Abstract
The aim of this study was to evaluate the effects of high pressure processing (HPP; 550 MPa/10 min) and high temperature short time (HTST; 110 °C/8.6 s) on microorganism, ascorbic acid, total phenols, antioxidant capacity, color, enzyme activity, and rheological behavior in red grapefruit juice during 30 days of storage at 4 °C. After HPP, the total plate count (TPC) and yeast and molds (Y & M) in red grapefruit juice were significantly decreased by 4.83 and 4.15 log cycles and showed to be microbiologically safe during storage. The activity of pectin methylesterase (PME) and peroxidase (POD) was only inactivated by 22.5 % and increased by 10.4 % by HPP, but completely inactivated by HTST. For all the other quality properties (such as total phenols, ascorbic acid, antioxidant capacity, color) except cloud stability studied in this article, their retention was significantly better in the HPP-treated samples than in the HTST-treated samples during storage, which were closer to natural grapefruit juice. A longer shelf life was observed in HPP-treated grapefruit juice compared to HTST-treated ones.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Grapefruit (Citrus grandis L.) is one of the most popular fruits consumed and cultivated in the world, with a sweet and sour taste. According to the statistics of Food and Agriculture Organization of the United Nations, there were 0.29 million-ha harvest area and more than 8.04 million tonnes of grapefruit in 2012. The grapefruit is mainly eaten freshly or processed as juices, teas (such as honey citron tea), preserves, liquor, and vinegar (Feng 2008). The middle and outer layer of grapefruit peel is also reported to contain pectin and flavone, being a source of pectin, flavone, and dietary fiber (Burana-osota et al. 2010). The popularity of grapefruit among consumers could be attributed to its clear flavor, health-promoting properties, and high nutritional value (Uckoo et al. 2011, 2012; Bhimangouda et al. 2012). Nowadays, orange juice dominates the citrus juice drink market and grapefruit juice was rarely processed.
GuiFei red grapefruit has appeared in front of us since their introduction to China in 2000 from US state of Florida, which was a variety of natural sexual offspring of grapefruit (Citrus paradisis) and pomelo (Citrus maxima) (Zhang 2009). Among the several species of citrus fruits, GuiFei red grapefruit are oblate to suborbicular, presenting bright yellow in skin and pink in flesh (Labell 1993). The mature period is from late September to late October, indicating early pomelo varieties (Feng 2008). GuiFei red grapefruit (C. grandis L.) has better processing attributes based on its unique red color, abundant bioactive compounds, and relatively low levels of peroxidase (POD) activity (Uckoo et al. 2011). Color and taste are important indicators of grapefruit juice which are easily damaged by thermal processing attributing to the breakdown of anthocyanin as the result of thermal pasteurization (Clydesdale and Francis 1976). To keep the visual and taste appeal of grapefruit juice, novel processing technologies should be employed, which also avoid loss of antioxidant capacity and make microbiologically safe.
Thermal processing is the main way to produce fruit and vegetable juice, which has some unfavorable effects on the quality of the products (Krebbers et al. 2003). As an effective manner of non-thermal processing, high pressure processing (HPP) offers 100–1000-MPa pressure processing, using water as pressure transmitting medium, at room or mild process temperature (<60 °C) for foods. To date, numerous studies have demonstrated that HPP has advantage over destroying microorganisms and inactivating enzymes in fruits, with little effects on sensory properties and nutritional value (Knorr 1993; Oey et al. 2008). Therefore, HPP has been applied for many fruit and vegetable products such as orange juice (Timmermans et al. 2011), broccoli juice (Beveridge 2002), cucumber juice (Zhao et al. 2013), raspberry juice (Garcia-Palazon et al. 2004), apple juice (Baron et al. 2006), apple jam (Queiroz et al. 2014), strawberry pulps (Cao et al. 2011, 2012), avocado paste (Jacobo-Velázquez and Hernández-Brenes 2010), apricot nectars (Wang et al. 2012), and chopped onions (Sánchez-Moreno et al. 2010), while there was rare investigation on HPP in grapefruit juice.
