Abstract
The purpose of this study was to compare the effects of the non-thermal technologies of pulsed electric fields (PEF), high-voltage electrical discharges (HVED) and ultrasound (USN) on bioactive compounds (ascorbic acid, total carotenoids, total phenolic compounds and total anthocyanins) and antioxidant capacity of a fruit juice (papaya and mango) blend sweetened with Stevia rebaudiana. Experiments were carried out at two equivalent energy inputs (32–256 kJ/kg) for each technology. Principal component analysis (PCA) was used to understand the contribution of ascorbic acid, total carotenoids and oxygen radical absorbance capacity (ORAC) values. These parameters were better retained with PEF treatments. Nevertheless, the use of HVED and USN technologies cannot be ruled out, as they may enhance the contents of other bioactive compounds such as total phenolic compounds when HVED technology is applied at an energy input of 256 kJ/kg. The obtained data can contribute to the determination of optimum processing conditions for production of high nutritional quality liquid foods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last two decades, a growing demand for exotic fruits has been observed. Amongst these, mango and papaya are some of the most important, now ranked second and third of the total tropical fruit production, only behind banana production (Evans and Ballen 2012). Moreover, consumption of several mango- and papaya-derived products such as juices, purées and fresh-cut mango slices has increased in response to consumer’s demand for highly nutritious healthy foods (Rawson et al. 2011a). These products contain a large amount of bioactive compounds, including ascorbic acid, phenolic compounds and carotenoids, that have shown to be good contributors to the total antioxidant capacity of foods (Zulueta et al. 2009; Vijaya et al. 2010) and have been associated with a reduced risk of degenerative diseases such as cancer and coronary heart disease (Abuajah et al. 2014; Pistollato and Battino 2014).
Although thermal treatments have been traditionally used in the preservation of liquid foods due to their ability to inactivate microorganisms and spoilage enzymes, several chemical and physical changes may take place, especially when high temperatures (>100 °C) are used, impairing organoleptic properties and reducing bioactive compound content. Consequently, new processing technologies which can avoid microbial contamination and allow the obtainment of high-quality food with “fresh-like” characteristics and improved functionalities are required (Rawson et al. 2011a). In this line, the use of electrotechnologies, such as pulsed electric fields (PEF), high-voltage electrical discharges (HVED) and ultrasound (USN) processing, has been shown to be promising for liquid food preservation (Toepfl et al. 2006, 2007; Barba et al. 2012; Zulueta et al. 2013). Additionally, PEF and USN treatment can be combined with conventional preservative techniques, such as the use of green herbs with antimicrobial activity in order to enhance the lethal or inhibitory effect of these technologies on microorganisms (Ross et al. 2003) as well as their preservation of nutritional properties (Wang et al. 2008; Soria and Villamiel 2010; Boussetta and Vorobiev 2014). Such combinations enhance food preservation at lower individual treatment intensities (Ross et al. 2003). Moreover, HVED, which is a pulsed electric-based technology, has also the potential to be used for food preservation. However, the formation of reactive species (e.g. ozone) generated during the discharges makes necessary deeper studies about the effect of this technology in the nutritional properties of foods (Sarkis et al. 2015).
In two previous studies, Carbonell-Capella et al. (2013) and Barba et al. (2014) combined a non-thermal technology (high pressure processing) with a natural antimicrobial Stevia rebaudiana Bertoni (Siddique et al. 2014), obtaining interesting results regarding Listeria monocytogenes, polyphenol oxidase (PPO) and peroxidase (POD) inactivation. Moreover, a significant increase of bioactive compounds and antioxidant capacity was found. However, there is a need to study if other mild preservation technologies such as PEF, HVED and USN have the same positive effect regarding antioxidant compounds.
This manuscript discusses the effect of pulsed electric fields, high-voltage electrical discharges and ultrasound technology on bioactive compound retention and antioxidant capacity of a fruit juice blend based on mango and papaya sweetened with S. rebaudiana. S tevia 2.5 % (w/v) was selected as a low-calorie sweetener with antioxidant potential based on a previous study (Barba et al. 2014). Equivalent energy inputs were applied in order to compare the different technologies between them, and ascorbic acid, total carotenoids, total phenolic compounds, total anthocyanins and antioxidant capacity were investigated.
Materials and Methods
Sample Preparation
Mango (Mangifera indica) and papaya (Carica papaya) were purchased from a local supermarket (Valencia, Spain) at commercial maturity stage. Mango and papaya juices were extracted after appropriate washing of the fruits and filtered by means of a mesh sieve (pore size 0.297 mm).
Dried S. rebaudiana leaves were supplied by Anagalide S.A. (Spain) and stored at room temperature. A stock solution of 8.33 ± 0.01 % (w/v) was prepared according to Carbonell-Capella et al. (2015). One hundred millilitres of boiling distilled water was added to the dried leaves (8.33 g), and the mixture was covered and allowed to infuse for 30 min at 100 °C. The infusion was vacuum filtered using a Kitasato flask, a Büchner funnel, a vacuum pump (VDE 0530, KNF Neuberger GmbH, Germany) and a filter paper (Whatman® No. 1, Whatman International Ltd, UK), and the filtrate obtained was stored at 40 °C.
The fruit juice blend was prepared by mixing 50.8 % (v/v) of papaya juice, 19.3 % (v/v) of mango juice and completing volume up to 100 % with Stevia stock infusion, with a final Stevia concentration of 2.5 % (w/v). The final Stevia concentration (2.5 %) was selected according to a previous study (Belda-Galbis et al. 2014) which showed the highest antimicrobial activity at this concentration against Listeria innocua, a pathogen of great concern in minimally processed beverages because of its ubiquitous, psychotropic nature and because of its ability to grow in acidic environments.
