sputtered with gold with a JEOL JEC-1200 sputter-coater. Filters were subsequently attached to large SEM stubs using double-sided tape and coated with gold palladium. The samples were analyzed using a scanning electron microscope (Hitachi S4700) at 15 kV, and photomicrographs were obtained.
Statistical Analysis
All data are shown as mean ± standard deviation, and all statistical analyses were performed using SAS software. Analysis of variance was conducted, and the differences between the means were examined using Duncan’s multiple range tests. The results were considered statistically significant at p < 0.05. All samples were measured in triplicate.
Results and Discussion
Effect of HPE Parameter on Extraction Efficiency
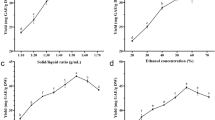
Figure 1 shows the CGA and caffeine extraction efficiencies at 400 MPa and 25 °C for 2.5 min using three solvents (methanol, ethanol, and water). The extraction efficiency of water reached 16.6%, which was significantly higher than that of methanol and ethanol (9.2% and 7.5%, respectively). The caffeine and CGA concentrations in the water extract were also higher than those in other solvents. Similar results were obtained in the study of Budryn et al. (2009), where a higher CGA content was found in the water extract than in the ethanol extract, although the caffeine content in the ethanol extract was higher than that of the water extract. The extractability of a solvent mainly depends on the following: the solubility of the compound in the solvent system, the mass transfer kinetics of the product, and the intensity of the solute/matrix interaction. The dielectric constant and polarity of water are obviously higher than those of alcohol. As there are no limitations on the use of water in future applications within the food and pharmaceutical industries, water extraction is considered a preferred extraction method. In addition, the method does not produce toxic waste, unlike organic solvent extraction methods (Upadhyay et al., 2012). Therefore, we selected water as the solvent for the HPE efficiency experiment to extract CGA from green coffee beans. Three variables, namely, pressure, temperature, and duration, were employed in the experiment, and nine conditions were, therefore, compared and analyzed (Table 1). The extraction was performed at 200 MPa, 400 MPa, and 600 MPa at 25 °C for 2.5 min. The results showed that higher CGA and caffeine extraction yields were obtained from HPE at 400 MPa than at 200 MPa, and this implied that a higher pressure was beneficial for improving the CGA extraction rate. However, when the pressure was increased to 600 MPa, there was no significant increase in the extraction rate compared to that at 400 MPa, as the cellular structure of the coffee beans was already fully destroyed and the CGA components had been extracted. Therefore, further experiments were conducted at 400 MPa. The use of different starting temperatures for extraction also affected the HPE efficiency. As the temperature was increased from 5 to 25 °C, the amounts of CGA and caffeine extracted increased from 4.32% and 3.27% to 7.48% and 5.66%, respectively. However, increasing the temperature to 50 °C provided no further improvements in the extraction rates of the crude extracts, CGA, or caffeine. Butiuk et al. (2021) observed the same trend in the extraction of yerba mate; a significant increase was observed in the CGA extraction rate when the extraction temperature was increased from 30 to 65 °C, but the rate reduced when the temperature was increased to 100 °C. Phenolic compounds are thermal sensitive, and certain important bioactive substances are likely to be degraded at a high temperature; therefore, the extraction temperature needs to be maintained under a certain limit (Silva et al., 2007). A comparison of the extraction durations used showed that an increase in the extraction duration increased the extraction efficiencies of CGA and caffeine, and the extraction rate was significantly higher at 2.5 min than at 1 min. However, no significant difference was observed with a 5-min extraction duration. These results suggest that the solute in the sample matrix was dissolved using high pressure, and the two target compounds in the extraction took approximately 2.5 min to reach equilibrium. Thus, it was not possible for any more CGA or caffeine to be dissolved, and the extended duration was not productive (Ahmad et al., 2021). Traditional HR extraction at 50 °C for 5 min provided 3.54% and 2.92% of CGA and caffeine, respectively, but twice the amount of CGA and caffeine was obtained using HPE extraction at 400 MPa at 25 °C within 2.5 min. Huang et al. (2013) found that HPE performed better than traditional extraction techniques (such as heat or solvent extraction) at lower temperatures, and thus, the extraction duration was reduced, the extraction efficiency was improved, thermal degradation was reduced, and fewer bioactive components were lost.
