Abstract
In order to reach a high volume reduction ratio (VRR) prior to drying of skim milk, a membrane cascade comprising of an ultrafiltration (UF) coupled with a nanofiltration (NF) can be applied. The present study investigated the impact of processing (filtration temperature, transmembrane pressure (TMP)) and product (feed pH) parameters on the NF of skim milk UF permeate. It could be shown that a low filtration temperature of 10 °C is more advantageous in terms of flux stability and rejection of the solute fraction as compared to higher filtration temperatures up to 45 °C. The solution pH did not affect permeate flux and lactose retention. However, in order to avoid calcium losses, it is more favorable to conduct the concentration at a pH of 6.8 instead of at a lower pH of 5. The application of a higher TMP (up to 4 MPa) enhances permeate flux and VRR as well as solute rejection during concentration of UF permeate. It was also shown that the retention of solutes decreases towards the end of the concentration process. As a consequence, the achievement of high final VRR must be weighed against increased product losses at the end.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
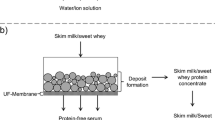
Every year, large quantities of skim milk are processed into powder. Since drying is very energy consuming, there is great interest in increasing the dry matter prior to drying by means of less energy-consuming processes like evaporation. The application of non-thermal processes like reverse osmosis (RO) as an additional pre-concentration step is even more energy efficient as compared to evaporation. However, the maximum volume reduction ratio (VRR) that can be reached by RO is limited due to deposition formation by the protein fraction and the rising osmotic pressure. Our experimental approach to reach higher VRR during pre-concentration is a membrane cascade as follows: in a first step, the protein fraction is enriched via ultrafiltration (UF) without limitation by the osmotic pressure. The UF permeate, hereinafter referred to as protein-free serum, containing the low molecular weight substances like lactose and milk salts, can be enriched in a second step via RO. The RO can then fulfill its real purpose, the enrichment of the solute fraction by overcoming its high impact on osmotic pressure, without being hindered by the deposit formation of the protein fraction. By mixing of both retentates, a whole skim milk concentrate can be obtained that can be further processed. A possible alternative to RO is the application of nanofiltration (NF). NF-membranes have a looser structure allowing the permeation of monovalent ions. Hence, the process is less limited by the osmotic pressure. Compared to RO, this may result in higher VRR at the same transmembrane pressure (TMP), or a lower TMP can be sufficient to reach a similar VRR. In order to obtain a highly enriched skim milk concentrate after mixing of the UF and the NF retentate, both process stages need to be optimized to achieve high VRR in each step.
To characterize the NF process, it is necessary to know the impact of the process parameters on the flux, the VRR as well as the retention of the solutes. Few studies already deal with the concentration of protein-free serum via NF (Suárez et al. 2009; Cuartas-Uribe et al. 2009; Atra et al. 2005). They investigated the impact of different TMP ranging from 1 to 2 MPa on flux and VRR. However, the influence of a TMP above 2 MPa was not examined, and other process parameters like pH and temperature were only investigated in non-concentrating mode (Rice et al. 2009a). Hence, a systematic investigation how relevant process parameters like pH, temperature, and TMP affect the maximum VRR and the flux during concentration, is still missing.
Besides flux and maximum VRR, rejection characteristics of the membrane also need to be taken into account. Usual NF-membranes have a molecular weight cut-off of 150 to 300 Da. This usually assures a sufficient rejection of the valuable compounds like lactose and divalent ions like calcium. Another benefit of NF-membranes is the depletion of unwanted monovalent ions like sodium and chloride. For uncharged molecules (like lactose), the rejection is mainly caused by sieving or size exclusion. The rejection of charged molecules (like ions) is caused by both sieving and electrostatic interactions (Tsuru et al. 2000; Childress and Elimelech 2000; Garba et al. 1999). The rejection can also be influenced by processing parameters, e.g., the pH value. Membrane charge has an impact on the electrostatic repulsion between charged molecules and the membrane. Furthermore, the internal structure of the membrane is affected by the dissociation of functional groups on the membrane that influences size exclusion effects (Childress and Elimelech 2000). Several authors investigated the retention behavior of NF-membranes for binary solutions of NaCl (Hussain et al. 2008; Xu and Lebrun 1999) or KCl (Rice et al. 2009b, 2011b; Suárez et al. 2009; Nilsson et al. 2008). They reported that the permeation was highest at the isoelectric point of the membrane (mIEP). In a ternary model solution, containing CaCl2 as well as KCl, no permeation maximum was observed in the pH range under consideration, which was explained by a shift in the mIEP to higher pH due to adsorption of calcium on the membrane (Rice et al. 2009b, 2011b). Protein-free serum, which is a more complex mixture, was not investigated. Regarding the impact of temperature, the rejection of neutral solutes decreases with increasing temperature due to increased diffusion through the membrane following an Arrhenius relationship (Nilsson et al. 2008; Ben Amar et al. 2007). Referring to the retention of ions, contradictory results can be found. Snow et al. (1996) measured that the NaCl rejection decreases with increasing temperature, whereas Mänttäri et al. (2002) reported that the retention of salts is temperature-independent. Because of the close coupling of the solution compounds and the process parameters, the interaction between them also must be considered. Milk salts, like, e.g., calcium phosphate, can dissociate or form complexes as a function of pH and temperature which may have an impact on the retention (Rice et al. 2010). Regarding the influence of the TMP, it is often reported that a higher retention can be observed at high pressures and hence high flux values due to a dilution effect (van der Horst et al. 1995; Nyström et al. 1995; Cuartas 2004; Suárez et al. 2006).
