Abstract
Objective
As shellfish preservation is still nowadays a challenge for the food industry, in this paper, the effectiveness of hyperbaric storage in preserving Atlantic razor clams (Ensis directus) was evaluated both at low (HS-LT) and at room temperature (HS-RT).
Methods
The study was carried out in two phases. In the first phase, razor clams were stored at different pressure levels, both at 5 °C and 20 °C, for 7 days to identify the most efficient storage pressure at both temperatures. Then, in the second phase of the study, HS-LT and HS-RT experiments were performed at these conditions for 14 days to compare the effectiveness of both methods.
Results
Microbial analysis after storage showed that, at 5 °C, a minimum pressure of 50 MPa was needed to prevent microbial growth in the samples, while 75 MPa were required at 20 °C. In the second phase of the study, results revealed that both HS-LT (50 MPa/5 °C) and HS-RT (75 MPa/20 °C) extended the microbial shelf-life of the razor clams to, at least, twice that achieved in conventional refrigeration, but quality decline was larger in the samples stored at 75 MPa and 20 °C.
Conclusions
HS-LT resulted more effective than HS-RT for the preservation of razor clams.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperbaric storage (HS) is a new technique of food preservation that consists in storing food under pressure, usually below 100 MPa, for long periods of time, even up to years in certain cases (Lemos et al. 2020). Pressure acts as an effective inhibitor for microbial growth, and depending on the level, it can either retard or prevent the proliferation of microorganisms. Hyperbaric storage has been tested in a wide temperature range, from low temperatures (HS-LT) close to 5 °C to room temperature (HS-RT) and several papers show that, in both cases, it can result more effective in extending the shelf-life of some foods than conventional refrigeration (Fidalgo et al. 2014, 2018, 2019; Otero et al. 2019).
The most important advantage of HS-RT over HS-LT is the power saving that no temperature control involves. In HS-RT, energy is only needed during compression, and once the storage pressure has been reached, no additional energy is required during storage. This low power consumption, together with the no use of refrigerants, contributes to reduce the CO2 total emissions, and thus, Bermejo-Prada et al. (2017) showed that the carbon footprint of HS-RT is considerably lower than that of conventional refrigeration (CR). However, some data in the literature suggest that HS-RT requires larger pressures than HS-LT to extend the shelf-life of foods. Thus, Lemos et al. (2017) stored watermelon juice at 50 MPa, either at 10 °C or at 25 °C, and found that, at 10 °C, the shelf-life of the juice could be extended to, at least, 21 days. By contrast, when the juice was stored at 50 MPa and 25 °C, it was spoiled after only 3 days. At this temperature, a pressure of 75 MPa was required to extend the juice shelf-life to 21 days.
Increasing pressure can allow the storage of food at room temperature, but in return, it requires thicker and, therefore, more expensive and harder to handle high-pressure vessels (Bermejo-Prada et al. 2017). Moreover, high storage pressures can also affect some quality attributes of foods, such as texture, color, or water-holding capacity (Matser et al. 2000; Montero and Gómez-Guillén 2004), especially when they are applied for long times. Therefore, when choosing the best option between HS-LT and HS-RT, not only economic and environmental criteria should be considered but also other factors such as the shelf-life extension and the effects on food quality.
Most papers in the literature focus either on HS-LT (Fidalgo et al. 2019; Otero et al. 2017) or on HS-RT (Fidalgo et al. 2014; Santos et al. 2020b), but to the best of our knowledge, only two studies compare the effectiveness of both methods at some particular conditions. Thus, Lemos et al. (2017) found that HS, both at 75 MPa/15 °C and at 75 MPa/25 °C, inhibited microbial growth in watermelon juice for, at least, 21 days, but color changes were smaller in the samples kept at 15 °C. By contrast, Santos et al. (2020a) did not find clear differences on the quality of raw meat stored either at 75 MPa/25 °C or at 60 MPa/10 °C for 60 days, probably because, at the conditions tested, the real potential of HS-LT could not be shown. To reveal this potential, lower storage temperatures, at least, as low as those employed in conventional refrigeration, should be tested. Moreover, to minimize pressure-induced quality losses, pressure should be set to the minimum level required to prevent microbial growth in the product during storage. This pressure depends not only on the storage temperature but also on some intrinsic food characteristics such as its composition, water activity, or pH (Otero 2019). Even though abundant for short processing times (1–15 min), data on the effects of pressure, applied for weeks or months, on food quality are very scarce in the literature and only available for a few products. Therefore, there is an urgent need to increase knowledge about the combined effects of pressure and temperature on food safety and quality during hyperbaric storage, especially in highly perishable and/or expensive foods because they could be the best candidates to introduce hyperbaric storage in the market.