Chemicals
Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylicacid), 2,2′-azinobis(3-ethylbenzothiazoline 6-sulfonate) (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), Folin–Ciocalteu reagent, fluorescein sodium salt and sodium metabisulfite were purchased from Sigma-Aldrich (Steinheim, Germany). Gallic acid was purchased from UCB (Brussels, Belgium). Hexane (LC grade) and potassium dihydrogen phosphate (KH2PO4) were purchased from Scharlau (Barcelona, Spain). Oxalic acid, acetic acid, sodium acetate, potassium persulphate (K2S2O8), sodium and disodium phosphate and 2,2′-azobis-(2-amidinopropane) dihydrochloride (AAPH) were purchased from Panreac (Barcelona, Spain). Ethanol, methanol, hydrochloric acid, sodium chloride (special grade), sodium carbonate anhydrous (Na2CO3), trichloroacetic acid and sodium sulphate proceeded from Baker (Deventer, The Netherlands). l(+)-ascorbic acid was obtained from Merck (Darmstadt, Germany).
PEF, HVED and USN Treatments
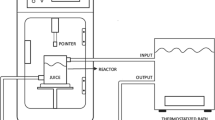
Electrical treatments in PEF and HVED modes were done using a high-voltage pulsed power 40 kV–10 kA generator (Tomsk Polytechnic University, Tomsk, Russia). The initial temperature before PEF or HVED treatments was ≈20 °C, and the temperature elevation after electrical treatment never exceeded 35 °C. Beverage temperature was controlled by a K-type thermocouple (±0.1 °C) connected to a data logger thermometer centre 305/306 (JDC Electronic SA, Yverdon-les-Bains, Switzerland). PEF treatments were carried out in a cylindrical batch treatment chamber between two plate electrodes of 95 cm2. The distance between electrodes was fixed to 2 cm with a corresponding electric field strength E of 25 kV/cm. Total treatment duration t t (t t = n × t i ) was changed by increasing the number of pulses n from 50 to 400. Time delay between sequential pulses was of Δt = 2 s. Exponential decay of voltage U ∝ exp (−t/t i ) with effective decay time t i ≈ 10.0 ± 0.1 μs was observed. Total specific energy input (W, kJ/kg) was chosen as a parameter to describe the treatment intensity. Two energy inputs were applied, which corresponded to samples PEF1 (32 kJ/kg) and PEF2 (256 kJ/kg). The energy input (W) of PEF treatment was calculated as shown in Eq. (1).
where W PEF is the pulse energy (kJ/pulse), n is the number of pulses and m is the product mass (kg). W PEF was determined from Eq. (2).
where U is the voltage (V) and I is the current strength (A).
For HVED treatments, the 1-L treatment chamber (inner diameter = 10 cm, wall thickness = 2.5 cm) was equipped with needle-plate geometry electrodes. The diameters of stainless steel needle and the grounded disk electrodes were of 10 and 35 mm, respectively. The distance between the electrodes was of 5 mm. Energy was stored in a set of low-inductance capacitors, which were charged by the high-voltage power supply. Electrical discharges were generated by electrical breakdown in water with a peak pulse voltage (U) of 40 kV. Damped oscillations were thus obtained over a total duration t i of ≈10 μs. The voltage (Ross VD45-8.3-A-K-A, Ross Engineering Corp., Campbell, CA, USA) and current (Pearson 3972, Pearson Electronics Inc., Campbell, CA, USA) measurement units were connected with a 108-Hz sampling system via an oscilloscope (Tektronix TDS1002, Beaverton, OR, USA). The software HPVEE 4.01 (Hewlett-Packard, Palo Alto, USA) was used for data acquisition. The energy input of HVED treatment was calculated as shown in Eqs. (1) and (2), where W PEF was substituted by W HVED. Total treatment duration (t t = n × t i ) was changed by increasing the discharge number n from 50 to 400. The discharge pulse duration t HVED was approximately 10 μs. The discharges were applied with a repetition rate of 0.5 Hz, which was imposed by the generator. In order to compare HVED treatments with PEF, equivalent energy inputs were used, obtaining sample HVED1 (32 kJ/kg) and HVED2 (256 kJ/kg).
For USN treatments, an ultrasonic processor UP 400S (Hielscher GmbH, Germany) which operates at 400 W and a frequency of 24 kHz was used. Amplitude, which could be adjusted from 20 to 100 %, was set at 100 %. The instrument can be used in cycle mode (0 ∼ 1), where a cycle setting of 1 means that the solution is sonicated without interruption, whereas with a cycle setting, for example, of 0.5 the solution is sonicated for 0.5 s and then sonication stops for 0.5 s. Hence, in cycle mode, the ratio of sound-emission time to cyclic pause time can be adjusted continuously from 0 to 100 % per second. In the present study, cycle was fixed at 1. The titanium sonotrode H14 with a diameter of 14 mm and a length of 100 mm was used to transmit ultrasound inside the sample. The sample was submerged in a cooling bath to avoid the heating induced by USN irradiation. The energy input of USN treatment was calculated as follows (Eq. (3)):
where t USN is the total treatment duration (s), m is the product mass (kg) and the generator power (400 J/s). In order to obtain equivalent energy inputs to the other treatments applied, total treatment duration was of 20 and 160 s, obtaining USN1 (32 kJ/kg) and USN2 (256 kJ/kg) samples.