However, literature data on the quality of red grapefruit, especially on the effect of HPP processing on the quality of red grapefruit, is very limited. Compared with other red grapefruit juice research, the quality of red grapefruit juice was studied systematically and comprehensively in the respect of microbial safety, physicochemical property, and rheological characteristics in this work. In this study, GuiFei red grapefruit (C. grandis L.) was selected for juice HPP processing. The objective of this work was to compare the impact of HPP and HTST treatments of grapefruit juice on microorganisms, total phenols, ascorbic acid, antioxidant capacity, color parameters, activity of pectin methylesterase (PME) and POD, and rheological feature after processing and during storage at 4 °C.
Materials and Methods
Chemicals
Analytical reagent chemicals, such as catechol, guaiacol, disodium hydrogen phosphate, sodium dihydrogen phosphate, and Folin-Ciocalteu reagent, were purchased from Beijing Yixiubogu Biological Technology Co., Ltd. (Beijing, China). Polyvinylpyrrolidone (PVPP), ascorbic acid, Tris(hydroxymethyl)aminomethan (Tris), (±)-6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylicacid (Trolox), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and 2,4,6-tri-2-pyridyl-1,3,5-triazine (TPTZ) were purchased from Beijing Baierdi Biological Technology Co., Ltd. (Beijing, China). Methyl alcohol and acetonitrile of HPLC grade were obtained from SK Chemical Co., Ltd. (Seoul, Korea). Plate count agar and rose Bengal medium were obtained from Beijing Aoboxing Biological Technology Co., Ltd. (Beijing, China). Other chemicals were provided by Beijing Yixiubogu Biological Technology Co., Ltd. (Beijing, China).
Preparation of Grapefruit Juice
In this study, the grapefruit variety “Guifei”, harvested at commercial maturity in October 2010, was obtained from a local market (Xinfadi) in Beijing (China). Fresh grapefruit were decorticated to get nearly 500 g of grapefruit meat and then were juiced with a screw extractor (GT6G7, Zhejiang Jixie Co., Zhejiang, China). The pomace had been sufficiently squeezed for three times. The squeezed grapefruit juice were filtered with four layers gauze and centrifuged at 2000 rpm for 10 min. Then the grapefruit juice was uniformly mixed, and part of it was filled into 60-mL EVOH plastic bottles for HPP and temporarily stored at 4 °C.
HPP and HTST Processing of Grapefruit Juice
The bottled grapefruit juice was processed by HPP (HHP-700, Baotou Kefa Co., Ltd., Inner Mongolia, China) at 550 MPa for 10 min at room temperature (approximately 25 °C) with an average pressurization rate of 120 MPa/min and releasing pressure rate of 550 MPa/min.
A tubular heat exchanger unit (Armfield FT74, HTST/UHT Processing Unit, Hampshire, England) was used for HTST (110 °C/8.6 s) treatment (Uemura and Inoue 2010). Then the juice was cooled to 20 °C and was aseptically transferred into aseptic 60-mL EVOH plastic bottles identical to the ones used for the HPP-treated samples.
According to our previous research studies, after HPP (550 MPa/10 min) and HTST treatments (110 °C/8.6 s), the counts of the total plating count (TPC) were less than 2.00 log 10 CFU/mL, and yeasts and molds (Y & M) were not detected which met the requirements of Chinese Drink Standard GB 10789–2007. Therefore, the grapefruit juice was processed by these treatments in this study.
Storage Conditions
The treated samples were stored at 4 ± 2 °C in darkness. Then sample analyses were carried out after 0, 3, 6, 9, 15, 20, 25, and 30 days of storage.
Microbial Analysis
To count viable microorganisms in grapefruit juice, TPC method was used. The samples were serially diluted with sterile 0.85 % NaCl solution, and 1.0 mL of each dilution was plated into duplicate plates of appropriate agar. Nutrient agar was used for counting the TPC after incubation at 36 ± 1 °C for 48 h and rose Bengal agar was used for counting the Y & M after incubation at 25–28 °C for more than 72 h. After incubation, the colonies were counted.
