Abstract
Fresh-cut fruits are susceptible for microbial contamination during handling and storage. Hence, there is a need for minimal processing of such foods using non-thermal technology that can inactivate both bacterial pathogens and undesirable enzymes while retaining the quality. In this regard, synergistic effect of light emitting diode (LED) based blue light (BL) and natural exogenous photosensitizer- curcumin (PS) on the inactivation of bacterial pathogens (Escherichia coli, Staphylococcus aureus), and enzymes (polyphenol oxidase, peroxidase, bromelain) of fresh-cut pineapple slices were evaluated. The effect of photodynamic treatment (PS+BL) on quality attributes like color, phenolics, flavonoids, ascorbic acid content and antioxidant activity was also investigated. The PS+BL treatment at optimized conditions resulted in 3 and 4 log reduction of E. coli and S. aureus, respectively. PS(100 μM)+BL treatment led to partial inactivation of polyphenol oxidase (33.5%) and peroxidase (25.7%), synergistically, but preserved desired enzyme bromelain. The PS+BL didn’t show any significant (p<0.05) consequence on color, phenolics, flavonoids and antioxidant activity, while it affected the ascorbic acid content negatively (reduced by ~30%). The current investigation showed that the photodynamic inactivation of E. coli and S. aureus using LED-based photosensitization in fresh-cut fruit slices could be used as a potential method for microbial control although some phytochemical losses.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tropical fruits, especially fresh-cut pineapple, are of great potential in global market, sold at all range of markets from farmer market to supermarket. In order to extend its shelf life without quality deterioration, low temperature storage is essential. Whereas, these fresh-cut fruits are often stored at ambient temperature in most of the regions worldwide, and are also exposed to air during distribution. This kind of inappropriate handling and storage are said to cause foodborne diseases and outbreaks, which has led to increased concern over the safety of consumption of fresh-cut fruits (Carstens et al. 2019). In the recent past, reports on the outbreaks associated with Escherichia coli O157:H7 and Salmonella in the USA are accounting for about 42.9 and 34.3% with the consumption of fresh cut-fruits and juices (Nüesch-Inderbinen and Stephan 2016). An outbreak occurred in USA in 1994 was associated with non-O157:H7 enterohemorrhagic E. coli (O11:H43) contamination in pineapple. Fresh-cut tropical fruits that are minimally processed are prone to attachment of pathogens in cut/injured surfaces and provided nutrients for the growth of food-borne pathogens (Gleeson and O’Beirne 2005). Few reports also found that Staphylococcus aureus is able to grow on pineapple slices having higher soluble solids content and aw and they even survive on low pH and storage temperatures of 5–25 °C (Lanciotti et al. 2001). Inactivation of quality deteriorating enzymes (oxidoreductases) that causes browning of the product like polyphenol oxidase (PPO) and peroxidase (POD) are crucial aspect during processing of fresh-cut fruits (Kathrin et al. 2020). Hence, ideal processing technique should address both microbial safety as well as reducing enzyme-based quality deterioration.
Non-thermal technologies are gaining focus in recent years, as an alternate to the thermal technologies, as they reduce microbial load as well as retain nutrients. One such non-thermal technology is light based processing for decontamination of foods (Koutchma 2009; Oms-Oliu et al. 2010; Bhavya and Hebbar 2017), and especially high energy wave that is blue region (400–500 nm) of the light spectrum has a huge scope for exploration. Recently, light-emitting diode (LED) are being used for blue light (BL) production as it saves energy and cost and is also environmentally friendly with high durability. Early studies with blue LED have shown potential application in food safety, especially for surface decontamination (Maclean et al. 2009; Ghate et al. 2017). However, prolonged exposure period was required for inactivation of food-borne pathogens. In order to reduce the processing time, many exogenous photosensitizers (PS) such as curcumin, chlorophyllin, hypericin, alpha-terthienyl, and 5-Aminolevulinic acid can aid the photodynamic inactivation (PDI) of microorganisms by BL (D’Souza et al. 2015). Further, PDI, a combination of PS (photoactive molecule) and specific wavelength of light (LED) is also being considered for food decontamination (Luksiene and Zukauskas 2009). The mechanism of microbial inactivation during PDI is through the formation of cytotoxic substances like reactive oxygen species (ROS) that damage the cells. These ROS result in creating biochemical and functional disturbances in cell system, especially targeting the cell membrane component by cross-linking, protein and lipid oxidation, inhibition of transport of metabolites, leakage of lysosomal enzymes, and increases the uptake of PS, finally causing cell death (Zerdin et al. 2009; Bertoloni et al. 1989).
The application of novel curcumin-mediated PDI in food products is gaining attention due to its biocompatibility and photoactive properties against wide range of microbes (Winter et al. 2013). In recent times, few reports on the use of PDI on fruits and vegetables are available (Al-Asmari et al. 2018; Aurum and Nguyen 2019; Tao et al. 2019). However, there are no comprehensive studies on the effect of photodynamic process on fresh-cut fruits in terms of microbial, enzyme and nutritional quality. Hence, the emphasis of the current study is on evaluating the efficacy of PS+BL treatment on surface decontamination of E. coli (Gram-negative bacteria) and S. aureus (Gram-positive bacteria) in fresh-cut pineapple slices. The study also focuses on the effect of non-thermal LED-based processing on the activity of enzymes like PPO, POD and bromelain. Further, retention of quality attributes like color, total phenolic content (TPC), total flavonoid content (TFC), ascorbic acid content, and antioxidant activity in treated cut-pineapple slices was also investigated in detail.