As shellfish preservation is, still nowadays, a challenge for the food industry, this paper focuses, for the first time in the literature, on hyperbaric storage of a bivalve mollusk. As no studies exist that compare HS at room and at usual chilling temperatures, the main objective of the paper was to examine the effectiveness of both methods in preserving Atlantic razor clams, a product with a typical shelf-life in conventional refrigeration of about 7 days from harvest (Khan and Liu 2019; Leavitt 2010). To do so, in the first phase of the study, the minimum pressure (Pmin) required to avoid microbial growth, both at 5 °C (HS-LT experiments) and at 20 °C (HS-RT experiments), was identified. Then, in the second phase of the study, HS-LT and HS-RT experiments were performed at Pmin(5 °C) and Pmin(20 °C), respectively, to compare the effectiveness of both methods in preserving razor clams for 14 days, that is, a time twice as long as the typical shelf-life of conventionally cold stored clams.
Materials and Methods
Sample
Before each storage experiment, a batch of Atlantic razor clams (Ensis directus), collected in the meridional coast of the North Sea in Holland (FAO Fishing Area 27.4.c), was acquired in a local market. At the moment of acquisition, the clams were alive and their mean shell length and weight were 11.4 ± 0.9 cm and 21.9 ± 5.0 g, respectively.
Storage Experiments
Twelve different batches of razor clams, acquired over a period of 4 months, were needed to complete all the experiments described in this paper. Just after receiving the razor clams at the laboratory, each batch was washed with tap water to remove residual sand on the shells, and microbial and physicochemical analyses were immediately performed to characterize the sample at day 0.
In the first phase of the study, sequential HS-LT and HS-RT experiments were conducted at increasing pressures to identify the Pmin required, in each method, to avoid microbial growth in the razor clams for 7 days, that is, the typical shelf-life in conventional refrigeration (Khan and Liu 2019; Leavitt 2010). This storage time was chosen because hyperbaric storage would be of interest only if the product shelf-life under pressure is longer than in conventional refrigeration. Before the HS experiments, the razor clams were packed in polyethylene bags filled with saline water (3.5% NaCl in water) to avoid shell cracking during compression. Saline water was used to mimic the natural habitat of the product and minimize potential osmotic phenomena.
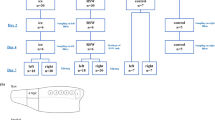
As HS-LT is expected to require lower Pmin than HS-RT, sequential experiments at 5 ± 2 °C were carried out first (Fig. 1), starting from 0.1 MPa (control experiments) and increasing pressure in 25 ± 2 MPa steps in successive experiments (HS-LT experiments). Control experiments at 0.1 MPa (C_5) were included to identify the real effects of pressure during storage. In these experiments, clams were packaged in saline water and kept at atmospheric pressure to reproduce exactly the same conditions as those of HS-LT experiments, except the pressure level. After each storage experiment, microbial analyses were performed to check whether microbial counts increased in the razor clams during storage or not. If did, in the next HS-LT experiment, pressure increased in 25 MPa. If no microbial growth was detected during storage, the pressure tested was identified as Pmin(5 °C), HS-LT experiments were stopped, and Pmin(5 °C) was used as the starting point to identify, also in a similar sequential way, Pmin in HS-RT experiments at 20 ± 2 °C.
Moreover, in the first phase of the study, the effectiveness of hyperbaric storage in preserving razor clams was compared with that of conventional refrigeration. In CR experiments, clams were stored on a tray, covered with a damp cloth to keep them moist but not wet, and maintained at 5 ± 2 °C for 7 days.
In the second phase of the study, HS-LT and HS-RT experiments were performed at the Pmin identified for each storage temperature (5 ± 2 °C and 20 ± 2 °C, respectively). In these experiments, the samples were stored for 14 days to compare the effectiveness of HS-LT and HS-RT in preserving razor clams for a period twice as long as the typical shelf-life that should be expected when the razor clams are conventionally cold stored.
HS experiments were carried out in a pilot-plant high-pressure storage system (model SV1, Institute of High Pressure Physics, Unipress Equipment Division, Poland) composed of two high-pressure stainless-steel vessels located in independent thermostatic chambers. The temperature during storage was monitored both inside the pressure vessels and at the thermostatic chambers by 4 T-type thermocouples. The pressure in each vessel was monitored by a pressure transducer (0–400 MPa, SH-1, WIKA, Germany). All sensor measurements were recorded every 30 s by a data acquisition system (MW100 Data Acquisition Unit, Yokogawa Electric Corporation, Tokyo, Japan). All the storage experiments were performed in triplicate.