Physicochemical Characteristics Analysis
Samples were equilibrated at 25 °C to measure pH, titrable acidity (TA), and total soluble solid (TSS). The pH value was measured by Thermo Orion 868 pH meter (Thermo Fisher Scientific, Inc., MA, USA). TSS was measured by WAY-2S digital Abbe refractometer (Shanghai Precision and Scientific Instrument Co., Shanghai, China), and the results were reported as °Brix. TA was measured by 842 GPD titrino, an automatic potentiometric titrator (Metrohm, Switzerland), and the results were expressed as percentages of citric acid content.
Determination of Browning Degree
The browning degree (BD) was determined using a spectrophotometric method described by Roig et al. (1999) with some modifications. Grapefruit juice was centrifuged at 10,000 rpm for 15 min at 4 °C with a refrigerated CF16RXII centrifuge (Hitachi Limited Company, Japan), then passed through a 0.45-μm cellulose nitrate membrane (Beijing Bomex Co., Beijing, China). The BD was analyzed by measuring the absorbance at 420 nm using Cary-50 ultraviolet-visible spectrophotometer (Varian Co., USA) at 25 ± 2 °C.
Determination of Cloud Stability
The cloud stability was measured by spectrophotometric method described by Rodrigo et al. (2003) with some modifications. Cloud stability was analyzed by transmittance at 660 nm using a UV-762 spectrophotometer (Shanghai Precision and Scientific Instrument Co., Shanghai, China) after the centrifugation of 4200 rpm for 15 min at 4 °C.
Color Assessment
The color value was evaluated at 25 ± 2 °C by the color measurement spectrophotometer (Hunter Lab Color Quest XE, Hunter Associates Laboratory, Inc., Virginia, USA) in the reflectance mode. The color value was expressed as L* (lightness; 0 = black, 100 = white), a* (−a* = greenness, +a* = redness), and b* (−b* = blueness, +b* = yellowness). The results were averaged and three measurements were performed. ∆E, which was calculated using the following equation, was used to express the total color difference by Eq. (1). L*0, a*0, and b*0 are the control values for untreated samples.
Determination of Total Phenols
Total phenols were measured by the Folin-Ciocalteu method described by Singleton et al. (1999). Five hundred microliters of fivefold of diluted grapefruit juice was mixed with 2 mL of Folin-Ciocalteu reagent (previously diluted tenfold with distilled water) and added with 1.8 mL of sodium carbonate (105.99 g/mol), then set at room temperature in the dark for 1 h. Absorption values were measured at 765 nm by a spectrophotometer (UV-726, Shanghai Precision and Scientific Instrument Co., Shanghai, China). The results were expressed as milligram of gallic acid equivalent (GAE) per 100 g of grapefruit juice.
HPLC Analysis of Ascorbic Acid
To extract and analyze ascorbic acid in grapefruit juice, the method proposed by Cao et al. (2012) was employed with some modifications. Fifty-gram grapefruit juice was mixed with 50-mL metaphosphoric acid (2.5 %) and incubated for 2 h at 4 °C, and then the mixture was centrifuged at 10,000 rpm for 15 min at 4 °C. The supernatant was collected after centrifugation.
After being passed through a 0.45-μm cellulose nitrate membrane, grapefruit juice was prepared to be tested. Ascorbic acid was separated and detected by a liquid chromatograph (LC-20AT) equipped with a UV-Vis detector (SPD-20AV), an auto sampler (SIL-20A), and a column oven (CTO-20A) from Shimadzu Co., Japan. The separation was performed using Sunfire TM C18, (4.6 × 250 mm i.d, 5-μm particle size) from Waters. The mobile phase was an isocratic solvent system which consisted of 95 % monopotassium phosphate (50 mM, pH = 3.0) and 5 % acetonitrile; aliquots of 20 μm were injected and the flow rate was 1 mL/min. The detection was conducted at 245 nm in absorbance mode at 30 °C. The calibration curves used for quantification were prepared from external standard. Results were showed as milligrams of ascorbic acid per 100 mL of grapefruit juice.