Materials and Methods
Chemicals and Reagents
Copper sulfate, Folin and Ciocalteu’s phenol (FC) reagent, ethylenediaminetetraacetic acid (EDTA), aluminium trichloride, hydrogen peroxide, metaphosphoric acid, ethanol, and sodium carbonate anhydrous, sodium potassium tartrate, sodium nitrate, sodium acetate, trichloro acetic acid (TCA), and sodium hydroxide were procured from Merck, Mumbai, India. Phosphate buffered saline (PBS) was purchased from Thermo Fisher Scientific, India. Catechol, guaiacol, curcumin, ascorbic acid, quercetin, L-cysteine hydrochloride, L-tyrosine, gallic acid monohydrate, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox), 2,4,6-Tri(2-pyridyl)-s-triazine (TPTZ), bovine serum albumin (BSA), casein, 2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), and 2,2′-Azobis(2-methylpropionamidine) dihydrochloride (AAPH) were obtained from Sigma-Aldrich, India. Tryptone soy broth (TSB) and agar-agar were procured from HiMedia Laboratory, India. Chemicals used for high-performance liquid chromatography (HPLC) analysis were of HPLC grade and other chemicals used in the study were of analytical grade.
LED-Based Processing System
A laboratory scale, LED processing system (batch type) developed at CSIR-CFTRI, Mysuru was used in the present study, and detailed description of the unit was reported by Bhavya and Hebbar (2019a). The unit mainly consisted of LED lamp (462 ± 3 nm), material holding tray, UV lamp, and temperature probe. The sample was placed in the sterile Petriplate under the LED lamp at a fixed distance (~ 7 cm). The photometric unit, luminance (Lux), was measured using digital light meter (Lutron-LX-101A, Lutron electronic enterprise Co. ltd, Taiwan, China). Further, the irradiance (W/cm2) of LED bulb was calculated using the photometric conversion (Eq. (1)) (Palmer 2010).
where, P = irradiance (W/cm2), L= luminance (lux), Km = maximum value of spectral luminous efficacy (683 lm/W), and V (λ) = photopic spectral function at the wavelength at 462 nm (0.0653).
The energy dosage provided for BL treatment was calculated using Eq. (2), suggested by Maclean et al. (2009).
where, E=dose (energy density) in J/cm2, P=irradiance (power density) in W/cm2, and t = time in seconds
Bacterial Inoculation and Its Inactivation in Fresh-Cut Pineapple
E. coli (ATCC 11775) and S. aureus (ATCC 12600) were obtained from Food Safety and Analytical Quality Control Laboratory at CSIR-CFTRI, and Mysuru and glycerol stocks of these cultures were stored at −80 °C till further use. Working cultures were prepared from the stock cultures in sterile TSB by consecutive (at least two) transfers after every 24 h and were incubated for 24 h at 37 ± 2 °C. Further, cell viability was maintained by subculturing daily, and the cells were centrifuged at 4000 ×g for 5 min at 4 °C. The pellet was washed twice with sterilized PBS (pH 7.4), and then the bacterial suspension was used for experiments by suspending the resultant pellet in PBS at an initial concentration of ~ 109 CFU/mL.
Fresh pineapples were procured from a local market in Mysuru, India. Then, pineapple surface was washed thoroughly with chlorine water in order to remove the surface microbial load and further was peeled and sliced using sterile stainless steel knife. The fruit slice was ~ 1-cm thick with dimension being ~ 5 × 4 × 1 cm. The bacterial culture, E. coli grown overnight (18 h), was inoculated (~ 7 log CFU/g) onto the surface of the fresh-cut pineapple slice (~ 10 g) placed in a sterile Petri dish. A similar protocol was used for S. aureus also. Further, PS (10 mM stock solution of curcumin in ethanol) was used to treat the inoculated pineapple slices at different concentrations (100 and 200 μM). Later, for the PDI treatment, the PS-treated slices were exposed to BL for a particular duration in the LED illumination chamber below the light source. Pineapple slices treated with PS (incubated in the dark), BL alone, and uninoculated slices were considered as controls. After the treatment, the slice was homogenized, serially diluted, and pour plated onto tryptone soya agar (TSA). The incubated TSA plates (37 ± 2 °C for 24 h) were manually counted for bacterial colonies, and the results were expressed as log CFU/g.
Determination of Enzyme Activity
Extraction of Enzymes
A 10 g of control/treated pineapple slices were macerated with 10 mL of 0.01 M sodium phosphate buffer (pH 7) for enzyme extraction. The obtained aqueous solution containing active enzymes was filtered through muslin cloth and centrifuged at 4000 ×g for 40 min at 4 °C. The supernatant was used for estimation of PPO, POD and bromelain activity. Protein content of supernatant was also determined using spectrophotometric method at 660 nm using Lowry’s method to calculate specific activity of each enzyme. The BSA (0.2 to 1 mg/mL) was used as standard to estimate the protein concentration of the extract.
Polyphenol Oxidase and Peroxidase Activity
The activity of PPO and POD was determined spectrophotometrically as described by Shewale and Hebbar (2017) with slight modification. For PPO assay, 0.1 mL of enzyme extract was added to 2.9 mL of substrate solution containing 0.05 M catechol in a 0.1 M phosphate buffer (pH 6.5). The increase in absorbance of reaction mixture was measured at 420 nm (UV-1800, Shimadzu Corporation, Japan) for 2 min at an interval of 0.5 min against the catechol solution (blank) at room temperature. The POD activity was determined using guaiacol and hydrogen peroxide as substrate. A 0.05 mL of enzyme extract was added to 3 mL of substrate solution containing 1.0 mL each of 15 mM guaiacol, 3 mM H2O2, and phosphate buffer (pH 6). The increase in absorbance of the reaction mixture was recorded at 470 nm for 3 min at 0.5 min interval. Both the PPO and POD activities were determined from the slope of the linear portion of the graph relating to change in absorbance with time. One unit of PPO/POD activity is defined as the amount of the enzyme that causes an increase in absorbance of 0.001/min and is expressed as Unit/mL. The enzyme inactivation was calculated by using the following equation (3):
where, At=enzyme activity of treated sample (U/mL) and A0=enzyme activity of control sample (U/mL).