Polarographic Determination of Ascorbic Acid
The beverage (5 mL) was diluted to 25 mL with the extraction solution (oxalic acid 1 %, w/v, trichloroacetic acid 2 %, w/v, sodium sulphate 1 %, w/v). After constant shaking at 4×g during 30 s in a vortex (VV3, VWR International, Spain), the solution was filtered through a folded filter (Whatman® no. 1). Oxalic acid (9.5 mL) 1 % (w/v) and 2 mL of acetic acid/sodium acetate 2 M buffer (pH = 4.8) were added to an aliquot of 0.5 mL of filtrate, and the solution was transferred to the polarographic cell. A Metrohm 746 VA Trace Analyser (Herisau, Switzerland) equipped with a Metrohm 747 VA stand was used for the polarographic determination (Carbonell-Capella et al. 2013).
Total Carotenoids
Extraction of total carotenoids was carried out in accordance with Lee and Castle (2001). Five millilitres of extracting solvent (hexane/acetone/ethanol, 50:25:25, v/v) was added to an aliquot of sample (2 mL) and centrifuged for 5 min at 3220×g at 5 °C. The top layer of hexane containing the colour was recovered and transferred to a 25-mL volumetric flask. The volume of recovered hexane was then adjusted to 25 mL with hexane. Total carotenoid determination was carried out on an aliquot of the hexane extract by measuring the absorbance at 450 nm. Total carotenoids were calculated according to Ritter and Purcell (1981) using an extinction coefficient of β-carotene, E 1 % = 2505.
Total Phenolic Compounds
Total phenols were determined according to the method reported by Georgé et al. (2005), with some modifications. Briefly, 50 mL of a mixture of acetone/water (7/3, v/v) was added to 10 mL of sample and centrifuged for 30 min at 3220×g. Mixture supernatants were then recovered by filtration (Whatman® no. 2, England) and constituted the raw extracts (REs). REs (2 mL) were settled on an Oasis cartridge (Waters). Interfering water-soluble components (reducing sugars, ascorbic acid) were recovered with 2 × 2 mL of distilled water. The recovered volume of the washing extract (WE) was carefully measured. In order to eliminate vitamin C, heating was carried out on the washing extract (3 mL) for 2 h at 85 °C and led to the heated washing extract (HWE). All extracts (RE, WE and HWE) were submitted to the Folin–Ciocalteu method, adapted and optimized (Barba et al. 2014). Gallic acid calibration standards with concentrations of 0, 100, 300, 500, 700 and 1000 ppm were prepared and 0.1 mL was transferred to borosilicate tubes. Three millilitres of 2 % (w/v) sodium carbonate solution and 100 μL of Folin–Ciocalteu reagent (1:1, v/v) were added to 100 μL of all gallic acid standard and sample tubes. The mixture was incubated for 1 h at room temperature. Absorbance was measured at 765 nm.
Total Anthocyanins
Total anthocyanins were determined using a modified method of Mazza et al. (1999). A tenfold diluted sample of 100 μL was mixed with 1700 μL of distilled water and 200 μL of 5 % (v/v) HCl. The sample was hold at room temperature for 20 min before measuring the absorbance at 520 nm. Calculations of total anthocyanins were based on cyanidin-3-glucoside (molar absorptivity 26900). All spectrophotometric analyses were performed using a UV–visible spectrophotometer Lambda 20 (Perkin-Elmer, Überlingen, Germany).
Total Antioxidant Capacity
TEAC Assay
Trolox-equivalent antioxidant capacity (TEAC) was measured using the method of Re et al. (1999) based on application of ABTS decolourisation assay (Sigma-Aldrich, Steinheim, Germany). The ABTS radical (ABTS•+) was generated using 440 μL of potassium persulfate (140 mM). The solution was diluted with ethanol (Baker, Deventer, The Netherlands) until an absorbance of 0.70 was reached at 734 nm. Once the radical was formed, 2 mL of ABTS•+ was mixed in a vortex (VV3, VWR International, Spain) for 30 s with 100 μL of extract and the sample was incubated for 60 min at 20 °C. Absorbance, A, was measured at the wavelength of 734 nm (Carbonell-Capella et al. 2013), and percentage of inhibition (% I) was calculated using the following formula (Eq. (4)):
Results were expressed as Trolox-equivalent values (mM TE) using a standard calibration curve of Trolox in the range of 50–250 μM.
ORAC Assay
The oxygen radical absorbance capacity (ORAC) assay used, with fluorescein as the “fluorescent probe”, was that described by Barba et al. (2014). The automated ORAC assay was carried out on a Wallac 1420 VICTOR2 multilabel counter (Perkin-Elmer, USA) with fluorescence filters, for an excitation wavelength of 485 nm and an emission wavelength of 535 nm. The measurements were made in plates with 96 white flat bottom wells (Sero-Wel, Bibby Sterilin Ltd., Stone, UK). The reaction was performed at 37 °C, as the reaction was started by thermal decomposition of AAPH in 75 mM phosphate buffer (pH 7.0). Calculations were done using Eq. (5), and results were expressed as mM TE:
where C Trolox is 20 μM, AUC is the area under the curve and K is the dilution factor.
DPPH Assay
Antioxidant capacity was also measured following the method described by Brand-Williams et al. (1995). The reaction was begun by adding 50 μL of a suitable dilution of sample to 1.45 mL of 2,2-diphenyl-1-picrylhydrazyl (DPPH)-coloured radical. The sample was incubated for 30 min at room temperature (T = 20 °C). Absorbance was measured at the wavelength of 515 nm, and percentage of inhibition (% I) was calculated using the following formula (Eq. (6)):
Results were expressed as mM TE using a standard calibration curve of Trolox in the range of 0.05−1 mM.
