Abstract
Eggs are an important, low-cost, high-protein food with a balanced nutritional composition. The sanitary quality of commercial eggs is a bottleneck that needs to be overcome through the use of technologies that guarantee the safety of the product. This work aimed to prepare, characterize, and evaluate the coating of commercial eggs by chitosan filmogenic solutions and 1st- and 5th-generation quaternary ammonium salt components (QACs). Egg shell properties such as thickness, morphology, and wettability were analyzed by scanning electron microscopy (SEM),TG/DSC, pHPZC, contact angle, color, and weight loss. Determinations of the minimal inhibitory concentration (MIC) and the minimal bactericidal concentration (MBC) with Salmonella spp., Escherichia coli, and Staphylococcus aureus were performed, as well as proliferation tests on eggshell surfaces. The results show a synergistic effect of the biopolymer in terms of antibacterial activity against all microorganisms tested. The composite of chitosan and ammonium quaternary ammonium films offers physical and biological safety to guarantee the integrity and microbiological quality of commercial eggs. Egg coating using bioactive chitosan films was shown to be a suitable method of preservation and a physical and microbiological barrier to minimize egg quality loss during storage, as well as to increase shelf life.
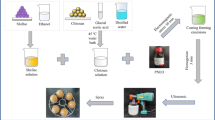
Graphical abstract

The use of chitosan and quaternary ammonium salt films offers a physical and biological safety to guarantee the integrity and microbiological quality of eggs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The egg is considered a complete food in nutritional terms because of the bioavailability of nutrients such as protein and its affordable price. The egg yolk lipid fraction is typically composed of 50% water, 34% lipids, and 16% protein. It contains 14 types of minerals, with sodium and calcium as the most abundant. Vitamins A, D, E, and K are found in egg yolk and B complex vitamin in albumen (El Ghamry et al. 2011; Lichovnikova and Br Poult 2007; Nascimento et al. 2014).
Egg production must follow health and quality criteria, requiring strict microbiological control, mainly due to possible egg contamination by salmonellosis or other microorganisms due to small-scale fractures in the eggshell (Messens et al. 2005). This may affect the nutritional and microbiological quality, consumer acceptability, and safe consumption of the product (Pereira et al. 2014). In this sense, the egg must be produced, processed, transported, and stored under appropriate conditions from laying to final consumption, including the conditions and protection of the product for sale (Mazzuco and Bertechini 2014).
According to the World Health Organization (WHO December 2015; WHO June 2019), 600 million people fall ill each year from consuming contaminated food. Diarrheal diseases are the most common illnesses resulting from unsafe food, and Salmonella is one of the four key global causes of these diseases (WHO February 2018). The consumption of contaminated eggs and egg products is the most frequent source of human salmonellosis (Han et al. 2013). Globally, Salmonella Enteritidis is considered to be one of the human enteropathogens most frequently associated with laying hens and contaminated eggs. This Salmonella serotype is predominantly isolated from eggs due to its greater ability to survive in the egg than other serotypes and serogroups (De Vylder et al. 2013; Kouam et al. 2018; Li et al. 2019; Webber et al. 2019).
In an attempt to reduce this contamination, different compounds have been proposed for egg treatment, e.g., chlorine-based compounds (hypochlorites and others) that are used as sanitizers (KNAPE et al. 1999). However, these compounds may have carcinogenic effects from the reaction of egg contents with chlorine (Bondoc 2014; Bondoc and Şindilar 2002). Sodium hypochlorite (NaClO) is the most widely used chlorine compound due to its availability, reactivity, and low cost (Estrela et al. 2002).
As an alternative to a safe sanitizing agent for egg surface treatment, quaternary ammonium salt components (QACs) are indicated (Brake and Sheldon 1990). Benzalkonium chloride (1st-generation quaternary) and other 2nd-, 3rd-, 4th-, and 5th-generation quaternary products are proposed commercial products for the surface treatment of eggs. Quaternary ammonium salts provide bioactivity, low toxicity, surface tension reduction, detergency, and tensoactivity that allow their active diffusion, spreading, and maintenance of concentration throughout the egg surface, due to the moisture in the polymeric film. Quaternary ammonium salts are bioactive surfactants with a broad activity spectrum for different microorganisms, from the 1st to 5th generations. The microbicidal action depends on the structure and concentration of molecules and/or mixtures that interact and/or permeate the cell surface of microorganisms, interfering with cell integrity and metabolism (Buffet-Bataillon et al. 2012; Forbes et al. 2017; Gadea et al. 2017; Gerba 2015; Soumet et al. 2016).
The efficiency of a sanitizing product also depends on the contact time, as well as the safe residual effect that this product has on the surface via the formation of films or molecular layers formed by electrostatic interactions of the product molecule with the product surface treated as a bioactive residual film (Pfuntner 2011). Thus, technologies and strategies that can prevent product contamination with formulations and use of the safe, easy handling, protective, inhibitory substances with antimicrobial activity are important and should be used for this purpose. In the case of eggs, these may be contaminated after laying (Gast et al. 2019). Coating with a bioactive film, oil, or quaternary ammonium salt sanitizer can be performed using natural combined polymers as a filmogenic solution to produce bioactive films that have protective, residual, and sanitizing effects for the maintenance of egg quality during transportation and storage, thereby increasing shelf life (Goy et al. 2016; KAVAS and KAVAS 2016; Nechita et al. 2015).
In this context, antimicrobial products in association with a polymeric solution can be applied to the egg surface by different processes after laying in the farms. This solution can reduce the surface tension, due to the antimicrobial surfactant and product mobility in the hydrated polymer film after drying, acting as a self-healing and bioactive barrier to reduce contamination and/or deterioration of the product (Beltrame et al. 2012). This bioactive film acts as an intelligent cover additional to the product packaging (eggshells) for maintaining and preserving the food quality of the product.
The eggshell surface is a natural barrier designed to prevent contamination and deterioration of the nutritional content. Technologies or formulations that protect or improve the action of this surface can allow even greater efficiency in poultry production, e.g., product quality conservation, besides guaranteeing in the packaging a washable protective biofilm on the eggshells (Ku and Ximenes 2016; Vaz et al. 2002).
The aim of this work was to develop bioactive polymeric films of chitosan enriched with QAC 1st and QAC 5th generation to coat commercial eggs during storage, in order to act as a protective microsealant, i.e., an intelligent biological barrier (by self-repair via the hydration, solubility, and flexibility of the polymer chain) to control bacteria such as Salmonella spp., Escherichia coli, and Staphylococcus aureus present on the eggshell surface.
Material and Methods
Raw Materials
Molecular weight technical chitosan with a deacetylation degree of 83% was obtained from Polymar (Fortaleza, CE, Brazil). The 1st-generation QAC with 50% activity was benzalkonium chloride (QAC 1st). Polybac QT 80 is a 5th-generation quaternary ammonium compound with 80% activity; it is a balanced mixture of n-alkyl dimethyl benzyl ammonium chloride + didecyl dimethyl ammonium chloride in a 70/30 proportion (QAC 5th). Both were purchased from PolyOrganic Technologic Ltda.
A total of 174 white-colored eggs were used to perform the analyses, obtained directly from a local farm located on the MS 379 km 03 highway, in the district of Panambi, city of Dourados, MS. Freshly laid eggs were chosen and transported in the morning.
Evaluation of Eggs and Eggshells
Eggs were weighed on a digital analytical balance and were classified into medium (50 to 55 g) and large (55 to 60 g) sizes (Brazil, 1991). The eggshells were separated for characterization and application of the films. The eggs were cleaned using detergents and sanitizers at the production facility. It is known that natural waxes that cover eggs and the internal cuticle are barrier against microorganisms. During cleaning, eggs lose this natural protection, so the application of a biofilm can replace this lost protection by sealing the surface and controlling porosity, which partially prevents the loss of gases and water, in addition to reducing the possibility of colonization by microorganisms from the surface, which can reduce the shelf life and viability of the eggs.
The eggshells were characterized by elemental analysis, spectroscopic techniques, scanning electron microscopy (SEM), thermogravimetric analysis (TGA) for composition analysis, morphology, film coating analysis, anf interactions/surface characteristics of quaternary ammonium compounds. For these analyses, circular eggshell samples were cut (radius of 1.10 cm) using a plaster filling to support the eggshells, made by mold marking, and cut by a Dremer Microrectific, Mondial model FMR-01. Standardizations were performed for the acrylic well plate experiments to analyze bactericidal activity regarding the size and equivalent cutting areas or were performed from a mean diameter or radius and standard deviation. For the antibiofilm activity of quaternary ammonium compounds, a complex of chitosan + QAC 1st and a complex of chitosan + QAC 5th were placed on circular eggshell samples with a total area of 1.10 cm2 cut using a Dremer Microrectific (Mondial model FMR-01). At least 12 samples (eggs) were analyzed for surface area (As) and standard deviation and volume (V) and standard deviation.
Preparation of Chitosan and Quaternary Ammonium Salts
The experiment was performed in triplicate, with three eggshell samples from different eggshells. The surfaces were treated with chitosan and 1%, 0.5%, and 0.25% QAC. The solutions were prepared with distilled water, acetic acid, and chitosan. The final volume was 200 mL and the concentrations were 1% acetic acid and 1% chitosan. The sample was stirred for 24 h at a temperature of 35 to 40 °C. In the next step, after stirring for 24 h, QAC 1st and QAC 5th were added as a function of volume at 1%, 0.5%, and 0.25%, both in a volume of 50 mL. After bepreparing the QAC and chitosan concentrations, eggshell samples were immersed in the solution for 5 s and placed in Petri dishes for drying at room temperature for 18 h.