Bromelain Activity
Bromelain activity was estimated as described by Chaurasiya et al. (2015) with slight modifications using the casein digestion unit (CDU) method. The assay was performed using casein (0.6 %) as a substrate in the presence of cysteine and EDTA (enzyme diluent). The test sample was made by adding 5 mL of casein solution, 0.2 mL of enzyme diluent, 0.6 mL of distilled water, 0.2 mL of enzyme extract, and the reaction was carried in water bath at 37 °C for 10 min. Further, the reaction was stopped by adding 5 mL of trichloroacetic acid (TCA) to the reaction mixture. The blank sample was prepared by heating 5 mL of casein solution for 10 min in a water bath maintained at 37 °C followed by subsequent addition of 5 mL of TCA, 0.2 mL of enzyme diluent, and 0.8 mL of distilled water. The test and blank sample were incubated for 30 min at room temperature. The TCA precipitates the unhydrolyzed substrate, and the precipitate was removed using Whatman No. 1 filter paper. The absorbance of the solubilized casein (filtrate) was read at 275 nm using a spectrophotometer. One unit of bromelain activity is defined as 1 μg of l-tyrosine released in 1 min per mL of sample when casein is hydrolyzed under the standard conditions of 37 °C at pH 7.0 for 10 min. The standard graph was prepared by using 10 to 100 μL of 1 mg/mL of tyrosine solution. The activity of the bromelain enzyme was calculated using Eqs. 4, 5, and 6:
Estimation of Color
The color parameters of control and treated pineapple slices were obtained using colorimeter (CM 5, Hunter Associates Laboratory, USA). The color coordinates, namely, L*, a*, and b*, represent lightness index, red-green, and yellow-blue color components, respectively. The calibrated colorimeter using black reference and standard white slab (L*=90.70, a*=1.08, b*=0.65) under illuminated conditions was used for the color estimation. The obtained values of L*, a*, and b* were used to calculate yellowness index (YI) and total color difference (∆E) using the following equations (7,8), as reported by Hirschler (2012):
where L*, a*, and b* were color values of treated pineapple slices, while L*ref, a*ref, and b*ref were the color values of control pineapple slices.
Total Phenolic Content
A 10-g pineapple slice was extracted with 30 mL of 80% aqueous methanol containing 1% HCl. Further, the mixture was kept in refrigerated condition for 24 h for proper extraction and then centrifuged at 4000 ×g for 20 min. The supernatant was collected and used for the estimation of total phenolic content (TPC), total flavonoid content (TFC), and antioxidant activity.
TPC was estimated by Folin-Ciocalteu method described by Shewale and Hebbar (2017). Briefly, extract (100 μL) and diluted FC reagent (500 μL) were added and mixed well. Further, 20% of sodium carbonate solution (400 μL) was added to the mixture after 5 min. After 60 min of incubation in dark, the absorbance of the reaction mixture was read at 760 nm. The TPC was calculated using standard curve of gallic acid (0.02 to 0.1 mg/mL), and the results were expressed as micrograms of gallic acid equivalents per gram (μg GAE/g) of pineapple.
Total Flavonoid Content
The TFC was determined according to Reis et al. (2012) with slight modification. In brief, an aliquot of 400 μL extract was diluted with 250 μL of distilled water followed by the addition of 20 μL of 5% NaNO2 solution and 50 μL of 10% AlCl3.6H2O. After 5 min, 150 μL of 1 M NaOH was added, and volume was made with distilled water (up to 1 mL). Further, the absorbance was recorded at 510 nm after 10 min using microplate reader (Epoch microplate reader, BioTek Instruments Inc., India). The TFC was expressed as μg of quercetin equivalents/g of pineapple.
DPPH Radical-Scavenging Ability
The DPPH radical-scavenging activity was performed according to Yen and Chen (1995). The DPPH radical stock solution (250 μM) was freshly prepared daily in ethanol. The DPPH solution (980 μL) was mixed with 20 μL of extract. The decrease in absorbance was read at 517 nm after 30 min of incubation at room temperature in the dark.
Trolox Equivalent Antioxidant Capacity
The trolox equivalent antioxidant capacity (TEAC) of treated/control pineapple slices was estimated according to Re et al. (1999). Briefly, ABTS radical was prepared by mixing 13.2 mg of potassium persulfate in 20 mL of 7 mM ABTS solution and incubated for 16 h in the dark at ambient temperature. The 20 μL of extract was mixed with 980 μL of ABTS diluted solution (diluted with ethanol to achieve a final absorbance value of 0.70 ± 0.01). After 4 min, the absorbance of the reaction mixture was measured at 734 nm. The TEAC of the pineapple slice was expressed as μg of trolox equivalent/g of pineapple.
Ferric Reducing Antioxidant Potential
The ferric reducing antioxidant potential (FRAP) was estimated as described by Benzie and strain (1996) using microtiter method. In brief, FRAP reagent consisting acetate buffer (300 mM, pH 3.6), ferric chloride solution (20 mM), and TPTZ (dissolved in 40 mM HCl) in the ratio of 10:1:1 was used in the assay. The extract (10 μL) was mixed with FRAP reagent (300 μL) and distilled water (30 μL). After 4 min, the absorbance of the reaction mixture was read at 593 nm. The FRAP results were represented as mg trolox equivalent/g of pineapple.