Statistical Analysis
All determinations were performed in triplicate. One-way analysis of variance and Pearson test were used for statistical analysis of the data using Statgraphics® Centurion XVI (Statpoint Technologies Inc., USA) (differences at p < 0.05 were considered significant). A principal component analysis based on the correlation matrix was performed to assess differences amongst the different treatments. Response variables were autoscaled prior to chemometric application, in accordance to Granato et al. (2015).
Results and Discussion
Effect of PEF, HVED and USN Treatment on Bioactive Compounds
To establish the effect of the non-thermal technologies of PEF, HVED and USN, two equivalent energy inputs (32 and 256 kJ/kg) were applied to a fruit juice blend (papaya and mango) sweetened with S. rebaudiana Bertoni, and in all cases, results were compared with the untreated beverage. Ascorbic acid, total carotenoids, total phenolic compounds, anthocyanins and total antioxidant capacity measured by TEAC, ORAC and DPPH method were determined.
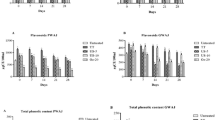
The ascorbic acid content in the fruit juice blend sweetened with Stevia was 18.5 ± 0.4 mg/100 mL (Table 1). These results are in close agreement with values of ascorbic acid obtained by Murillo et al. (2012) in mango and papaya. Immediately after treatment by PEF, HVED and USN, ascorbic acid retention was of 17–91 %. USN was the processing technique that better retained the ascorbic acid content of the fruit juice-Stevia blend (84–91 %), followed by PEF (80–83 %). However, after HVED treatment, ascorbic acid retention decreased to 17–23 %. This fact can be attributed to the formation of gaseous cavitation bubbles, as well as the emission of shock waves of high pressure and of high-intensity UV light when electrical discharges are applied in liquids (Boussetta and Vorobiev 2014). In any case, higher energy inputs caused higher ascorbic acid losses, independently of the treatment applied, only significant in the case of HVED treatment (Fig. 1), which may be caused by the increase of temperature at high energy levels, causing the degradation of this thermolabile vitamin. Nevertheless, our results were in accordance to those found by other previous studies which have reported the feasibility of USN and PEF to preserve ascorbic acid when they are used for liquid food preservation (Barba et al. 2012; Tiwari et al. 2008a, b; Zulueta et al. 2013).
Regarding total carotenoids, untreated sample exhibited a yield of 676.1 ± 3.5 μg/100 mL, higher than in a previous study of a mango, papaya and orange beverage sweetened with Stevia (Carbonell-Capella et al. 2013). After PEF1 processing, total carotenoids were significantly higher (p < 0.05), with values of 800.9 ± 3.5 μg/100 mL (Fig. 2). This may be due to carotenoids being released or leaching of other minerals or solid substances into the juice as a result of the pulsed electric fields (Roohinejad et al. 2014). In this line, Torregrosa et al. (2005) found that the application of various PEF treatments in orange–carrot juice produces a significant increase in carotenoid concentrations as treatment time increases. Moreover, Zulueta et al. (2010) also found a slight increase in the concentration of the extracted carotenoids when they applied PEF (25 kV/cm, 80 μs) in an orange juice-milk beverage. However, after PEF2 treatment at an energy input of 256 kJ/kg, total carotenoid diminished significantly (p < 0.05). Behaviour of carotenoids after pulsed electric treatments is complex and, although other authors have also seen that at higher field intensities, carotenoid content is reduced, this is not yet well understood. For instance, vitamin A content was higher than in the pasteurized juice after Torregrosa et al. (2005) applied PEF treatment at different field intensities (25, 30, 35 and 40 kV/cm) and only with electric fields of 25 and 30 kV/cm. When using HVED technology, total carotenoids decreased, possibly because of the formation of reactive species generated during the treatment. In this case, HVED2 treatment led to the beverage with the lowest content in total carotenoids (46 %). Boussetta et al. (2011) also observed a negative effect of HVED in antioxidant compounds above an energy value of 80 kJ/kg. Carotenoid content after ultrasound processing was of 429.1–486.5 μg/100 mL. Differently from other technologies, higher treatment time of ultrasound led to a higher carotenoid content. A possible explanation for this increase may be (1) the ability of ultrasound to enhance disruption of cell walls, which might have facilitated the release of bound carotenoid contents, or (2) the rupture of carotenoid-protein binding, thus facilitating the extractability of carotenoids. These results are in close agreement to those obtained by Abid et al. (2014), who found a significant improvement in carotenoid content of USN-treated apple juices, and Rawson et al. (2011b), who found a slight increase in lycopene content in USN-treated watermelon juice at low amplitude level.
The contents of total phenolic compounds (TPC) in the untreated fruit juice-Stevia blend were 2685.6 ± 3.5 mg/L, in accord with literature data in an exotic fruit-oat beverage with Stevia (Carbonell-Capella et al. 2015). Phenolic concentration after PEF2 and HVED2 treatments was significantly higher than that of control (Fig. 3), which indicated that extractability of TPC may be increased by the release of solutes into the solvent because of the irreversible pores in cell membranes caused by the use of electrical fields. In support to these results, Hsieh and Ko (2008) obtained an increase in the amount of TPC when carrot juice was treated at 100 kV/m with high-voltage electrostatic field. On the other hand, although there is no clear trend regarding TPC behaviour after PEF, Morales-de la Peña et al. (2010) demonstrated the ability of PEF to increase the concentration of TPC in fruit juice–soymilk beverages (35 kV/cm, 800–1400 μs). These authors attributed this phenomenon to biochemical reactions during the PEF processing, which led to the formation of new phenolic compounds: significant effects on cell membranes or in phenolic complexes with other compounds, releasing some free phenolic compounds after PEF processing and a possible inactivation of PPO after PEF treatment, preventing further loss of phenolic compounds. Nevertheless, after PEF1 and HVED1 treatments, non-significant changes (p > 0.05) in total phenolic compounds were found, confirming results obtained by Chen et al. (2014) in blueberry juice. After the fruit juice blend sweetened with Stevia was treated by USN technology, non-significant differences were obtained when compared with untreated fruit juice-Stevia, independently of the energy input applied. Results are in accord with those obtained by Martínez-Flores et al. (2014), who did not find significant differences in phenolic compounds between control and thermo-sonicated carrot juice.