Antioxidant Properties of Extracts
As shown in Table 2, the TPC concentration range was 3.34–4.57 mg GA/g in the coffee extracts obtained with different HPE conditions. All three operating conditions had an impact on the amount of TPC extracted. When the initial extraction temperature was 25 °C, there was a significant increase in the amount of TPC obtained with an increase in extraction pressure and duration. However, increasing the temperature to 50 °C resulted in a slight decrease in TPC, and this was probably related to the degradation of other phenolic components at the higher temperature (Upadhyay et al., 2012). In this study, the TFC showed the same trends as those of TPC. The optimal extraction pressure of TFC was 400 MPa, and the amount of TFC increased significantly with the extraction duration and reached 2.52 mg quercetin equivalents/g at 6 min. However, temperature did not appear to have a significant impact on the amount of TFC extracted. Nevertheless, both the extraction rates of TPC and TFC obtained through HPE were significantly higher than the 3.02 mg GA/g and 1.24 mg quercetin equivalents/g obtained by heat reflux extraction, respectively. The free radical-scavenging activity in the extract was measured through the percentage inhibition of DPPH free radicals (Table 2). The free radical-scavenging activity of the heat reflux extract was 65.9%, while that of the HPE extract ranged from 64.2 to 81.5%. The extract obtained at 25 °C and 600 MPa for 2.5 min had the highest scavenging activity, and the extract obtained at 50 °C and 400 MPa had the lowest scavenging capacity (with no significant difference from that of the HR extracts). Caffeine in coffee reduces oxidative stress and protects the antioxidant system in hypoxia-induced lung epithelial cells. In human skin fibroblasts, caffeine is an inhibitor of lipid peroxidation products induced by hydrogen peroxide, and it reduces lipid peroxidation and reactive oxygen species (Tiwari et al., 2014). The free radical-scavenging activity is related to the bioactive compounds, including not only CGA and its derivatives but also caffeine, theophylline, theobromine, cafestol, kahweol, tocopherols, and trigonelline. These compounds have strong antioxidant potentials, and they exhibit various health-promoting effects. For example, they have anticancer properties and are known to inhibit hyperglycemia, hyperinsulinemia, and hyperlipidemia (Jeszka‑Skowron et al., 2016). Hu et al. (2015) used HPE, HR extraction, ultrasonic extraction, and Soxhlet extraction to extract CGA from the flower buds of Lonicera japonica. The shortest extraction duration was obtained with HPE, and this process provided the highest CGA concentration. In addition, there were no significant differences between the DPPH-scavenging ability of the extract produced, and those of the other extracts, which indicated the advantages of the high extraction rate, short extraction duration, and the low energy consumption of HPE (Khan et al., 2019).
Inhibition of α-Glucosidase and α-Amylase
Alpha-amylase and α-glucosidase are key enzymes affecting the digestion and absorption of starch and other carbohydrates. α-Amylase hydrolyzes starch into dextrins and oligosaccharides by hydrolyzing the α-D-1,4-glycosidic bond, while α-glucosidase hydrolyzes dextrins and oligosaccharides into glucose, which is then transported to blood vessels; it increases postprandial blood sugar, which can result in obesity or diabetes. The inhibition of α-amylase and α-glucosidase activities reduces starch digestibility and delays starch degradation and glucose absorption (Papoutsis et al., 2019). In this study, the HPE extracts obtained using different extraction conditions showed varying α-glucosidase and α-amylase inhibition levels due to the different CGA and caffeine concentrations obtained, as shown in Fig. 2. In extracts obtained at temperatures below 25 °C for more than 2.5 min at 400 MPa, the inhibition rates of α-glucosidase and α-amylase reached a minimum of 27.8% and 26.7%, respectively, and these results were significantly higher than those of the heat reflux extracts (22% and 10%, respectively). Oboh et al. (2015) previously demonstrated the inhibitory effect of CGA on α-amylase (IC50 of 3.68 μg/mL) and α-glucosidase (4.98 μg/mL), and Zheng et al. (2020) reported the inhibitory effect of CGA on α-amylase (IC50 of 0.498 mg/mL). However, Wang et al. (2022) found that the positive effects from inhibiting phenolic acids and flavonoids on α-glucosidase and α-amylase were due to the specific functional patterns of the hydroxyl group, which formed hydrogen bonds with specific amino acids at the active site of enzymes. In this study, the coffee extract obtained at 25 °C and 600 MPa for 2.5 min had the highest inhibitory effects on α-glucosidase and α-amylase, and this result was likely related to the high TPC and TFC contents. CGA may have a low binding affinity to α-glucosidase and α-amylase, which could be the reason for the low α-glucosidase and α-amylase inhibition rates in some of the extracts that had a high CGA concentration. Therefore, it is more likely that the inhibitory effect of CGA on α-amylase and α-glucosidase is associated with non-competitive inhibition and competitive inhibition, respectively; this has been attributed to the structural differences between α-amylase and α-glucosidase (Nyambe-Silavwe et al., 2018). In this respect, Alongi et al. (2019) found that adding milk to coffee followed by homogenization at a high pressure of 150 MPa increased the bioaccessibility of CGA from nearly 25 to > 50% because the milk fat promoted the formation of CGA microencapsules, which reduced the susceptibility to degradation during digestion and also enhanced the inhibition of α-glucosidase.
