Abstract
The experimental sensitivity analysis of the novel ohmic assisted drying system (OAD: simultaneous use of ohmic heating and convectional air drying), which was developed as an alternative to the traditional method, was carried out for the first time. Within the scope of the study, changes in drying time, drying characteristics and some quality parameters were examined by changing the controlled parameters (applied voltage, temperature and air velocity) of the new OAD system. For this purpose, potato, which is an important commercial food in the European continent, was used as raw material. The optimum levels of OAD were calculated as approximately 60 °C for drying temperature, 112 V for applied voltage and any value between 1.07 and 1.98 m/s for air velocity to minimise drying time, browning index and to maximise effective diffusivity corresponding to accelerated drying period, b* colour value. According to the results, a 32–38% improvement was achieved in drying time compared to the traditional method. In addition, no negative effect was observed on the quality properties of potato slices dried under optimum conditions compared to the control samples. Moreover, it appears as an advantage that the new method provides partial pre-gelatinisation of starch molecules, which is important for the food industry. OAD will also save both time and economy by removing the need for the blanching stage in drying processes due to the obtained significant enzyme inactivation. Considering the results, it is seen that the newly developed technique has a promising potential both for the food industry and the literature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Foods tend to undergo some chemical and microbiological deteriorations after harvesting, especially during the supply and storage chain. These undesired changes originate from high water activity/content of food materials. As a result of these changes, the nutritional and sensorial properties of foods are weakened, and the formation of some detrimental compounds are also inevitable. Therefore, various preservation methods have been developed to prolong the shelf-life of food materials. Among all preservation methods, drying is known as one of the well-known and oldest techniques (Akpınar, 2006). Besides, due to being high energy/time-consuming technology (Moses et al., 2014; Raghavan et al., 2005; Ratti, 2001), manufacturers/researchers try to develop novel methods to improve the efficiency of traditional drying methods like convective drying (CD) and drying under sunlight (SD). Despite their common use in the food industry, both CD and SD have some disadvantages. For example, the use of SD is dependent on climate conditions so the final product may suffer from low quality and lack of standardisation (Ceylan et al., 2006). Although CD is helpful to overcome these problems, the CD is very time and energy-intensive and still leads to losses in nutrients and quality (Canizares & Mauro, 2015; Liu et al., 2014; Turgut et al., 2018). The studies, which were conducted to overcome these problems, have mostly focused on energy efficiency, time requirement and product quality (Andreou et al., 2021; Arikan et al., 2012; Bi et al., 2014; Fernandes et al., 2015; Gong et al., 2020; Liu et al., 2020; Wang et al., 2019; Zhao et al., 2014; Zhou et al., 2018). In those studies, researchers mostly tried to combine different and novel food processing techniques such as ultrasound, microwave, infrared, pulsed electric field, etc., to shorten the drying time of the food products. Similarly, ohmic heating (OH), another novel and volumetric food processing technique, has been successfully adopted as a pre-treatment to improve the drying performance of solid food materials (Icier, 2010; Lebovka et al., 2006; Wang & Sastry, 2000; Zhong & Lima, 2003). Apart from drying, OH is also evaluated for different purposes such as tempering (Seyhun et al., 2013), bread making (Bender et al., 2019), juice concentration (Fadavi et al., 2020) etc. So far as we are aware, the simultaneous use of ohmic heating with convective drying for solid food materials (briefly ohmic assisted drying, OAD) was introduced to the literature for the first time with our previous study (Turgut et al., 2021). In this study, a deeper understanding of the underlying mechanisms was also evaluated using the developed mechanistic model.
In brief, the mechanism of ohmic heating is based on the passage of electric current through food material which shows electrical resistance resulting in volumetric heat generation. The advantages of ohmic heating over other methods can be listed as (i) rapid heating and uniform temperature profile, (ii) instant shut down and no residual heat transfer, (iii) being environmentally friendly, (iv) low maintenance cost and (v) high energy conversion efficiency (Sakr & Liu, 2014). In addition, the positive effects of ohmic heating have been previously shown on the drying performance of solid food materials when used as a pre-treatment method (Lebovka et al., 2006; Wang & Sastry, 2000; Zhong & Lima, 2003). It helps to achieve a higher retention ratio of nutrients, vitamins, and bioactive compounds in the final dried food product (Icier, 2010). However, to the best of our knowledge, there is no available study assessing the sensitivity of the final dried product quality properties against OAD (simultaneous ohmic heating with convective drying) parameters and how they can be controlled/optimised to meet consumers’/producers’ demands. To guarantee the optimal quality of dried products, the OAD process should be designed carefully. Response surface methodology (RSM) can be used for this purpose. RSM is commonly used to analyse experimental data for the optimisation of process parameters. That is to ensure the best possible product specifications and understand the relationship between response and explanatory variables (Bi et al., 2014; Eren & Kaymak-Ertekin, 2007; Icier, 2010).
Therefore, the objective of the present study is (i) to assess the sensitivity of OAD system efficiency and product quality against process variables, (ii) to find optimal process conditions using RSM, and (iii) to compare quality properties of dried potato slices produced using OAD and CD.
Material and Methods
Preparation of Drying Material
Potatoes (Solanum tuberosum cv. Agria) were used for the experiments as a model food material and potato tubers were purchased from a local producer in Isparta, Turkey. Special care was given to select defect-free tubers in a similar shape, size, and colour. After the purchase, the potatoes were let at room temperature (at around 20 °C in the dark) for a day to equilibrate their temperature with the environment temperature. All the drying experiments were finished within 10 days to prevent/limit the arise of possible variations among the tubers, and the same storage conditions were ensured during the experiments. Potato tubers were sliced to form rectangular prisms (45 × 45x5 mm) before drying trials.
Drying Procedure
Experiments were performed in a tunnel type hot air dryer (Eraktek Innovation, Konya, Turkey). The dryer was equipped with a custom-engineered/produced lab-scale electrode apparatus (ohmic cell). The developed ohmic cell is seen in Fig. 1a with attached potato slices. Each sample holder has six stainless steel needle-shaped electrodes (3 mm in diameter) and the whole system has 16 sample loading capacity. The electrodes are attached to a cylindrical polyethylene base to ensure electrical isolation and high-temperature resistance (approximately until 150–180 °C). The ohmic cell is powered with an AC power supply (max. operation 400 V-10 A AC at 50–60 Hz) (Eraktek Innovation, Konya, Turkey).
(a) Ohmic cell with inserted potato slices and (b) illustration of tunnel drying equipped with ohmic heating apparatus (Turgut et al., 2021)
Before each drying trial, the oven with the ohmic cell was left working for almost 30 min to attain set conditions (temperature and flow rate) in the drying chamber (for details about process conditions please see the experimental design in Tables 1 and 2). Then, fresh-cut potato slices were immediately attached to the electrodes as shown in Fig. 1a. The ohmic cell was placed on an in-situ weighed drying tray (KERN, KB 3600-2 N, Balingen, Germany) to monitor the real-time weight change of a whole bunch of samples. As soon as the ohmic cell was placed into the dryer, the power supply was turned on and alternating electric current at constant voltage was given during drying without any interruption (applied voltage levels were given in Tables 1 and 2). The constant voltage level was adjusted using a manual variac and digital multi-meter (measuring volt and amper) (Eraktek Innovation, Konya, Turkey). The initial moisture content of the potato tubers was determined using a moisture analyser (KERN, DBS 60–3, Balingen, Germany) before each trial. To follow the change in moisture content during drying, the weight of samples was recorded in one-minute intervals. The moisture content was calculated on dry basis (g moisture/g dry matter).
Experimental Design
Optimisation of OAD conditions was conducted using RSM. The independent variables were temperature (\({x}_{1}\)), air velocity (\({x}_{2}\)) and applied voltage (\({x}_{3}\)). The real and corresponding coded values of the independent variables were given in Table 1. A rotational central composite design was used. Each variable was tested at 5 levels and the total number of runs was 20 including 6 replicates in the central point. The design table and corresponding response variables used for optimisation (drying time, b*, browning index and effective diffusivity of accelerated drying region) were given in Table 2. The accelerated drying region was previously defined as the period of OAD drying in which a rise in sample temperature and evaporation rate was accelerated by ohmic heating (Turgut et al., 2021). The experimental data were fitted to a full quadratic regression model to define the variability in studied responses with process parameters (Eq. 1).
where \(Z\) is the dependent (response) variables which were given in Table 2, \({\beta }_{0}\) is the model constant (intercept), \({\beta }_{i}\) is the linear coefficient (main effect), \({\beta }_{ii}\) is the quadratic coefficient, and \({\beta }_{ij}\) is the two factors interaction coefficient. Multiple, adjusted and predicted coefficients of determination (\({R}^{2}\), \({R}_{adj}^{2}\) and\({R}_{pred}^{2}\), respectively) were used for model evaluation and adequacy. The creation of experimental design and data analysis was conducted using R language with RStudio (Version 1.1.383). The rsm package (ccd function for central composite design and lm function for regression analysis) was used with coded data (with coded.data function) (Lenth, 2009). After regression model development with full quadratic equation, it was further simplified using the Akaikes’ Information Criterion (AIC) in mixed stepwise elimination using step function (James et al., 2013). The lack of fit value for each model was calculated using alr3 package (Weisberg, 2018). The optimisation was performed by desirability (Kuhn, 2016) and genoud (Mebane Jr. & Sekhon, 2011) functions. As an initial search pattern, a data frame consisting of 150 × 3 random numbers changing between -1/+1 was used. The graphics were produced using plotly package (Sievert, 2020).