Most of the studies mentioned above investigated the rejection of solutes in model solutions (e.g., Nilsson et al. 2008; Mänttäri et al. 2002; Nyström et al. 1995; Rice et al. 2011b; van der Horst et al. 1995). As protein-free serum is a complex mixture of different ions and neutral solutes that may influence the rejection of each other (Bargeman et al. 2005; Bouchoux et al. 2005) and because of the fact that the findings are partly contradictory, those results are not sufficient to predict the behavior of protein-free serum which will also be used in industrial-scale dry milk production. Protein-free serum was only used by few authors to investigate the impact of the VRR on rejection of lactose at different temperatures (Atra et al. 2005) or on the rejection of salts (Suárez et al. 2009, 2006). The influence of TMP on solute rejection was only investigated in non-concentrating mode (Suárez et al. 2006; Cuartas-Uribe et al. 2009). To our knowledge, no data exist dealing with the impact of pH and TMP on the retention of solutes during concentration of protein-free serum.
To evaluate and optimize the NF stage of the membrane cascade, the filtration as well as the retention behavior must be known. Therefore, the purpose of this study was to investigate the impact of different processing parameters in the range of settings applicable in large-scale plants in order to achieve high flux levels and VRR considerably above normal current operations during the concentration of protein-free serum. Besides, the impact of these parameters on the rejection of neutral (e.g., lactose) and charged solutes (e.g., mono- and divalent ions) was determined. First, the reproducibility of the experiments was checked at reference conditions (10 °C, pH 6.8, and TMP = 2 MPa). Then, the impact of temperature (10–45 °C), pH (5.0–6.8), and TMP (1–4 MPa) on flux, VRR and retention of solutes was investigated. This was done not only to describe the process behavior and possible interactions but also to assess the optimal processing parameters.
Materials and Methods
Experimental Set-up
A piston diaphragm pump (Hydracell G10-X, Verder Deutschland GmbH, Haan, Germany) was used to pump the filtration fluid into the filtration module. The pressure was adjusted by a throttle valve at the module outlet. The mean transmembrane pressure in MPa was calculated according to Eq. (1):
where p 1 is the pressure at the module inlet and p 2 is the pressure at the module outlet in MPa. p 1 and p 2 were measured by pressure transducers (Schneider Electric GmbH, Ratingen, Germany). The effective pressure difference Δp eff in MPa can be calculated according to Eq. (2):
where Δπ is the transmembrane osmotic pressure difference in MPa, hence, the difference between the osmotic pressure in the retentate and permeate. For a dilute solution, the osmotic pressure π can be calculated according to:
where c is the concentration of the solute in g m−3, R the universal gas constant in J mol−1 K−1, T the absolute temperature in K, and MW the molecular weight in g mol−1. The retentate was completely recycled to the feed tank and the permeate was collected in a separate container. Magnetic-inductive flowmeters were used to measure the permeate (Endress + Hauser Messtechnik GmbH & Co. KG, München, Germany) and retentate flow rates (Danfoss GmbH, Offenbach am Main, Germany). A tubular heat exchanger kept the fluid at a constant filtration temperature.
Membrane and Cleaning Procedure
The spiral wound NF-membrane module (DK2540F1072/30, GE Power & Water, Fairfield, USA) had a membrane area of 2.6 m2. The approximate cut-off of the thin-film membrane was 150–300 Da. The membrane was cleaned after each experiment according to the following procedure: before cleaning, the system was flushed with demineralized water. The first cleaning step was carried out using a combination of 0.3% enzymatic (Ultrasil 67, Ecolab, Monheim am Rhein, Germany) and 0.2% alkaline (Ultrasil 69, Ecolab, Monheim am Rhein, Germany) cleaning agents for 30 min at 45 °C in a closed circuit. This was followed by a flushing with demineralized water and a subsequent acid cleaning with Ultrasil 75 (Ecolab, Monheim am Rhein, Germany) at 0.2% and 45 °C for 30 min. At the end of the acid cleaning, the system was rinsed with demineralized water until a pH of 7 was reached.
Test Fluid
The protein-free serum was produced from pasteurized skim milk using a spiral wound module (GR81PE-6338/30, Alfa Laval, Glinde, Germany) with a nominal cut-off of 10 kDa. The skim milk was purchased from a local dairy and the filtration was carried out at a temperature of 50 °C, a TMP of 200 kPa, and a feed flow rate of 15,000 L h−1. The average composition of the protein-free serum was: dry matter: 6.4 (±0.46) %; lactose: 53.9 (±3.7) g L−1; calcium: 347 (±28.7) mg L−1; sodium: 465 (±27.5) mg L−1. The protein-free serum was stored maximum 4 days at 4 °C before use.