Determination of PPO, POD, and PME Activity
PPO Activity Assay
The polyphenoloxidase (PPO) extraction and activity determination was conducted by Wang et al. (2008), with minor modifications. Twenty-five milliliters of grapefruit juice was homogenized with 50 mL of 0.2 M phosphate buffer of polyvinylpyrrolidone (PVPP) (pH 6.5) for 4 h at 4 °C and centrifuged at 10,000 rpm for 15 min at 4 °C. The supernatants were used for further testing.
PPO activity was measured using a continuous spectrophotometric method (UV-726, Shanghai Precision and Scientific Instrument Co., Shanghai, China) with the reaction substrate of 2-mL catechol solution (0.07 M). Substrate was incubated for 10 min at 30 °C and then was mixed with 1 mL of grapefruit juice PPO enzyme extraction, and the absorption curve of time curve during 0.1 s at 470 nm for 15 min was immediately determined. The slope of linear port of absorption curve was used for the calculation of enzyme activity. Enzyme activity unit (U) was defined as the 0.001 unit of change caused by 1 mL enzyme extraction in 1 min.
POD Activity Assay
The extraction of POD was as same as the method described in “PPO Activity Assay.”
POD activity was also measured using a continuous spectrophotometric method (UV-726, Shanghai Precision and Scientific Instrument Co., Shanghai, China). The reaction substrate of POD was 2 mL of 1.0 % (v/v) guaiacol (diluted by 0.2 M pH 6.5 phosphate buffer) and 0.2 mL of 1.5 % (v/v) hydrogen peroxide. Substrate was incubated at 30 °C for 10 min and then was mixed with 0.4 mL grapefruit juice POD enzyme extraction, and the absorption curve of time curve during 0.1 s at 470 nm for 1 min was immediately determined. The slope of linear port of absorption curve was used for the calculation of enzyme activity.
PME Activity Assay
To extract PME, 25 mL of grapefruit juice was mixed with 50 mL of 0.2 M Tris-HCl buffer (pH 8, including 0.1 M NaCl) for 12 h at 4 °C and centrifuged at 10,000 rpm for 15 min at 4 °C (Cao et al. 2011, 2012). The supernatants were used for further testing.
PME activity was assayed by an automatic potentiometric titrator (842 GPD titrino, Metrohm, Switzerland) measuring the release ratio of pectic acid at pH 7.5 and 30 °C described by Sampedroa et al. (2008) with some modifications. Forty milliliters of pectin solution was incubated in 30 °C thermostatic water bath until pH was regulated to 7.5. The PME extract (5 mL) was added to the substrate, and pH was readjusted to 7.5 by 0.05 M NaOH. Then NaOH was continuously added in 25 min to calculate amount of usage. The slope of the linear portion of the curve of V NaOH (mL) versus t (minutes) was used to calculate the PME activity by Eq. (2) as follows:
where C NaOH is the NaOH concentration (0.05 M) and V Sample is the sample volume (5 mL of PME extract).
Determination of Antioxidant Capacity
·DPPH Assay
The ·DPPH assay was described by Brand-William et al. (1995) with some modifications. The reaction started by adding 100 μL of fourfolds of diluted grapefruit juice to the cuvette containing 4 mL of methanol solution (0.14 mol/L) of the free radical (·DPPH). The mixture was set in the dark at room temperature for 45 min and then measured the decrease in the absorption at 517 nm. Determinations were conducted by a spectrophotometer (UV-726, Shanghai Precision and Scientific Instrument Co., Shanghai, China). Trolox solutions were used for calibration within the range of 100–1000 μmol/L, while the baseline was corrected by methanol.