Analysis
Calculation of Effective Diffusivity
The effective moisture diffusivity (Deff) was calculated to obtain information about the mechanism of moisture transfer and the complexity of the drying process. It was defined by Fick’s second law with the assumption that diffusion is the only physical mechanism controlling the transfer of water molecules to the sample surface. Potato slices were considered as infinite slabs (since other directions were large enough compared to the thickness) with uniform initial moisture distribution. Thus, moisture movement took place only through the thickness. The analytical solution of Crank (1975) for Fick’s second law was used to calculate Deff values of different drying regions during OAD. The moisture ratio (MR) of the sample was calculated using the following equation.
where Mt is the moisture content of the material at any time (g water/g dry solid); Mi is the initial moisture content of the material before drying (g water/g dry solid) and Me is the equilibrium moisture content of potato samples (g water/g dry solid). The Me was assumed to be zero for drying since it is relatively small compared to Mt and Mi and accepted to be negligible (Doymaz, 2004; Karacabey et al., 2016).
The MR is described in Eq. 3.
where Deff is the effective moisture diffusivity (m2/min), L is the half-thickness of the slab (m) (drying from both sides), MR is the moisture ratio (Eq. 2) and t is the drying time (min). For long-term drying, only the first term of Eq. 3 was used to explain the drying procedure. The final equation to calculate the Deff is given below (Eq. 4) and the effective moisture diffusivity was calculated by fitting ln(MR) vs. time data to the proposed mathematical expression.
Enzyme Activity
The peroxidase activity of potato samples was determined using the method proposed by Cemeroğlu (2013) after small modifications. A 2 g of sample was homogenised with 8 mL of 0.1 M Na-phosphate buffer (pH 7.0) for 1 min at 4 °C. The filtrate was centrifuged at 13,500 rpm for 20 min at 4 °C. The supernatant (50 µL), distilled water (1.1 mL) and reactive solution (1 mL, 1:1 mixture of 0.5% H2O2 and 0.5% guaiacol) were mixed. The increase in absorbance of the mixture read at 470 nm was recorded for 3 min intervals. One unit of enzyme activity was defined as the amount that caused a change of 0.01 in absorbance per minute. The results were given as unit/g dry matter.
Browning Index
The browning index was determined using the procedure described by Mudahar et al. (1990) and Leeratanarak et al. (2006). The sample (2 g) was extracted with 10 mL of 2% acetic acid solution and sonicated for 5 min. The mixture was centrifuged at 4000 rpm for 10 min. An aliquot of the filtrate was mixed with an equal volume of ethanol and centrifuged again at 13,500 rpm for 5 min. The absorbance of the extract was measured at 420 nm using a spectrophotometer (C-7100S, Peak Instruments Inc., USA) using a 1 cm cell. The results were expressed in optical density.
Starch Gelatinisation Degree
The colourimetric method reported by Wootton et al. (1971) and modified by Dalla Rosa et al. (1989) was used to determine the degree of starch gelatinisation in dried potatoes. Briefly, 0.5 g of the dried sample (sample A) and an equivalent amount of fresh potato in terms of dry matter (sample B) were taken. Sample A was homogenised in 25 mL of distilled water for 1 min. It was then centrifuged at 4000 rpm. The supernatant (150 µL) was mixed with 1840 µL distilled water and 10 µL of iodine solution (4 g of potassium iodide and 1 g of iodine in 100 mL of distilled water). The absorbance of the mixture was read at 600 nm and recorded. Sample B was cooked in an autoclave at 135 °C for one hour to ensure complete starch gelatinisation and then same procedure was followed. The degree of gelatinisation (GD, %) was calculated using Eq. 5.
where AA and AB values were the corresponding absorbance values obtained for samples A and B, respectively.
Ascorbic Acid
Ascorbic acid contents of fresh and dried samples were determined by the method described by Giovanelli et al. (2002) with some modifications. Briefly, 2 g of potato sample was homogenised with 10 mL of 4.5% metaphosphoric acid for 1 min, then the mixture was centrifuged at 4000 rpm for 20 min at 4 °C. The supernatant was filtered through a 0.45 µm membrane filter and injected into HPLC.
Detection and quantification were carried out using Agilent 1260 Infinity HPLC system equipped with an autosampler, Agilent XDB C18 column (250 mm × 4.6 mm inner diameter, 5 µm particle size) and a diode array detector (DAD). The flow rate was 0.8 mL/min, injection volume was 10 µL, the column temperature and detection wavelength were set at 30 °C and 245 nm, respectively. Isocratic elution of mobile phase (HPLC grade distilled water, pH adjusted to 2.2 with phosphoric acid) was carried out and the data were integrated and analysed using Agilent ChemStation for LC 3D Systems software. Ascorbic acid was identified by comparison with the retention time and UV–Vis spectrum of an authentic standard (Sigma-Aldrich; Oakville, ON, Canada). Quantitation was performed using an external calibration curve. The result was expressed as mg ascorbic acid/g dry matter.
Total Phenolic Content
Phenolics were extracted from potato samples using the method of Ahmed et al. (2010) with some modifications and total phenolic content was determined using the method described by Singleton and Rossi (1965). Briefly, the potato sample (0.1 g) was homogenised in 10 mL 75% methanol twice and filtered through Whatman No. 2 filter paper. The extract (1.5 mL) was mixed with 940 µL distilled water and 200 µL Folin-Ciocalteu reagent. Following, 600 μL of saturated Na2CO3 solution and 760 μL distilled water were added in order and vortexed. The absorbance of the mixture was measured using a spectrophotometer (C-7100S, Peak Instruments Inc., USA) at 765 nm after 2 h incubation in the dark at room temperature. The result was calculated using the gallic acid calibration curve and expressed as mg gallic acid equivalent/g dry matter.
Colour and Texture
Colour values of samples were determined using a portable colour meter (NH310, Shenzhen 3NH Technology, China) in CIE Colour Scale (L*, a*, b*). Moreover, total colour change (ΔE) was calculated as follows.
The textural properties of potato slices, i.e. firmness (F) and elasticity (E), were determined using a texture analyser (TA-XT Plus, Texture Stable Micro Systems, Surrey, UK) equipped with a 5 kg load cell. Each sample was punctured from 5 different positions with a 2 mm diameter stainless steel cylinder probe. The penetration speed was set at 1 mm/s. The results were given in N and mm, respectively, for F and E.
Rehydration Ratio and Leaching Loss
The rehydration ability and leaching loss during rehydration were evaluated by immersing potato slices in distilled water at 60 °C until a stable balance was attained (during 240 min) (Cunningham et al., 2008b). The potato:water ratio was 1:100 (w:v). The rehydration ratio (RR) of the sample was then calculated using Eq. 7.
where mt was the weight of samples at any time and mi was the initial weight (g), respectively.
To calculate leaching loss, soaking water was evaporated in a preheated oven at 105 °C for 24 h (AOAC, 2005). The results were given as g dry residue/g dry matter of sample.
Scanning Electron Microscope Images
Scanning electron microscopy images (SEM) of dried potato samples were captured using Fei Quanta FEG 250 (Czechia) (capable of 1.4 resolution and × 106 magnification) without a conductive coating using Gaseous ESEM (GSED) detector.
Statistical Analysis
All statistical analyses were conducted using R language with RStudio (Version 1.1.383). Details about the design of optimisation experiments, model development and assessment were given in detail under the “Experimental Design” section. Apart from it, variance analysis and statistical comparison of validation trials results were made using aov function. All results for validation and control samples were compared in terms of treatments. Comparison of means was done with Tukey HSD test using TukeyHSD function (p < 0.05) (Abdi & Williams, 2010). Letter denotation for significantly different groups was made using multcompLetters function in multcompView library (Graves et al., 2019). The cor and cor.test functions were used for given correlation coefficients and relation significance results.