Oxygen Radical Absorbance Capacity
The oxygen radical absorbance capacity (ORAC) was estimated as described by Ou et al. (2001) with slight modification. Briefly, a stock solution of FL (44 mg) in PBS (100 mL of 75 mM, pH 7) was prepared and stored in dark at 4 °C. The working solution of FL (78 nM) and AAPH radical (221 mM) were freshly prepared daily in PBS. A standard of trolox (10 μM) was prepared freshly from the stock of 1 mM solution (stored at -20 °C). The supernatant (extracted in 80% aqueous methanol) was diluted by 250 times in PBS. The assay was performed in black well plates by adding 50 μL of FL and 50 μL of sample (diluted supernatant) or blank (PBS) or standard (Trolox). Further, the plate was incubated at 37 °C for 10 min prior to the addition of 25 μL of AAPH radical, and immediately the fluorescence was read until relative fluorescence intensity (FI%) was < 5% of the initial reading at an interval of 5 min. The fluorescence of the mixture was measured using multimode plate reader (Spark, Tecan Trading AG, Switzerland) at an excitation and emission wavelength of 485 and 535 nm, respectively. The ORAC results were calculated using the following equation (9) and were represented as μM trolox equivalent,
where Ctrolox is the concentration of trolox (μM), k the sample dilution factor, and AUC the area under the fluorescence curve.
Ascorbic Acid Content
The extraction for ascorbic acid estimation was followed as described by Hernández et al. (2006). Briefly, 10 g sample was crushed and extracted in 50 mL of 3% metaphosphoric acid—8% acetic acid solution. The extract was then centrifuged at 5000 ×g for 20 min at 4 °C, and the supernatant was filtered through 0.45-μm syringe filter for further use. The ascorbic acid estimation was performed using HPLC method as described by Bhavya and Hebbar (2019a). Briefly, Shimadzu LC-10A HPLC model with C18 Ascentis column (5 μm particle, 4.6 mm × 250 mm ID) coupled with PDA detector was used. The following method was used: mobile phase, 0.005 N sulfuric acid; flow rate, 1 mL/min; run time, 20 min; injection volume, 10 μL; and detection wavelength, 245 nm. Ascorbic acid content in the pineapple slices were quantified using ascorbic acid standard calibration curve (0.1–1 mg/mL) and the results were represented as mg of ascorbic acid/g of pineapple.
Statistical Analysis
Statistical analysis was performed using GraphPad prism software (version 5), and significance between the groups was determined by one-way ANOVA with Tukey’s post t test. Value of p < 0.05 was considered as significant difference between the groups. The principal component analysis was performed using the XL-Stat software. All the values are represented as mean of three independent experiments done in triplicates.
Results and Discussion
Surface Decontamination of Fresh-Cut Pineapple by Photosensitizer and Blue Light
The irradiance and fluence used in the present study were 6.34 ± 0.05 mW/cm2 and 50 J/cm2, respectively. The initial temperature of pineapple slice was 27 °C and reached a maximum of 35 °C at the end of PDI processing.
In the present study, the effect of PS and BL in combination was investigated on E. coli and S. aureus inoculated fresh-cut pineapple slices. Further, the effect of two different PS concentrations (100 and 200 μM) on the inactivation of these bacterial pathogens was also studied. The PS treatment at100 and 200 μM reduced E. coli counts by 1.18 ± 0.16 and 1.70 ± 0.12 log CFU/g, respectively (Fig.1a), while the corresponding values for S. aureus were 1.13 ± 0.05 and 1.78 ± 0.25 log CFU/g (Fig.1b). The BL treatment (50 J/cm2) showed a marginal effect on the inactivation of E. coli and S. aureus (0.46 ± 0.30and 1.17 ± 0.01 log CFU/g, respectively). During combinational treatment (PS+BL), higher concentration of PS tested showed higher inactivation of E. coli by 3.17 ± 0.29 log CFU/g. Aurum and Nguyen (2019) reported reduction of E. coli by 2.4 log CFU/g in grapes illuminated (465 nm) at 36.3 J/cm2 fluence and curcumin at a concentration of 1.6 mM. The difference in inactivation of E. coli in the present study in comparison to the above-reported literature could be due to the critical factors that dictate the effectiveness of light-based processing such as fluence applied and photosensitizer concentration used. Further, a comprehensive study focusing on the effect of different process variables is necessary to understand the factors governing the inactivation of microbial population. In the current study, concentration of PS during combinational treatment did not have significant (p > 0.05) difference on inactivation of S. aureus. In addition, a maximum reduction of S. aureus count by 4.21 ± 0.13 log CFU/g with PS(200 μM)+BL treatment was observed. These results indicate that PS (curcumin) and BL (462 nm) treatment in combination had a synergistic effect on E. coli and S. aureus inactivation. Further, it can also be noticed that E. coli (Gram-negative bacteria) was more resistant to PDI than S. aureus (Gram-positive bacteria). This significant difference between surface decontamination of E. coli and S. aureus on fresh-cut pineapple can be contributed to structural difference in their cell membranes. Similarly, Penha et al. (2017) also reported an inactivation of E. coli and S. aureus by 1.29 and 3.27 log CFU/mL of PBS, respectively, when PDI treatment was applied at a fluence of 139 J/cm2 (470 nm) in the presence of curcumin (75 μM). Our previous studies on maximum recovery diluent (in vitro) also have shown that treatment with PS (20 μM) and BL (13 J/cm2) inactivated E. coli and S. aureus by 6 log CFU/mL (Bhavya and Hebbar 2019b). This difference in inactivation of bacterial pathogens between the current investigation and the above-reported literature (in vitro studies) could be attributed to the limited penetration of BL in food matrix, and also, higher curcumin concentration is required in food matrix to induce ROS. The mechanism of action on the inactivation of these bacteria during PS+BL treatment is attributed to the damage of membrane permeability leading to cell death in the presence of ROS (Bhavya and Hebbar 2019b).