In the untreated fruit juice-Stevia blend, the concentration of total anthocyanins was 11.8 ± 1.4 mg/L, which can be compared with previous results in an exotic fruit-oat beverage sweetened with Stevia (Carbonell-Capella et al. 2015). A high retention of anthocyanin content was obtained immediately after PEF, HVED and USN treatments (94–110 %). Total anthocyanins increased after applying PEF, HVED and USN treatments at an energy input of 256 kJ/kg with respect to fruit juice-Stevia blends treated at an energy input of 32 kJ/kg, although differences were not significant (p > 0.05) (Fig. 4). Results indicate a high stability of anthocyanins to processing conditions. This was also observed in a study carried out by Guo et al. (2014), where PEF processed and unprocessed pomegranate juice had similar concentrations of total anthocyanins. Furthermore, Pérez-Ramírez et al. (2015) found that Stevia addition decreased the degradation rate of anthocyanins in a roselle beverage. By contrast, Barba et al. (2012) did observe a statistically significant increase of total anthocyanin immediately after blueberry juice was treated with pulsed electric fields at 36 kV/cm during 100 μs. Odriozola-Serrano et al. (2009) also reported greater anthocyanin retention in strawberry juice during PEF treatment in bipolar pulse mode compared to monopolar mode, showing that anthocyanin retention during PEF processing is influenced by polarity, treatment time and frequency employed. Meanwhile, total anthocyanins did not undergo any change when apple juice was sonicated at different treatment times in the study carried out by Abid et al. (2014). Similarly, significant retention of anthocyanin content (>94 %) was observed by Tiwari et al. (2009) in blackberry juice at the same amplitude conditions of our study although with a treatment duration of 10 min, indicating stability of anthocyanin during sonication.
Effect of PEF, HVED and USN Treatment on Total Antioxidant Capacity
Total antioxidant capacity (TAC) measured as TEAC and DPPH values in the untreated beverage were 23.9 ± 0.9 and 24.3 ± 0.5 mM TE, respectively, in the range of previous studies in mixture of fruits with Stevia (Criado et al. 2014). A good ABTS scavenging was already cited for a fruit juice mixture (mango, papaya and orange) sweetened with S. rebaudiana (Carbonell-Capella et al. 2013). As shown in Table 2, the increase in the ABTS and DPPH scavenging capacity of the fruit juice-Stevia blend was only significant in HVED-treated samples. This may be due to the ability of these techniques to enhance disruption of cell walls and thus facilitate the release of antioxidant compounds, increasing total antioxidant capacity measured with these methods. A positive impact of HVED on TEAC and DPPH values was observed in fruit juice-Stevia blends treated at an energy input of 256 kJ/kg, whilst non-significant changes (p > 0.05) were found immediately after PEF and USN treatment with regard to the control sample. Grimi et al. (2014) observed that HVED provided a more powerful disintegration of Nannochloropsis sp. microalgae suspensions than application of PEF, whilst Rajha et al. (2014) obtained that HVED lead to higher cell damage than PEF and USN for the same energy input due to the cavitation phenomena and pressure shock waves induced by HVED. Consequently, the beverage treated by HVED2 would be better homogenized, which could explain the higher TAC values obtained. However, its applications in the food and pharmaceutical oriented industry can be reduced because of possible contamination of the treated product by chemical products of electrolysis, free reactive radicals, etc. (Sarkis et al. 2015). In accordance with the present study, TEAC values were not significantly modified in PEF-treated orange juice-milk beverage in the research carried out by Zulueta et al. (2013). Moreover, Morales-de la Peña et al. (2010), immediately after PEF processing of fruit juice–soymilk beverage, did not observe significant changes in antioxidant capacity (TEAC and DPPH, respectively) in comparison with the untreated beverage. By contrast, Martínez-Flores et al. (2014) obtained an increase in the antioxidant capacity measured with DPPH assay due to the effect of ultrasound in carrot juice. It is noteworthy that although TEAC and DPPH are two different procedures used to measure the free radical-scavenging of food products, values obtained in the fruit juice-Stevia blend were similar.
ORAC value in the control fruit juice-Stevia blend was 30.8 ± 0.9 mM. This value is consistent with previous studies of a fruit juice mixture (papaya, mango and orange) sweetened with S. rebaudiana (Carbonell-Capella et al. 2013) and comparable to the results obtained in different fruit formulations (smoothies, fruit purees, concentrates and juices) by Müller et al. (2010). The ANOVA analysis confirmed an increase of TAC when the fruit juice-Stevia blend was treated by PEF and USN (116.4–121.7 %), independently of the energy input and time treatment, in comparison with the untreated fruit juice-Stevia blend. This increase in TAC may be related to an increased extractability of some of the antioxidant components following PEF and USN processing. HVED treatment did not result in an increase of ORAC values in comparison with the untreated fruit juice-Stevia blend, independently of the energy input applied. The different TAC levels obtained from the assays may reflect a relative difference in the ability of antioxidant compounds in the beverage to quench aqueous peroxyl radicals and to reduce ABTS and DPPH free radical in in vitro systems (Zulueta et al. 2009).