Results and Discussion
RSM model Development of OAD System
To optimise the OAD system by RSM, 20 trials were conducted with different combinations of process parameters (temperature (\(T\)), air velocity (\(v\)) and applied voltage (\(V\))) using central composite design (rotational) (Table 3). Multivariate regression analysis was performed and main, second-order and interaction effects of explanatory variables on drying time (DT), effective diffusivity during accelerated drying period (Deff,2), enzyme activity (peroxidase) (EA), browning index (BI) and b* colour value were examined. The regression analysis shows that the quadratic model (after elimination of parameters that do not contribute to models according to the AIC number) can adequately explain the process parameters dependent variabilities in these responses. By mixed elimination, consistency and accuracy of the proposed models were improved and overfitting was avoided (James et al., 2013). In Table 3, information corresponding to the statistically meaningful models was given for process optimisation. Apart from the models in Table 3, the model belonging to C* was also found as significant (p < 0.05). However, it was not included in optimisation since C* is in a very meaningful correlation with b* (r≈1 with p < 0.001). So keeping one of them in the optimisation procedure is enough.
The adequacy of the developed models was tested using the analysis of variance. The models having low p-values (p < 0.001, Table 3) are highly significant and they could be used for feature prediction and optimisation procedures. The quality of the models was also assessed by the coefficient of determination (\({R}^{2}\)), adjusted and predicted \({R}^{2}\) values (\({R}_{adj}^{2}\) and \({R}_{pred}^{2}\), respectively). The high values for these parameters are the sign of accurate predictions for DT, Deff,2, EA, BI and b*. Moreover, the insignificant lack-of-fit value (p < 0.05) confirms that the developed models could safely be used to predict the output variables.
Besides the general model performance, the other important point is to evaluate the significance of model parameters. They were assessed with low p-values and their contributions were tested using AIC numbers (AIC number improves the goodness of fit but it also includes a penalty term for parameter number to keep the model as simple as possible and to avoid overfitting problem) (James et al., 2013).
Effect of OAD System on Drying Time and Effective Moisture Diffusivity
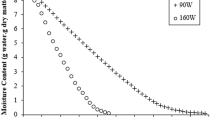
The multiple regression model developed to explain the dependency of DT on OAD parameters shows high accuracy (\({R}^{2}\)=88.34%) with p < 0.001. Figure 2 was plotted to show the effects of temperature and voltage on DT. While temperature had a higher (p < 0.001) and voltage had a relatively lower (p < 0.1) influences on DT, air velocity did not play any role in the model. As the temperature increased, DT linearly decreased which is coincident with the negative linear term effect of temperature (p < 0.001) in the corresponding regression model (Table 3). This favourable influence of increasing temperature on DT may be attributed to the accelerated heat and mass (moisture) transfers around the drying material. Although there is weaker importance of voltage’s main effect term, the second-order term of voltage (p < 0.001) and voltage-temperature interaction (p < 0.001) reveal how much the voltage is important for DT of potato slices in the OAD system. As can be seen from Fig. 2, the curve effect of voltage on DT is compatible with its second-order term in the model. At low voltage levels (approximately for the values lower than 70 V), an increase in DT was observed due to the frail heating capability of the electrical current. Increasing voltage (approximately up to 100 V) resulted in shorter DT values. However, a further increase in voltage caused an increased DT. This may be due to the over-shot effect which is seen at higher voltages. The occurrence of over-shot close to electrodes may limit the electric current passing through the material leading to inefficiency of ohmic treatment (Turgut et al., 2021).
For the current drying system, three effective diffusivity coefficients (Deff,1, Deff,2, Deff,3 (m2/min)) were defined for three corresponding regions of a drying curve. A sample drying curve, showing which regions correspond to Deff,1, Deff,2 and Deff,3 values, was presented in Fig. 3. In Fig. 3, Deff,1 corresponds to the drying period from t = 0 to the time point at which the effect of ohmic heating starts to appear (product temperature was not higher than the surrounding air), (ii) Deff,2 was considered for the period when the heating process was dominated by ohmic treatment (product temperature was higher than the surrounding air) and lastly (iii) Deff,3 was the period that was in between the end of the ohmic heating and the final of the drying (product temperature decreases around to surrounding air temperature). Since any significant regression model could not be developed for Deff,1 and Deff,3, any associated data were not presented in this paper. But briefly, all Deff,2 values were higher than Deff,1 and Deff,3, except the drying trials conducted at 33 and 50 V. This was because ohmic heating did not demonstrate any clear heating effect due to insufficient voltage levels. Thus, Deff,2 values were assumed to be 0 for the trials conducted at 33 and 50 V. The regression model developed to predict Deff,2 values can adequately explain its variation depending on process parameters (\({R}^{2}\)=93.45% and p < 0.001). Although all the main effect terms contribute to the model according to the AIC number, only \(V\) (p < 0.001) and \(T\) (p < 0.05) coefficients were found as significant. Among model parameters, only the velocity term shows a negative impact on diffusivity. Regarding second-order and interactions terms, \(V\times V\), \(T\times v\) and \(T\times V\) have p-values less than 0.1. From these results, it can be seen that the most efficient parameter on Deff,2 is the applied voltage gradient of ohmic heating, and it is followed by drying air temperature. The contributions of the different processing parameters were graphically represented through three-dimensional (3D) response surface plots (Fig. 4a-c). From these figures, it can be concluded that the temperature and the air velocity showed weaker impacts on Deff,2 compared to the voltage level. The voltage level positively affects Deff,2 regardless of other process parameters but at higher temperatures and higher air velocities, a synergetic effect appears. Interestingly, at moderate or lower voltage levels, higher temperatures and velocities cause slight reductions in diffusivity. This may be due to (i) the loss of the suppressive impact of ohmic heating at low voltages (as known, ohmic heating was dominant over other heat transfer phenomena when it is active Goullieux & Pain, 2005; Jun & Sastry, 2005)) or (ii) the non-existence of accelerated drying region at low voltages. In brief, the combination of higher voltage gradients with higher temperature and velocity is superior to maximise mass transfer.
Effect of OAD System on Enzyme Activity and Visual Properties
The visual properties of food products are so important that appearance and the first impression of foods are the major criteria determining consumer willingness (Hutchings, 1977). In the present study, statistically meaningful regression models were developed for two different visual properties which were BI (\({R}^{2}\)=86.68% and p < 0.001) and b* (\({R}^{2}\)=88.63% and p < 0.001) (please see Table 3). As discussed before, since the correlation coefficient between b* and C* is around 1, the model information which belongs to C* was not shared here and was not considered in the optimisation. The main and interaction terms contributed to the regression models developed for both BI and b*.
Regarding BI, \(T\times v\) and \(T\times v\) terms were eliminated from the equation according to the AIC number. On the other hand, the quadratic term of applied voltage (\(V\times V\)) has statistically significant importance for the regression model (p < 0.001). From Fig. 5, the applied voltage is the most effective explanatory variable on BI and it is followed by temperature. Particularly, the use of high voltages (≈105 V) and high drying temperatures caused minimum browning in potato samples (Fig. 5a). The curve-like changes in BI against voltage gradient is clear especially at air temperatures approximately less than 50 °C (Fig. 5a). On the other hand, at low voltage levels (< 70 V), the increase of the temperature proportionally and negatively changed BI. OAD at voltage levels less than 70 V can be accepted like convective drying in terms of browning since the effect of ohmic heating was not apparent. As a result, the activity of oxidative enzymes causing browning took place. However, the use of higher voltages causing fast heating and high internal/volumetric temperature decreased enzyme activity and BI (for details please see “Optimisation, validation and discussion”). Therefore, as seen from Fig. 5b, for the voltage levels higher than 70 V, BI reduced to its minimum. On the other hand, air velocity showed an adverse effect on BI. But it was weaker than that was observed for voltage and temperature.
Regarding the b* value, the structure of the regression model is very similar to that for BI. The only difference is the presence of the second-order term for velocity (\(v\times v\)) instead of \(T\times v\) interaction (existing in the model for BI). However, the significance of \(v\times v\) in the model developed for b* is lower than its effect on BI. The terms of \(V\) (p < 0.001) and \(V\times V\) (p < 0.001) as well as \(v\) (p < 0.01) show the greatest contributions to the model. From Fig. 6, it can be concluded that all the model parameters are effective on the b* value. Besides its distinct effects, voltage has strong interactions with temperature and air velocity (Fig. 6a-c). Temperature and air velocity have individual weak influences on b* value compared to voltage do (Fig. 6c), however, their effects are promoted by voltage (Fig. 6a and b). Supporting the second-order term of the voltage in the model, the curvature effect of voltage is seen in Fig. 6a. On the other hand, for air velocity and temperature, linear changes in b* values are apparent (Fig. 6a-c).