Evolution of the Microbial Load During Storage
Samples were analyzed for total viable bacteria (TVB), H2S-producing microorganisms, luminescent colonies, total aerobic mesophiles (TAM), enterobacteria, lactic acid bacteria, and anaerobic sulfite-reducing bacteria (presumptive Clostridium perfringens) before and immediately after storage. Briefly, 10 g of razor clam flesh was collected in a vertical laminar-flow cabinet (mod. AV 30/70 Telstar, Madrid, Spain) and placed in a sterile plastic bag (BBAG-03, Corning Gosselin 400 mL, USA) with 90 mL of buffered 0.1% peptone water (Panreac, Barcelona, Spain). After 1-min processing in a Stomacher blender (model Colworth 400, Seward, London, UK), tenfold serial dilutions were prepared in buffered peptone water and duplicates of the dilutions were plated and incubated as follows: total viable bacteria, H2S-producing microorganisms, and luminescent bacteria on spread plates of Iron Agar (Lyngby, Madrid, Spain) 1% NaCl at 15 °C for 5 days; total aerobic mesophiles on pour plates of Plate Count Agar, PCA (Panreac, Barcelona, Spain) at 30 °C for 72 h; lactic acid bacteria on double-layered plates of MRS Agar (Merck, Germany) at 30 °C for 72 h; enterobacteria on double-layered plates of Violet Red Bile Glucose Agar (VRBG, Panreac, Barcelona, Spain) at 30 °C for 48 h; and anaerobic sulfite-reducing clostridia on double-layered plates of Tryptose Sulfite Cycloserine Agar (bioMèrieux, Marcy l’Etoile, France) at 37 °C for 48 h. Plate counts were expressed as the decimal logarithm of colony-forming units (CFU) per gram of razor clam flesh (log10 CFU·g−1). The detection limit was 1 log10 CFU·g−1 in pour plates and 2 log10 CFU·g−1 in spread plates. In each replicated experiment, microbial determinations were performed in 3 individuals for each experimental condition.
Effect of the Storage Conditions on Physicochemical Parameters
Several physicochemical parameters were employed as quality indicators and evaluated in both raw and cooked razor clams. Thus, weight change after storage, water content, water-holding capacity, and pH were determined in the raw samples, while cooking loss, firmness, and color were measured in the cooked razor clams. Cooking of the samples was performed in a saturated steam oven (Rational, Combi-Master CM 6, Croßküchentechnik GmbH, Landsberg a. Lech, Germany) at 100 °C for 3 min. In each replicated experiment and for each storage condition, physicochemical attributes were evaluated in 3 individuals in the raw samples and in 9 individuals in the cooked ones.
Weight Change After Storage
The weight change after storage (%) was determined as the percent of weight gained or lost after storage according to Eq. (1):
where Wbs and Was are the weight of the razor clams, superficially dried with a soft paper, before and after storage, respectively.
Water Content (WC)
The water content (%) was evaluated in the razor clams by determining the mass loss (%) in about 5 g of the central part of the flesh after oven drying at 105 °C until a constant weight was reached.
Water-Holding Capacity (WHC)
For each determination, two portions of about 2 cm were cut from the extremes of the flesh of each sample, weighed, and centrifuged at 2200 × g and 4 °C for 10 min. After centrifugation, the sample was superficially dried with a soft paper and weighed again. WHC was expressed as the percent of water retained per 100 g of water present in the sample prior to centrifuging according to:
where Mbc and Mac are the masses (g) of the flesh portions before and after centrifugation, respectively, and WC is the water content in the sample prior to centrifuging.
pH
pH was measured in flesh homogenates (5 g of flesh in 50 mL of distilled water) at room temperature with a pH meter (pH-Burette 24 1S, Crison Instruments, Barcelona, Spain). For each individual, two homogenates were obtained and the corresponding pH measurements were averaged.
Cooking Loss (CL)
Cooking loss (%) was determined as the percentage of mass loss according to Eq. (3):
where Mbck and Mack are the masses (g) of the razor clams, superficially dried with a soft paper, before and after cooking, respectively.
Firmness
The firmness of the cooked samples was evaluated by a Warner-Bratzler test. A Texture Analyser (TA-XTPlus, Stable Micro System Ltd., Surrey, UK), equipped with a V-shaped Warner-Bratzler blade, was employed. The samples were sheared (2 mm/s crosshead speed, 5 kg load cell) perpendicular to the fibers and the maximum force (N) was recorded.
Color
The color of the flesh of the cooked razor clams was characterized as described previously by Otero et al. (2019). L*, a*, and b* color parameters were measured with a CM-3500d spectrophotometer managed with the color data software CM-S100w SpectraMagic™ (Konica Minolta, Tokyo, Japan). For each individual, two measurements at different locations were performed and the obtained L*, a*, and b* values were averaged.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics v. 24.0.0.1 for Windows (IBM Corp., Armonk, NY, USA). After checking the prerequisites of normality and homogeneity of variances, a one-way analysis of variance (ANOVA) was performed. Significant differences among means were determined by a Tukey-b multiple range test in those cases in which the prerequisite of homogeneity of variances was fulfilled. Otherwise, a Tamhane’s post hoc test was employed. The significance level was set at 5%.