When the possible correlation (Pearson test) between the various parameters that contribute to antioxidant capacity (ascorbic acid, total carotenoids, total phenolic compounds and total anthocyanins) was studied for the different non-thermally treated fruit juice-Stevia blends, a positive correlation between total carotenoids and total phenolic compounds with TEAC values (p = 0.0496 and p = 0.0172, respectively) and between anthocyanins and DPPH values (p = 0.0006) was observed, reflecting the importance of using different methods to measure total antioxidant capacity in foodstuff as different bioactive compounds may be better represented by one or another assay. In this line, Bishi et al. (2015) found a significant correlation between phenol content and antioxidant capacity measured with TEAC assay in 41 Indian peanut cultivars. In addition, Paz et al. (2014) observed a positive correlation between total phenolic compounds and antioxidant capacity measured with DPPH method in mango pulp. Moreover, in model juices prepared exclusively with purified plum extract, a high correlation between total anthocyanins and TEAC was found (Hernández-Herrero and Frutos 2015).
Principal Component Analysis (PCA)
In a way of comparing the different treatments applied to the fruit juice-Stevia beverage, a principal component analysis was effectuated. The biplot for the first two principal components (accounting for 78 % of the total variance, with an eigenvalue > 1) is shown in Fig. 5. PEF1 and PEF2 samples are separated from the others, whereas HVED1- and USN1-treated samples and HVED2- and USN2-treated samples are clustered together.
The PCA allowed discovering which parameters seemed to drive the whole distribution. Carotenoids and ascorbic acid seemed to be the key elements influencing the bioactive compound and antioxidant capacity distribution. Furthermore, PCA indicated that ORAC might also be an important component for antioxidant interactions, as it has been well-known (Zulueta et al. 2009).
Focusing on these three parameters (total carotenoids, ascorbic acid and ORAC values), PEF-treated fruit juice-Stevia beverage at an energy input of 32 kJ/kg was the treatment that better enhanced TC, AA and ORAC values, clearly separated from the rest of the samples and from untreated beverage and followed by PEF treatment at an energy input of 256 kJ/kg (Fig. 6). Consequently, it could be concluded that of all treatments, PEF technology resulted in the highest retention of bioactive compounds and antioxidant capacity with regard to the quality of the fruit juice-Stevia beverage, always taking into account that the use of one or other technology will highly depend on which compound is pretended to be enhanced.
Conclusions
The non-thermal technologies discussed in the present study (PEF, HVED and USN) are processing technologies which can enhance bioactive compound retention and antioxidant capacity in the analysed fruit juice (papaya and mango)-Stevia blend. A high recovery of ascorbic acid, total carotenoids and ORAC values was obtained after PEF treatments. Nevertheless, HVED and USN processing technologies were also found to be useful for the retention of certain bioactive compounds. The results obtained in the present work may be used by food producers in order to obtain new functional foods rich in bioactive compounds and antioxidant capacity. However, further studies evaluating the impact of PEF, HVED and USN technology on microbiological safety, sensorial parameters and storage stability of liquid foods are required.
References
Abid, M., Jabbar, S., Wu, T., Hashim, M. M., Hu, B., Lei, S., & Zeng, X. (2014). Sonication enhances polyphenolic compounds, sugars, carotenoids and mineral elements of apple juice. Ultrasonics Sonochemistry, 21(1), 93–97.
Abuajah, C. I., Ogbonna, A. C., & Osuji, C. M. (2014). Functional components and medicinal properties of food: a review. Journal of Food Science and Technology, 52(5), 2522–2529.
Barba, F. J., Jäger, H., Meneses, N., Esteve, M. J., Frígola, A., & Knorr, D. (2012). Evaluation of quality changes of blueberry juice during refrigerated storage after high-pressure and pulsed electric fields processing. Innovative Food Science & Emerging Technologies, 14, 18–24.
Barba, F. J., Criado, M. N., Belda-Galbis, C. M., Esteve, M. J., & Rodrigo, D. (2014). Stevia rebaudiana Bertoni as a natural antioxidant/antimicrobial for high pressure processed fruit extract: processing parameter optimization. Food Chemistry, 148, 261–267.
Belda-Galbis, C. M., Pina-Pérez, M. C., Espinosa, J., Marco-Celdrán, A., Martínez, A., & Rodrigo, D. (2014). Use of the modified Gompertz equation to assess the Stevia rebaudiana Bertoni antilisterial kinetics. Food Microbiology, 38, 56–61.
Bishi, S. K., Lokesh, K., Mahatma, M. K., Khatediya, N., Chauhan, S. M., & Misra, J. B. (2015). Quality traits of Indian peanut cultivars and their utility as nutritional and functional food. Food Chemistry, 167, 107–114.
Boussetta, N., & Vorobiev, E. (2014). Extraction of valuable biocompounds assisted by high voltage electrical discharges: a review. Comptes Rendus Chimie, 17(3), 197–203.
Boussetta, N., Vorobiev, E., Deloison, V., Pochez, F., Falcimaigne-Cordin, A., & Lanoiselle, J. L. (2011). Valorisation of grape pomace by the extraction of phenolic antioxidants: application of high voltage electrical discharges. Food Chemistry, 128(2), 364–370.
Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology, 28(1), 25–30.
Carbonell-Capella, J. M., Barba, F. J., Esteve, M. J., & Frígola, A. (2013). High pressure processing of fruit juice mixture sweetened with Stevia rebaudiana Bertoni: optimal retention of physical and nutritional quality. Innovative Food Science & Emerging Technologies, 18, 48–56.