Optimisation, Validation and Discussion
By using RSM, it is possible to determine the optimal combination(s) of input parameters that produce the desired reduction in drying time and minimise undesired changes in food material. So, in the current study, the optimisation was aimed to minimise DT, BI, while maximising Deff,2 and b*. The OAD system parameters were optimised using desirability (Kuhn, 2016) and genoud (Mebane Jr. & Sekhon, 2011) libraries for the R language. During the optimisation efforts, process parameters were kept within the studied ranges as given in Table 1. However, since the air velocity (\(v\)) did show no or very slight impact on output variables, more than one optimum parameter combination arose leading to the target overall desirability of 1. So, by initialising a data matrix consisting of randomly attained different levels of input variables (in the range of -1 to +1 as coded values), 150 optimal combinations in total were produced which all came up with the overall desirability which means all targeted outputs could be satisfied. It was seen that the levels for temperature and applied voltage were almost constant for all optimal combinations. But despite that, the air velocity was varying in a wide range (from -0.66 to +0.74 with coded values) still achieving overall desirability of 1 since air velocity was not that effective on optimised outputs. So, validation trials were conducted for three different optimum combinations of input parameters where only air velocity changed notably to reveal the prediction accuracy of the developed regression model. So, (i) optimal levels of input variables, (ii) predicted outputs with confidence and prediction intervals and (ii) corresponding experimental results are given in Table 4. Moreover, in the same table (Table 4), there are experimental results for control samples (potatoes dried at the same temperature and air velocity levels of optimal conditions without ohmic heating application) to demonstrate the effect of ohmic heating on system efficiency and product quality. The control drying trials were conducted using an ordinary drying tray, not the ohmic cell, to objectively reflect the difference between ordinary convective drying and the OAD process.
As shown in Table 4, when the potato slices were dried in optimum conditions, the experimental results for optimised variables (DT (\({Z}_{1}\)), Deff,2 (\({Z}_{2}\)), BI (\({Z}_{3}\)) and b* (\({Z}_{4}\))) were close to the predicted results by corresponding models. This verifies that the developed regression models can reflect real-life results for the OAD system. Besides, with the use of the OAD system, 32–38% reduction (variation due to air velocity changes) in DT was achieved for the samples dried using OAD under optimal conditions compared to control samples (Table 4 and Fig. 2). There could be several reasons to get faster and shorter drying with the use of OAD and with the increase of \(V\). One of them is the release of water from the cell walls as a result of tissue damage in potatoes during electrical treatment even at very low voltage levels (Lebovka et al., 2005, 2006, 2007). Moreover, the enhanced diffusivity of water through the tissue associated with those structural changes expedites water removal (Lebovka et al., 2006). As a result, the drying rate increases. However, there should be more than those when ohmic heating is applied simultaneously (not as a pre-treatment). As known, ohmic heating is a volumetric heating method like microwave application. In volumetric heating methods, the temperature of the all heating domain increases too fast and homogeneously compared to conductive and convective heating (Rakesh et al., 2012). So, potato slices were internally heated very rapidly with the ohmic heating application. Consequently, (i) the water in tissue evaporates faster, (ii) vapour diffuses through the material easily compared to water in liquid form, (iii) internal pressure increases with the evaporation and it pushes water and vapour throughout the sample volume (Turgut et al., 2021). Thus, the potatoes treated with OAD dried faster than control equivalents and as the applied voltage increased, DT decreased due to faster heating and higher available temperature levels. On the other hand, possible structural deformation during ohmic heating may explain why Deff,2 and Deff,3 (data not given for Deff,3) increased being proportional to the voltage levels.
Before processing foods, they generally undergo a gentle blanching to inactivate enzymes causing undesired changes. Inactivation of enzymes with blanching (due to denaturation of proteins at high temperatures) helps producers to ensure the desired colour, texture and flavour of potatoes during further processes like drying, frying, canning, storage and it also improves drying performance (Leeratanarak et al., 2006; Moreno-Perez et al., 1996; Pimpaporn et al., 2007; Reis, 2017). One of the blanching alternatives applied before drying is ohmic heating and its positive effects on products were previously reported (Icier, 2010; Lebovka et al., 2006; Zhong & Lima, 2003). However, ohmic heating technology was always used as a pre-treatment before food drying. But in our study, it was integrated into the drying system to gain some additional advantages. These can be listed as (i) the requirement for additional blanching/heating step was omitted and (ii) amount of juice/wastewater was avoided (if blanching was used) since most of the ohmic heating pre-treatments in the literature were carried out in water medium which was then discarded and not used (Icier, 2010; Icier et al., 2017; Jaeger et al., 2016). According to the results obtained from our study, all EA results are similar among those for products produced in the OAD system and they are significantly lower than control samples (p < 0.05). This was due to the higher temperatures reached during ohmic heating. Thus, it can be concluded that the use of OAD may remove the requirement for the blanching step before drying potato slices since high-temperature levels are achieved during OAD serving the same purpose. Regarding BI and L* values, both are important variables for product quality assessment. Because they affect the visual quality of a food product leading to consumers’ purchasing behaviours. These variables are dependent on the browning reactions taking place during drying. In our study, the correlation coefficient between EA and BI was calculated as 0.60 with p = 0.005 indicating that the majority of undesired browning of potato colour during OAD originated from enzymatic browning reactions. In other words, browning was more intense if EA was higher. The enzymatic browning reactions in foods mainly take place due to the activity of polyphenol oxidase (PPO) and peroxidase (POD) enzymes. The activity of POD is usually used to understand whether sufficient blanching is achieved or not. Because POD is ubiquitous and known to be the most heat‐resistant food enzyme (Ganthavorn et al., 1991). So, proper inactivation of POD is enough to slow down/inhibit undesired enzymatic changes in food materials since it means that other deteriorative enzymes should have already been inactivated. With the use of OAD, an 82–84% reduction in POD activity was achieved compared to control samples (convective drying without ohmic heating). This reduction corresponded to 88–91% compared to the POD activity of fresh samples. That level of reduction is sufficient to consider OAD as a method for discarding the requirement of blanching pre-treatment to ensure food materials. This activity loss is equivalent to the level (70–90% inactivation of POD) achieved with classical blanching (Gomes et al., 2018; Kim et al., 2020). As the products of browning reactions increase in the food material, BI increases and L* decreases. From Table 4, the BI of OAD samples was significantly lower than the corresponding control samples. An increment in air velocity slightly decreased BI. The trends observed for L* were similar to those of BI but the difference between OAD and control samples was less notable.
Regarding the other colour properties, a* value is considered as an indicator of browning reactions in general (Leeratanarak et al., 2006), and according to our results, the correlation coefficient between a* and EA of OAD potatoes was 0.51 with p = 0.02. This fact indicated that a* had a moderate dependency on enzymatic browning. a* value of the control sample was slightly higher than the OAD equivalents. Thus, the higher a* value obtained for the control samples showed that OAD plays a role that slows down browning reactions. However, this effect may not be only due to enzyme inactivation but also shorter DTs of OAD samples. It is known that drying causes an increase in the b* value of potatoes and this is attributed to the proportional increase in carotenoid concentration (Park, 1987; Pimpaporn et al., 2007). Carotenoids are known as relatively heat stable colour pigments in foods (Lin et al., 1998). The carotenoid content of food materials does not show a significant reduction after heat treatments such as microwaves (Choi & Okos, 1986; Lin et al., 1998; Yan et al., 2013). On the contrary, these studies show that microwave-assisted vacuum drying reduces the drying time of foods and it is beneficial in the protection of carotenoids compared to the air-drying method. Therefore, it can be concluded that the loss of carotenes during drying can be attributed to limited oxidation. As a consequence, Schadle et al. (1983) explained the limited loss of carotenoids during freeze-drying with avoided oxidation under vacuum. In our study, the inactivation of enzymes and reduction of drying time with the use of OAD could prevent carotenoids from oxidation. As a result, the higher carotenoid concentration of OAD samples could lead to higher b* values than control equivalents. The higher b* values being relevant to the yellowness of food materials are beneficial in terms of nutritional and visual aspects. So we aimed to maximise the yellowness of dried samples which is more attractive for consumers in optimisation. The total colour change of OAD samples is higher than control ones, although the difference between them is statistically insignificant (p > 0.05). But one should remember that b* values of OAD samples were greater than both control and fresh potatoes (data is not given). So, this difference enhanced the ΔE of OAD treated samples in a favourable manner.