α-Glucosidase and α-amylase inhibition activity of extracts isolated via HPE and HR. Values represent mean ± standard deviation (n = 3). HPE, high pressure-assisted extraction; HR, heat reflux. A, B, C, and D: the different letters associated with α-glucosidase inhibition indicate significant statistical differences. a, b, c, and d: the different letters associated with α-amylase inhibition indicate significant statistical differences (p > 0.05)
Antibacterial Activity of Extracts
The results of the zone of inhibition test showed that coffee extracts obtained under high pressure inhibited food pathogens (Table 3). The MICs of coffee extracts obtained at different HPE conditions against the gram-positive bacteria S. aureus and L. innocua were 27.5–37.3 mg/mL and 30.5–43.3 mg/mL, respectively, while the MICs of the HR extract against S. aureus and L. innocua were 39.2 mg/mL and 44.5 mg/mL, respectively, which were significantly higher than those of all the HPE extracts. However, the MICs of the HPE extracts against gram-negative bacteria were significantly higher than those against gram-positive bacteria. The MICs for E. coli and S. enterica ranged between 35.5 and 45.6 mg/mL and between 41.9 and 49.7 mg/mL, respectively, although these were lower than MICs of the HR extracts (45.6 mg/mL and 51.7 mg/mL, respectively). This result suggested that the extraction temperature had no effect on antibacterial activity of the coffee extract and that the MIC was reduced when the extraction duration and pressure were increased; this implication agrees with those of our previous reports. Duangjai et al. (2016) found that CGA and caffeine were the main components of the water extract of coffee pulp, and these showed antibacterial activity against both gram-positive bacteria (S. aureus and S. epidermidis) and gram-negative bacteria (Pseudomonas aeruginosa and E. coli). In particular, the MIC of S. epidermidis was the lowest at only 4.69 mg/mL. Tasew et al. (2020) obtained different antibacterial activity results from green coffee bean extracts, with an MIC against S. typhimurium of 15.62 mg/mL, which was lower than that of E. coli (31.25 mg/mL) and S. aureus (31.25 mg/mL). Due to the different levels of antibacterial activity of phenolic acids, CGA, and caffeine in coffee and the differences in the extraction conditions, the mechanisms used to destroy the biological structure of bacteria were also different, which resulted in the coffee extracts having different inhibition efficiencies against each type of bacteria. In general, the antibacterial activity is mediated through the inactivation of the cytoplasmic membrane and the inhibition of intracellular and extracellular enzymes. However, it is difficult for hydrophobic compounds to absorb the outer membrane of gram-negative bacteria, which is composed of phospholipids; this results in a poorer antibacterial activity against gram-negative bacteria (Runti et al., 2015). The data obtained in this study show that the high-pressure extracts with higher total phenol, CGA, and caffeine contents provided better antibacterial activity against gram-positive bacteria.