Experimental Procedure
Before each experiment, a conditioning step was carried out using a 0.1% Ultrasil 69 solution (Ecolab, Monheim am Rhein, Germany) for 20 min at a temperature of 45 °C, followed by rinsing with demineralized water. Afterwards, the pure water flux was measured. Before filling the protein-free serum (V = 100 L) into the feed tank, it was heated to the filtration temperature and the pH was adjusted. To check the reproducibility of the experiments, a TMP of 2 MPa, a temperature of 10 °C, and pH 6.8 were set as reference conditions and the experiment was performed in fourfold determination. Based on those reference settings, the impact of the filtration temperature was investigated by varying it between 10 and 45 °C. To determine the influence of the pH, it was varied from 5.0 to 6.8. The effect of the TMP was investigated by performing filtration experiments at different TMPs from 1 to 4 MPa. All experiments were conducted at a feed flow rate of 1100 L h−1. The filtration was stopped when the flux dropped below 2 L m−2 h−1.
Retentate and Permeate Analysis
The dry matter of the samples was determined by weighing before and after microwave drying using a SMART Turbo (CEM GmbH, Kamp-Lintfort, Germany). The lactose content was quantified via HPLC according to a method described by (Schmitz-Schug 2014). The content of sodium and calcium was examined by means of a flame photometer (ELEX 6361, Eppendorf AG, Hamburg, Germany). The measurement was conducted at a wave length of 589 nm for sodium and 622 nm for calcium. An acetylene-air mixture was used as burnable gas. The cations could be quantified after performing a one-point calibration with a standard containing sodium and calcium, each at a concentration of 150 mg L−1.
Calculations
The rejection coefficient R of lactose and the cations (in %) was calculated according to Eq. (4):
where c Per and c Ret are the concentrations of the components in the permeate and retentate in g L−1, respectively.
The permeate flux J in L m−2 h−1 can be calculated according to Darcy’s law (5):
where η is the dynamic permeate viscosity in Pa s, R T the total and R M and R F the membrane and fouling resistances in m−1, respectively.
Statistical Analysis
Statistical analyses and creation of the artwork were carried out using R (R Core Team (2014); R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/). To support the findings with respect to the underlying physical and chemical relationships, the logarithm of the response was used. This results in a linear statistical model where the influence of the different explanatory variables is multiplicative on the response. If not stated otherwise, we always started with the statistical model in the sense of linear regression expressed in Eq. (6).
Depending on the problem under consideration, y stands for flux and rejection, whereas the independent variables x 1 and x 2 stand for either dry matter or flux and temperature, either dry matter or flux and transmembrane pressure, either dry matter or flux and pH, respectively. To illustrate the approach, taking for example x 1 as temperature the factor \( {e}^{\frac{\beta_3}{x_1}} \) expresses an Arrhenius-like relationship which makes not only the interpretation in the sense of the best fit, but also with regard to the physical and chemical effects feasible. The respective \( {\beta}_i \) are the slopes and \( {\beta}_o \) the intercept of the linear statistical model resulting in a multiplicative constant in the chosen model. The relative quality of the statistical models was measured using the R standard implementation of the Akaike information criterion (AIC):
with the residual sum of squares RSS, n the number of cases and p the number of parameters into account. The simplest model is the one with the smallest AIC (Akaike 1974, Venables and Ripley 2002). If interactions were significant, the statistical models without interactions were also checked whether a simpler model could explain the data satisfactorily. When appropriate to support the discussion of the underlying effects, the 95% prediction interval was chosen to illustrate the range where the result of a single independent experiment could be found in 95% of all independent runs. Whenever illustrative, the coefficients β i are given with the standard error. P values for the coefficients β i refer to the null hypothesis β i = 0, so in case of significance, β i ≠ 0. Note, the P values stand for the probability to obtain a β i far more greater than the observed one under the assumption that the respective β i is zero. So the smaller the P value, the more unrealistic is the assumption β i = 0 and thus β i ≠ 0 is more reasonable.
Results and Discussion
Reproducibility
In order to check the reproducibility, the experiment at reference conditions (TMP = 2 MPa, 10 °C, pH 6.8) was carried out in fourfold determination. As β2 was found to be 0.01 at a P value of 0.95, the run number has no significant influence on the experiment. Furthermore, it can be seen from Fig. 1 that all values lie within the 95% prediction interval and the experiment shows a good reproducibility. The statistical model log(response) = β 0 + β 1drymatter + β 2run was also used to check whether there is an influence of the run number on the rejection of sodium, calcium and lactose. β2 was found to be 0.014 (P = 0.39), −0.0009 (P = 0.41), and −9.8e-05 (P = 0.68), respectively. Hence, it is obvious for all cases that there is no influence of the run number on the experiment.
NF-Flux and Rejection of Solutes for Various Process Temperatures
The results of the filtrations performed with protein-free serum at different temperatures and a TMP of 2 MPa and pH 6.8 are depicted as a function of dry matter in Fig. 2. It can be seen that the initial flux rises with increasing temperature. This can be attributed to a lower permeate viscosity with increasing temperature. According to Darcy’s law (see Eq. (5)) a lower permeate viscosity results in higher flux values. Even though the experiments of the present study were performed in concentration mode, regarding the initial flux values, they are in good accordance to the results of Rice et al. (2009a) obtained in non-concentrating mode. The authors also found a rising initial flux with increasing temperature during NF of protein-free serum and explained it by viscosity effects (Rice et al. 2009a). The similar findings can be attributed to the fact that the operation mode hardly influences the beginning, but especially the following course of the filtration. During concentration, the flux decreases with increasing dry matter. One reason for the flux reduction is the increasing osmotic pressure with rising VRR that reduces the effective pressure difference. However, note that the dependency is of exponential type \( J={e}^{\beta_0}{e}^{\beta_1\mathrm{drymatter}}{e}^{\beta_2\mathrm{temperature}} \) for the temperatures of 20, 30, and 45 °C whereas the data for 10 °C could be best described using a linear dependency. This is a hint to different underlying mechanisms. Rice et al. (2009a) investigated the impact of temperature on flux decline over time at constant feed concentration, i.e., in non-concentrating mode. They found a steep flux decline within the first 40 min of NF of protein-free serum at a temperature of 50 °C, whereas they observed only a small flux decline over time at an operating temperature of 10 °C. A possible explanation for this behavior is the deposition of calcium phosphate crystals on the membrane at 50 °C due to its inverse solubility. This deposition can cause severe scaling on the membrane and lower the flux. Hence, the strong flux decline observed in the present study during the concentration of protein-free serum at elevated temperatures can probably also be attributed to precipitating calcium phosphate. In the present study, the scaling might even be more pronounced due to the increasing concentration of calcium phosphate during filtration that further supports the crystallization. At the lower temperature (10 °C), the flux drop with increasing dry matter is less remarkable due to less scaling.