In the case of RR and LL, compared to other analysed parameters, the difference between the drying process at optimal conditions of OAD and control one decreased for RR and disappeared for LL. The RR capability of OAD samples is higher than the control samples but the only significant difference is between O2 and the corresponding control sample (C2) (Table 4). This can be attributed to the changes in the physical properties of the potato tissue and starch gelatinisation (Cunningham et al., 2008b; Mazza, 1983). In these studies, blanching was applied before drying and it was noted that structural changes at the cellular level in potatoes (due to blanching pre-treatment) reduced resistance to water diffusion/flow through the cell membrane/wall. Therefore, dried samples pre-processed by blanching show higher rehydration ability (Cunningham et al., 2008b; Nieto et al., 2001). Additionally, the ohmic heating dependent starch gelatinisation and structural changes have been reported for potato samples (Lebovka et al., 2005, 2006) which may be responsible for the higher RR values of potato samples processed by OAD. Besides, microwave application, which is another volumetric heating method, is known to be beneficial to obtain dried potato products with higher rehydration ability compared to convective drying (Cunningham et al., 2008b; Khraisheh et al., 2004; Yan et al., 2013). This is generally related to the limited shrinkage and superior porosity of microwave dried samples. However, in our study, the difference between volume changes of OAD and control samples did not vary from each other. Thus, it is not reasonable to explain the higher RR of dried potato slices by OAD with an analogy to volumetric changes observed in the microwave. Because of that, another potential reason for higher RR values has been sought. As an answer, the SGD of samples is defined. The results we have are clearer and they are indicating differences between drying techniques. OAD dried samples contained more gelatinised starch (p < 0.05). As known, pre-gelatinisation is a technique used by the food industry to give starch molecules the ability to dissolve even in cold water (Mitch, 1984). However, the hydration ability of ungelatinised starch is very poor at levels below its gelatinisation temperature (Gallay & Puddington, 1943). Thus, pre-gelatinisation of potato starch during OAD may be responsible for the slight increase in RR. It has been previously reported that blanching (which is applied before drying) changes the physical properties of potato tissue, gelatinisation characteristics, the loss of soluble solids, which promotes gelatinisation and water transfer, and increases rehydration kinetics (Cunningham et al., 2008a). Thanks to these positive features it brings to the product, the OAD system may also be beneficial for the convenience food industry (soup powders, dough mixtures, etc.) since it supplies partial pre-gelatinisation for starchy foods which need further drying before being sold. The leach of soluble materials from the solid matrix to the rehydration medium is an important quality factor for the dried food industry. Because it produces important losses of vitamins, amino acids, etc. (Lewicki, 1998). Since there is no significant difference between the LL values of OAD and convectional dried samples, we can say that OAD does not have any negative effect on LL.
It is well known that ascorbic acid is a very unstable compound against exposure to heat, oxygen, moisture and light (Ahmed et al., 2010; Khraisheh et al., 2004). Therefore, loss of ascorbic acid is inevitable to some extent during drying as the consequences of high temperature and the presence of oxygen (Wang et al., 1992). This sensitivity prompts scientists to consider ascorbic acid in their studies (Khraisheh et al., 2004; Turgut et al., 2018). In our study, loss of ascorbic acid content of potato slices was also considered to this extent. Our results indicated that the validation trial at the highest air velocity was the only trial (p < 0.05) where the loss in AA was lower than the corresponding control, whereas the samples produced by other trials had higher AA than those corresponding control ones had (p < 0.05). The degradation of ascorbic acid is a complex process and there are many different factors effective on it (Kirk et al., 1977). Khraisheh et al. (2004) have already explained the details of these possible degradation inducers as time, temperature, sample’s moisture content, the viscosity of liquids in the sample and the presence of endogenous antioxidants. In our case, the temperature was one of the important factors. Because depending on process conditions, the sample's internal temperature increased over the boiling point of water for a short period during OAD treatment (Turgut et al., 2021). As the voltage decreases, temperature increment is also expected to slow down. This should play a significant role in the final AA content. Another possible important factor is drying time. The effect of shortened drying time could easily be seen for validations carried at intermediate and lowest air velocity levels. For these samples, the AA content was higher than those the controls had. This showed that the shortened drying time as a result of the use of OAD can compensate for the negative effect of high temperature on AA. However, the validation sample dried at the highest velocity (O3) had lower AA content than the corresponding control, which might be due to the negative effect of low moisture content on the stability of vitamin C. In the low moisture contents of the drying products, the degradation rate of ascorbic acid increases as a result of increased oxidation. In the end, a lower amount of vitamin C remains in the final product (Khraisheh et al., 2004). Since the moisture content of these samples reaches the lower levels much faster than the control group, the rate of ascorbic acid degradation due to oxidation may increase and result in a lower AA value. Nonetheless, the AA content of samples processed with OAD was still within the commercially acceptable range. Wang et al. (1992) reported that the loss of ascorbic acid during dehydrated flake production from raw potatoes was between 30–100%. With the use of commercial drying equipment, this loss was maintained approximately at 50%. On the other hand, AA retention was between 56–58% and 47–71% for OAD and control samples, respectively. Therefore, it can be concluded that although high-temperature levels came about during OAD, AA loss was still in the acceptable range. Similar to ascorbic acid, the total phenolic content of food material decreases after the processes like blanching, ohmic heating and drying. The TPC retention after ohmic and conventional blanching was reported as 63–89% and 50–65%, respectively (Icier, 2010). Compared to these values, TPC retention of all validation trials changed between 60–80% and 66–82% for OADs and controls, respectively. Therefore, we can say that although high temperatures were attained during OAD, there was no clear trend in both AA and TPC results between OAD and control samples. This can be attributed to the short drying time and/or enhanced extraction efficiency of these bioactive compounds due to thermal and electro permeation impacts (Rodrigues et al., 2019).
Regarding product quality, textural properties are one of the most important features, as their impacts on consumer perception begin even at the first bite. Moreover, they are useful to have an idea about the resistance to possible physical deteriorations during handling, processing and storage of the products (Vega-Gálvez et al., 2012). The results regarding the textural properties of the potato samples are presented in Table 3. There was no significant difference among the samples in terms of elasticity (p > 0.05). However, some differences were detected between some firmness values. All the OAD samples had slightly higher firmness values compared to their control equivalents. This is due to the hard nature of the starch gel. Similarly, Wang et al. (2010) pointed to the developed mechanical resistance of dried potato slices against deformation in the case of starch gelatinisation. On the other hand, as the air velocity decreases, an increasing trend for both F and E values drew attention in the validation trials. These results were surprising as the tissue structure became more rigid and harder as the velocity increased. It is called the case hardening effect and it is mainly caused by the rapid removal of moisture from the outer layers of drying foods (Askari et al., 2009). However, the situation observed in our study may be related to the relative puffing effect which occurs during OAD drying. This puffing effect was previously reported for microwave drying. Since both microwave treatment and ohmic heating supplies volumetric heating, rapid evaporation of water in the material and rapid removal of vapour causes tissue expansion and shorter drying time (Giri & Prasad, 2007). This provides a puffy texture to the final product. A similar increase in the sample’s internal pressure was previously demonstrated by Turgut et al. (2021). Therefore, it may be expected that tissue expansion also occurs during OAD when ohmic heating is active. After ohmic heating lost its effect, the temperature and pressure of the domain decreased over time. This decrement may cause a gradual reduction in tissue expansion and structural collapse started. Therefore, we observed that as DT increased, more durable and elastic material against deformation occurred. These ideas were also supported by SEM images (please see Fig. 7) of potato samples processed with OAD and CD. When Fig. 7 was inspected, any noticeable difference in final cellular volumes or shrinkage could not be observed among the samples as it was previously reported for microwave supported drying systems (Askari et al., 2009; Giri & Prasad, 2007).
In Fig. 8, SEM images of the dried potato samples were given for O2 and C2, respectively, for OAD and CD applications. In those figures, SEM images taken from the inner and outer sections of the samples were given separately to show how ohmic heating affected the structure. Starch globules of the O2 sample disappeared compared to the control equivalent (C2). Similarly, as the applied voltage lowered, starch globules remained more apparent after OAD. These changes were no doubt due to the starch gelatinisation which took place during ohmic heating. Normally, starch globules present in cells of potato tissue can easily be observed from SEM images (Cheyne et al., 2005). However, as a result of gelatinisation (water uptake, expansion and rupture) during the processes like blanching, they lose their visibility (Barreto et al., 2019; Liu & Shi, 2006). The differences between the morphological features of starch globules among the SEM images were not limited to the effect of OAD. There were also variations between different potatoes and in potato tubers. It has been previously determined that starch globules in the cells of samples taken from the centre of potato tubers contain fewer and smaller starch globules compared to the samples obtained from the regions close to the peel/outer layer. The remaining volumes in the cells are filled with water (Nguyen et al., 2018). For this reason, there were some differences in size and amount of starch globules between the SEM images given in the present study.
Perspective
In the present study, the effects of the ohmic assisted convective drying on drying time and some quality parameters of potato slices were evaluated. Moreover, the optimum values for system parameters of air velocity, drying temperature and applied voltage level, were determined. In our previous study, a mechanistic understanding of the OAD system was presented (Turgut et al., 2021). According to the results obtained from both studies, the system is effective in reducing the drying time of solid food materials compared to convective drying. Moreover, it helps to protect some quality parameters of the dried potatoes such as colour (especially inhibition of browning reactions), phenolic content, enzyme inactivation.
OAD ensures an enzyme inactivation at an equivalent level to the blanching application. Additionally, it causes partial starch gelatinisation which might be useful for the convenience food industry. With these advantages, the OAD can be considered as a useful method to eliminate the need for blanching for enzyme inactivation or pre-cooking (where a lot of wastewater is produced) before the convective drying. In that way, OAD may assist the inevitable adaptation/change of the food industry for the economic use of water resources due to the climate change, whose effects we are feeling (Coles & Hall, 2012).