Microstructure Changes of Green Coffee Bean
To understand the effect of HPE on the microstructure of coffee beans, a scanning electron microscope was used to observe the cellular structure of unextracted coffee beans and that of coffee beans extracted by both hot reflux extraction and HPE. Figure 3a-c show the morphology of the unpressurized coffee beans observed via SEM (magnification 300–1000 times). The cells were relatively closely connected in an orderly manner, with obvious intracellular organelle spacing. Figure 3d, e, and f show coffee beans extracted via hot reflux extraction. The intercellular compartments were partially damaged and showed evidence of irregular and sudden shrinkage. However, the patterns of most of the cell compartments were similar to those of the control group. Figure 3g-i show coffee beans obtained via HPE. Although the basic cellular structure was visually distinguishable, the intracellular pores were significantly compressed following high-pressure treatment, and the volume was noticeably reduced. The cell pores were irregular with significant intracellular tissue ruptures and were squeezed into irregular or loose porous floccules. According to the Le Chatelier’s principle, the volume of the system tended to decrease during pressurization, as the extraction solvent entered the cells and made contact with bioactive components. These components were then dissolved in the solvent and extracted out of the cell. During HPE, the pressurized cells also exhibited increased solvent permeability. The higher the water pressure, the greater was the amount of solvent that entered the cell pores and the greater were the amounts of biological compounds dissolved in the solvent; this suggests that there was an increase in the dissolved functional components of cells, which improved the extraction efficiency. An equilibrium concentration of the solvent between the inside and outside of the cell was established when the pressure is maintained. When the pressure was suddenly reduced, the cell wall was disrupted to release an extract that contained high concentrations of solutes (Xi et al., 2017). In HPE studies of broccoli seeds (Xing et al., 2019) and Lonicera japonica (Hu et al., 2015), the extraction efficiencies showed increasing trends because of the damage conferred to the sample cell structure, owing to the pressure applied, as observed via SEM.
Conclusions
Extraction is the most crucial step during the separation of functional components. The condition of the parameters associated with the extraction method affects not only the extraction rate of the index components but also the operating costs and potential use in commercialized production. The results of this study prove that HPE improves the extraction efficiency of the functional components in coffee and that the extraction rates of total phenols, flavonoids, phenolic acids, and caffeine are higher using this process, compared with those obtained from traditional heat reflux extraction. Scanning electron microscopic observations showed that the high-pressure treatment damages the microstructure of coffee cells, which is believed to increase the permeability of the extraction solution in coffee bean cells, accelerate entry of the extraction solution into the cells, improve the solubility of the internal components, and shorten the extraction duration. The improved efficiency of extracting functional ingredients further enhanced the functionality of the extract. For example, the coffee extract obtained through HPE in this study showed a superior free radical-scavenging activity, a higher ability to inhibit sugar-decomposing enzymes, and a higher antibacterial activity than the heat reflux extract. HPE has been shown to help recover the indicator functional components from natural products and is suitable for sample extraction and preparation methods that can be used in studies on health supplements and drugs. When scaling up from laboratory equipment to commercial mechanized manufacturing, the parameters, conditions, and HPE extraction principles would remain identical, and they would not be affected by the capacity of the HPE equipment. In addition, HPE can be conducted at room temperature, and this is more suitable for the extraction of heat-sensitive active ingredients than traditional high-temperature extraction methods. Furthermore, the shortened extraction duration also has the advantage of providing low energy consumption. The results of this study and the associated advantages show that HPE should be developed as a future potential alternative to traditional solid–liquid extraction.
Data Availability
Research data are not shared.
References
Alongi, M., Calligaris, S., & Anese, M. (2019). Fat concentration and high-pressure homogenization affect chlorogenic acid bioaccessibility and α-glucosidase inhibitory capacity of milk-based coffee beverages. Journal of Function Foods, 58, 130–137.
Ahmad, I., Syakfanaya, A. M., Azminah, A., Saputri, F. C., & Munim, A. (2021). Optimization of betaine-sorbitol natural deep eutectic solvent-based ultrasound-assisted extraction and pancreatic lipase inhibitory activity of chlorogenic acid and caffeine content from Robusta green coffee beans. Heliyon, 7, e07702.