Effect on Enzyme Activity
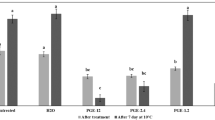
The effect of treatments on PPO, POD and bromelain enzyme activity was analyzed and results are presented in Fig. 2. The specific activity of PPO and POD in fresh-cut pineapple slices were 367.7 ± 66.8 and 546.3 ± 95.1 U/mg of protein, respectively. It was noticed that the BL treatment inactivated PPO and POD by 7.8 and 17.9%, respectively (Fig. 2a and b). This could be due to the modification of protein structure by photo-oxidation as explained by Manzocco et al. (2009) in their studies on the effect of UV-C and visible light on PPO in model system and apple derivatives. Similarly, Müller et al. (2014) also studied the effect of UV-B (290 to 315 nm) and UV-C (254 nm) on the inactivation of PPO in apple juice. They observed ~ 20 and 22% reduction of PPO by UV-C and UV-B treatment, respectively, and attributed this change to the greater effect of light on the enzyme activity with decreasing wavelength. In the present study, increase in concentration of PS from 100 to 200 μM reduced the inactivation of POD and PPO by 17.8 and 23.7%, respectively. Higher concentration of curcumin might have activated PPO, resulting in reduced degree of inactivation. In the same way, Muthukumaran and Rajalakshmi (2014) also reported that higher concentration of curcumin (0.8 μg/mL) increased the activity of banana PPO by 50% compared with control. In the current investigation, pineapple slices treated with PS+BL showed higher degree of PPO inactivation compared with PS and BL alone. This could be due to the direct photo-oxidation of the enzymes caused by absorption of radiation as well as indirect photo-oxidation mediated by ROS modifying the overall conformation of proteins structure, thus making the enzymes inactive (Davies and Truscott 2001; Manzocco et al. 2009; Tao et al. 2019). In the present investigation, PS (100 μM)+BL showed 33.5 and 25.7% inactivation of PPO and POD, respectively, in pineapple slices. From Fig. 2a, it can be noticed that PS (100 μM)+BL had higher degree of PPO inactivation (33.5%) and least specific activity (103 ± 45 U/mg of protein) among the studied treatments. This degree of inactivation was lower than the reported for in vitro PS+BL treatment of crude extract of PPO (48%) and POD (51%) from fresh-cut apples (Tao et al. 2019). They suggested that the efficacy of enzyme inactivation in the food matrix by PS+BL treatment may be lower than that in vitro due to the limitation of penetration effect. In the present work, PS (100 μM)+BL showed significantly higher inactivation of PPO (19.7%) and POD (74.7%) than PS (200 μM)+BL (Fig. 2). This could be due to the protective effect of curcumin at a higher concentration against the PPO denaturalization by BL illumination. Pigments like melanin and curcumin having maximum absorption at 425 nm might absorb most of radiant energy leading to a slower inactivation of PPO (Falguera et al. 2012). Similarly, Müller et al. (2014) and Falguera et al. (2012) reported protective effect of pigments against the PPO denaturalization by UV radiation in grape juice and different model solutions. The results of the present study suggest that the synergistic effect of PS and BL was more effective at lower concentration of PS on enzyme inactivation (PPO and POD).
Pineapple is the best-known source of bromelain, a protein-digesting enzyme that acts as an anti-inflammatory and antibiotic potentiating agent (Chaurasiya et al. 2015). Hence, it is important to investigate the effect of PDI on bromelain activity. The specific activity of bromelain in fresh-cut pineapple slices was found to be in range of 120–130 CDU/mg protein. All the studied treatments showed a marginal increase in bromelain activity by ~2–15% (Fig. 2c). PS (100 μM)+BL resulted in highest bromelain activity (140.55 CDU/mg), in turn having 15.5% activation of enzyme. As there are no literature reports on the effect of PDI on the bromelain activity, the probable reason for the activation could be as discussed below. The increase in bromelain activity could be possibly due to the inactivation of inhibitors of bromelain and variation of other factors such as pH, hydrophobic interactions with the PS might activate bromelain (Ota et al. 1961; Verma et al. 2016). Further studies are required to understand the exact mechanism of activation of bromelain during PDI.
Effect on Color Attributes
Color is an essential quality parameter that influences the consumer’s acceptance/preference of the food (Pathare et al. 2013). It can be seen from Table 1 that BL-treated pineapple slices showed a slight increase in L* and decrease in b* values, which resulted in lower YI. The change in lightness and yellowness could be due to the degradation of color caused by the exposure of pigments (β-carotene) to the light during processing. It is reported in a study conducted by Ghate et al. (2017) that β-carotene pigment also has maximum absorption peaks at 450 and 478 nm, which is in the illumination range of blue LED used in the present study. A comparable observation was also made by Ghate et al. (2017) when pineapple slices were illuminated with LED at 460 nm. They also reported that generally, YI values for pineapple slices were above 70, which is similar to the values obtained in our study. As expected, PS-treated pineapple slices had significantly (p < 0.05) higher a* and b* values as well as greater YI and ∆E, which could be attributed to the addition of curcumin. The reduced yellowness in PS+BL combination compared with PS alone could be due to photo-oxidative degradation of curcumin resulting in photo-bleaching. No significant difference (p < 0.05) in YI values of PS+BL-treated slices was observed compared with control. This could be due to the degradation caused by BL is compensated by the residual PS (remaining PS after photo-bleaching). This is also reflected in terms of lowest ∆E values of combination processing (PS+BL).