Results and Discussion
Storage Experiments for 7 Days
In the first phase of this study, razor clams were stored at different pressure levels for 7 days to identify the minimum pressure required, both at 5 °C (HS-LT experiments) and at 20 °C (HS-RT experiments), to avoid microbial growth during storage. Moreover, a group of razor clams was conventionally cold stored at 5 °C to compare the effectiveness of hyperbaric storage in preserving razor clams with that of classical refrigeration.
Effect of the Storage Conditions on the Microbial Load
Figure 2 shows the evolution of TVB, TAM, and H2S-producing microorganisms in the razor clams stored at different conditions. Positive log10 (N/N0) values in Fig. 2 mean microbial growth during storage, while negative log10 (N/N0) values mean microbial inactivation. After 7 days of storage, growth of TVB, TAM, and/or H2S-producing microorganisms was detected in all the samples, except in those stored at 50 MPa/5 °C and at 75 MPa/20 °C. By contrast, during this period, luminescent colonies, lactic acid bacteria, and anaerobic sulfite-reducing clostridia did not increase significantly (p > 0.05) at any of the storage conditions tested in this paper, while enterobacteria showed a slight increase only in the conventionally refrigerated samples (Table 1).
Evolution of (a) total viable bacteria (TVB), (b) total aerobic mesophiles (TAM), and (c) H2S producing microorganisms (H2S-PROD) in Atlantic razor clams stored at different conditions.  : conventionally refrigerated at 5 °C,
: conventionally refrigerated at 5 °C,  : immersed in saline water at 5 °C, and
: immersed in saline water at 5 °C, and  : immersed in saline water at 20 °C. Positive log10 (N/N0) values mean microbial growth, while negative log10 (N/N0) values mean microbial inactivation during storage. Data are mean values obtained from replicated experiments (n = 3 replicated experiments × 3 individuals = 9) and vertical bars represent standard error. Different letters indicate significant differences among means due to the storage conditions. Significance level: 5%
: immersed in saline water at 20 °C. Positive log10 (N/N0) values mean microbial growth, while negative log10 (N/N0) values mean microbial inactivation during storage. Data are mean values obtained from replicated experiments (n = 3 replicated experiments × 3 individuals = 9) and vertical bars represent standard error. Different letters indicate significant differences among means due to the storage conditions. Significance level: 5%
Figure 2 reveals that hyperbaric storage, whichever the pressure and temperature conditions, was more effective than conventional refrigeration in extending the shelf-life of the razor clams. Thus, after 7 days of storage, TVB and TAM increased in CR samples by more than 3 log10 units and counts close to 7 log10 CFU/g; that is, the typical acceptability limit in seafood products (ICMSF and Swanson 2011), were reached (data not shown). By contrast, microbial counts in all the samples stored under pressure remained well below this limit. The inhibitory effect of hyperbaric storage on TAM growth was probably not only due to the pressure/temperature conditions during storage but also to the saline water in which the razor clams were immersed. In fact, under these anaerobic and saline storage conditions, no significant TAM growth was detected in the samples kept at 0.1 MPa (C_5 samples) either, while mesophilic microflora increased by more than 3 log10 units when the samples were stored at aerobic conditions (CR samples). By contrast, the saline water seems not to affect the growth of TVB and H2S-producing microorganisms, and thus, similar log10 (N/N0) values were observed in all the samples stored at atmospheric pressure (CR and C_5 samples).
Temperature during hyperbaric storage determined the minimum pressure required to avoid microbial growth in the samples, and as expected, the higher the storage temperature, the larger Pmin. At 5 °C, hyperbaric storage at 25 MPa did not produce significant effects (p > 0.05) on log10 (N/N0) values compared to those observed at 0.1 MPa, and a minimum pressure of 50 MPa was needed to inhibit the growth of TVB, TAM, and H2S-producing microorganisms completely. However, at 20 °C, this pressure level was not enough to guarantee no TVB and TAM growth in the razor clams and a minimum pressure of 75 MPa was required. At 75 MPa and 20 °C, microbial growth was not only inhibited but significant damage was also detected on the microorganisms, and thus, after 7 days of storage, TVB, TAM, and H2S-producing microorganisms reduced by 1.6, 1.7, and 0.6 log10 units, respectively.