Besides flux, the rejection of the solutes by the membrane is also an important evaluation criterion. In Fig. 3, the rejection of sodium, as an example for a monovalent positively charged ion, is plotted against the dry matter in the retentate for filtrations at a TMP of 2 MPa and pH 6.8 at various operational temperatures. The rejection values for 10, 20, and 30 °C can be best described by a temperature-independent statistical model. The rejection of sodium at a process temperature of 45 °C cannot be fitted by the same temperature-independent statistical model indicating that other mechanisms are involved in the rejection at this temperature. However, the values for 45 °C lie within the prediction interval of the temperature-independent statistical model. Hence, it can be assumed that the temperature is finally not relevant. Therefore, the findings of Snow et al. (1996), who observed a clear impact of temperature on the rejection of sodium during the NF of a model solution, were not found for the rejection behavior when concentrating protein-free serum. However, the results of the present study correspond well with the findings of Mänttäri et al. (2002) who reported a temperature-independent retention of salts during NF of a model solution.
Rejection of sodium as a function of the dry matter in the retentate for various process temperatures (* 10 °C, □ 20 °C, ○ 30 °C, and △ 45 °C) at a transmembrane pressure of 2 MPa and pH 6.8. The bold dashed line shows the temperature independent statistical model for 10, 20, and 30 °C and the thin dashed lines represent the corresponding prediction interval. The bold straight line indicates the statistical model for 45 °C
It can be seen from Fig. 3 that low rejection values were detected at a process temperature of 45 °C and dry matters of more than 10% as well as for lower temperatures (20 and 30 °C) and dry matters of more than 18%. A possible explanation for the low rejection is the low flux level at these dry matters. It was also reported by other research groups that the retention of sodium increases with increasing flux. They investigated the impact of TMP on rejection and explained the increasing retention by the increasing permeate flux that induces dilution effects in the permeate (Nyström et al. 1995; Cuartas 2004; Suárez et al. 2006). Hence, low flux values during concentration may induce low rejection values. According to the model of the microporous NF-membrane, the mass transport of ions through the membrane takes place convectively through the pores and diffusively through the membrane. At low flux values the convective part of the mass transfer is comparably low, whereas the diffusive part is unaffected and becomes more important. Thus, the ion transport is almost exclusively diffusive. The ions desorb at the permeate side and are solved in a small amount of permeate. This results in a comparably high permeate concentration and, hence, in a low rejection at low flux values. Therefore, the low flux values during the filtration at 45 °C and at the end of the filtrations at lower temperatures can be a possible explanation for the low rejection values. To confirm this assumption the rejection of sodium is plotted against the flux (see Fig. 4). It can be seen that the rejection decreases with decreasing flux. Again, the values for 45 °C lie within the prediction interval of the temperature-independent model for lower temperatures (10 to 30 °C) indicating that the impact of the temperature is not relevant. Hence, it can be assumed that the variation of sodium rejection can be explained by the variation of the flux instead of the variation of the temperature.
Rejection of sodium versus the NF-permeate flux for various process temperatures (* 10 °C, □ 20 °C, ○ 30 °C, △ 45 °C) at a transmembrane pressure of 2 MPa and pH 6.8. The dashed lines show the calculated values (bold) and prediction interval (thin) for the temperature independent statistical model for the data for 10, 20, and 30 °C. The bold straight line indicates the statistical model for 45 °C
Since calcium is a valuable compound in protein-free serum, its rejection at different operational temperatures was also investigated and found to be between 99 and 97% (data not shown). The rejection of calcium is independent of temperature and rises with increasing dry matter and decreasing flux as already shown for sodium. Compared to monovalent ions the rejection is much higher. This contradicts the Donnan exclusion mechanism after which the permeation of calcium should be higher compared to sodium. The negatively charged membrane repels co-ions and attracts counter-ions. Pursuant to the Donnan theory the greater attraction for divalent counter-ions should lead to a lower rejection of calcium than sodium. This effect is enhanced by a preferential rejection of the monovalent sodium compared to the divalent calcium together with the co-ions to maintain electroneutrality. However, preferential monovalent ion permeation has been reported earlier (Rice et al. 2011b; Garcia-Aleman and Dickson 2004; Schaep et al. 1999; Román et al. 2009; Suárez et al. 2009). One suggested explanation for the higher retention of magnesium compared to sodium observed by Garcia-Aleman and Dickson (2004) is a shift in the mIEP due to the adsorption of the divalent ion on the membrane. This may lead to charge inversion. A positive charge of the membrane causes a stronger repulsion of divalent and a preferential permeation of monovalent ions (Garcia-Aleman and Dickson 2004). Rice et al. (2011a) already showed that a shift in mIEP may arise when filtering salt solutions. However, in the present study it is rather unlikely that such a charge inversion occurs as shown later for the results of the pH-dependent salt retention (see section 3.3). Another proposed explanation for the preferential monovalent ion permeation is the difference in hydrated ion size between magnesium and sodium (Garcia-Aleman and Dickson 2004). As calcium is approximately 1.24 times larger than sodium in terms of hydrated ion size (Marcus 1994), steric effects might also be the reason for the higher divalent ion’s rejection in the present study. It can be noted that electrostatic as well as steric effects need to be taken into account when evaluating ion rejection of NF-membranes.