Alyahya, H. K., Subash-Babu, P., Salamatullah, A. M., Hayat, K., Albader, N., Alkaltham, M. S., Ahmed, M. A., Arzoo, S., & Bourhia, M. (2021). Quantification of chlorogenic acid and vanillin from coffee peel extract and its effect on α-amylase activity, immunoregulation, mitochondrial oxidative stress, and tumor suppressor gene expression levels in H2O2-induced human mesenchymal stem cells. Frontiers in Pharmacology, 12, 760242.
Belay, A., Ture, K., Redi, M., & Asfaw, A. (2008). Measurement of caffeine in coffee beans with UV/vis spectrometer. Food Chemistry, 108, 310–315.
Budryn, G., Nebesny, E., Podsędek, A., Żyżelewicz, D., Materska, M., Jankowski, S., & Janda, B. (2009). Effect of different extraction methods on the recovery of chlorogenic acids, caffeine and Maillard reaction products in coffee beans. European Food Research and Technology, 228, 913–922.
Butiuk, A. P., Maidana, S. A., Adachi, O., Akakabe, Y., Martos, M. A., & Hours, R. A. (2021). Optimization and modeling of the chlorogenic acid extraction from a residue of yerba mate processing. Journal of Applied Research on Medicinal and Aromatic Plants, 25, 100329.
Cho, A. R., Park, K. W., Kim, K. M., Kim, S. Y., & Han, J. (2014). Influence of roasting conditions on the antioxidant characteristics of Colombian coffee (Coffea Arabica L.) beans. Journal of Food Biochemistry, 38, 271–280.
Duarte, G. S., Pereira, A. A., & Farah, A. (2010). Chlorogenic acids and other relevant compounds in Brazilian coffees processed by semi-dry and wet post-harvesting methods. Food Chemistry, 118, 851–855.
Duangjai, A., Suphrom, N., Wungrath, J., Ontawong, A., Nuengchamnong, N., & Yosboonruang, A. (2016). Comparison of antioxidant, antimicrobial activities and chemical profiles of three coffee (Coffea arabica L.) pulp aqueous extracts. Integrative Medicine Research, 5, 324–331.
Farah, A., & Santos, T.F. (2015). The coffee plant and beans: An introduction. In Preedy, V. R. Coffee in health and disease prevention. Elsevier. 5–10.
Getachew, A. T., & Chun, B. S. (2016). Influence of hydrothermal process on bioactive compounds extraction from green coffee bean. Innovative Food Science and Emerging Technologies, 38, 24–31.
Hu, W., Guo, T., Jiang, W. J., Dong, G. L., Chen, D. W., Yang, S. L., & Li, H. R. (2015). Effects of ultrahigh pressure extraction on yield and antioxidant activity of chlorogenic acid and cynaroside extracted from flower buds of Lonicera japonica. Chinese Journal of Natural Medicines, 13, 445–453.
Huang, H. W., Hsu, C. P., Yang, B. B., & Wang, C. Y. (2013). Advances in the extraction of natural ingredients by high pressure extraction technology. Trends in Food Science & Technology, 33, 54–62.
Jeszka-Skowron, M., Sentkowska, A., Pyrzyńska, K., & De Peña, M. P. (2016). Chlorogenic acids, caffeine content and antioxidant properties of green coffee extracts: Influence of green coffee bean preparation. European Food Research and Technology, 242, 1403–1409.
Khan, S. A., Aslam, R., & Makroo, H. A. (2019). High pressure extraction and its application in the extraction of bio-active compounds: A review. Journal of Food Process Engineering, 42, e12896.
Lou, Z., Wang, H., Zhu, S., Ma, C., & Wang, Z. (2011). Antibacterial activity and mechanism of action of chlorogenic acid. Journal of Food Science, 76, 398–403.
Li, H., Chen, B., & Yao, S. (2005). Application of ultrasonic technique for extracting chlorogenic acid from Eucommia ulmodies Oliv. (E. ulmodies). Ultrasonics Sonochemistry, 12, 295–300.
Navarra, G., Moschetti, M., Guarrasi, V., Mangione, M.R., Militello, V. & Leone, M. (2017). Simultaneous determination of caffeine and chlorogenic acids in green coffee by UV/Vis spectroscopy. Journal of Chemistry, 2017, ID 6435086.
Nyambe-Silavwe, H., & Williamson, G. (2018). Chlorogenic and phenolic acids are only very weak inhibitors of human salivary α-amylase and rat intestinal maltase activities. Food Research International, 113, 452–455.