The Pmin(5 °C) and Pmin(20 °C) values identified in this paper for razor clams are similar to those previously reported in the literature for several fish species. Thus, as observed in the present paper, Fidalgo et al. (2018) showed that, at room temperature, a pressure of, at least, 75 MPa was needed to avoid microbial growth in Atlantic salmon samples for 10 days, while Fidalgo et al. (2019) proved that, at 5 °C, a pressure of only 50 MPa was needed. In this sense, Otero et al. (2017) and Otero et al. (2019) also reported that, at 5 °C, a storage pressure of 50 MPa was enough to maintain microbial counts unaltered in hake loins and Atlantic mackerel fillets for, at least, 7 and 12 days, respectively.
Effect of the Storage Conditions on Physicochemical Parameters
At day 0, all the razor clams were alive, and therefore, their shells were tightly closed (Fig. 3). After 7 days of storage, all the razor clams died, whichever the storage conditions, and the shells opened. The clam flesh remained stuck to the shell in all the samples stored at atmospheric pressure (CR and C_5 samples), while it detached from the shell in all the samples stored under pressure. This behavior has been previously described in the literature and attributed to the pressure-induced denaturation of the adductor muscle proteins (Xuan et al. 2018; Yi et al. 2013).
Figure 3 also reveals that, depending on the storage medium (air in CR samples or saline water in C_5, HS_LT, and HS_RT samples), the appearance of the clam flesh was very different. Thus, the flesh of the samples stored in saline water increased its length and showed a glossy and swollen appearance, while the clams stored in air lost their juicy aspect. In any case, these differences disappeared after cooking.
Table 2 shows the evolution of some physicochemical parameters evaluated in the razor clams before and after cooking. All the storage conditions produced significant effects on the razor clams before cooking, either on the weight, the water content, the water-holding capacity, or the pH.
During conventional refrigeration, the clams reduced their weight by more than 10%, while all the other physicochemical attributes remained stable for 7 days. Weight losses during conventional cold storage of bivalves are well documented in the literature as they can have important economic consequences. For example, as observed in the present paper, Buzin et al. (2011) detected weight losses of 10.5% in oysters stored at 3 °C for 10 days. Some studies show that these weight losses could be avoided by storing the bivalves in water (Ekanem and Achinewhu 2006). The data in Table 2 corroborate this statement and reveal that storage in saline water reduced the weight losses observed in the clams, especially when they were kept under pressure. In this case, even small weight gains were detected.
Storage in saline water increased the water content in the muscle, whichever the pressure and temperature conditions, but it reduced the water-holding capacity, except in the samples kept at 50 MPa and 5 °C. These results suggest that the water absorbed during storage was weakly retained by the muscle and it was easily liberated by centrifugation. However, phenomena of partial solubilization and/or denaturation of myofibrillar proteins, induced either by the saline water or by the pressure and temperature conditions during storage, could be also implied. Many researchers have described WC increases (%) in bivalve shellfish after pressure processing (100-800 MPa) for some minutes, even when the product is packed without liquid inside the pouch (Briones-Labarca et al. 2012; Cruz-Romero et al. 2004; Yi et al. 2013). These changes are usually attributed to the infusion of the intervalval liquor and/or the medium in which the bivalves are immersed into their tissue that acquires a glossy and swollen appearance (Cruz-Romero et al. 2004; Yi et al. 2013), but other phenomena such as solubilization and/or leaching of some flesh constituents into the intervalval fluid during expansion should not be neglected.
Pressure also had significant effects on the pH of the razor clams kept in saline water. After bivalves death, the typical pH evolution is characterized by an initial decrease, due to the generation of lactic acid from glycogenolysis under anoxic conditions, that is later reversed as a result of the decomposition of nitrogenous compounds by enzymatic reactions and microbial activity (Ashie et al. 1996; Briones-Labarca et al. 2012). In this paper, the initial pH decline was only observed in C_5 samples probably because the saline water, that simulated the natural habitat of the razor clams, could delay their death. Moreover, as previously described, the saline water also reduced microbial growth in these samples and this could also contribute to delay pH increases. When the razor clams were stored for 7 days either at 25-50 MPa/ 5 °C or at 50 MPa/ 20 °C, no changes were observed in the pH, but it significantly increased in HS-RT samples kept at 75 MPa and 20 °C. Similar results were observed by Fidalgo et al. (2018) who also observed a pH increase in salmon samples stored at 75 MPa/25 °C for 6 days, but not in those maintained at 50–60 MPa/25 °C. As microbial growth was completely inhibited in HS-RT samples kept at 75 MPa (“Effect of the Storage Conditions on the Microbial Load”), some mechanisms other than microbial activity should be responsible of this pH increase. In this sense, several authors in the literature have shown that pH of bivalves increases immediately after processing at 100–800 MPa for some minutes and the higher the pressure and the longer the treatment, the larger the pH increase (Cruz-Romero et al. 2004; Xuan et al. 2018; Yi et al. 2013). This pH change has been attributed to conformational changes associated with pressure-induced denaturation and unfolding of proteins, resulting in the decrease of the number of exposed acidic groups (Ma and Ledward 2004).