Although any results were not shared in the present paper, OAD might be beneficial for decreasing the energy consumption of drying operations. Among the controlled drying methods used today, the most used and the most studied drying method is convective drying. It is thought that 85% of the currently used drying methods are based on hot air drying (Mujumdar & Beke, 2003). Although convective drying is a widespread and old technology, heating the air used in these systems using fossil fuels is a serious problem. In addition, although there are systems developed to recycle the used air, it is generally released directly to the environment at the end of the process. (Raghavan et al., 2005). For these reasons, convective drying systems are considered energy inefficient. It is aimed to reduce greenhouse gas emissions with the pressure of societies and governments. Towards this aim, there is an increasing effort to enhance the number of energy-efficient drying systems. For these reasons, hybrid or combined drying systems are being developed (Kudra & Mujumdar, 2009). At that point, OAD may arise as an alternative approach. We believe its combination with convective drying can be helpful to reduce the energy requirement and carbon footprint of drying processes since ohmic heating is known as one of the most energy-friendly heating technologies with almost 90% energy efficiency (Akkara & Kayaardı, 2014). However, for a deeper understanding of the energy perspective, detailed energy-exergy calculations and a life cycle assessment should be carried out.
Besides its advantages, the OAD system in the present form carries some disadvantages, as well. One of them is non-uniform electric current distribution in the sample domain because of the use of needle-type electrodes. It may cause some ignorable heterogeneities in product properties, but those can be overcome with the use of electrodes in different geometries. The second negation is that the OAD is a batch system. Thus, the OAD system may not be very well suitable for mass production with its present form. However, when it is considered that the adaptation of ohmic heating to industrial use took about 100 years after the first patent was taken (Goullieux & Pain, 2005), we can foresee that the OAD will become suitable for continuous industrial systems in the near future thanks to the ongoing efforts and studies. Last but not least, the third concern is the geometric properties of the potatoes used. Due to the existing electrode configuration, irregularities in product geometry may have adverse effects on system performance and quality standardization of the dried product. However, with the development of continuous ohmic systems as mentioned above, it is thought that this problem can be solved in the future.
Conclusion
In the present study, the simultaneous use of ohmic heating and convective drying in combination (OAD) was studied as a novel drying method. Process parameters (drying temperature, air velocity and applied voltage) were investigated for minimizing the drying time of potato slices and its effects on some quality characteristics of the final product. Drying air temperature and applied voltage was found considerably effective on the outputs where drying air velocity did not show a very notable impact in the studied range. As a result, a 32–38% reduction in drying time was achieved with the use of OAD. Moreover, 88–91% peroxidase inactivation was achieved with OAD which in turn removes the need for blanching before drying processes.
On the other hand, drying characteristics and products quality of the potato slices dried at optimal conditions were compared with their control equivalent. Compared to control equivalents, the OAD system did not show any adverse effect on dried potato quality. On the contrary, most of the studied quality features of samples produced using OAD were generally superior to the control ones had. Moreover, starch gelatinisation occurred during OAD was higher than control ones which may make OAD produced starchy food materials preferable for the convenience food industry. In brief, due to its positive impact on drying characteristics and product properties, the OAD system is a promising alternative to the conventional drying process in the food industry.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdi, H., & Williams, L. J. (2010). Tukey’s Honestly Significant Difference (HSD) Test. In N. J. Salkind (Ed.), Encyclopedia of Research Design (pp. 1-5). Thousand Oaks, CA: Sage.
Ahmed, M., Akter, M. S., & Eun, J.-B. (2010). Peeling, drying temperatures, and sulphite-treatment affect physicochemical properties and nutritional quality of sweet potato flour. Food Chemistry, 121(1), 112–118. https://doi.org/10.1016/j.foodchem.2009.12.015
Akkara, M., & Kayaardı, S. (2014). Effect of advanced preservation techniques on meat quality. Akademik Gıda, 12(4), 79–85.
Akpınar, E. K. (2006). Mathematical modelling of thin layer drying process under open sun of some aromatic plants. Journal of Food Engineering, 77(4), 864–870. https://doi.org/10.1016/j.jfoodeng.2005.08.014
Andreou, V., Dimopoulos, G., Tsonas, T., Katsimichas, A., Limnaios, A., Katsaros, G., & Taoukis, P. (2021). Pulsed electric fields-assisted drying and frying of fresh zucchini. Food and Bioprocess Technology. https://doi.org/10.1007/s11947-021-02705-z
AOAC. (2005). Official methods of analysis of AOAC International. AOAC International.
Arikan, M. F., Ayhan, Z., Soysal, Y., & Esturk, O. (2012). Drying characteristics and quality parameters of microwave-dried grated carrots. Food and Bioprocess Technology, 5(8), 3217–3229. https://doi.org/10.1007/s11947-011-0682-8
Askari, G. R., Emam-Djomeh, Z., & Mousavi, S. M. (2009). An investigation of the effects of drying methods and conditions on drying characteristics and quality attributes of agricultural products during hot air and hot air/microwave-assisted dehydration. Drying Technology, 27(7–8), 831–841. https://doi.org/10.1080/07373930902988106
Barreto, I. M. A., Tribuzi, G., Marsaioli Junior, A., Carciofi, B. A. M., & Laurindo, J. B. (2019). Oil–free potato chips produced by microwave multiflash drying. Journal of Food Engineering, 261, 133–139. https://doi.org/10.1016/j.jfoodeng.2019.05.033
Bender, D., Gratz, M., Vogt, S., Fauster, T., Wicki, B., Pichler, S., & Schoenlechner, R. (2019). Ohmic heating—a novel approach for gluten-free bread baking. Food and Bioprocess Technology, 12(9), 1603–1613. https://doi.org/10.1007/s11947-019-02324-9
Bi, J., Chen, Q., Zhou, Y., Liu, X., Wu, X., & Chen, R. (2014). Optimization of short- and medium-wave infrared drying and quality evaluation of jujube powder. Food and Bioprocess Technology, 7(8), 2375–2387. https://doi.org/10.1007/s11947-013-1245-y
Canizares, D., & Mauro, M. A. (2015). Enhancement of quality and stability of dried papaya by pectin-based coatings as air-drying pretreatment [journal article]. Food and Bioprocess Technology, 8(6), 1187–1197. https://doi.org/10.1007/s11947-015-1483-2
Cemeroğlu, B. S. (2013). Gıda analizleri. Bizim Grup Basımevi.
Ceylan, İ., Aktaş, M., & Doğan, H. (2006). Apple drying at kiln by solar energy. Journal of Polytechnic, 9(4), 289–294. https://doi.org/10.2339/2006.9.4.289-294
Cheyne, A., Barnes, J., Gedney, S., & Wilson, D. I. (2005). Extrusion behaviour of cohesive potato starch pastes: II. Microstructure–process interactions. Journal of Food Engineering, 66(1), 13–24. https://doi.org/10.1016/j.jfoodeng.2004.02.036
Choi, Y., & Okos, M. R. (1986). Thermal properties of liquid foods-review. In M. Okos (Ed.), Physical and Chemical Properties of Food. American Society of Agricultural Engineers.
Coles, N., & Hall, P. (2012, 27–29 Oct. 2012). Water, energy and food security. 2012 IEEE Conference on Technology and Society in Asia (T&SA),
Crank, J. (1975). The mathematics of diffusion. Clarendon Press.
Cunningham, S. E., McMinn, W. A. M., Magee, T. R. A., & Richardson, P. S. (2008a). Effect of processing conditions on the water absorption and texture kinetics of potato. Journal of Food Engineering, 84(2), 214–223. https://doi.org/10.1016/j.jfoodeng.2007.05.007
Cunningham, S. E., McMinn, W. A. M., Magee, T. R. A., & Richardson, P. S. (2008b). Experimental study of rehydration kinetics of potato cylinders. Food and Bioproducts Processing, 86(1), 15–24. https://doi.org/10.1016/j.fbp.2007.10.008
Dalla Rosa, M., Lerici, C. R., Pinnavaia, G., & Cencic, L. (1989). Sul grado di gelatinizzazione dell'amido in alimenti diversi. Tecnica Molitoria.