Antimicrobial Activity QAC 1st, QAC 5th, Acetic Acid, Chitosan, and Complexes
To evaluate the antimicrobial activity, four bacteria from the American Type Culture Collection (ATCC, Rockville, MD, USA) were used for the assays: Escherichia coli (ATCC 25922), Salmonella Typhimurium (ATCC 14028), Salmonella Enteritidis (ATCC 13076), and Staphylococcus aureus (ATCC 25923). All species were stored at − 20 ± 2 °C in nutrient broth (Merck KGaA, Darmstadt, Germany) with 20% (v/v) glycerol. Prior to each assay, species were subcultured from the frozen stock suspension onto nutrient agar (Merck KGaA, Darmstadt, Germany) plates. The plates were incubated for 18–24 h at 37 °C to start the tests. After incubation, a suspension of inoculum was prepared containing 1 to 2 × 108 CFU mL−1.
The antibacterial activity of quaternary ammonium compounds, acetic acid, chitosan, complex of chitosan + QAC 1st, and and complex of chitosan + QAC 5th was determined by broth microdilution method in 96-well polystyrene plates according to the methodology described in Clinical Laboratory Standards Institute (CLSI, 2012) standard M7-A9. The concentrations used were QAC 1st (0.00002–4.8%) and QAC 5th (0.00002–4.8%), acid acetic (0.00391–2%), chitosan (0.00391–2%), complex chitosan (0.25–1.5%) + QAC 1st (0.000004–0.00234%), and complex of chitosan (0.25–1.5%) + QAC 5th (0.00001–0.00469%). For the positive control, the antibiotic ampicillin was used (AMP, Sigma-Aldrich, São Paulo, BRA) and for the negative control, Mueller-Hinton broth was used (Merck KGaA, Darmstadt, Germany). The MIC was considered as the lowest concentration of the compound at which the microorganisms did not show visible growth after incubation. A visual reading was performed to determine if there was growth of the microorganism. The MIC was determined by adding 50 μL of 0.1% triphenyltetrazolium chloride solution (TTC, VETEC/SIGMA-ALDRICH, São Paulo, BRA) to the wells for 30 min (Panghal et al. 2011).
For the MBC evaluation, a 10 μL aliquot from each microplate well was transferred to a Mueller-Hinton agar (Himedia, Mumbai) plate. The plates were incubated at 37 °C for 24 h. MBC was determined as the lowest concentration that showed no bacterial growth. Tests were performed in duplicate in three independent trials (Bagiu et al. 2012).
Inhibition of Biofilm Formation on Eggshells
The antibiofilm activity of quaternary ammonium compounds, complex of chitosan + QAC 1st, and complex of chitosan + QAC 5th were performed according to Trevisan et al. (2018) with adaptations. Circular eggshell samples with a total area of 1.10 cm2 were used as adhesion surfaces, which were first washed individually by immersion in neutral detergent for 1 h, rinsed with sterilized distilled water, and dried and cleaned with 70% alcohol (v/v). After cleaning, the circular eggshell samples were washed again with sterile distilled water, dried, and autoclaved at 121 °C for 15 min prior to testing according to Uchida et al. (2015) with adaptations. The concentrations of QAC 1st, QAC 5th, a complex of chitosan + QAC 1st, and a complex of chitosan + QAC 5th used in this test were based on the MIC results.
Based on the results of the antibacterial activity test on planktonic cells, Salmonella Enteritidis, a major cause of egg-borne human salmonellosis, was selected for the examination of quaternary ammonium compounds, the complex of chitosan + QAC 1st, and the complex of chitosan + QAC 5th on biofilm formation in eggshells.
To evaluate the effect of quaternary ammonium compounds, the complex of chitosan + QAC 1st, and the complex of chitosan + QAC 5th on biofilm formation in eggshells, first Salmonella Enteritidis (ATCC 13076) was inoculated into trypticasein soybean broth (TSB, Himedia, Mumbai) at 37 °C for 18–24 h with shaking at 120 rpm. Afterwards, the bacteria was centrifuged at 3000g for 10 min and washed three times with phosphate-buffered saline (PBS). Afterwards, a suspension of inoculum was prepared containing 1.5 × 108 CFU mL−1.
The assay was performed with concentrations of QAC 1st (0.00014–0.00463%), QAC 5th (0.00029–0.00937%), the complex of chitosan + QAC 1st (0.00237–0.00937%), and the complex of chitosan + QAC 5th (0.00466–0.01875%) diluted in TSB and simultaneously added to the wells in 24-well polystyrene plates with the inoculum where eggshells had been deposited. The microplates were incubated while shaking at 50 rpm min−1 for 24 h at 37 °C. Subsequently, the S. Enteritidis biofilms in eggshells were washed three times with PBS to remove non-adhered cells. Finally, the eggshells were removed from the well using sterile tweezers and introduced individually into a tube with 10 mL of 0.9% solution saline and the S. Enteritidis biofilms on eggshells were removed by sonication. The activities of QAC 1st, QAC 5th, the complex of chitosan + QAC 1st, and the complex of chitosan + QAC 5th against biofilm formation were examined by counting the viable cells. The total number of CFUs per unit area eggshell was expressed in log CFU mL−1. The experiment was conducted at three different times. Statistical analyses were performed by one-way ANOVA and the Tukey test using Graph Pad Prism 7.0 statistical software.
SEM and Dispersive Energy Spectroscopy
Eggshell thickness and morphology were analyzed by scanning electron microscopy (SEM). A Zeiss Supra 35VP field effect electron gun (FEG-SEM) microscope was used, working at 3 to 5 keV and spot 3. To prepare the samples, small fragments of film fixed on carbon tape were placed on adhesive in order to fix the sample position at angles of 180°, 90°, and 45°; then, the samples were oven dried at 40 °C for 12 h. This analytical procedure allowed the samples to be free of moisture. The samples were coated with a thin layer of gold using a Balzers Sputter Coater SCD 004 for 90 s. This gold foil assists in conducting electrons that reach the surface of the material, contributing to an interference-free image of better quality and resolution.
Analyses of 2D energy-dispersive X-ray detector, EDX, were performed with 2D mapping working at 25 keV and spot 4. This accessory device was essential in the characterization study of the dispersion of carbon, calcium, and sodium in the sample. When the electron beam strikes the material, the electrons in the inner layer of atoms are removed. The atoms of the levels just above occupy the position of the removed electron, and in this process, the electron releases quantized energy indicating the difference in the energy levels of the layer. A detector installed in the SEM vacuum chamber measured the energy associated with the emission of this electron. Since the electrons of a given atom have distinct energies, it was possible at the point of incidence of the beam to determine which chemical elements were present at that location and to identify the chemical composition in the observed area. The graph was plotted by analyzing the energy released from the emission C Kα, O Kα, Mg Kα, P Kα, S Kα, Cl Kα, and Ca Lα1.
Optical Contact Angle
The static optical contact angle (OCA) was measured using the drop method, which is the standard method for measuring contact angles. A drop of liquid was deposited on the eggshell surface with and without the polymeric film coatings under a diffused light source. The drop contour was optically adjusted. All samples were dried at room temperature in a vacuum oven for at least 1 h before measurements. Measurements were performed using the sessile drop method with a contact angle measuring system (DataPhysics, San Jose, CA, USA), using model optical contact angle 15 (OCA 15), equipped with a video charge-coupled device (CCD) camera, and sca 20 software. Images were taken every 2 s over 3 min after deposition of an 8-μL drop of Milli-Q water. Droplet profiles were fitted using the Young-Laplace mathematical function. The water contact angle of each sample was calculated by extrapolating the time-dependent curve to zero. Results are the average of two measurements on three independent samples. Samples had to meet the requirements: (a) size > 0.5 × 0.5 cm2, (b) chemically homogeneous, (c) flat and physically homogeneous (roughness not greater than 0.1 μm), (d) no reaction with the liquid or altered the sample surface, and (e) maintained under controlled temperature and humidity. The Young-Laplace calculation method/algorithm was used due to its precision.
Results and Discussion
Surface Morphology and Properties
SEM images show that the eggshells have a thickness of 370 μm; three regions of the eggshells are highlighted (Fig. 1). The first region is the external surface of the egg, which has a rough surface with spherical cavities (Fig. 1(a)). Eggshell morphology may undergo changes related to biological transformation and factors related to the physiological cycle of the bird, such as feeding, rearing environment, management practices, and other sanitary procedures (Al-Ajeeli et al. 2016).
The second region is the cross-section of the shell, more specifically the 90° angle image of the eggshells and in the fracture region, where we can observe the prevalence of a larger number of spherical cavities, quite possibly water bubbles that were trapped in the formation process of the shell, which after a time dried and gave rise to homogeneous-sized spherical cavities around 450 nm (Fig. 1(b)). The cavities are evenly distributed in a ratio of 6 cavities/10 μm2. The outer surface also has cavities that are harder to spot due to roughness and organic material that covers part of the cavities.
The third region is the inner shell membrane (about 20 μm) that is in direct contact with the albumen; this surface is covered with a protein film in the form of long fibers 2 μm in thickness (Fig. 1(c)).
The outer membrane is about 50 μm thick and is located between the inner membrane and the calcified part of the shell. Both inner and outer membranes are made up of organic fibers lying parallel to the egg surface and are easily visible using scanning electron microscopy (Fig. 1(c)). La Scala Jr et al. (2000) showed that the film is not compact and pores can be seen in the fiber structure. The structure of the protein film is composed of a polymeric network of glycoproteins, mucoproteins, collagen, and mucopolysaccharides, which has bactericidal activity and is the second protective barrier of the egg against microorganism penetration (Nascimento et al. 2014; Nys et al. 2004, 2001, 1991; Wang et al. 2002).