Carbonell-Capella, J. M., Buniowska, M., Esteve, M. J., & Frígola, A. (2015). Effect of Stevia rebaudiana addition on bioaccessibility of bioactive compounds and antioxidant activity of beverages based on exotic fruits mixed with oat following simulated human digestion. Food Chemistry, 184, 122–130.
Chen, J., Tao, X. Y., Sun, A. D., Wang, Y., Liao, X. J., Li, L. N., & Zhang, S. (2014). Influence of pulsed electric field and thermal treatments on the quality of blueberry juice. International Journal of Food Properties, 17(7), 1419–1427.
Criado, M. N., Barba, F. J., Frígola, A., & Rodrigo, D. (2014). Effect of Stevia rebaudiana on oxidative enzyme activity and its correlation with antioxidant capacity and bioactive compounds. Food and Bioprocess Technology, 7(5), 1518–1525.
De La Peña, M., Salvia-Trujillo, L., Rojas-Graü, M. A., & Martín-Belloso, O. (2010). Impact of high intensity pulsed electric field on antioxidant properties and quality parameters of a fruit juice-soymilk beverage in chilled storage. LWT - Food Science and Technology, 43(6), 872–881.
Evans, E. A., & Ballen, F. H. (2012). An overview of global papaya production, trade, and consumption. Resource document. University of Florida. http://edis.ifas.ufl/fe913. Accessed 19 Dec 2014.
Georgé, S., Brat, P., Alter, P., & Amiot, M. J. (2005). Rapid determination of polyphenols and vitamin C in plant-derived products. Journal of Agricultural and Food Chemistry, 53(5), 1370–1373.
Granato, D., Karnoppc, A. R., & Rutha, S. M. (2015). Characterization and comparison of phenolic composition, antioxidant capacity and instrumental taste profile of juices from different botanical origins. Journal of the Science of Food and Agriculture, 95(10), 1997–2006.
Grimi, N., Dubois, A., Marchal, L., Jubeau, S., Lebovka, N. I., & Vorobiev, E. (2014). Selective extraction from microalgae Nannochloropsis sp. using different methods of cell disruption. Bioresource Technology, 153, 254–259.
Guo, M., Jin, T. Z., Geveke, D. J., Fan, X., Sites, J. E., & Wang, L. (2014). Evaluation of microbial stability, bioactive compounds, physicochemical properties, and consumer acceptance of pomegranate juice processed in a commercial scale pulsed electric field system. Food and Bioprocess Technology, 7(7), 2112–2120.
Hernández-Herrero, J. A., & Frutos, M. J. (2015). Influence of rutin and ascorbic acid in colour, plum anthocyanins and antioxidant capacity stability in model juices. Food Chemistry, 173, 495–500.
Hsieh, C. W., & Ko, W. C. (2008). Effect of high-voltage electrostatic field on quality of carrot juice during refrigeration. LWT - Food Science and Technology, 41(10), 1752–1757.
Lee, H. S. & Castle, W. S. (2001). Seasonal changes of carotenoid pigments and color in Hamlin, Earlygold, and Budd blood orange juices. Journal of Agriculture and Food Chemistry, 49, 877–882
Martínez-Flores, H. E., Garnica-Romo, M. G., Bermúdez-Aguirre, D., Pokhrel, P. R., & Barbosa-Cánovas, G. V. (2014). Physico-chemical parameters, bioactive compounds and microbial quality of thermo-sonicated carrot juice during storage. Food Chemistry, 172, 650–656.
Mazza, G., Fukumoto, L., Delaquis, P., Girard, B., & Ewert, B. (1999). Anthocyanins, phenolics, and color of Cabernet Franc, Merlot, and Pinot Noir wines from British Columbia. Journal of Agricultural and Food Chemistry, 47(10), 4009–4017.
Muller, L., Gnoyke, S., Popken, A. M., & Bohm, V. (2010). Antioxidant capacity and related parameters of different fruit formulations. LWT - Food Science and Technology, 43(6), 992–999.
Murillo, E., Britton, G. B., & Durant, A. A. (2012). Antioxidant activity and polyphenol content in cultivated and wild edible fruits grown in Panama. Journal of Pharmacy and Bioallied Sciences, 4(4), 313–317.
Odriozola-Serrano, I., Soliva-Fortuny, R., & Martín-Belloso, O. (2009). Impact of high-intensity pulsed electric fields variables on vitamin C, anthocyanins and antioxidant capacity of strawberry juice. LWT - Food Science and Technology, 42(1), 93–100.
Paz, M., Gullon, P., Barroso, M. F., Carvalho, A. P., Domingues, V. F., Gomes, A. M., Beckerc, H., Longhinottic, E., & Delerue-Matosa, C. (2014). Brazilian fruit pulps as functional foods and additives: evaluation of bioactive compounds. Food Chemistry, 172, 462–468.
Pérez-Ramírez, I. F., Castaño-Tostado, E., Ramírez-de León, J. A., Rocha-Guzmán, N. E., & Reynoso-Camacho, R. (2015). Effect of stevia and citric acid on the stability of phenolic compounds and in vitro antioxidant and antidiabetic capacity of a roselle (Hibiscus sabdariffa L.) beverage. Food Chemistry, 172, 885–892.
Pistollato, F., & Battino, M. (2014). Role of plant-based diets in the prevention and regression of metabolic syndrome and neurodegenerative diseases. Trends in Food Science and Technology, 40(1), 62–81.
Rajha, H. N., Boussetta, N., Louka, N., Maroun, R. G., & Vorobiev, E. (2014). A comparative study of physical pretreatments for the extraction of polyphenols and proteins from vine shoots. Food Research International, 65, 462–468.