Effect on Phenolic and Flavonoid Content
The effect of light based treatments on TPC and TFC is presented in Fig. 3a and b, respectively. It can be clearly noticed that there was a significant (p<0.05) decrease (~20%) in the TPC of fresh-cut pineapple slices treated with BL, while PS+BL treatment did not show any significant difference compared to control. Al-Asmari et al. (2018) also did not find any change in TPC of fresh dates, even when treated with the combination of curcumin (1400 μM) and BL (420 nm). In our previous study, 16% reduction in TPC of orange juice treated with PS (100 μM)+BL(70 J/cm2) was observed (Bhavya and Hebbar 2019a). In contrary, Tao et al. (2019) mentioned an increase in TPC of fresh-cut apples treated with curcumin (2 μmol/L) and BL illumination at 420 nm. This could be due to the combined effect of increasing oxidative stress as well as inhibition of PPO activity accelerating the accumulation of phenolics.
In the present investigation, it was also observed that there was no significant difference (p < 0.05) in TFC of pineapple slices treated with BL and PS (100 μM)+BL compared with control. But surprisingly, a significant increase in TFC by 200% was observed compared with control in PS (200 μM)+BL-treated pineapple slices. This increase could be attributed to the presence of residual curcumin present in the slices after PS+BL treatment.
Effect on Ascorbic Acid Content
The effect of PDI treatment (PS+BL) on the ascorbic acid content of fresh-cut pineapple slices is presented in Fig. 3c. Reduction of ascorbic acid content during BL treatment was minimum (decrease by ~ 15%) than control, while it was ~ 40 and 30% decrease during combinational treatment (PS+BL) at 100 and 200 μM of PS concentration, respectively. This reduction can be attributed to the oxidation of ascorbic acid in the presence of free radicals (ROS) that are produced during PDI process. In our previous experiments also, a reduction of 60% of ascorbic acid content was found in orange juice treated with PS+BL compared with BL (Bhavya and Hebbar 2019a).
Effect on Antioxidant Activity
From the results of FRAP assay, it can be seen that the PDI treatment at the highest concentration tested did not show any significant change (p < 0.05) compared with control (Fig. 4a). While, in case of BL alone and PS (100 μM)+BL treatment, antioxidant activity of treated pineapple slices was reduced by ~ 15% than control. In our previous study, we observed higher reduction (~ 64%) in antioxidant activity (FRAP) of orange juice treated with PS+BL, compared with that of control, which could be attributed to decrease in ascorbic acid content (Bhavya and Hebbar 2019a). In the present study, although the ascorbic content was decreased, the antioxidant activity was preserved, indicating limited contribution of ascorbic acid to its total antioxidant activity in pineapple.
The TEAC analysis revealed no significant difference (p> 0.05) in antioxidant activity of the pineapple slices treated with BL or PS+BL, compared with that of control (Fig. 4b). Further, the same trend was also observed in the DPPH radical scavenging assay, showing no difference in the antioxidant activity of treated pineapple slices (Fig. 4c). In ORAC assay, it was also observed that there was no significant difference (p>0.05) in antioxidant activity of pineapple slices treated with BL and PS (100 μM)+BL compared with control (Fig. 4d), while significant increase in antioxidant activity was noticed (~ 49%) in PS (200 μM)+BL-treated pineapple slices, as compared with control. This can be explained by the presence of residual curcumin, an antioxidant present in the sample after PS+BL treatment. A similar increase in the total antioxidant activity of fresh-cut apples treated with curcumin and BL was noted by Tao et al. (2019). Even Luksiene and Paskeviciute (2011) reported 19% higher total antioxidant activity in strawberries treated with PDI, which was related to the improved cellular capability to detoxify ROS.
Principal Component Analysis
Principal component analysis (PCA) is a powerful mathematical tool used to identify clusters among the data, reduce the data dimensionality and for visualization of underlying pattern in the experimental data (Ahmadian-Kouchaksaraie et al. 2016). PCA was performed with the correlation matrix and the results are presented in Fig. 5. The first two principal components (PC) majorly account for 81.91% of total variance, where PC1 and PC2 explained variances of 51.42 and 30.49%, respectively. The PC1 was mainly associated with TPC, FRAP, ABTS, YI, and ∆E, while the PC2 was defined by TFC, ascorbic acid content, and ORAC. From the biplot, it was evident that control was located in the lower right quadrant, indicating that it contained higher TPC, FRAP, and YI. While PS (200 μM)+BL was situated in the upper right quadrant along with ABTS, TFC, and ORAC, demonstrating that these values were high due to the addition of PS (curcumin). Further, PS (100 μM)+BL placed close to origin had low contribution of PC scores (PC1 and PC2 to be −0.339 and 0.011, respectively), suggesting that there was no significant effect on the overall quality parameters compared to other treatments. BL was falling in the upper left quadrant along with ∆E values, indicating greater color difference compared with other treatments. From the correlation matrix (Table 2), TPC showed positive correlation with FRAP and ABTS (r = 0.889 and 0.714, respectively). In addition, YI showed good correlation with ABTS (0.934), TPC (r = 0.906), and FRAP (r = 0.766), suggesting that addition of curcumin contributes to the polyphenols, and antioxidant activity. Further, ORAC values were not significantly correlated with TPC (r =−0.018), while it was highly correlated with TFC (r = 0.937), and a similar observation was also made by Mamelona et al. (2007).