Treating the outer surface of the eggshells with chitosan + quaternary ammonium salts allowed the formation of a thin coating that covered the entire surface, serving as a barrier against colonization and the proliferation of microorganisms. A qualitative analysis of the presence of the film is seen in Fig. 2, which shows the surface with microcracks and microfractures containing numerous spherical cavities up to 450 nm in size distributed evenly over the entire inner surface of the shell egg (Fig. 2a). Figure 2b shows the external surface of the control egg, with an irregular surface presenting numerous cavities. In Fig. 2c, the outer surface of an eggshell treated with chitosan polymer + quaternary ammonium is observed. When compared with the three surfaces, Fig. 2c shows covered cavities, which in some cases do not appear clearly. The EDS analysis confirmed the qualitative observations.
Figure 3 shows the EDS graph of the eggshell fracture surface and the chitosan + quaternary ammonium salt–coated surface. We can observe that the composition of both surfaces was similar, containing energy released from the emissions C Kα, O Kα, Mg Kα, P Kα, and Ca Kα. It is possible to clearly observe, after zooming in the region between 0.2 and 0.8 KeV, peaks referring to the emission of C Kα and O Kα. The graph makes it clear that there is a greater amount of carbon on the surface of the chitosan + quaternary–treated eggshell as compared to the surface of the control eggshell. Figure 3b shows the atomic percentage values of carbon, oxygen, and calcium. It can be seen that the surface treated with the polymer contained 39.93% carbon and the control had 19.57% carbon. This is a qualitative indication of the presence of the chitosan polymeric coating on the egg surface. The EDS results corroborate the reduction in contact angle results.
Figure 3a shows that eggshells are basically composed of calcium carbonate (CaCO3) and small amounts of other minerals such as magnesium carbonate (MgCO3), calcium phosphate (Ca3(PO4)2), and organic substances. Previous studies (Hunton 2005) showed that shells are composed of about 97% calcium carbonate, and most of the research on shell quality has concentrated on this proportion. This is because the percentage of calcium is related to the hens’ diet (Ketta and Tůmová 2016).
Optical Contact Angle (θ°)
The contact angle measurements (θ) of the eggshell samples are summarized in Table 1. The reference sample (fresh shell, untreated) is shown in Fig. 4d and Table 1 with the indication 0%. The contact angle measured was 61°. The other eggshell samples were treated (chitosan + QAC 1st and QAC 5th film coatings at different concentrations, i.e., 0.25%, 0.50%, and 1% (v/v)) and showed reduced contact angle values (θ), indicating greater wettability or hydrophilicity. Figure 4a shows images of water droplets on the surface of the eggshell samples without and with chitosan + quaternary ammonium coatings at 0%, 0.25%, 0.50%, and 1% (v/v).
a Contact angle (θ) measurements on the surface of eggshells with and without the chitosan + quaternary ammonium film coating at 0%, 0.25%, 0.50%, and 1% (v/v). b Image of an uncoated eggshell (0% biopolymer). c Eggshell coated with the biopolymer film with 0.25% quaternary ammonium. d Eggshell coated with the biopolymer film with 0.5% quaternary ammonium. e Eggshell coated with the biopolymer film with 1% quaternary ammonium
Image analysis of contact angles (θ) and evaporation time (tevap.) of the water drop (Table 1) in Fig. 4b–e showed the behavior (scattering) of the water drop on the egg surface after spontaneous deposition on the surface of the egg without and with chitosan + QAC 1st and QAC 5th at different concentrations (0%, 0.25%, 0.50%, and 1% (v/v)). The scattering of the drop of water deposited on the egg surface indicates the increased hydrophilicity of the coated surface, and the contact angle variations (θ) indicated that the wettability (hydrophilicity) increased with increased concentrations of quaternary ammonium salts, i.e., QAC 1st and QAC 5th in the polymeric coating from 0 to 1.0% (v/v). Thus, the surface became more wettable (hydrophilic) as the quaternary ammonium salt concentration increased from 0 to 1.0% (v/v) in the chitosan coating.
The drying behavior of the drop of water spread on the eggshell surface was assessed. The contact angle (θ) and total evaporation time results are shown in Table 1.
Figure 4a shows the mean values and standard deviations (θ° ± SD) in graphical form showing the increased wettability of the eggshell surface without and with chitosan + quaternary ammonium coating as the quaternary ammonium film concentration increased.
These contact angle (θ) values show interesting water surface activity on the uncoated commercial eggshell surfaces coated with the chitosan coatings containing QAC 1st and QAC 5th.
QAC 1st and QAC 5th have surfactant activity, which improves surface wettability by reducing the contact angle (θ) as a function of the concentration of quaternary ammonium salts present in the chitosan coating. The surface of the chitosan-coated eggshells + QAC 1st and QAC 5th became more wettable (increased surface hydrophilicity allowing quaternary ammonium salt scattering via polymer chains). This property shown by the polymeric coating + quaternary ammonium salts is important in film restoration if it is damaged because it is self-regenerating. This is important in the stocking process and/or egg placement in shelf packs due to temperature and relative humidity variation in the environment, which due to the formation and maintenance of a surface water film allows the availability of quaternary ammonium films in the coating and the induction of more efficient microbiological control (bio)activity due to the availability of molecules in the film for biological control. This is more efficient than a dry coating. The values of the experiments showed the dependence of the contact angle (θ) (wettability) of the egg surface on the concentration of the quaternary ammonium films in relation to the uncoated commercial eggshell surface.
The water drop drying time values showed that increasing the film concentration of 1st- and 5th-generation quaternary ammonium compounds, in addition to facilitating water drop spreading on the surface of commercial eggs, reduced the water drop evaporation time. This likely occurred due to the diffusion of the quaternary ammonium molecules from the coating to the water drop, reducing intermolecular interactions between water molecules, improving their scattering on the surface, reducing interactions, and providing greater surface area of the water film for the evaporation of the drop of water.
Shelf Life and Weight Loss
Egg quality can be measured by various methods, direct or indirect (Roberts 2004). Direct methods include measuring shell breaking strength such as impact fracture force, puncture force, or quasi-static compression. Indirect methods include specific gravity and non-destructive deformation. In commercial operations, eggs are either candled using light to detect cracks, fractures, and other defects or they pass through an electronic crack detector for egg breakage detection.
Numerous parameters have been proposed to evaluate egg quality (Romao et al. 2008; Taha et al. 2019; Uyanga et al. 2020; Walayat et al. 2020; Yang et al. 2020). One of the most direct methods is weight loss through the evaporation of water (Grochowska et al. 2019; Luo et al. 2020; Vlčková et al. 2019). Stadelman (1986) related the weight loss during the storage period with the loss of water from albumen to the environment due to the permeability of eggshells to vapor and gases. Mass loss is directly related to other parameters such as eggshell percentage, thickness, strength, and density. So, we can evaluate the quality of eggs by monitoring for mass losses (Ketta and Tůmová 2016).
An egg starts losing water through its membrane and shell pores to the environment from the time it is laid. Water and gas loss depend on several parameters like temperature, airflow, and relative humidity (RH) during storage. The longer the storage period, the more critical these factors become, especially at room temperature. The drier the atmosphere, the greater the water loss, so egg vitality is also lost, affecting hatchability and chick quality (Weder and Belitz 2003a, b). In Brazil, 92% of in natura commercialized eggs are exposed to room temperature, being refrigerated only in the consumer’s house (Beltrame et al. 2012; Oliveira et al. 2009). Thus, the development of technology to guarantee and extend the quality of eggs is urgent.
The weight loss fraction during the storage period is shown in Fig. 5. No differences were found in the final weight of the eggs covered with chitosan with or without quaternary ammonium salts. The eggs coated with the polymer progressively lost weight during storage following a first-degree equation with R2 = 0.9948, as shown Fig. 5 (y = a – αx, with values of a = 0.2392 and α = 0.1659). The graph shows that the eggs presented a mass loss average of 1% over 6 days. The eggs stored for 30 days showed a weight loss of 4.6% for all samples containing the biopolymer coating. The egg weight decreased with the duration of storage, as reported by Schmidt et al. (2009). However, the present study demonstrated 50% less weight loss with the biopolymer coating than reported in the literature under the same experimental conditions.
The eggs with no coating progressively lost weight during storage following a first-degree equation with R2 = 0.9981, as shown in Fig. 5 (y = a − αx, with values of a = 0.3192 and α = 0.14351). The graph shows that the eggs presented a mass loss average of 2% over 6 days. Panda and Singh (1990) showed weight loss in eggs stored at room temperature, obtaining a result of 9.25 g after 21 days of storage, while Samli et al. (2005) presented an average weight loss of 9.20% after a period of 31 days.
The egg weight loss observed during storage in the present study followed the expected pattern and was lower than that found in previous studies (Reijrink et al. 2009) on eggs stored at 20 to 27 °C and unspecified relative humidity and for eggs stored at room temperature (25 °C) and 80% RH (González-Redondo 2010). The 60% decrease in the weight loss fraction during the storage period seen in the eggs covered by the biopolymer can increase egg quality, because it slows down loss of albumen height and maintains the antimicrobial activity of proteins (Kouame et al. 2019; Pires et al. 2020; Uyanga et al. 2020). The developed biofilm is capable of protecting eggs from moisture loss through evaporation. In Fig. 3, the biofilm formed by chitosan and the quaternary ammonium salts acted as a film on the surface of eggs, mainly on microfractures and pores. The performance of the biofilm allowed for an increase in the shelf life of the product, as it acted as a physical and/or biologically active barrier against microorganisms and mass loss.