Storage for 7 days also produced significant changes (p < 0.05) in the physicochemical attributes evaluated after cooking (Table 2). At day 0, cooking losses of about 30% were detected in all the samples. These losses include not only water, soluble proteins, and fats from the flesh but also the intervalval fluid. As previously commented, after 7 days of storage, the valves of the razor clams opened and the intervalval fluid was lost before cooking. As a result, cooking losses significantly decreased in CR samples. By contrast, CL increased in all the samples stored in saline water, except in those kept at 50 MPa and 5 °C. These results are probably a consequence of the water absorbed during storage that, as occurred when centrifuging, was easily liberated during cooking. Moreover, after storage, all the razor clams became significantly softer, especially those maintained in saline water, and significant color changes occurred. Thus, lightness and redness increased, whichever the conditions tested, while yellowness increased significantly only in CR and HS-RT samples kept at 75 MPa. In any case, these color changes can be considered small in practical terms as Fig. 3 clearly shows.
Storage Experiments for 14 Days: Comparison of the Effectiveness of HS-LT and HS-RT in Preserving Razor Clams
The results in “Storage Experiments for 7 Days” showed that the minimum pressure needed to avoid microbial growth in the razor clams depended on the storage temperature and it was 50 MPa at 5 °C, but 75 MPa at 20 °C. Therefore, in the second phase of this study, new HS experiments were performed at these conditions for 14 days.
After 14 days of storage, significant reductions (p < 0.05) were observed in TVB, TAM, H2S-producing microorganisms, and anaerobic sulfite-reducing clostridia in all the samples, whichever the storage conditions (Table 3), while no changes were observed in luminescent colonies, lactic acid bacteria, and enterobacteria that remained close to or under the detection limits. Even though, after 7 days, storage at 75 MPa/20 °C produced larger inactivation of TVB and TAM than storage at 50 MPa/5 °C (“Effect of the Storage Conditions on the Microbial Load”), the data in Table 3 show that, after 14 days of storage, both storage conditions produced similar effects on the microbial load of the razor clams. In this sense, Santos et al. (2020a) also described similar reductions of TAM, LAB, and enterobacteria counts in pork meat after 60 days of storage either at 60 MPa/10 °C or at 75 MPa/25 °C.
However, HS-LT and HS-RT did not produce identical effects on all the physicochemical parameters. After 14 days of storage, the appearance of HS-LT and HS-RT samples, both before and after cooking, was similar to the naked eye (Fig. 4) and also weight and WC increases (Table 4), but all the other physicochemical attributes presented significant differences. Before cooking, the samples stored at 75 MPa and 20 °C showed the largest changes on WHC and pH. Thus, a WHC reduction of 12.8% was detected in HS-RT samples, but of only 3.5% in HS-LT samples. In the same way, pH increased by 5.6% in the samples stored at 75 MPa/20 °C but no changes occurred in those maintained at 50 MPa/5 °C. These results, that showed the same trend in the 7-day storage experiments, could be attributed to larger denaturation and unfolding of proteins at 75 MPa/20 °C than at 50 MPa/5 °C. Accordingly, cooking losses and changes in redness and yellowness after cooking were also larger in HS-RT samples. By contrast, HS-LT samples showed the largest changes in lightness and firmness. Thus, these samples were significantly lighter and softer than those stored at 75 MPa and 20 °C probably because their CL was lower.
These results confirm previous data in the literature that suggest that HS-RT produces larger changes on food quality than HS-LT. For example, in Atlantic salmon, Fidalgo et al. (2019) found no changes in color parameters during storage at 60 MPa/10 °C for up to 50 days, while Fidalgo et al. (2018) reported significant increases in lightness after only 3 days of storage at 75 MPa/25 °C. Moreover, Santos et al. (2020a) reported larger pH increases in pork meat pieces stored at 75 MPa/25 °C than in those kept at 60 MPa/10 °C. As previously mentioned, all these changes could be related to larger protein denaturation at 75 MPa. In this sense, Fidalgo et al. (2020) showed that storage of Atlantic salmon at 75 MPa and 25 °C affected the stability of both myofibrillar and sarcoplasmic proteins to a larger extent than storage at 60 MPa and 10 °C.
Conclusions
The results obtained in this paper show that hyperbaric storage, both at low and at room temperature, is more effective than conventional refrigeration in extending the shelf-life of the razor clams. The temperature during hyperbaric storage determined the minimum pressure needed to avoid microbial growth in the samples and the higher the storage temperature, the larger the pressure. Thus, a storage pressure of 75 MPa was needed at room temperature, while only 50 MPa were required at 5 °C. Even though both storage conditions were effective in extending the microbial shelf-life of the razor clams to, at least, twice that achieved in conventional refrigeration, the quality decline after 14 days of storage was larger in the samples stored at 75 MPa and 20 °C.