Conclusion
E. coli and S. aureus were inactivated (3–4 log CFU/g) synergistically, when pineapple slices were treated with PDI process (PS+BL). Even, PS+BL treatment showed a synergistic effect on inactivation of PPO (33.5%) and POD (25.7%) at PS concentration of 100 μM, but preserved desirable enzyme, bromelain. There was a significant change in color, in terms of YI and ∆E when slices were treated with PS. While, there was no change in the color attributes of pineapple slices treated with PS+BL. The PDI process, PS(100 μM)+BL(50 J/cm2) had no significant effect on TPC, TFC, and antioxidant activity compared with control samples. The PS+BL treatment had a negative effect on ascorbic acid content (30–40% reduction) of the pineapple slices due to the formation of ROS. The results suggest that the effect of combination treatment (PS+BL) on fresh-cut pineapple slices had greater ability to reduce microbial while having lesser impact on quality. Therefore, blue LED-based photosensitization process can be used as an effective preservation method for fresh-cut fruits. Future work on sensory evaluation, shelf-life extension studies, and process scale-up of PDI treated cut-fruits are of great interest.
References
Ahmadian-Kouchaksaraie, Z., Niazmand, R., & Najafi, M. N. (2016). Optimization of the subcritical water extraction of phenolic antioxidants from Crocus sativus petals of saffron industry residues: Box-Behnken design and principal component analysis. Innovative Food Science & Emerging Technologies, 36, 234–244.
Al-Asmari, F., Mereddy, R., & Sultanbawa, Y. (2018). The effect of photosensitization mediated by curcumin on storage life of fresh date (Phoenix dactylifera L.) fruit. Food Control, 93, 305–309.
Aurum, F. S., & Nguyen, L. T. (2019). Efficacy of photoactivated curcumin to decontaminate food surfaces under blue light emitting diode. Journal of Food Process Engineering, 42(3), e12988.
Benzie, I. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry, 239(1), 70–76.
Bertoloni, G., Reddi, E., Gatta, M., Burlini, C., & Jori, G. (1989). Factors influencing the haematoporphyrin-sensitized photoinactivation of Candida albicans. Microbiology, 135(4), 957–966.
Bhavya, M. L., & Hebbar, H. U. (2017). Pulsed light processing of foods for microbial safety. Food Quality and Safety, 1(3), 187–202.
Bhavya, M. L., & Hebbar, H. U. (2019a). Sono-photodynamic inactivation of Escherichia coli and Staphylococcus aureus in orange juice. Ultrasonics Sonochemistry, 57, 108–115.
Bhavya, M. L., & Hebbar, H. U. (2019b). Efficacy of blue LED in microbial inactivation: Effect of photosensitization and process parameters. International Journal of Food Microbiology, 290, 296–304.
Carstens, C., Salazar, J. K., & Darkoh, C. (2019). Multistate outbreaks of foodborne illness in the United States associated with fresh produce from 2010 to 2017. Frontiers in Microbiology, 10, 2667.
Chaurasiya, R. S., Sakhare, P. Z., Bhaskar, N., & Hebbar, H. U. (2015). Efficacy of reverse micellar extracted fruit bromelain in meat tenderization. Journal of Food Science and Technology, 52(6), 3870–3880.
Davies, M. J., & Truscott, R. J. (2001). Photo-oxidation of proteins and its role in cataractogenesis. Journal of Photochemistry and Photobiology B: Biology, 63(1-3), 114–125.
D’Souza, C., Yuk, H. G., Khoo, G. H., & Zhou, W. (2015). Application of light‐emitting diodes in food production, postharvest preservation, and microbiological food safety. Comprehensive Reviews in Food Science and Food Safety, 14(6), 719–740.
Falguera, V., Pagán, J., Garza, S., Garvín, A., & Ibarz, A. (2012). Inactivation of polyphenol oxidase by ultraviolet irradiation: Protective effect of melanins. Journal of Food Engineering, 110(2), 305–309.
Ghate, V., Kumar, A., Kim, M. J., Bang, W. S., Zhou, W., & Yuk, H. G. (2017). Effect of 460 nm light emitting diode illumination on survival of Salmonella spp. on fresh-cut pineapples at different irradiances and temperatures. Journal of Food Engineering, 196, 130–138.
Gleeson, E., & O’Beirne, D. (2005). Effects of process severity on survival and growth of Escherichia coli and Listeria innocua on minimally processed vegetables. Food Control, 16(8), 677–685.
Hernández, Y., Lobo, M. G., & González, M. (2006). Determination of vitamin C in tropical fruits: A comparative evaluation of methods. Food Chemistry, 96(4), 654–664.
Hirschler, R. (2012). Whiteness, yellowness, and browning in food colorimetry. Color in Food: Technological and Psychophysical Aspects. Editorial JL Caivano & Buera MP EE. UU, 93–104.
Kathrin, V., Snehasis, C., Bhalerao, P. P., Reinhold, C., Frank, J., & Steingass, C. B. (2020). Effect of pulsed light treatment on natural microbiota, enzyme activity, and phytochemical composition of pineapple (Ananas comosus [L.] Merr.) juice. Food and Bioprocess Technology, 13(7), 1095–1109.
Koutchma, T. (2009). Advances in ultraviolet light technology for non-thermal processing of liquid foods. Food and Bioprocess Technology, 2(2), 138–155.
Lanciotti, R., Sinigaglia, M., Gardini, F., Vannini, L., & Guerzoni, M. E. (2001). Growth/no growth interfaces of Bacillus cereus, Staphylococcus aureus and Salmonella enteritidis in model systems based on water activity, pH, temperature and ethanol concentration. Food Microbiology, 18(6), 659–668.
Luksiene, Z., & Paskeviciute, E. (2011). Novel approach to the microbial decontamination of strawberries: chlorophyllin-based photosensitization. Journal of Applied Microbiology, 110(5), 1274–1283.