Figure 5 depicts the rejection of lactose, as a neutral solute, during the filtration at a TMP of 2 MPa and pH 6.8 as a function of the dry matter for various operational temperatures. It is obvious that the rejection decreases as dry matter and temperature rises. One possible reason for the decreasing rejection with increasing dry matter is the decreasing flux as already explained above. The increasing dry matter may also have an impact on the rising permeation since a decrease in rejection with increasing concentration was already reported earlier and is typical for charged membranes (Xu and Lebrun 1999). These reasons may explain the decrease in rejection with rising dry matter but not the difference between 10, 20, and 30 °C at a dry matter of 20% and a similar flux. The mass transport of neutral solutes like lactose through NF-membranes takes place mainly by diffusion. It has already been demonstrated for simple model solutions that the permeation of neutral solutes increases with rising temperature due to increased diffusion according to Arrhenius (Nilsson et al. 2008; Ben Amar et al. 2007; Tsuru et al. 2000; Mänttäri et al. 2002). Atra et al. (2005) also found a decreasing lactose retention during the filtration of protein-free serum with increasing temperature from 30 to 50 °C, without further discussing this effect. The diffusivity of maltose, a sugar of comparable molecular weight like lactose, for instance, increases from 0.474·10−9 m2 s−1 to 0.957·10−9 m2 s−1 by elevating the temperature from 20 to 50 °C (Tsuru et al. 2000). Thus, the decreasing lactose rejection with increasing temperature in the present study can presumably also be explained by an enhanced diffusive mass transport with increasing temperature. Furthermore, it can be concluded that the observed trend of an increasing retention with decreasing temperature from 50 to 30 °C by Atra et al. (2005) continues with a further temperature decrease down to 10 °C as shown by the present study. In addition, it can be derived from the statistical model that the change in the logarithm of the lactose rejection is 0.19 ± 0.023 (P < 0.001) per 1 % dry matter and for the Arrhenius part the slope is −85.4 ± 9.1 (P < 0.001) per one degree Celsius. Note that since the range of both dry matter and temperature is almost the same (without units), the change in lactose rejection is for both dry matter and temperature almost the same over the observed range. Hence, the statistical model indicates that both factors have a comparable influence on the rejection of lactose.
Rejection of lactose as a function of the dry matter in the retentate for various process temperatures (* 10 °C, □ 20 °C, ○ 30 °C, △ 45 °C) at a transmembrane pressure of 2 MPa and pH 6.8. The lines stand for the calculated values for the logarithm of the rejection indirect proportional to the dry matter and proportional to the temperature
NF-Flux and Rejection of Solutes for Different Feed pH Values
In Fig. 6, the logarithm of the permeate flux during the NF of protein-free serum at different starting pH values (10 °C, TMP = 2 MPa) is plotted against the retentate dry matter. The calculated values are directly shown in the obtained linear relationship. No impact of solution pH on the flux could be observed in the investigated pH range. This observation is supported by a P value of 0.81. This contradicts the expectation of a rising flux due to less scaling owing to the increased solubility of salts with decreasing pH. Rice et al. (2009a) found a significant effect of pH on the NF of protein-free milk serum in non-concentrating mode. The authors compared the filtration at pH 5.5 and pH 8.3 and observed a higher flux and less flux decline at pH 5.5 due to the higher solubility of the milk salts, especially calcium phosphate. In the present study, the pH was only varied between 5 and 6.8. Hence, it is conceivable that the pH shift is not big enough to observe an influence on permeate flux. A further explanation is the increased dissociation of salts with decreasing pH. An increased dissociation increases the osmotic pressure and, hence, lowers the effective pressure difference. This effect could compensate the impact of the increased solubility of the salt and explain the absence of an effect on the permeate flux.