Oboh, G., Agunloye, O.M., Adefegha, S.A., Akinyemi, A.J., & Ademiluyi, A.O. (2015). Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. Journal of Basic and Clinical Physiology and Pharmacology, 26165–170.
Zheng, Y., Yang, W., Sun, W., Chen, S., Liu, D., Kong, X., Tian, J., & Ye, X. (2020). Inhibition of porcine pancreatic α-amylase activity by chlorogenic acid. Food Chemistry, 64, 103587.
Oboh, G., Ogunsuyi, O. B., Ogunbadejo, M. B., Adefegha, S. A. (2016). Influence of gallic acid on α-amylase and α-glucosidase inhibitory properties of acarbose. Journal of Food and Drug Analysis, 24, 627–634.
Papoutsis, K., Zhang, J., Bowyer, M. C., Brunton, N., Gibney, E. R., & Lyng, J. (2019). Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chemistry, 338, 128119.
Runti, G., Pacor, S., Colomban, S., Gennaro, R., Navarini, L., & Scocchi, M. (2015). Arabica coffee extract shows antibacterial activity against Staphylococcus epidermidis and Enterococcus faecalis and low toxicity towards a human cell line. LWT Food Science & Technology, 62, 108–114.
Silva, E. M., Rogez, H., & Larondelle, Y. (2007). Optimization of extraction of phenolics from Inga edulis leaves using response surface methodology. Separation and Purification Technology, 55, 381–387.
Shimada, K., Fujikawa, K., Yahara, K., & Nakamura, T. (1992). Antioxidative properties of xanthone on the auto oxidation of soybean in cylcodextrin emulsion. Journal of Agricultural and Food Chemistry, 40, 945–948.
Tang, Z., Zang, S., & Zhang, X. (2012). Detection of chlorogenic acid in honeysuckle using infrared-assisted extraction followed by capillary electrophoresis with UV detector. Journal of Chromatographic Science, 50, 76–80.
Tasew, T., Mekonnen, Y., Gelana, T., Redi-Abshiro, M., Chandravanshi, B.S., Ele, E., Mohammed, A.M., & Mamo, H. (2020) In vitro antibacterial and antioxidant activities of roasted and green coffee beans originating from different regions of Ethiopia. International Journal of Food Science, 8490492.
Tiwari, K. K., Chu, C., Couroucli, X., Moorthy, B., & Lingappan, K. (2014). Differential concentration-specific effects of caffeine on cell viability, oxidative stress, and cell cycle in pulmonary oxygen toxicity in vitro. Biochemical and Biophysical Research Communications, 450, 1345–1350.
Upadhyay, R., Ramalakshmi, K., & Rao, L. J. M. (2012). Microwave-assisted extraction of chlorogenic acids from green coffee beans. Food Chemistry, 130, 184–188.
Wang, S., Li, Y., Huang, D., Chen, S., Xia, Y., & Zhu, S. (2022). The inhibitory mechanism of chlorogenic acid and its acylated derivatives on α-amylase and α-glucosidase. Food Chemistry, 372, 131334.
Xi, J. (2017). Ultrahigh pressure extraction of bioactive compounds from plants—A review. Critical Reviews in Food Science and Nutrition, 57, 1097–1106.
Xing, J. J., Cheng, Y. L., Chen, P., Shan, L., Ruan, R., Li, D., & Wang, L. J. (2019). Effect of high-pressure homogenization on the extraction of sulforaphane from broccoli (Brassica oleracea) seeds. Powder Technology, 358, 103–109.
Funding
This research work was supported by the Ministry of Science and Technology, MOST 109–2221-E-150–022, Taiwan, Republic of China.
Author information
Authors and Affiliations
Contributions
H-WH and Y-HL finish the experiment and interpreted the results. C-YW as a corresponding author supervised the experiment and drafted the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, YH., Huang, HW. & Wang, CY. Effects of High Pressure-Assisted Extraction on Yield, Antioxidant, Antimicrobial, and Anti-diabetic Properties of Chlorogenic Acid and Caffeine Extracted from Green Coffee Beans. Food Bioprocess Technol 15, 1529–1538 (2022). https://doi.org/10.1007/s11947-022-02828-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02828-x