Antimicrobial Activity
Quaternary ammonium compounds of the 1st and 5th generations showed bacteriostatic and bactericidal activity against Escherichia coli, Salmonella Enteritidis, Salmonella Typhimurium, and Staphylococcus aureus, with MICs ranging from 0.00014 to 0.00937% mL−1, data shown in Table 2. The same concentrations showed bacteriostatic and bactericidal activity. QAC 5th inhibited the growth of Escherichia coli, Staphylococcus aureus, and Salmonella Typhimurium at lower concentrations (i.e., 0.00117%, 0.00117%, and 0.00468%) than QAC 1st (0.00234%, 0.00234%, and 0.00937%). In contrast, Salmonella Enteritidis was more sensitive to QAC 1st (0.00014%) than QAC 5th (0.00029%).
Acetic acid, chitosan, and chitosan + quaternary ammonium compound complexes showed bacteriostatic and bactericidal activity against Salmonella Enteritidis (Table 3). The synergistic activity of chitosan and other compounds has been shown; however, in this study, we showed that there was an improvement in the activity of QACs when chitosan was utilized. The synergistic effect chitosan + QAC 1st and QAC 5th was shown when we compared the MIC values against Salmonella Enteritidis for QAC 1st and QAC 5th and the chitosan + QAC 1st and QAC 5th complexes, i.e., 0.0000046 to 0.00014% mL−1 and 0.0000091 to 0.00029% mL−1, respectively. These values indicate a considerable decrease in the concentration (by two log units) of the QACs, and the QACs were about 30 times more diluted but presented the same activity as QAC 1st and QAC 5th alone.
The consumption of eggshells contaminated with microorganisms has frequently led to outbreaks. Egg contamination by Salmonella is extremely important, because this microorganism is one of the main causes of food poisoning in humans and Salmonella Enteritidis is a major cause of egg-borne human salmonellosis (Whiley and Ross 2015).
Thus, eggshell sanitation is a fundamental and common practice to eliminate or lower the microbial load on the surface of eggs and to reduce the probability of contamination with this pathogen. Several strategies such as UV radiation, electrolysed water, whole egg pasteurization, ionizing radiation, lactic acid, and sanitizers have been studied for sanitizing egg surfaces and reducing the population of microorganisms on eggshells (Howard et al. 2012). However, some factors such as the effective elimination of the microorganisms, safety to humans and the environment, energy savings, emission reductions, low cost, and preservation of the intregrity of eggshells should be considered when selecting the most appropriate sanitizing method, thus benefiting both the public and industry.
The use of quaternary ammonium compounds has been shown to be a promising reducer of microorganisms on the surface of eggs in our study as well as in previous studies (Li et al. 2013). The antimicrobial activity of quaternary ammonium groups is attributed to their ionic part combined with a lipophilic chain (Muñoz-Bonilla and Fernández-García 2015). Palermo et al. (2011) found that the alkyl chain can penetrate the cell, causing lysis and death.
Chitosan’s antimicrobial activity may be related to its cationic nature (Rabea et al. 2003). The presence of charged groups in the polymer backbone and their ionic interactions with bacterial cell wall constituents suggests the occurrence of peptidoglycan hydrolysis in the microorganism’s cell wall, provoking disturbances in metabolism, leading to microorganism death. The charges present in chitosan chains are generated by the protonation of amino groups when in acid medium, or they may be introduced via structural modification. Structural modification can be achieved by a methylation reaction, resulting in a quaternized derivative with a higher polymeric charge density, and these charges provide efficient antimicrobial activity (Fernandes et al. 2018; Goy et al. 2009; Mendes-Gouvêa et al. 2018; Souza et al. 2018; Takamiya et al. 2016). In addition, Bhale et al. (2003) showed that a chitosan coating improves the shelf life of eggs, at a low cost, and with no toxicity, which makes this an effective method for use in food.
The literature points out that the principal application of chitosan is in food packaging for the extended shelf life of fruit food products (Poverenov et al. 2014; Rabea et al. 2003; Saleh et al. 2020; Saxena et al. 2013), vegetables (Martins et al. 2013), flour (Lamas et al. 2016; Malmo et al. 2013; Walayat et al. 2020), emulsions (Ghaderi-Ghahfarokhi et al. 2016; Samsalee and Sothornvit 2020), and fresh meat (Amjadi et al. 2019; Cai et al. 2014; Cianca et al. 2020; Diao et al. 2020; Wang et al. 2017). Applications have focused on dehydration and antibacterial activity against food-borne pathogenic bacteria (Shiroodi et al. 2016) and fungi (Pagliarulo et al. 2016). It is important to mention that there are no similar papers to our work, demonstratung how our study is different from those that have already been published and fill a knowledge gap in this topic.
In combination, chitosan and QAC can provide antimicrobial activity because chitosan is an efficient polycationic polymer that competes with bivalent cations by binding to the outer layer of microorganisms; it is also efficient as a coating matrix (Vu et al. 2011). The quaternary ammonium salts provide antimicrobial activity against a broad spectrum of Gram-positive and Gram-negative bacteria. Thus, the evidence suggests that treatment with quaternary ammonium salts and chitosan can be used on eggshells as an effective method to eliminate and control Salmonella.
Inhibition of Biofilm Formation
The activity of QAC 1st and QAC 5th and their chitosan complexes against Salmonella Enteritidis biofilm formation is observed in Fig. 6. Quaternary ammonium salts were able to inhibit biofilm formation at concentrations of 0.00464% mL−1 for QAC 1st and 0.00938% mL−1 for QAC 5th. When the chitosan + QAC 5th complex was used, it showed a reduction in the concentration capable of inhibiting the formation of biofilms, i.e., at a concentration of 0.00466/1% mL−1.
An important feature of Salmonella spp. is the ability to form biofilms on different surfaces present in food processing environments (Barbosa et al. 2016; Monteiro et al. 2012a, b, 2013, 2014a, b, 2015; Steenackers et al. 2012; Takamiya et al. 2016). Biofilm formation has become a major problem in a wide range of food industries. These are associated with spoilage and food-borne diseases which can result in huge economic losses and threaten food security (Gibson et al. 1999).
Salmonella is able to adhere and form biofilms on diverse surfaces (Oliveira et al. 2006) including eggshells (Pande et al. 2016). During biofilm formation, microorganisms attach to biotic and abiotic surfaces where they manage to grow as sources of permanent contamination, releasing biofilm fragments formed of bacteria. They may compromise the microbiological quality and safety of food products (Clímaco et al. 2018; Mead 2004; EFSA Panel on Biological Hazards 2014).
Biofilms embedded in a surface are more resistant than planktonic bacteria (Nguyen and Yuk 2013). Biofilm resistance may be due to different factors, such as inhibition of disinfecting agent diffusion by extracellular polymeric substances (EPS), physiological heterogeneity caused by nutrient, and oxygen gradients generated in the biofilm. Therefore, cleaning and disinfection processes are the most important activities for biofilm control in the food industry. Without proper treatment by disinfectant application, microorganisms can colonize and persist on food contact surfaces (Mah and O’Toole 2001).
The experimental results of this study show that the QAC 1st and QAC 5th had similar activity against Salmonella Enteritidis biofilm formation. A previous study showed a 98% reduction in microbiological contamination of eggshells sprayed with a quaternary ammonium solution (Brake and Sheldon 1990). Webber et al. (2019) in their study on Salmonella Enteritidis biofilms showed that there was a reduction of 3.562 log10 UFC mL−1 after 5 min of contact with quaternary ammonium salts (1%). On the other hand, the effect of concentration upon the log cell reduction was 3 log cell reductions on Salmonella biofilms with 1% chitosan (Orgaz et al. 2011). The QAC 1st and 5th generation + chitosan coatings also showed activity against Salmonella Enteritidis biofilm formation. The synergistic effect remained at the same concentrations for the 1st quaternary ammonium salts and there was an improvement in the activity of the 5th quaternary ammonium salts together with chitosan.
The QAC 1st and QAC 5th data with or without chitosan coatings showed similar activity against Salmonella Enteritidis biofilm formation, with biofilm formation inhibited at concentrations about 0.00464% mL−1. These data suggest analyzing the structure and distribution of antibacterial material (QAC) on the surface of each of the samples. The schematic diagram in Fig. 7 shows the synergistic efffect of the formulation containing chitosan + QAC and the potential of this material. Figure 7a shows that 100% of the concentration QAC that covers the surface of the eggshells is available to act as a bactericide. However, in Fig. 7b, it shows a thicker biopolymer covering on the surface of the eggshells formed by chitosan + QAC, where the quaternary ammonium salt is homogeneously distributed in the polymer so that a much QAC smaller fraction is available in the surface to provide bactericidal action against Salmonella biofilms. When we analyzed the data from this perspective, it was clear that there was an improvement in the activity of the chitosan + QAC coating.
Chitosan coatings in eggs have been shown to be a method of conservation, acting as a physical and biological barrier in order to minimize quality loss during storage, enabling shelf life to be extended by preventing the loss of water and carbon dioxide through the pores of the shell. These factors are responsible for changes in internal quality. Moreover, chitosan forms a barrier to microorganisms (Liu et al. 2007). Thus, the synergistic effect improves the microbial safety, surface integrity, and internal quality of eggs.
Synergistic Effect of Chitosan and Quaternary Ammonium Complexes
The results show the synergistic effect of biopolymer/quaternary ammonium complexes with an improvement in activity in conjunction with chitosan against biofilm formation by Salmonella spp., Escherichia coli, and Staphylococcus aureus. Eggs coated with bioactive chitosan films have improved physical and microbiological barriers to minimize egg quality loss during storage, as well as to increase egg shelf life by preventing colonization by microorganisms, which are responsible for changes in egg quality.