Rawson, A., Tiwari, B. K., Patras, A., Brunton, N., Brennan, C., Cullen, P. J., & O’Donnell, C. (2011a). Effect of thermosonication on bioactive compounds in watermelon juice. Food Research International, 44(5), 1168–1173.
Rawson, A., Patras, A., Tiwari, B. K., Noci, F., Koutchma, T., & Brunton, N. (2011b). Effect of thermal and non thermal processing technologies on the bioactive content of exotic fruits and their products: review of recent advances. Food Research International, 44(7), 1875–1887.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26(9–10), 1231–1237.
Ritter, E. D., & Purcell, A. E. (1981). Carotenoid analytical methods. In X. Bauernfeind (Ed.), Carotenoids as colorants and vitamin A precursors (pp. 815–923). New York: Academic.
Roohinejad, S., Everett, D. W., & Oey, I. (2014). Effect of pulsed electric field processing on carotenoid extractability of carrot purée. International Journal of Food Science and Technology, 49(9), 2120–2127.
Ross, A. I. V., Griffiths, M. W., Mittal, G. S., & Deeth, H. C. (2003). Combining nonthermal technologies to control foodborne microorganisms. International Journal of Food Microbiology, 89(2–3), 125–138.
Sarkis, J. R., Boussetta, N., Tessaro, I. C., Marczak, L. D. F., & Vorobiev, E. (2015). Application of pulsed electric fields and high voltage electrical discharges for oil extraction from sesame seeds. Journal of Food Engineering, 153, 20–27.
Siddique, A. B., Rahman, S. M. M., Hossain, M. A., & Rashid, M. A. (2014). Phytochemical screening and comparative antimicrobial potential of different extracts of Stevia rebaudiana Bertoni leaves. Asian Pacific Journal of Tropical Disease, 4(4), 275–280.
Soria, A. C., & Villamiel, M. (2010). Effect of ultrasound on the technological properties and bioactivity of food: a review. Trends in Food Science and Technology, 21(7), 323–331.
Tiwari, B. K., Muthukumarappan, K., O’Donnell, C. P., & Cullen, P. J. (2008a). Effects of sonication on the kinetics of orange juice quality parameters. Journal of Agricultural and Food Chemistry, 56(7), 2423–2428.
Tiwari, B. K., O’Donnell, C. P., Patras, A., & Cullen, P. J. (2008b). Anthocyanin and ascorbic acid degradation in sonicated strawberry juice. Journal of Agricultural and Food Chemistry, 56(21), 10071–10077.
Tiwari, B. K., ODonnell, C. P., & Cullen, P. J. (2009). Effect of sonication on retention of anthocyanins in blackberry juice. Journal of Food Engineering, 93(2), 166–171.
Toepfl, S., Mathys, A., Heinz, V., & Knorr, D. (2006). Review: potential of high hydrostatic pressure and pulsed electric fields for energy efficient and environmentally friendly food processing. Food Reviews International, 22(4), 405–423.
Toepfl, S., Heinz, V., & Knorr, D. (2007). High intensity pulsed electric fields applied for food preservation. Chemical Engineering and Processing: Process Intensification, 46(6), 537–546.
Torregrosa, F., Cortés, C., Esteve, M. J., & Frígola, A. (2005). Effect of high-intensity pulsed electric fields processing and conventional heat treatment on orange-carrot juice carotenoids. Journal of Agricultural and Food Chemistry, 53(24), 9519–9525.
Vijaya, C., Sreeramulu, D., & Raghunath, M. (2010). Antioxidant activity of fresh and dry fruits commonly consumed in India. Food Research International, 43(1), 285–288.
Wang, Y., Wang, B., & Li, L. (2008). Keeping quality of tomato fruit by high electrostatic field pretreatment during storage. Journal of the Science of Food and Agriculture, 88(3), 464–470.
Zulueta, A., Esteve, M. J., & Frígola, A. (2009). ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chemistry, 114(1), 310–316.
Zulueta, A., Esteve, M. J., & Frígola, A. (2010). Ascorbic acid in orange juice-milk beverage treated by high intensity pulsed electric fields and its stability during storage. Innovative Food Science & Emerging Technologies, 11(1), 84–90.
Zulueta, A., Barba, F. J., Esteve, M. J., & Frígola, A. (2013). Changes in quality and nutritional parameters during refrigerated storage of an orange juice-milk beverage treated by equivalent thermal and non-thermal processes for mild pasteurization. Food and Bioprocess Technology, 6(8), 2018–2030.
Acknowledgments
This research project was supported by the Spanish Ministry of Science and Technology and European Regional Development Funds (AGL2010-22206-C02-01) in the framework of the COST Action TD1104: European network for development of electroporation-based technologies and treatments (EP4Bio2Med). Carbonell-Capella, J.M. holds an award from the Spanish Ministry of Education (AP2010-2546). Buniowska, M. thanks Podkarpacie Marshal’s Office for Ph.D. scholarship number 8.2.2/IV.26/217/11/U/155/12 of RSI Project for Podkarpacie Region, and Poland. Barba, F.J. wishes to thank the Valencian Autonomous Government (Consellería d’Educació, Cultura i Esport. Generalitat Valenciana) for the postdoctoral fellowship of the VALi+d programme (APOSTD/2013/092).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carbonell-Capella, J.M., Buniowska, M., Barba, F.J. et al. Changes of Antioxidant Compounds in a Fruit Juice-Stevia rebaudiana Blend Processed by Pulsed Electric Technologies and Ultrasound. Food Bioprocess Technol 9, 1159–1168 (2016). https://doi.org/10.1007/s11947-016-1706-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-016-1706-1