Our results show that HS at temperatures similar to those usually employed in conventional refrigeration allows reducing the storage pressure to levels that minimize quality losses significantly, and therefore, HS-LT results more effective than HS-RT for the preservation of razor clams. Future research works should include sensorial analysis to assess whether consumers can detect the differences observed in the quality of HS-LT and HS-RT samples or not. Moreover, economic, logistic, and environmental criteria should be considered to compare not only the effectiveness but also the efficiency of both methods.
References
Ashie, I. N. A., Smith, J. P., & Simpson, B. K. (1996). Spoilage and shelf-life extension of fresh fish and shellfish. Critical Reviews in Food Science and Nutrition, 36(1–2), 87–121. https://doi.org/10.1080/10408399609527720.
Bermejo-Prada, A., Colmant, A., Otero, L., & Guignon, B. (2017). Industrial viability of the hyperbaric method to store perishable foods at room temperature. Journal of Food Engineering, 193, 76–85. https://doi.org/10.1016/j.jfoodeng.2016.08.014.
Briones-Labarca, V., Perez-Won, M., Zamarca, M., Aguilera-Radic, J. M., & Tabilo-Munizaga, G. (2012). Effects of high hydrostatic pressure on microstructure, texture, colour and biochemical changes of red abalone (Haliotis rufecens) during cold storage time. Innovative Food Science and Emerging Technologies, 13(JANUARY), 42–50. https://doi.org/10.1016/j.ifset.2011.09.002.
Buzin, F., Baudon, V., Cardinal, M., Barillé, L., & Haure, J. (2011). Cold storage of Pacific oysters out of water: Biometry, intervalval water and sensory assessment. International Journal of Food Science and Technology, 46(9), 1775–1782. https://doi.org/10.1111/j.1365-2621.2011.02686.x.
Cruz-Romero, M., Smiddy, M., Hill, C., Kerry, J. P., & Kelly, A. L. (2004). Effects of high pressure treatment on physicochemical characteristics of fresh oysters (Crassostrea gigas). Innovative Food Science and Emerging Technologies, 5(2), 161–169. https://doi.org/10.1016/j.ifset.2004.01.002.
Ekanem, E. O., & Achinewhu, S. C. (2006). Mortality and quality indices of live west African hard-shell clams (Galatea paradoxa, Born) during wet and dry postharvest storage. Journal of Food Processing and Preservation, 30(3), 247–257. https://doi.org/10.1111/j.1745-4549.2006.00052.x.
Fidalgo, L. G., Santos, M. D., Queirós, R. P., Inácio, R. S., Mota, M. J., Lopes, R. P., Gonçalves, M. S., Neto, R. F., & Saraiva, J. A. (2014). Hyperbaric storage at and above room temperature of a highly perishable food. Food and Bioprocess Technology, 7(7), 2028–2037. https://doi.org/10.1007/s11947-013-1201-x.
Fidalgo, L. G., Lemos, Á. T., Delgadillo, I., & Saraiva, J. A. (2018). Microbial and physicochemical evolution during hyperbaric storage at room temperature of fresh Atlantic salmon (Salmo salar). Innovative Food Science & Emerging Technologies, 45, 264–272. https://doi.org/10.1016/j.ifset.2017.11.003.
Fidalgo, L. G., Castro, R., Trigo, M., Aubourg, S. P., Delgadillo, I., & Saraiva, J. A. (2019). Quality of fresh Atlantic salmon (Salmo salar) under hyperbaric storage at low temperature by evaluation of microbial and physicochemical quality indicators. Food and Bioprocess Technology, 12(11), 1895–1906. https://doi.org/10.1007/s11947-019-02346-3.
Fidalgo, L. G., Delgadillo, I., & Saraiva, J. A. (2020). Autolytic changes involving proteolytic enzymes on Atlantic salmon (Salmo salar) preserved by hyperbaric storage. LWT - Food Science and Technology, 118(June 2019), 108755. https://doi.org/10.1016/j.lwt.2019.108755.
International Commission on Microbiological Specifications for Foods (ICMSF), & Swanson, K. M. (2011). Fish and seafood products. In Microorganisms in Foods (Vol. 8). Boston, MA: Springer. https://doi.org/10.1007/978-1-4419-9374-8_10.
Khan, B. M., & Liu, Y. (2019). Marine mollusks: food with benefits. Comprehensive Reviews in Food Science and Food Safety, 18(2), 548–564. https://doi.org/10.1111/1541-4337.12429.