The synergistic effect of chitosan and quaternary ammonium films offers improved physical and biological safety to guarantee the integrity and microbiological quality of commercial eggs and a decreased weight loss fraction during storage (Fig. 8). The biofilm is capable of protecting eggs from the production to commercialization stages, preventing eggs from losing moisture mass through evaporation. A biofilm could extend the shelf life of eggs in the world’s poorest and hottest regions, where they spoil faster and are vulnerable to microorganisms without adequate food storage conditions. The new technology could help to control Salmonella, a bacterium that infects about 1% of chicken eggs and can cause severe gut infections, with symptoms including fever, nausea, and vomiting. It can even cause death in some cases. Salmonella is especially harmful in lower-income countries.
This biofilm is a social technology that can be used to meet hunger needs in the world’s developing regions, which do not have proper sanitary infrastructure for the conservation and maintenance of eggs, as well as fruits, vegetables, and other food types. The biofilm may also be used to coat other food packages, providing greater mechanical resistance and protection against microorganisms. It should offer a real gain for the egg producer, making them more competitive in the market and at the same time it should prevent contamination by microorganisms that cause intestinal infections.
Conclusion
The experimental results shown in the present study indicate that the quaternary ammonium complexes of the 1st (QAC 1st) and 5th (QAC 5th) generations + chitosan showed biological activity against biofilm formation by Salmonella Enteritidis. A synergistic effect was found at the same concentrations for QAC 1st and QAC 5th, which showed a slight improvement in activity in conjunction with chitosan. Egg coating with bioactive chitosan films has been shown to provide a physical and microbiological barrier to minimize egg quality loss during storage, as well as to increase egg shelf life by preventing water loss and colonization by microorganisms, which are responsible for changes in egg quality. These composite chitosan and quaternary ammonium films improve physical and biological safety to guarantee the integrity and microbiological quality of commercial eggs. These biofilms are capable of sealing and protecting eggs from the production to commercialization stages, preventing eggs from losing moisture mass through evaporation during storage. The bioactive coating improves eggshell structure and maintains the egg composition, its nutritional qualities, and microbiological quality prior to consumption. Egg coating with bioactive chitosan films has been shown to be a suitable method of preservation by provding a physical and microbiological barrier to minimize egg quality loss during storage, as well as to increase egg shelf life by preventing of colonization by microorganisms, which are responsible for changes in egg quality.
Abbreviations
- QAC 1st:
-
1st generation of quaternary ammonium salt compounds
- QAC 5th:
-
5th generation of quaternary ammonium salt compounds
- FAPESP:
-
Fundação de Amparo à Pesquisa do Estado de São Paulo
- CEPID:
-
Centro de Pesquisa, Inovação e Difusão
- INCTMN:
-
Instituto Nacional de Ciência e Tecnologia dos Materiais em Nanotecnologia
- CNPq:
-
Conselho Nacional de Desenvolvimento Científico e Tecnológico
- MIC:
-
Minimal inhibitory concentration
- MBC:
-
Minimal bactericidal concentration
References
Al-Ajeeli, M. N., Taylor, T. M., Alvarado, C. Z., & Coufal, C. D. (2016). Comparison of eggshell surface sanitization technologies and impacts on consumer acceptability. Poultry Science, 95(5), 1191–1197. https://doi.org/10.3382/ps/pew014.
Amjadi, S., Emaminia, S., Nazari, M., Davudian, S. H., Roufegarinejad, L., & Hamishehkar, H. (2019). Application of reinforced ZnO nanoparticle-incorporated gelatin bionanocomposite film with chitosan nanofiber for packaging of chicken fillet and cheese as food models. Food and Bioprocess Technology, 12(7), 1205–1219. https://doi.org/10.1007/s11947-019-02286-y.
Bagiu, R. V., Vlaicu, B., & Butnariu, M. (2012). Chemical composition and in vitro antifungal activity screening of the Allium ursinum L. (Liliaceae). International Journal of Molecular Sciences, 13(2), 1426–1436. https://doi.org/10.3390/ijms13021426.
Barbosa, D. B., Agostinho Hunt, A. M., Berretta, A., Rodrigues de Camargo, E., Gorup, L. F., Monteiro, D. R., Fernandes, G. L., Fernandes, R. A., & Kirker, K. R. (2016). The importance of preventing and controlling biofilm in wounds: biofilm models and nanotechnology in antibiofilm approaches. Wound Healing Biomaterials. https://doi.org/10.1016/B978-1-78242-456-7.00004-0.
Beltrame, C. A., Kubiak, G. B., Lerin, L. A., Rottava, I., Mossi, A. J., de Oliveira, D., Cansian, R. L., Treichel, H., & Toniazzo, G. (2012). Influence of different sanitizers on food contaminant bacteria: effect of exposure temperature, contact time, and product concentration. Food Science and Technology, 32(2), 228–232.
Bhale, S., No, H. K., Prinyawiwatkul, W., Farr, A. J., Nadarajah, K., & Meyers, S. P. (2003). Chitosan coating improves shelf life of eggs. Journal of Food Science,. https://doi.org/10.1111/j.1365-2621.2003.tb05776.x.
Bondoc, I. (2014). EUROINVENT 2015, Silver Medal for the handbook: Control of products and food of animal origin (Controlul produselor si alimentelor de origine animala), Universitary Textbook, 1st ed. Iasi: Ion Ionescu de la Brad, Publishing.
Bondoc, I., & Şindilar, E. V. (2002). Controlul sanitar veterinar al calităţii şi salubrităţii alimentelor. Iasi: Editura “Ion Ionescu de la Brad”.
Brake, J., & Sheldon, B. W. (1990). Effect of a quaternary ammonium sanitizer for hatching eggs on their contamination, permeability, water loss, and hatchability. Poultry Science, 69(4), 517–525. https://doi.org/10.3382/ps.0690517.
Buffet-Bataillon, S., Tattevin, P., Bonnaure-Mallet, M., & Jolivet-Gougeon, A. (2012). Emergence of resistance to antibacterial agents: the role of quaternary ammonium compounds—a critical review. International Journal of Antimicrobial Agents, 39(5), 381–389. https://doi.org/10.1016/j.ijantimicag.2012.01.011.
Cai, L., Li, X., Wu, X., Lv, Y., Liu, X., & Li, J. (2014). Effect of chitosan coating enriched with ergothioneine on quality changes of Japanese sea bass (Lateolabrax japonicas). Food and Bioprocess Technology, 7(8), 2281–2290. https://doi.org/10.1007/s11947-013-1215-4.
Cianca, L. O. A., Nakasse, T. S. L., Damasceno, Y. W., Silva, T. G. Q., Gorup, L. F., Silva, V. D., Toffoli, L. M. N., & Bellini, M. Z. (2020). Caracterização físico-química de biocurativos dérmo-epidérmicos de Quitosana, Xantana e Beta-Glucana. Brazilian J. Heal. Rev., 3, 5631–5650. https://doi.org/10.34119/bjhrv3n3-132.
Clímaco, W. L. d. S., Melo, É. d. F., Vaz, D. P., Saldanha, M. M., Pinto, M. F. V. d. S., Fernandes, L. C. C., Baião, N. C., Oliveira, L. G. d., Sant Anna, F. M. d., Souza, M. R. d., & Lara, L. J. C. (2018). Eggshell microbiology and quality of hatching eggs subjected to different sanitizing procedures. Pesquisa Agropecuária Brasileira, 53, 1177–1183.
De Vylder, J., Raspoet, R., Dewulf, J., Haesebrouck, F., Ducatelle, R., & Van Immerseel, F. (2013). Salmonella Enteritidis is superior in egg white survival compared with other Salmonella serotypes. Poultry Science, 92(3), 842–845. https://doi.org/10.3382/ps.2012-02668.
Diao, X., Huan, Y., & Chitrakar, B. (2020). Extending the shelf life of ready-to-eat spiced chicken meat: garlic aqueous extracts-carboxymethyl chitosan ultrasonicated coating solution. Food and Bioprocess Technology, 13(5), 786–796. https://doi.org/10.1007/s11947-020-02428-7.
El Ghamry, A., El, A., Hewida, M., Yassein, S. A., & El Mallah, G. M. (2011). Evaluation of dietary calcium requirements in Fayoumi laying hens. Iranian Journal of Applied Animal Science, 1, 81–86.
Estrela, C., Estrela, C. R. A., Barbin, E. L., Spanó, J. C. E., Marchesan, M. A., & Pécora, J. D. (2002). Mechanism of action of sodium hypochlorite. Brazilian Dental Journal 13(2), 113–117. https://doi.org/10.1590/S0103-64402002000200007.
Fernandes, R. A., Berretta, A. A., Torres, E. C., Buszinski, A. F. M., Fernandes, G. L., Mendes-Gouvêa, C. C., De Souza-Neto, F. N., Gorup, L. F., De Camargo, E. R., & Barbosa, D. B. (2018). Antimicrobial potential and cytotoxicity of silver nanoparticles phytosynthesized by pomegranate peel extract. Antibiotics, 7(3), 7. https://doi.org/10.3390/antibiotics7030051.
Forbes, S., Cowley, N., Humphreys, G., Mistry, H., Amézquita, A., & McBain, A. J. (2017). Formulation of biocides increases antimicrobial potency and mitigates the enrichment of nonsusceptible bacteria in multispecies biofilms. Applied and Environmental Microbiology, 83(7), e03054–e03016. https://doi.org/10.1128/aem.03054-16.