The rejection of sodium as a function of the dry matter in the retentate during the NF at different starting pH values (10 °C, TMP = 2 MPa) is depicted in Fig. 7. The rejection decreases with rising dry matter during the concentration due to the increasing retentate concentration and the decreasing flux as already explained in section 3.2. Furthermore, it can be seen that the rejection increases with increasing pH (0.25 ± 0.023% per one pH unit). At the mIEP, the uncharged functional groups result in an increase in pore size and no repulsion between the membrane and the charged solutes. Both effects induce a low rejection of charged solutes at the mIEP. With increasing or decreasing pH the pore size decreases and the charge of the membrane increases inducing a higher retention of charged solutes (Childress and Elimelech 2000). The IEP of the membrane used could not be measured but according to literature the mIEP can be estimated to be in the range from 3.5 to 5.5 (Rice et al. 2009b, 2011a; Hussain et al. 2008). At pH values above the mIEP, the membrane is negatively charged and rejects anions like chloride. Cations are also retained due to electroneutrality (Hussain et al. 2008; Rice et al. 2011b). Hence, the increasing rejection of sodium with increasing pH may be attributed to steric (decrease in pore size) as well as electrostatic effects (increased repulsion). The results of the present study correspond surprisingly well with investigations of various authors on the retention behavior of NF-membranes for binary mixtures of NaCl (Hussain et al. 2008; Xu and Lebrun 1999) or KCl (Rice et al. 2009b, 2011b; Suárez et al. 2009; Nilsson et al. 2008) that also reported a rejection minimum at the mIEP. For a ternary model solution containing CaCl2 as well as KCl, it was shown that the rejection minimum of KCl at the former mIEP disappeared and permeation increases with increasing pH from 5 to 7. This was explained by a shift in the mIEP to higher pH values due to adsorption of calcium to the negatively charged sites on the membrane (Rice et al. 2009b, 2011b). Based on the results, it can be assumed that no shift in mIEP occurred during NF of protein-free serum in the present study. This demonstrates that the use of simplified model solutions may help to understand the complex processes during NF. However, it does not always allow the prediction of the behavior of more complex solutions like protein-free serum.
Rejection of sodium plotted against the dry matter in the retentate for various starting pH values (■ 5.0, ▲ 6.0, ♦ 6.5, ● 6.8) at 10 °C and a transmembrane pressure of 2 MPa. The lines show the calculated values by the statistical model relating the logarithm of the sodium rejection linear with both dry matter and pH
Figure 8 shows the rejection of calcium plotted against the dry matter in the retentate during the NF at different starting pH values (10 °C, TMP = 2 MPa). The rejection of calcium decreases during concentration with increasing dry matter and decreasing flux. To rate the influence on either dry matter or flux, both were used to explain the calcium rejection. In both statistical models, almost the same coefficient of determination slightly greater than 0.75 could be obtained with almost the same residual standard error. However, only the influence of dry mass was significant (P < 0.1), whereas the influence of the flux was not (P = 0.143). The effect of pH on calcium rejection is comparable to the impact on the rejection of sodium. The rejection decreases with decreasing pH due to larger pores and less repulsion. It also has to be taken into account that calcium forms complexes with rising pH in the presence of phosphate that are rejected because of size effects. This was already shown for model solutions (Rice et al. 2009b, 2011b). Their findings can be transferred to protein-free serum as shown by the present study.
The rejection of lactose is above 99.5% and remains unaffected by the pH of the solution (data not shown). Nilsson et al. (2008) observed an effect of pH on the retention of glucose during the NF of a solution containing glucose and salt. This was attributed to a combined effect of pH and the presence of salts on the membrane retention characteristics due to membrane swelling. In contrast to glucose, no impact of salt on the retention behavior of sucrose could be observed when filtering a sucrose/NaCl mixture. The presence of salts results in a slight increase of the mean pore size of the membrane. This affects the glucose retention more extensively than the sucrose retention due to the higher molecular weight of sucrose compared to glucose (Vellenga and Trägårdh 1998; Bargeman et al. 2005). Hence, the lack of an effect of pH on the retention of lactose can probably be explained by the higher molecular weight of lactose compared to glucose.
NF-Flux and Rejection of Solutes for Various Transmembrane Pressures
The permeate flux during the NF of protein-free serum at 10 °C, pH 6.8 and at different TMP as a function of the dry matter in the retentate and as a function of the TMP are depicted in Figs. 9 and 10, respectively. It can be seen that the overall flux level and the maximum dry matter increases with increasing TMP. This is due to the fact that the effective pressure difference increases with rising TMP and, hence, the osmotic pressure can be more easily overcome. Comparing these results to literature, a slightly higher maximum dry matter of approx. 25% compared to 20% can be obtained during concentration experiments at 2 MPa by Suárez et al. (2009). A higher TMP was not investigated by the authors. In the present study, it could be shown that a maximum dry matter content of approx. 33% can be reached by applying a higher TMP of 4 MPa. From Fig. 10, it can be seen that the flux does not increase linearly with TMP but the curve levels off with rising TMP. This can be attributed to a decrease in effective pressure difference due to concentration polarization at the membrane surface. This effect is even more pronounced at higher dry matters. Compared to literature, the flux values at a dry matter of 7% are in good agreement with results reported by Cuartas-Uribe et al. (2009) for the NF of protein-free whey serum and findings obtained by Suárez et al. (2006) for the NF of protein-free serum at different TMP in non-concentrating mode. Note, to obtain a reasonable fit for both Figs. 9 and 10, a different statistical model, namely J = β 0 + β 1 ∙ DMR + β 2 ∙ p + β 3 ∙ DMR2 + β 4 ∙ p 2 + β 5 ∙ DMR ∙ p was applied.