Leavitt, D. F. (2010). Biology of the Atlantic jacknife (razor) clam (Ensis directus Conrad, 1843). Northeastern Regional Aquaculture Center Publication No. 217-2010. http://fisheries.tamu.edu/files/2013/09/NRAC-Publication-No.-217-2010-–-Biology-of-the-atlantic-jacknife-razor-clam-ensis-directus-conrad-1843.pdf. Accessed 6 Oct 2020.
Lemos, Á. T., Ribeiro, A. C., Fidalgo, L. G., Delgadillo, I., & Saraiva, J. A. (2017). Extension of raw watermelon juice shelf-life up to 58 days by hyperbaric storage. Food Chemistry, 231, 61–69. https://doi.org/10.1016/j.foodchem.2017.03.110.
Lemos, Á. T., Ribeiro, A. C., Delgadillo, I., & Saraiva, J. A. (2020). Preservation of raw watermelon juice up to one year by hyperbaric storage at room temperature. LWT - Food Science and Technology, 117(September 2019), 108695. https://doi.org/10.1016/j.lwt.2019.108695.
Ma, H. J., & Ledward, D. A. (2004). High pressure/thermal treatment effects on the texture of beef muscle. Meat Science, 68(3), 347–355. https://doi.org/10.1016/j.meatsci.2004.04.001.
Matser, A. M., Stegeman, D., Kals, J., & Bartels, P. V. (2000). Effects of high pressure on colour and texture of fish. High Pressure Research, 19(1–6), 109–115. https://doi.org/10.1080/08957950008202543.
Montero, P., & Gómez-Guillén, M. C. (2004). High-pressure applications on myosystems. In G. V. Barbosa-Cánovas, M. S. Tapia, & M. P. Cano (Eds.), Novel Food Processing Technologies (pp. 311–342). Boca Raton, FL, USA: CRC Press. Taylor & Francis Group.
Otero, L. (2019). Hyperbaric storage at room temperature for fruit juice preservation. Beverages, 5(3), 49. https://doi.org/10.3390/beverages5030049.
Otero, L., Pérez-Mateos, M., & López-Caballero, M. E. (2017). Hyperbaric cold storage versus conventional refrigeration for extending the shelf-life of hake loins. Innovative Food Science and Emerging Technologies, 41, 19–25. https://doi.org/10.1016/j.ifset.2017.01.003.
Otero, L., Pérez-Mateos, M., Holgado, F., Márquez-Ruiz, G., & López-Caballero, M. E. (2019). Hyperbaric cold storage: Pressure as an effective tool for extending the shelf-life of refrigerated mackerel (Scomber scombrus, L.). Innovative Food Science and Emerging Technologies, 51(May 2018), 41–50. https://doi.org/10.1016/j.ifset.2018.05.003.
Santos, M. D., Castro, R., Delgadillo, I., & Saraiva, J. A. (2020a). Improvement of the refrigerated preservation technology by hyperbaric storage for raw fresh meat. Journal of the Science of Food and Agriculture, 100(3), 969–977. https://doi.org/10.1002/jsfa.10083.
Santos, M. D., Delgadillo, I., & Saraiva, J. A. (2020b). Extended preservation of raw beef and pork meat by hyperbaric storage at room temperature. International Journal of Food Science and Technology, 55(3), 1171–1179. https://doi.org/10.1111/ijfs.14540.
Xuan, X. T., Cui, Y., Lin, X. D., Yu, J. F., Liao, X. J., Ling, J. G., & Shang, H. T. (2018). Impact of high hydrostatic pressure on the shelling efficacy, physicochemical properties, and microstructure of fresh razor clam (Sinonovacula constricta). Journal of Food Science, 83(2), 284–293. https://doi.org/10.1111/1750-3841.14032.
Yi, J., Xu, Q., Hu, X., Dong, P., Liao, X., & Zhang, Y. (2013). Shucking of bay scallop (Argopecten irradians) using high hydrostatic pressure and its effect on microbiological and physical quality of adductor muscle. Innovative Food Science and Emerging Technologies, 18, 57–64. https://doi.org/10.1016/j.ifset.2013.02.010.
Acknowledgements
The authors thank Ignacio Rodríguez, technician at ICTAN-CSIC, Yaritza Clemente, Bachelor student, and Marta Blázquez, Master student, for their assistance in the lab work and their help in obtaining and processing part of the data.
Funding
This work was partially supported by the State Programme for Knowledge Generation and Scientific and Technologic Strengthening of the R&D&i system of the Spanish Ministry of Science, Innovation, and Universities through the MALTA CONSOLIDER TEAM Research Network (RED2018-102612-T).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Otero, L., Pérez-Mateos, M. Hyperbaric Storage of Atlantic Razor Clams: Effect of the Storage Conditions. Food Bioprocess Technol 14, 530–541 (2021). https://doi.org/10.1007/s11947-021-02596-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-021-02596-0