Doymaz, İ. (2004). Convective air drying characteristics of thin layer carrots. Journal of Food Engineering, 61(3), 359–364. https://doi.org/10.1016/S0260-8774(03)00142-0
Eren, İ., & Kaymak-Ertekin, F. (2007). Optimization of osmotic dehydration of potato using response surface methodology. Journal of Food Engineering, 79(1), 344–352. https://doi.org/10.1016/j.jfoodeng.2006.01.069
Fadavi, A., Salari, S., Mansouri, A., & Hoseini, S. (2020). Effects of vacuum and juice concentration on electrical conductivity by the ohmic method: A case study of sour cherry. Food and Bioprocess Technology, 13(7), 1146–1153. https://doi.org/10.1007/s11947-020-02463-4
Fernandes, F. A. N., Rodrigues, S., Cárcel, J. A., & García-Pérez, J. V. (2015). Ultrasound-assisted air-drying of apple (Malus domestica L.) and its effects on the vitamin of the dried product. Food and Bioprocess Technology, 8(7), 1503–1511. https://doi.org/10.1007/s11947-015-1519-7
Gallay, W., & Puddington, I. E. (1943). The hydration of starch below the gelatinization temperature. Canadian Journal of Research, 21b(9), 179–185. https://doi.org/10.1139/cjr43b-024
Ganthavorn, C., Nagel, C. W., & Powers, J. R. (1991). Thermal inactivation of asparagus lipoxygenase and peroxidase. Journal of Food Science, 56(1), 47–49. https://doi.org/10.1111/j.1365-2621.1991.tb07972.x
Giovanelli, G., Zanoni, B., Lavelli, V., & Nani, R. (2002). Water sorption, drying and antioxidant properties of dried tomato products. Journal of Food Engineering, 52(2), 135–141. https://doi.org/10.1016/S0260-8774(01)00095-4
Giri, S. K., & Prasad, S. (2007). Drying kinetics and rehydration characteristics of microwave-vacuum and convective hot-air dried mushrooms. Journal of Food Engineering, 78(2), 512–521. https://doi.org/10.1016/j.jfoodeng.2005.10.021
Gomes, C. F., Sarkis, J. R., & Marczak, L. D. F. (2018). Ohmic blanching of Tetsukabuto pumpkin: Effects on peroxidase inactivation kinetics and color changes. Journal of Food Engineering, 233, 74–80. https://doi.org/10.1016/j.jfoodeng.2018.04.001
Gong, C., Liao, M., Zhang, H., Xu, Y., Miao, Y., & Jiao, S. (2020). Investigation of hot air–assisted radio frequency as a final-stage drying of pre-dried carrot cubes. Food and Bioprocess Technology, 13(3), 419–429. https://doi.org/10.1007/s11947-019-02400-0
Goullieux, A., & Pain, J.-P. (2005). Ohmic heating In D.-W. Sun (Ed.), Emerging technologies for food processing (pp. 469–505). Academic Press. https://doi.org/10.1016/B978-012676757-5/50020-7
Graves, S., Piepho, H. P., Selzer, L., & Dorai-Raj, S. (2019). Visualizations of paired comparisons. Retrieved 01.05. from https://cran.r-project.org/web/packages/multcompView/multcompView.pdf
Hutchings, J. B. (1977). The importance of visual appearance of foods to the food processor and the consumer. Journal of Food Quality, 1(3), 267–278. https://doi.org/10.1111/j.1745-4557.1977.tb00945.x
Icier, F. (2010). Ohmic blanching effects on drying of vegetable byproduct. Journal of Food Process Engineering, 33(4), 661–683. https://doi.org/10.1111/j.1745-4530.2008.00295.x
Icier, F., Cokgezme, O. F., & Sabanci, S. (2017). Alternative thawing methods for the blanched/non-blanched potato cubes: Microwave, ohmic, and carbon fiber plate assisted cabin thawing. Journal of Food Process Engineering, 40(2), e12403. https://doi.org/10.1111/jfpe.12403
Jaeger, H., Roth, A., Toepfl, S., Holzhauser, T., Engel, K.-H., Knorr, D., . . . Steinberg, P. (2016). Opinion on the use of ohmic heating for the treatment of foods. Trends in Food Science & Technology, 55, 84–97. https://doi.org/10.1016/j.tifs.2016.07.007
James, G., Witten, D., Hastie, T., & Tibshirani, R. (2013). Linear model selection and regularization. In An introduction to statistical learning: with applications in R (pp. 203–264). Springer New York. https://doi.org/10.1007/978-1-4614-7138-7_6
Jun, S., & Sastry, S. (2005). Modeling and optimization of ohmic heating of foods inside a flexible package. Journal of Food Process Engineering, 28(4), 417–436. https://doi.org/10.1111/j.1745-4530.2005.00032.x
Karacabey, E., Turan, M. S., Özçelik, Ş, & G., Baltacıoğlu, C., & Küçüköner, E. (2016). Optimisation of pre-drying and deep-fat-frying conditions for production of low-fat fried carrot slices. Journal of the Science of Food and Agriculture, 96(13), 4603–4612. https://doi.org/10.1002/jsfa.7678
Khraisheh, M. A. M., McMinn, W. A. M., & Magee, T. R. A. (2004). Quality and structural changes in starchy foods during microwave and convective drying. Food Research International, 37(5), 497–503. https://doi.org/10.1016/j.foodres.2003.11.010
Kim, A.-N., Lee, K. Y., Rahman, M. S., Kim, H.-J., Chun, J., Heo, H. J., & Choi, S.-G. (2020). Effect of water blanching on phenolic compounds, antioxidant activities, enzyme inactivation, microbial reduction, and surface structure of samnamul (Aruncus dioicus var kamtschaticus). International Journal of Food Science & Technology, 55(4), 1754–1762. https://doi.org/10.1111/ijfs.14424
Kirk, J., Dennison, D., Kokoczka, P., & Heldman, D. (1977). Degradation of ascorbic acid in a dehydrated food system. Journal of Food Science, 42(5), 1274–1279. https://doi.org/10.1111/j.1365-2621.1977.tb14477.x
Kudra, T., & Mujumdar, A. S. (2009). Advanced drying technologies. CRC Press.
Kuhn, M. (2016). The desirability package. Retrieved 15.05 from https://cran.r-project.org/web/packages/desirability/vignettes/desirability.pdf
Lebovka, N. I., Praporscic, I., Ghnimi, S., & Vorobiev, E. (2005). Does electroporation occur during the ohmic heating of food? Journal of Food Science, 70(5), E308–E311. https://doi.org/10.1111/j.1365-2621.2005.tb09969.x
Lebovka, N. I., Shynkaryk, M. V., & Vorobiev, E. (2006). Drying of potato tissue pretreated by ohmic heating. Drying Technology, 24(5), 601–608. https://doi.org/10.1080/07373930600626677
Lebovka, N. I., Shynkaryk, N. V., & Vorobiev, E. (2007). Pulsed electric field enhanced drying of potato tissue. Journal of Food Engineering, 78(2), 606–613. https://doi.org/10.1016/j.jfoodeng.2005.10.032
Leeratanarak, N., Devahastin, S., & Chiewchan, N. (2006). Drying kinetics and quality of potato chips undergoing different drying techniques. Journal of Food Engineering, 77(3), 635–643. https://doi.org/10.1016/j.jfoodeng.2005.07.022
Lenth, R. V. (2009). Response-Surface Methods in R, Using rsm. 2009, 32(7), 17. https://doi.org/10.18637/jss.v032.i07
Lewicki, P. P. (1998). Effect of pre-drying treatment, drying and rehydration on plant tissue properties: A review. International Journal of Food Properties, 1(1), 1–22. https://doi.org/10.1080/10942919809524561
Lin, T. M., Durance, T. D., & Scaman, C. H. (1998). Characterization of vacuum microwave, air and freeze dried carrot slices. Food Research International, 31(2), 111–117. https://doi.org/10.1016/S0963-9969(98)00070-2
Liu, L., Wang, Y., Zhao, D., An, K., Ding, S., & Wang, Z. (2014). Effect of carbonic maceration pre-treatment on drying kinetics of chilli (Capsicum annuum L.) flesh and quality of dried product [journal article]. Food and Bioprocess Technology, 7(9), 2516–2527. https://doi.org/10.1007/s11947-014-1253-6
Liu, Y., & Shi, Y.-C. (2006). Phase and state transitions in granular starches studied by dynamic differential scanning calorimetry. Starch - Stärke, 58(9), 433–442. https://doi.org/10.1002/star.200500448
Liu, Z.-L., Staniszewska, I., Zielinska, D., Zhou, Y.-H., Nowak, K. W., Xiao, H.-W., & Zielinska, M. (2020). Combined hot air and microwave-vacuum drying of cranberries: Effects of pretreatments and pulsed vacuum osmotic dehydration on drying kinetics and physicochemical properties. Food and Bioprocess Technology, 13(10), 1848–1856. https://doi.org/10.1007/s11947-020-02507-9
Mazza, G. (1983). Dehydration of carrots. International Journal of Food Science & Technology, 18(1), 113–123. https://doi.org/10.1111/j.1365-2621.1983.tb00249.x
Mebane Jr., W. R., & Sekhon, J. S. (2011). Genetic optimization using derivatives: The rgenoud package for R. 2011, 42(11), 26. https://doi.org/10.18637/jss.v042.i11
Mitch, E. L. (1984). Potato starch: Production and uses. In Starch: Chemistry and technology (pp. 479–490). Elsevier.