Gadea, R., Fernández Fuentes, M. Á., Pérez Pulido, R., Gálvez, A., & Ortega, E. (2017). Effects of exposure to quaternary-ammonium-based biocides on antimicrobial susceptibility and tolerance to physical stresses in bacteria from organic foods. Food Microbiology, 63, 58–71. https://doi.org/10.1016/j.fm.2016.10.037.
Gast, R. K., Regmi, P., Guraya, R., Jones, D. R., Anderson, K. E., & Karcher, D. M. (2019). Contamination of eggs by Salmonella Enteritidis in experimentally infected laying hens of four commercial genetic lines in conventional cages and enriched colony housing. Poultry Science, 98(10), 5023–5027. https://doi.org/10.3382/ps/pez222.
Gerba, C. P. (2015). Quaternary ammonium biocides: efficacy in application. Applied and Environmental Microbiology, 81(2), 464–469. https://doi.org/10.1128/aem.02633-14.
Ghaderi-Ghahfarokhi, M., Barzegar, M., Sahari, M. A., & Azizi, M. H. (2016). Nanoencapsulation approach to improve antimicrobial and antioxidant activity of thyme essential oil in beef burgers during refrigerated storage. Food and Bioprocess Technology, 9(7), 1187–1201. https://doi.org/10.1007/s11947-016-1708-z.
Gibson, H., Taylor, J. H., Hall, K. E., & Holah, J. T. (1999). Effectiveness of cleaning techniques used in the food industry in terms of the removal of bacterial biofilms. Journal of Applied Microbiology, 87(1), 41–48. https://doi.org/10.1046/j.1365-2672.1999.00790.x.
González-Redondo, P. (2010). Effect of long-term storage on the hatchability of red-legged partridge (Alectoris rufa) eggs. Poultry Science, 89(2), 379–383. https://doi.org/10.3382/ps.2009-00408.
Goy, R. C., de Britto, D., & Assis, O. B. G. (2009). A review of the antimicrobial activity of chitosan. Polímeros, 19(3), 241–247.
Goy, R. C., Morais, S. T. B., & Assis, O. B. G. (2016). Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Revista Brasileira de Farmacognosia, 26(1), 122–127. https://doi.org/10.1016/j.bjp.2015.09.010.
Grochowska, E., Kinal, A., Sobek, Z., Siatkowski, I., & Bednarczyk, M. (2019). Field study on the factors affecting egg weight loss, early embryonic mortality, hatchability, and chick mortality with the use of classification tree technique. Poultry Science, 98(9), 3626–3636. https://doi.org/10.3382/ps/pez180.
Han, J., Gokulan, K., Barnette, D., Khare, S., Rooney, A., Deck, J., Nayak, R., Stefanova, R., Hart, M., & Foley, S. (2013). Evaluation of virulence and antimicrobial resistance in Salmonella enterica serovar Enteritidis isolates from humans and chicken- and egg-associated sources. Foodborne Pathogens and Disease, 10(12), 10–1015. https://doi.org/10.1089/fpd.2013.1518.
Howard, Z. R., O’Bryan, C. A., Crandall, P. G., & Ricke, S. C. (2012). Salmonella Enteritidis in shell eggs: current issues and prospects for control. Food Research International, 45(2), 755–764. https://doi.org/10.1016/j.foodres.2011.04.030.
Hunton, P. (2005). Research on eggshell structure and quality: an historical overview. Brazilian Journal of Poultry Science, 7(2), 67–71. https://doi.org/10.1590/S1516-635X2005000200001.
Kavas, N., & Kavas, G. (2016). Physical-chemical and antimicrobial properties of egg white protein powder films incorporated with orange essential oil on Kashar cheese. Food Science and Technology, 36(4), 672–678.
Ketta, M., & Tůmová, E. (2016). Eggshell structure, measurements, and quality-affecting factors in laying hens: a review. Czech Journal of Animal Science, 61, 299–309. https://doi.org/10.17221/46/2015-cjas.
Knape, K. D., Carey, J. B., Burgess, R. P., Kwon, Y. M., & Ricke, S. C. (1999). Comparison of chlorine with an iodine-based compound on eggshell surface microbial populations in a commercial egg washer. Journal of Food Safety, 19(3), 185–194. https://doi.org/10.1111/j.1745-4565.1999.tb00244.x.
Kouam, M. K., Biekop, M. H. F., Katte, B., & Teguia, A. (2018). Salmonella status of table eggs in commercial layer farms in Menoua Division, West region of Cameroon. Food Control, 85, 345–349. https://doi.org/10.1016/j.foodcont.2017.09.037.
Kouame, Y. A. E., Nideou, D., Kouakou, K., & Tona, K. (2019). Effect of guinea fowl egg storage duration on embryonic and physiological parameters, and keet juvenile growth. Poultry Science, 98(11), 6046–6052. https://doi.org/10.3382/ps/pez264.
Ku, S., & Ximenes, E. (2016). Salmonella in shell eggs: mechanisms, prevention and detection. Journal of Nutrition & Food Sciences, 06(01). https://doi.org/10.4172/2155-9600.1000455.
La Scala, N., Jr., Boleli, I. C., Ribeiro, L. T., Freitas, D., & Macari, M. (2000). Distribuição do Tamanho de Poros em Cascas de Ovos Determinada pela Porosimetria de Mercúrio. Brazilian Journal of Poultry Science, 2, 177–181. https://doi.org/10.1590/S1516-635X2000000200007.
Lamas, A., Anton, X., Miranda, J. M., Roca-Saavedra, P., Cardelle-Cobas, A., Ibarra, I. S., Franco, C. M., & Cepeda, A. (2016). Technological strategies for the development of egg-derived products with reduced content of cholesterol. Food and Bioprocess Technology, 9(1), 81–90. https://doi.org/10.1007/s11947-015-1599-4.
Li, F., Weir, M. D., & Xu, H. H. K. (2013). Effects of quaternary ammonium chain length on antibacterial bonding agents. Journal of Dental Research, 92(10), 932–938. https://doi.org/10.1177/0022034513502053.
Li, Z., Guo, R., Wang, F., Geng, S., Kang, X., Meng, C., Gu, D., Jiao, X., & Pan, Z. (2019). Inactivation of Salmonella Enteritidis on eggshells by lactic acid spray. Food Control, 104, 201–207. https://doi.org/10.1016/j.foodcont.2019.04.046.
Lichovnikova, M., & Br Poult, S. (2007). The effect of dietary calcium source, concentration and particle size on calcium retention, eggshell quality and overall calcium requirement in laying hens. Br Poult Sci, 48(1), 71–5. https://doi.org/10.1080/00071660601148203.
Liu, J., Tian, S., Meng, X., & Xu, Y. (2007). Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Biology and Technology, 44(3), 300–306. https://doi.org/10.1016/j.postharvbio.2006.12.019.
Luo, W., Xue, H., Xiong, C., Li, J., Tu, Y., & Zhao, Y. (2020). Effects of temperature on quality of preserved eggs during storage. Poultry Science, 99(6), 3144–3157. https://doi.org/10.1016/j.psj.2020.01.020.
Mah, T.-F. C., & O’Toole, G. A. (2001). Mechanisms of biofilm resistance to antimicrobial agents. Trends in Microbiology, 9(1), 34–39. https://doi.org/10.1016/s0966-842x(00)01913-2.
Malmo, C., La Storia, A., & Mauriello, G. (2013). Microencapsulation of Lactobacillus reuteri DSM 17938 cells coated in alginate beads with chitosan by spray drying to use as a probiotic cell in a chocolate soufflé. Food and Bioprocess Technology, 6(3), 795–805. https://doi.org/10.1007/s11947-011-0755-8.
Martins, J. T., Bourbon, A. I., Pinheiro, A. C., Souza, B. W. S., Cerqueira, M. A., & Vicente, A. A. (2013). Biocomposite films based on κ-carrageenan/locust bean gum blends and clays: physical and antimicrobial properties. Food and Bioprocess Technology, 6(8), 2081–2092. https://doi.org/10.1007/s11947-012-0851-4.
Mazzuco, H., & Bertechini, A. G. (2014). Critical points on egg production: causes, importance and incidence of eggshell breakage and defects. Ciência e Agrotecnologia, 38(1), 7–14.
Mead, G. C. (2004). Microbiological quality of poultry meat: a review. Brazilian Journal of Poultry Science, 6(3), 135–142. https://doi.org/10.1590/S1516-635X2004000300001.
Mendes-Gouvêa, C. C., do Amaral, J. G., Fernandes, R. A., Fernandes, G. L., Gorup, L. F., Camargo, E. R., Delbem, A. C. B., & Barbosa, D. B. (2018). Sodium trimetaphosphate and hexametaphosphate impregnated with silver nanoparticles: characteristics and antimicrobial efficacy. Biofouling, 34(3), 34–308. https://doi.org/10.1080/08927014.2018.1437146.
Messens, W., Grijspeerdt, K., & Herman, L. (2005). Eggshell penetration by Salmonella: a review. World's Poultry Science Journal, 61(1), 71–86. https://doi.org/10.1079/WPS200443.
Monteiro, D. R., Negri, M., Silva, S., Gorup, L. F., Camargo, E. R. D., Oliveira, R., Barbosa, D. B., & Henriques, M. (2014a). Adhesion of Candida biofilm cells to human epithelial cells and polystyrene after treatment with silver nanoparticles. Colloids and Surfaces B: Biointerfaces, 114, 410–412. https://doi.org/10.1016/j.colsurfb.2013.10.027.
Monteiro, D. R., Silva, S., Negri, M., Gorup, L. F., De Camargo, E. R., Oliveira, R., & Barbosa, D. B. (2012a). Silver colloidal nanoparticles: effect on matrix composition and structure of Candida albicans and Candida glabrata biofilms. Journal of applied Microbiology, 114(4), 1175–1183. https://doi.org/10.1111/jam.12102.