NF-permeate flux of protein-free serum as a function of the dry matter in the retentate for various transmembrane pressures (+ 1 MPa, × 2 MPa, ◇ 3 MPa, ▽ 4 MPa) at 10 °C and pH 6.8. The lines show the calculated values by the statistical model J = β 0 + β 1 ∙ DMR + β 2 ∙ p + β 3 ∙ DMR2 + β 4 ∙ p 2 + β 5 ∙ DMR ∙ p
Figure 11 shows the rejection of sodium as a function of the dry matter in the retentate during the NF at various TMP (10 °C, pH 6.8). The decrease in rejection during concentration can probably be attributed to the increasing dry matter and the decreasing flux (see section 3.2). Furthermore, it can be seen that the overall rejection increases with increasing TMP. This can be explained by the higher overall flux values that induce a dilution of the transmitted solutes as already explained in section 3.2. The same behavior was found for the rejection of calcium and lactose (data not shown).
Rejection of sodium plotted against the dry matter in the retentate for various transmembrane pressures (+ 1 MPa, × 2 MPa, ◇ 3 MPa, ▽ 4 MPa) at 10 °C and pH 6.8. The solid lines show the calculated values by the statistical model of the logarithm of the flux linearly dependent on dry matter and transmembrane pressure
Conclusion
The filtration temperature has a marked impact on the permeate flux. Due to microbiological reasons, filtrations in the dairy industry are usually conducted at temperatures ≤10 or ≥45–50 °C. The filtration at 45 °C shows the highest initial flux but also a strong flux decline during the concentration. The flux decline can probably be attributed to the precipitation of calcium phosphate on the membrane. At a temperature of 10 °C the flux is more stable. No relevant impact of temperature on the rejection of sodium and calcium could be observed, whereas the retention of lactose decreases with increasing temperature. Hence, taking into account the permeate flux as well as the rejection of the solutes, it is more favorable to conduct the filtration at a low temperature of 10 °C. No effect of solution pH on permeate flux and lactose retention was observed. In contrast, the retention coefficient of sodium and calcium decreases with decreasing pH (from 6.8 to 5.0) due to increasing pore size and less repulsion. As calcium is a valuable compound, its retention shall be maximized. Hence, it is advantageous to conduct the filtration at a pH of 6.8 instead of at a lower pH. Regarding the impact of TMP, it was found that permeate flux, VRR, and solute rejection can be increased with increasing TMP. Therefore, the concentration of protein-free serum via NF should be carried out at a TMP of 4 MPa. Furthermore, it was shown that the retention of solutes decreases towards the end of the filtration. Hence, the achievement of high final VRR must be weighed against increased final product losses.
References
Akaike, H. (1974). An new look at statistical model identification. IEEE Transactions on Automatic Control. doi:10.1109/TAC.1974.1100705.
Atra, R., Vatai, G., Bekassy-Molnar, E., & Balint, A. (2005). Investigation of ultra- and nanofiltration for utilization of whey protein and lactose. Journal of Food Engineering. doi:10.1016/j.jfoodeng.2004.04.035.
Bargeman, G., Vollenbroek, J. M., Straatsma, J., Schroën, C. G. P. H., & Boom, R. M. (2005). Nanofiltration of multi-component feeds. Interactions between neutral and charged components and their effect on retention. Journal of Membrane Science. doi:10.1016/j.memsci.2004.05.022.
Ben Amar, N., Saidani, H., Deratani, A., & Palmeri, J. (2007). Effect of temperature on the transport of water and neutral solutes across nanofiltration membranes. Langmuir. doi:10.1021/la060268p.
Bouchoux, A., Balmann, H. R.-d., & Lutin, F. (2005). Nanofiltration of glucose and sodium lactate solutions: variations of retention between single- and mixed-solute solutions. Journal of Membrane Science. doi:10.1016/j.memsci.2005.03.002.
Childress, A. E., & Elimelech, M. (2000). Relating nanofiltration membrane performance to membrane charge (electrokinetic) characteristics. Environmental Science & Technology. doi:10.1021/es0008620.
Cuartas, B. (2004). Separation of mineral salts and lactose solutions through nanofiltration membranes. Food Science and Technology International. doi:10.1177/1082013204045883.
Cuartas-Uribe, B., Alcaina-Miranda, M. I., Soriano-Costa, E., Mendoza-Roca, J. A., Iborra-Clar, M. I., & Lora-García, J. (2009). A study of the separation of lactose from whey ultrafiltration permeate using nanofiltration. The Third Membrane Science and Technology Conference of Visegrad Countries (PERMEA)), doi: 10.1016/j.desal.2007.11.086.
Garba, Y., Taha, S., Gondrexon, N., & Dorange, G. (1999). Ion transport modelling through nanofiltration membranes. Journal of Membrane Science. doi:10.1016/S0376-7388(99)00085-X.
Garcia-Aleman, J., & Dickson, J. M. (2004). Permeation of mixed-salt solutions with commercial and pore-filled nanofiltration membranes: membrane charge inversion phenomena. Journal of Membrane Science. doi:10.1016/j.memsci.2004.02.036.
Hussain, A. A., Nataraj, S. K., Abashar, M. E. E., Al-Mutaz, I. S., & Aminabhavi, T. M. (2008). Prediction of physical properties of nanofiltration membranes using experiment and theoretical models. Journal of Membrane Science. doi:10.1016/j.memsci.2007.11.005.
Mänttäri, M., Pihlajamäki, A., Kaipainen, E., & Nyström, M. (2002). Effect of temperature and membrane pre-treatment by pressure on the filtration properties of nanofiltration membranes. Desalination. doi:10.1016/S0011-9164(02)00390-9.