Moreno-Perez, L. F., Gasson-Lara, J. H., & Ortega-Rivas, E. (1996). Effect of low temperature-long time blanching on quality of dried sweet potato. Drying Technology, 14(7–8), 1839–1857. https://doi.org/10.1080/07373939608917177
Moses, J. A., Norton, T., Alagusundaram, K., & Tiwari, B. K. (2014). Novel drying techniques for the food industry. Food Engineering Reviews, 6(3), 43–55. https://doi.org/10.1007/s12393-014-9078-7
Mudahar, G. S., Toledo, R. T., & Jen, J. J. (1990). A response surface methodology approach to optimize potato dehydration process. Journal of Food Processing and Preservation, 14(2), 93–106. https://doi.org/10.1111/j.1745-4549.1990.tb00831.x
Mujumdar, A. S., & Beke, J. (2003). Grain drying: Basic principles. In A. Chakraverty, A. S. Mujumdar, G. S. V. Raghavan, & H. S. Ramaswamy (Eds.), Handbook of Postharvest Technology: Cereals, Fruits, Vegetables, Tea, and Spices. Marcel Dekker, Inc.
Nguyen, T. K., Mondor, M., & Ratti, C. (2018). Shrinkage of cellular food during air drying. Journal of Food Engineering, 230, 8–17. https://doi.org/10.1016/j.jfoodeng.2018.02.017
Nieto, A., Castro, M. A., & Alzamora, S. M. (2001). Kinetics of moisture transfer during air drying of blanched and/or osmotically dehydrated mango. Journal of Food Engineering, 50(3), 175–185. https://doi.org/10.1016/S0260-8774(01)00026-7
Park, Y. W. (1987). Effect of freezing, thawing, drying, and cooking on carotene retention in carrots, broccoli and spinach. Journal of Food Science, 52(4), 1022–1025. https://doi.org/10.1111/j.1365-2621.1987.tb14266.x
Pimpaporn, P., Devahastin, S., & Chiewchan, N. (2007). Effects of combined pretreatments on drying kinetics and quality of potato chips undergoing low-pressure superheated steam drying. Journal of Food Engineering, 81(2), 318–329. https://doi.org/10.1016/j.jfoodeng.2006.11.009
Raghavan, G. V., Rennie, T. J., Sunjka, P. S., Orsat, V., Phaphuangwittayakul, W., & Terdtoon, P. (2005). Overview of new techniques for drying biological materials with emphasis on energy aspects. Brazilian Journal of Chemical Engineering, 22(2), 195–201. https://doi.org/10.1590/S0104-66322005000200005
Rakesh, V., Datta, A. K., Walton, J. H., McCarthy, K. L., & McCarthy, M. J. (2012). Microwave combination heating: Coupled electromagnetics- multiphase porous media modeling and MRI experimentation. AIChE Journal, 58(4), 1262–1278. https://doi.org/10.1002/aic.12659
Ratti, C. (2001). Hot air and freeze-drying of high-value foods: A review. Journal of Food Engineering, 49(4), 311–319. https://doi.org/10.1016/S0260-8774(00)00228-4
Reis, F. R. (2017). Impact of blanching on the performance of subsequent drying. In New Perspectives on Food Blanching (pp. 123–136). Springer.
Rodrigues, R. M., Genisheva, Z., Rocha, C. M. R., Teixeira, J. A., Vicente, A. A., & Pereira, R. N. (2019). Ohmic heating for preservation, transformation and extraction. In F. Chemat & E. Vorobiev (Eds.), Green Food Processing Techniques (1st ed., pp. 159–192). Academic Press
Sakr, M., & Liu, S. (2014). A comprehensive review on applications of ohmic heating (OH). Renewable and Sustainable Energy Reviews, 39, 262–269. https://doi.org/10.1016/j.rser.2014.07.061
Schadle, E. R., Burns, E. E., & Talley, L. J. (1983). Forced air drying of partially freeze-dried compressed carrot bars. Journal of Food Science, 48(1), 193–196. https://doi.org/10.1111/j.1365-2621.1983.tb14821.x
Seyhun, N., Ramaswamy, H. S., Zhu, S., Sumnu, G., & Sahin, S. (2013). Ohmic tempering of frozen potato puree. Food and Bioprocess Technology, 6(11), 3200–3205. https://doi.org/10.1007/s11947-012-1002-7
Sievert, C. (2020). Interactive web-based data visualization with R, plotly, and shiny. Chapman and Hall/CRC. https://plotly-r.com
Singleton, V. L., & Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16(3), 144–158. http://www.ajevonline.org/content/16/3/144.abstract
Turgut, S. S., Küçüköner, E., Feyissa, A. H., & Karacabey, E. (2021). A novel drying system – simultaneous use of ohmic heating with convectional air drying: System design and detailed examination using CFD. Innovative Food Science & Emerging Technologies, 102727. https://doi.org/10.1016/j.ifset.2021.102727
Turgut, S. S., Küçüköner, E., & Karacabey, E. (2018). Improvements in drying characteristics and quality parameters of tomato by carbonic maceration pretreatment. Journal of Food Processing and Preservation, 42(2), e13282. https://doi.org/10.1111/jfpp.13282
Vega-Gálvez, A., Ah-Hen, K., Chacana, M., Vergara, J., Martínez-Monzó, J., García-Segovia, P., . . . Di Scala, K. (2012). Effect of temperature and air velocity on drying kinetics, antioxidant capacity, total phenolic content, colour, texture and microstructure of apple (var. Granny Smith) slices. Food Chemistry, 132(1), 51–59. https://doi.org/10.1016/j.foodchem.2011.10.029
Wang, J., Xiao, H.-W., Ye, J.-H., Wang, J., & Raghavan, V. (2019). Ultrasound pretreatment to enhance drying kinetics of kiwifruit (Actinidia deliciosa) slices: Pros and cons. Food and Bioprocess Technology, 12(5), 865–876. https://doi.org/10.1007/s11947-019-02256-4
Wang, R., Zhang, M., & Mujumdar, A. S. (2010). Effects of vacuum and microwave freeze drying on microstructure and quality of potato slices. Journal of Food Engineering, 101(2), 131–139. https://doi.org/10.1016/j.jfoodeng.2010.05.021
Wang, W.-C., & Sastry, S. K. (2000). Effects of thermal and electrothermal pretreatments on hot air drying rate of vegetable tissue. Journal of Food Process Engineering, 23(4), 299–319. https://doi.org/10.1111/j.1745-4530.2000.tb00517.x
Wang, X. Y., Kozempel, M. G., Hicks, K. B., & Seib, P. A. (1992). Vitamin C stability during preparation and storage of potato flakes and reconstituted mashed potatoes. Journal of Food Science, 57(5), 1136–1139. https://doi.org/10.1111/j.1365-2621.1992.tb11282.x
Weisberg, S. (2018). Package ‘alr3’ Retrieved 20.05 from https://cran.r-project.org/web/packages/alr3/alr3.pdf
Wootton, M., Weeden, D., & Munk, N. (1971). Rapid method for the estimation of starch gelatinization in processed foods. Food Technol Australia.
Yan, W.-Q., Zhang, M., Huang, L.-L., Mujumdar, A. S., & Tang, J. (2013). Influence of microwave drying method on the characteristics of the sweet potato dices. Journal of Food Processing and Preservation, 37(5), 662–669. https://doi.org/10.1111/j.1745-4549.2012.00707.x
Zhao, D., An, K., Ding, S., Liu, L., Xu, Z., & Wang, Z. (2014). Two-stage intermittent microwave coupled with hot-air drying of carrot slices: Drying kinetics and physical quality. Food and Bioprocess Technology, 7(8), 2308–2318. https://doi.org/10.1007/s11947-014-1274-1
Zhong, T., & Lima, M. (2003). The effect of ohmic heating on vacuum drying rate of sweet potato tissue. Bioresource Technology, 87(3), 215–220. https://doi.org/10.1016/S0960-8524(02)00253-5
Zhou, X., Xu, R., Zhang, B., Pei, S., Liu, Q., Ramaswamy, H. S., & Wang, S. (2018). Radio frequency-vacuum drying of kiwifruits: Kinetics, uniformity, and product quality. Food and Bioprocess Technology, 11(11), 2094–2109. https://doi.org/10.1007/s11947-018-2169-3
Acknowledgements
This research was financially supported by “Süleyman Demirel University Scientific Research Projects Office”, Turkey (Project ID: FDK-2019-6978). Sebahattin Serhat Turgut is supported under postgraduate scholarship (2211-C) of “The Scientific and Technical Research Council of Turkey (TÜBİTAK)”. The authors thank all supporting organisations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Turgut, S.S., Karacabey, E. & Küçüköner, E. A Novel System—the Simultaneous Use of Ohmic Heating with Convective Drying: Sensitivity Analysis of Product Quality Against Process Variables. Food Bioprocess Technol 15, 440–458 (2022). https://doi.org/10.1007/s11947-022-02765-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02765-9