Monteiro, D. R., Silva, S., Negri, M., Gorup, L. F., de Camargo, E. R., Oliveira, R., Barbosa, D. B., & Henriques, M. (2013). Antifungal activity of silver nanoparticles in combination with nystatin and chlorhexidine digluconate against Candida albicans and Candida glabrata biofilms. Mycoses, 56(6), 672–680. https://doi.org/10.1111/myc.12093.
Monteiro, D. R., Silva, S., Negri, M., Gorup, L. F., De Camargo, E. R., Oliveira, R., Barbosa, D. B., & Henriques, M. (2012b). Silver nanoparticles: influence of stabilizing agent and diameter on antifungal activity against Candida albicans and Candida glabrata biofilms. Letters in Applied Microbiology, 54(5), 383–391. https://doi.org/10.1111/j.1472-765X.2012.03219.x.
Monteiro, D. R., Takamiya, A. S., Feresin, L. P., Gorup, L. F., de Camargo, E. R., Delbem, A. C. B., Henriques, M., & Barbosa, D. B. (2015). Susceptibility of Candida albicans and Candida glabrata biofilms to silver nanoparticles in intermediate and mature development phases. Journal of Prosthodontic Research, 59(1), 42–48. https://doi.org/10.1016/j.jpor.2014.07.004.
Monteiro, D. R., Takamiya, A. S., Feresin, L. P., Gorup, L. F., De Camargo, E. R., Delbem, A. C. B., Henriques, M., & Barbosa, D. B. (2014b). Silver colloidal nanoparticle stability: influence on Candida biofilms formed on denture acrylic. Medical Mycology, 52(6), 627–635. https://doi.org/10.1093/mmy/myu021.
Muñoz-Bonilla, A., & Fernández-García, M. (2015). The roadmap of antimicrobial polymeric materials in macromolecular nanotechnology. European Polymer Journal, 65, 46–62. https://doi.org/10.1016/j.eurpolymj.2015.01.030.
Nascimento, G. R. d, Murakami, A. E., Guerra, A., Ospinas-Rojas, I. C., Ferreira, M. F. Z., & Fanhani, J. C. (2014). Effect of different vitamin D sources and calcium levels in the diet of layers in the second laying cycle. Brazilian Journal of Poultry Science, 16, 37–42. https://doi.org/10.1590/1516-635x160237-42.
Nechita, P., Bobu, E., Parfene, G., Dinica, R.-M., & Balan, T. (2015). Antimicrobial coatings based on chitosan derivatives and quaternary ammonium salts for packaging paper applications. Cellulose Chemistry and Technology, 49, 625–632.
Nguyen, H. D. N., & Yuk, H.-G. (2013). Changes in resistance of Salmonella Typhimurium biofilms formed under various conditions to industrial sanitizers. Food Control, 29(1), 236–240. https://doi.org/10.1016/j.foodcont.2012.06.006.
Nys, Y., Gautron, J., Garcia-Ruiz, J. M., & Hincke, M. T. (2004). Avian eggshell mineralization: biochemical and functional characterization of matrix proteins. Comptes Rendus Palevol, 3(6–7), 549–562. https://doi.org/10.1016/j.crpv.2004.08.002.
Nys, Y., Gautron, J., McKee, M. D., Garcia-Ruiz, J. M., & Hincke, M. T. (2001). Biochemical and functional characterisation of eggshell matrix proteins in hens. World's Poultry Science Journal, 57(4), 401–413. https://doi.org/10.1079/wps20010029.
Nys, Y., Zawadzki, J., Fau-Gautron, J., Fau-Mills, Gautron J., & A. D., Mills, A. D., Poult, S. (1991). Whitening of brown-shelled eggs: mineral composition of uterine fluid and rate of protoporphyrin deposition. Poultry Science, 70(5), 1236–1245. https://doi.org/10.3382/ps.0701236
Oliveira, G. E., Figueiredo, T. C., Souza, M. R., Oliveira, A. L., Cançado, S. V., & Gloria, M. B. A. (2009). Bioactive amines and quality of egg from Dekalb hens under different storage conditions. Poultry Science, 88(11), 2428–2434. https://doi.org/10.3382/ps.2009-00028.
Oliveira, K., Oliveira, T., Teixeira, P., Azeredo, J., Henriques, M., & Oliveira, R. (2006). Comparison of the adhesion ability of different Salmonella Enteritidis serotypes to materials used in kitchens. Journal of Food Protection, 69(10), 2352–2356. https://doi.org/10.4315/0362-028x-69.10.2352.
Orgaz, B., Lobete, M. M., Puga, C. H., & San Jose, C. (2011). Effectiveness of chitosan against mature biofilms formed by food related bacteria. International Journal of Molecular Sciences, 12(1), 817–828. https://doi.org/10.3390/ijms12010817.
Pagliarulo, C., Sansone, F., Moccia, S., Russo, G. L., Aquino, R. P., Salvatore, P., Di Stasio, M., & Volpe, M. G. (2016). Preservation of strawberries with an antifungal edible coating using peony extracts in chitosan. Food and Bioprocess Technology, 9(11), 1951–1960. https://doi.org/10.1007/s11947-016-1779-x.
Palermo, E. F., Lee, D.-K., Ramamoorthy, A., & Kuroda, K. (2011). Role of cationic group structure in membrane binding and disruption by amphiphilic copolymers. The Journal of Physical Chemistry. B, 115(2), 366–375. https://doi.org/10.1021/jp1083357.
Panda, B., & Singh, R. P. (1990). Developments in processing quail meat and eggs. World's Poultry Science Journal, 46(3), 219–234. https://doi.org/10.1079/wps19900022.
Pande, V. V., McWhorter, A. R., & Chousalkar, K. K. (2016). Salmonella enterica isolates from layer farm environments are able to form biofilm on eggshell surfaces. Biofouling, 32(7), 699–710. https://doi.org/10.1080/08927014.2016.1191068.
Panghal, M., Kaushal, V., & Yadav, J. P. (2011). In vitro antimicrobial activity of ten medicinal plants against clinical isolates of oral cancer cases. Annals of Clinical Microbiology and Antimicrobials, 10(1), 21. https://doi.org/10.1186/1476-0711-10-21.
Pereira, A. S., dos Santos, T. T., & Coelho, A. F. S. (2014). Quality of eggs sold in different commercial establishments and the study of the conditions of storage. Food Science and Technology, 34(1), 82–87.
Pires, P. G. S., Leuven, A. F. R., Franceschi, C. H., Machado, G. S., Pires, P. D. S., Moraes, P. O., Kindlein, L., & Andretta, I. (2020). Effects of rice protein coating enriched with essential oils on internal quality and shelf life of eggs during room temperature storage. Poultry Science, 99(1), 604–611. https://doi.org/10.3382/ps/pez546.
Poverenov, E., Danino, S., Horev, B., Granit, R., Vinokur, Y., & Rodov, V. (2014). Layer-by-layer electrostatic deposition of edible coating on fresh-cut melon model: anticipated and unexpected effects of alginate-chitosan combination. Food and Bioprocess Technology, 7(5), 1424–1432. https://doi.org/10.1007/s11947-013-1134-4.
Rabea, E. I., Badawy, M. E. T., Stevens, C. V., Smagghe, G., & Steurbaut, W. (2003). Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules, 4(6), 1457–1465. https://doi.org/10.1021/bm034130m.
Reijrink, I. A. M., Meijerhof, R., Kemp, B., Graat, E. A. M., & van den Brand, H. (2009). Influence of prestorage incubation on embryonic development, hatchability, and chick quality. Poultry Science, 88(12), 2649–2660. https://doi.org/10.3382/ps.2008-00523.
Roberts, J. R. (2004). Factors affecting egg internal quality and egg shell quality in laying hens. The Journal of Poultry Science, 41(3), 161–177. https://doi.org/10.2141/jpsa.41.161.
Romao, J. M., Moraes, T. G. V., Teixeira, R. S. C., Cardoso, W. M., & Buxade, C. C. (2008). Effect of egg storage length on hatchability and weight loss in incubation of egg and meat type Japanese quails. Brazilian Journal of Poultry Science, 10(3), 143–147. https://doi.org/10.1590/S1516-635X2008000300001.
Saleh, G., El Darra, N., Kharroubi, S., & Farran, M. T. (2020). Influence of storage conditions on quality and safety of eggs collected from Lebanese farms. Food Control, 111, 107058. https://doi.org/10.1016/j.foodcont.2019.107058.
Samli, H. E., Agma, A., & Senkoylu, N. (2005). Effects of storage time and temperature on egg quality in old laying hens. Journal of Applied Poultry Research, 14(3), 548–553. https://doi.org/10.1093/japr/14.3.548.
Samsalee, N., & Sothornvit, R. (2020). Characterization of food application and quality of porcine plasma protein-based films incorporated with chitosan or encapsulated turmeric oil. Food and Bioprocess Technology, 13(3), 488–500. https://doi.org/10.1007/s11947-020-02411-2.
Saxena, A., Saxena, T. M., Raju, P. S., & Bawa, A. S. (2013). Effect of controlled atmosphere storage and chitosan coating on quality of fresh-cut jackfruit bulbs. Food and Bioprocess Technology, 6(8), 2182–2189. https://doi.org/10.1007/s11947-011-0761-x.
Schmidt, G. S., Figueiredo, E. A. P., Saatkamp, M. G., & Bomm, E. R. (2009). Effect of storage period and egg weight on embryo development and incubation results. Brazilian Journal of Poultry Science, 11(1), 1–5.