Marcus, Y. (1994). A simple empirical model describing the thermodynamics of hydration of ions of widely varying charges, sizes, and shapes. Biophysical Chemistry. doi:10.1016/0301-4622(94)00051-4.
Nilsson, M., Trägårdh, G., & Östergren, K. (2008). The influence of pH, salt and temperature on nanofiltration performance. Journal of Membrane Science. doi:10.1016/j.memsci.2007.12.059.
Nyström, M., Kaipia, L., & Luque, S. (1995). Fouling and retention of nanofiltration membranes. Journal of Membrane Science. doi:10.1016/0376-7388(94)00196-6.
Rice, G., Barber, A., O’Connor, A., Stevens, G., & Kentish, S. (2009a). Fouling of NF membranes by dairy ultrafiltration permeates. Journal of Membrane Science. doi:10.1016/j.memsci.2008.12.048.
Rice, G. S., Kentish, S. E., O'Connor, A. J., Barber, A. R., Pihlajamäki, A., Nyström, M., et al. (2009b). Analysis of separation and fouling behaviour during nanofiltration of dairy ultrafiltration permeates. International Membrane Science and Technology Conference 2007, doi: 10.1016/j.desal.2007.10.046.
Rice, G., Barber, A., O’Connor, A., Stevens, G., & Kentish, S. (2010). A theoretical and experimental analysis of calcium speciation and precipitation in dairy ultrafiltration permeate. International Dairy Journal. doi:10.1016/j.idairyj.2010.03.004.
Rice, G., Barber, A. R., O’Connor, A. J., Pihlajamaki, A., Nystrom, M., Stevens, G. W., et al. (2011a). The influence of dairy salts on nanofiltration membrane charge. Journal of Food Engineering. doi:10.1016/j.jfoodeng.2011.06.028.
Rice, G., Barber, A. R., O’Connor, A. J., Stevens, G. W., & Kentish, S. E. (2011b). Rejection of dairy salts by a nanofiltration membrane. Separation and Purification Technology. doi:10.1016/j.seppur.2011.03.022.
Román, A., Wang, J., Csanádi, J., Hodúr, C., & Vatai, G. (2009). Partial demineralization and concentration of acid whey by nanofiltration combined with diafiltration. Desalination. doi:10.1016/j.desal.2007.12.054.
Schaep, J., Vandecasteele, C., Mohammad, A. W., & Bowen, W. R. (1999). Analysis of the salt retention of nanofiltration membranes using the Donnan–Steric partitioning pore model. Separation Science and Technology. doi:10.1081/ss-100100819.
Schmitz-Schug, I. (2014). Improving the nutritional quality of dairy powders—analyzing and modeling lysine loss during spray drying as influenced by drying kinetics, thermal stress, physical state and molecular mobility. München: Verl. Dr. Hut.
Snow, M. J. H., de Winter, D., Buckingham, R., Campbell, J., & Wagner, J. (1996). New techniques for extreme conditions: high temperature reverse osmosis and nanofiltration. Proceedings of the 1st Symposium of the European Desalination Society 'Desalination for Europe', doi: 10.1016/0011-9164(96)00058-6.
Suárez, E., Lobo, A., Álvarez, S., Riera, F. A., & Álvarez, R. (2006). Partial demineralization of whey and milk ultrafiltration permeate by nanofiltration at pilot-plant scale. The Second Membrane Science and Technology Conference of Visegrad Countries (PERMEA 2005), doi: 10.1016/j.desal.2005.12.028.
Suárez, E., Lobo, A., Alvarez, S., Riera, F. A., & Álvarez, R. (2009). Demineralization of whey and milk ultrafiltration permeate by means of nanofiltration. The Third Membrane Science and Technology Conference of Visegrad Countries (PERMEA). doi:10.1016/j.desal.2007.11.087.
Tsuru, T., Izumi, S., Yoshioka, T., & Asaeda, M. (2000). Temperature effect on transport performance by inorganic nanofiltration membranes. AICHE Journal. doi:10.1002/aic.690460315.
van der Horst, H., Timmer, J., Robbertsen, T., & Leenders, J. (1995). Use of nanofiltration for concentration and demineralization in the dairy industry: model for mass transport. Journal of Membrane Science. doi:10.1016/0376-7388(95)00041-A.
Vellenga, E., & Trägårdh, G. (1998). Nanofiltration of combined salt and sugar solutions: coupling between retentions. Desalination. doi:10.1016/S0011-9164(98)00219-7.
Venables, W. N., & Ripley, B. D. (2002). Modern applied statistics with S. New York: Springer.
Xu, Y., & Lebrun, R. E. (1999). Investigation of the solute separation by charged nanofiltration membrane: effect of pH, ionic strength and solute type. Journal of Membrane Science. doi:10.1016/S0376-7388(99)00005-8.
Acknowledgement
We gratefully thank Michael Reitmaier and Christian Gottwald for experimental support. This research project was supported by the German Ministry of Economics and Technology (via AiF) and the FEI (Forschungskreis der Ernährungsindustrie e.V., Bonn). Project AiF 16836 N.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meyer, P., Petermeier, J., Hartinger, M. et al. Concentration of Skim Milk by a Cascade Comprised of Ultrafiltration and Nanofiltration: Investigation of the Nanofiltration of Skim Milk Ultrafiltration Permeate. Food Bioprocess Technol 10, 469–478 (2017). https://doi.org/10.1007/s11947-016-1836-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-016-1836-5