Luksiene, Z., & Zukauskas, A. (2009). Prospects of photosensitization in control of pathogenic and harmful micro-organisms. Journal of Applied Microbiology, 107(5), 1415–1424.
Maclean, M., MacGregor, S. J., Anderson, J. G., & Woolsey, G. (2009). Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light-emitting diode array. Applied and Environmental Microbiology, 75(7), 1932–1937.
Mamelona, J., Pelletier, E., Girard-Lalancette, K., Legault, J., Karboune, S., & Kermasha, S. (2007). Quantification of phenolic contents and antioxidant capacity of Atlantic sea cucumber, Cucumaria frondosa. Food Chemistry, 104(3), 1040–1047.
Manzocco, L., Quarta, B., & Dri, A. (2009). Polyphenoloxidase inactivation by light exposure in model systems and apple derivatives. Innovative Food Science & Emerging Technologies, 10(4), 506–511.
Müller, A., Noack, L., Greiner, R., Stahl, M. R., & Posten, C. (2014). Effect of UV-C and UV-B treatment on polyphenol oxidase activity and shelf life of apple and grape juices. Innovative Food Science & Emerging Technologies, 26, 498–504.
Muthukumaran, P., & Rajalakshmi, N. (2014). Modulation of Banana Polyphenol Oxidase (PPO) activity by naturally occurring compounds. International Journal of Pharmaceutical Research and Allied Sciences, 3, 41–44.
Nüesch-Inderbinen, M., & Stephan, R. (2016). Fresh fruit and vegetables as vehicles of bacterial foodborne disease: A review and analysis of outbreaks registered by proMED-mail associated with fresh produce. Journal of Food Safety and Food Quality, 67(2), 32–39.
Oms-Oliu, G., Martín-Belloso, O., & Soliva-Fortuny, R. (2010). Pulsed light treatments for food preservation. A review. Food and Bioprocess Technology, 3(1), 13.
Ota, S., Fu, T. H., & Hirohata, R. (1961). Studies on bromelain. The Journal of Biochemistry, 49(6), 532–537.
Ou, B., Hampsch-Woodill, M., & Prior, R. L. (2001). Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. Journal of Agricultural and Food Chemistry, 49(10), 4619–4626.
Palmer, J. M. (2010). Radiometry and photometry: Units and conversions. Handbook of Optics, 3, 7–1.
Pathare, P. B., Opara, U. L., & Al-Said, F. A. J. (2013). Colour measurement and analysis in fresh and processed foods: a review. Food and Bioprocess Technology, 6(1), 36–60.
Penha, C. B., Bonin, E., da Silva, A. F., Hioka, N., Zanqueta, É. B., Nakamura, T. U., de Abreu Filho, B. A., Campanerut-Sá, P. A. Z., & Mikcha, J. M. G. (2017). Photodynamic inactivation of foodborne and food spoilage bacteria by curcumin. LWT- Food Science and Technology, 76, 198–202.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cationde colorization assay. Free Radical Biology and Medicine, 26(9-10), 1231–1237.
Reis, S. F., Rai, D. K., & Abu-Ghannam, N. (2012). Water at room temperature as a solvent for the extraction of apple pomace phenolic compounds. Food Chemistry, 135(3), 1991–1998.
Shewale, S. R., & Hebbar, H. U. (2017). Effect of infrared pretreatment on low-humidity air drying of apple slices. Drying Technology, 35(4), 490–499.
Tao, R., Zhang, F., Tang, Q. J., Xu, C. S., Ni, Z. J., & Meng, X. H. (2019). Effects of curcumin-based photodynamic treatment on the storage quality of fresh-cut apples. Food Chemistry, 274, 415–421.
Verma, S., Dixit, R., & Pandey, K. C. (2016). Cysteine proteases: modes of activation and future prospects as pharmacological targets. Frontiers in Pharmacology, 7, 107.
Winter, S., Tortik, N., Kubin, A., Krammer, B., & Plaetzer, K. (2013). Back to the roots: photodynamic inactivation of bacteria based on water-soluble curcumin bound to polyvinylpyrrolidone as a photosensitizer. Photochemical & Photobiological Sciences, 12(10), 1795–1802.
Yen, G. C., & Chen, H. Y. (1995). Antioxidant activity of various tea extracts in relation to their antimutagenicity. Journal of Agricultural and Food Chemistry, 43(1), 27–32.
Zerdin, K., Horsham, M. A., Durham, R., Wormell, P., & Scully, A. D. (2009). Photodynamic inactivation of bacterial spores on the surface of a photoactive polymer. Reactive and Functional Polymers, 69(11), 821–827.
Acknowledgments
The authors thank the Director, CSIR-CFTRI for his support and co-operation. Bhavya ML thank UGC, New Delhi for her senior research fellowship. Authors also thank Dr. Poornima Priyadarshini C G, Department of Molecular Nutrition, CSIR-CFTRI for her help in extending facilities for microbial trials.
Author information
Authors and Affiliations
Contributions
Bhavya: Conceptualization, Methodology, Data curation, Investigation, Writing- Original draft preparation. Shewale: Data curation, Writing- Original draft preparation. Rajoriya: Data curation, Formal analysis. Hebbar: Conceptualization, Supervision, Writing- Reviewing and Editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhavya, M.L., Shewale, S.R., Rajoriya, D. et al. Impact of Blue LED Illumination and Natural Photosensitizer on Bacterial Pathogens, Enzyme Activity and Quality Attributes of Fresh-Cut Pineapple Slices. Food Bioprocess Technol 14, 362–372 (2021). https://doi.org/10.1007/s11947-021-02581-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-021-02581-7