EFSA Panel on Biological Hazards (BIOHAZ). (2014). Scientific Opinion on the public health risks of table eggs due to deterioration and development of pathogens. EFSA J, 12(7), 3782. https://doi.org/10.2903/j.efsa.2014.3782.
Shiroodi, S. G., Nesaei, S., Ovissipour, M., Al-Qadiri, H. M., Rasco, B., & Sablani, S. (2016). Biodegradable polymeric films incorporated with nisin: characterization and efficiency against Listeria monocytogenes. Food and Bioprocess Technology, 9(6), 958–969. https://doi.org/10.1007/s11947-016-1684-3.
Soumet, C., Méheust, D., Pissavin, C., Le Grandois, P., Frémaux, B., Feurer, C., Le Roux, A., Denis, M., & Maris, P. (2016). Reduced susceptibilities to biocides and resistance to antibiotics in food-associated bacteria following exposure to quaternary ammonium compounds. Journal of Applied Microbiology, 121(5), 1275–1281. https://doi.org/10.1111/jam.13247.
Souza, J. A. S., Barbosa, D. B., Berretta, A. A., Do Amaral, J. G., Gorup, L. F., De Souza Neto, F. N., Fernandes, R. A., Fernandes, G. L., Camargo, E. R., Agostinho, A. M., & Delbem, A. C. B. (2018). Green synthesis of silver nanoparticles combined to calcium glycerophosphate: antimicrobial and antibiofilm activities. Future Microbiol, 13(3), 13–357. https://doi.org/10.2217/fmb-2017-0173.
Stadelman, W. J. (1986). Preservation of quality in shell eggs. In W. J. Stadelman & O. Cotterill (Eds.), Egg Sci. Technol.
Steenackers, H., Hermans, K., Vanderleyden, J., & De Keersmaecker, S. C. J. (2012). Salmonella biofilms: an overview on occurrence, structure, regulation and eradication. Food Research International, 45(2), 502–531. https://doi.org/10.1016/j.foodres.2011.01.038.
Taha, A. E., El-Tahawy, A. S., Abd El-Hack, M. E., Swelum, A. A., & Saadeldin, I. M. (2019). Impacts of various storage periods on egg quality, hatchability, post-hatching performance, and economic benefit analysis of two breeds of quail. Poultry Science, 98(2), 777–784. https://doi.org/10.3382/ps/pey468.
Takamiya, A. S., Monteiro, D. R., Bernabé, D. G., Gorup, L. F., Camargo, E. R., Gomes-Filho, J. E., Oliveira, S. H. P., & Barbosa, D. B. (2016). In vitro and in vivo toxicity evaluation of colloidal silver nanoparticles used in endodontic treatments. Journal of Endodontia, 42(6), 953–960. https://doi.org/10.1016/j.joen.2016.03.014.
Trevisan, D. A. C., da Silva, A. F., Negri, M., de Abreu Filho, B. A., Machinski Junior, M., Patussi, E. V., et al. (2018). Antibacterial and antibiofilm activity of carvacrol against Salmonella enterica serotype Typhimurium. Brazilian Journal of Pharmaceutical Sciences, 54(1), e17229. https://doi.org/10.3390/ijerph120302543.
Uchida, N. S., Grespan, R., Piovezan, M., Ferreira, E. C., Júnior, M. M., Cuman, R. K. N., & Mikcha, J. M. G. (2015). Effect of carvacrol on Salmonella Saintpaul biofilms on stainless steel surface. Tropical Journal of Pharmaceutical Research, 13(12), 2021. https://doi.org/10.4314/tjpr.v13i12.11.
Uyanga, V. A., Onagbesan, O. M., Oke, O. E., Abiona, J. A., & Egbeyale, L. T. (2020). Influence of age of broiler breeders and storage duration on egg quality and blastoderm of Marshall broiler breeders. Journal of Applied Poultry Research, 29(3), 535–544. https://doi.org/10.1016/j.japr.2020.03.001.
Vaz, F., Machado, P., Rebouta, L., Mendes, J. A., Lanceros-Méndez, S., Cunha, L., Nascimento, S. M. C., Goudeau, P., Rivière, J. P., Alves, E., & Sidor, A. (2002). Physical and morphological characterization of reactively magnetron sputtered TiN films. Thin Solid Films, 420-421, 421–428. https://doi.org/10.1016/S0040-6090(02)00812-X.
Vlčková, J., Tůmová, E., Míková, K., Englmaierová, M., Okrouhlá, M., & Chodová, D. (2019). Changes in the quality of eggs during storage depending on the housing system and the age of hens. Poultry Science, 98(11), 6187–6193. https://doi.org/10.3382/ps/pez401.
Vu, K. D., Hollingsworth, R. G., Leroux, E., Salmieri, S., & Lacroix, M. (2011). Development of edible bioactive coating based on modified chitosan for increasing the shelf life of strawberries. Food Research International, 44(1), 198–203. https://doi.org/10.1016/j.foodres.2010.10.037.
Walayat, N., Xiong, Z., Xiong, H., Moreno, H. M., Li, Q., Nawaz, A., Zhang, Z., Wang, P., & Niaz, N. (2020). The effectiveness of egg white protein and β-cyclodextrin during frozen storage: functional, rheological and structural changes in the myofibrillar proteins of Culter alburnus. Food Hydrocolloids, 105, 105842. https://doi.org/10.1016/j.foodhyd.2020.105842.
Wang, X., Ford, B. C., Praul, C. A., & Leach, R. M. (2002). Collagen X expression in oviduct tissue during the different stages of the egg laying cycle. Poultry Science, 81(6), 805–808. https://doi.org/10.1093/ps/81.6.805.
Wang, Y., Xia, Y., Zhang, P., Ye, L., Wu, L., & He, S. (2017). Physical characterization and pork packaging application of chitosan films incorporated with combined essential oils of cinnamon and ginger. Food and Bioprocess Technology, 10(3), 503–511. https://doi.org/10.1007/s11947-016-1833-8.
Webber, B., de Oliveira, A. P., Pottker, E. S., Daroit, L., Levandowski, R., dos Santos, L. R., Nascimento, V. P. d., & Rodrigues, L. B. (2019). Salmonella Enteritidis forms biofilm under low temperatures on different food industry surfaces. Ciência Rural, 49(7). https://doi.org/10.1590/0103-8478cr20181022.
Weder, J. K. P., Belitz, H.-D., (2003a). Protein interactions and reactions involved in food processing, in: Caballero, B.B.T.-E. of F.S. and N. (Second E. (Ed.),. Academic Press, Oxford, pp. 4841–4847. https://doi.org/10.1016/B0-12-227055-X/00983-4.
Weder, J.K.P., Belitz, H.-D., (2003b). Protein food sources, in: Caballero, B.B.T.-E. of F.S. and N. (Second E. (Ed.),. Academic Press, Oxford, pp. 4818–4824. https://doi.org/10.1016/B0-12-227055-X/00979-2.
Whiley, H., & Ross, K. (2015). Salmonella and eggs: from production to plate. International Journal of Environmental Research and Public Health, 12(3), 2543–2556. https://doi.org/10.3390/ijerph120302543.
Yang, Y., Geveke, D. J., & Niemira, B. A. (2020). Quality of radio frequency pasteurized shell eggs during extended storage under normal and moderate abuse conditions. Food Control, 116, 107330. https://doi.org/10.1016/j.foodcont.2020.107330.
Web References
Pfuntner A (2011) Saneantes e desinfetantes: os produtos químicos da prevenção. Food Safety Magazine,11:111-222; https://www.foodsafetymagazine.com/magazine-archive1/junejuly-2017/clean-up-that-food-an-update-on-sanitizers-and-disinfectants/. Accessed 26 June 2020.
WHO. 2015. News release, GENEVA, https://www.who.int/news-room/detail/03-12-2015-who-s-first-ever-global-estimates-of-foodborne-diseases-find-children-under-5-account-for-almost-one-third-of-deaths. Accessed 20 Sept 2020.
WHO. (2018). Salmonella (non-typhoidal). https://www.who.int/news-room/fact-sheets/detail/salmonella-(non-typhoidal). Access in June 26 of 2020.
WHO. (2019). 23 million people falling ill from unsafe food each year; https://www.euro.who.int/en/media-centre/sections/press-releases/2019/23-million-people-falling-ill-from-unsafe-food-each-year-in-europe-is-just-the-tip-of-the-iceberg#:~:text=Every%20minute%2C%2044%20people%20%E2%80%93%20more,per%20 year%20lose%20their%20lives. Access in semtember 20 of 2020.
Acknowledgments
The authors give special thanks to PolyOrganic Technologic Ltda, São Paulo, SP, Polymar Indústria e Comércio Ltda, Fortaleza, CE, Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT), and Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES). Special thanks to Márcio Roberto da Silva Oliveira for their contributions in contact angle analyses.
Funding
This work was supported by FAPESP (grant numbers 2012/07067-0,2013/23572-0; 2016/019405; and 2013/07296); CEPID (2013/07296-2); INCTMN (2008/57872-1); and CNPq (573636/2008-7, 435975/2018-8, and 421648/2018-0).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Synopsis
Towards a green biopackaging material for eggs, natural polymeric antimicrobial coatings were developed and investigated to improve sanitary quality and extend shelf life.
Rights and permissions
About this article
Cite this article
Almeida e Silva, T., Gorup, L.F., de Araújo, R.P. et al. Synergy of Biodegradable Polymer Coatings with Quaternary Ammonium Salts Mediating Barrier Function Against Bacterial Contamination and Dehydration of Eggs. Food Bioprocess Technol 13, 2065–2081 (2020). https://doi.org/10.1007/s11947-020-02545-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-020-02545-3