FRAP Assay
The FRAP assay was described by Benzie and Strain (1996) with some modifications. Prepared FRAP solution was consisted of 25 mL of 0.3 M acetate buffer (pH 3.6), 2.5 mL of 10 mM TPTZ (dissolved in 40 mM HCl), and 2.5 mL of 20 mM ferric chloride. Four milliliters of FRAP solution was mixed with 100 μL of fourfolds of diluted grapefruit juice at 37 °C. After setting in dark for 10 min, the ferric reducing ability of samples was measured by monitoring the increase of absorbance at 593 nm with a spectrophotometer (UV-726, Shanghai Precision and Scientific Instrument Co., Shanghai, China). Trolox solutions within the range of 100–1000 μmol/L were used for calibration.
The results expressing as radical scavenging activity of ·DPPH and FRAP were calculated by Eq. (3) as follows:
where A 1 is the absorbance at 517 or 593 nm of the control and A 2 is the absorbance in presence of grapefruit juice extract.
Determination of Rheological Measurement
Rheological property was measured immediately after HPP treatments by using a controlled shear rate AR 550 rheometer (TA Instruments, Waters Co., USA) as previously reported by Xiao et al. (2012). Rheological measurements were performed in triplicate by cone head concentric cylinder (stator radius = 15.00 mm, rotor radius = 14.00 mm, immersion altitude = 42.00 mm, space = 5920 μm). The grapefruit juice (15 mL) was placed at 25 °C provided by circulating water.
The analysis software owned by the instrument could be used for automatic data processing. The shear stress and shear rate data were conducted by curve fitting analysis, and specific fitting model were Bingham model, Power Law model, and Herschel-Bulkley model. Choose the best fitting model according to the regression coefficient (R 2) and the residual sum of squares (RSS).
Determination of Particle Size Distribution Measurement
The particle size distribution (PSD) values were determined by a LS 230 particle size analyzer (Beckman Coulter, Inc. Miami, FL, USA) as reported by Xiao et al. (2012) with some modifications. A laser light with a wavelength of 750 nm was used to determine the particles from 0.375 to 2000 μm by light diffraction in triplicate. Distilled water was poured into the sample cell at the rate of 8 L/min to clean instrument three to five times; after that the cell was filled full. The grapefruit juice was added to the cell until the concentration reached 8–12 %.
The volume-based mean diameter D [4, 3] (Eq. 4) and area-based mean diameter D [3, 2] (Eq. 5) are recorded. The D [4, 3] is mainly influenced by large particles, while the D [3, 2] is majorly influenced by smaller particles (Augusto et al. 2012).
where n i is the number of particles with diameter d i .
Statistical Analysis
All experiments were performed in triplicate. The data were handled using the Statistical Package for the Social Sciences (SPSS 17.0, Chicago, IL, USA) software for analysis of variance and Duncan’s test. Significance was established at P < 0.05. Results were expressed by mean ± S.D. All data were drawn by Origin Pro 9.2.
Results and Discussion
The pH (3.48), TSS (12.96 °Brix), and TA (0.89 %) showed no significant change after HPP and HTST and during 30 days of storage at 4 °C, and the shear stress and viscosity of HPP- and HTST-treated samples also did not exhibit significant difference (data not shown).
Microbiological Analysis
As shown in Table 1, the initial counts of TPC and Y & M in grapefruit juice were 4.83 and 4.15 log10 CFU/mL, respectively, and they were all below detection limit (=1 CFU/mL) after HPP and HTST. The inactivation of TPC and Y & M by HPP was reported in grapefruit juice (Uckoo et al. 2013), orange juice (Timmermans et al. 2011), apple juice (Landl et al. 2010), blueberry juice (Barba et al. 2012), pepper juice (Hernández-Carrión et al. 2014), and other fruit and vegetable products. Uckoo et al. (2013) found that the TPC and Y & M of grapefruit (Citrus paradisi Macfad) juice (pH 3.0–3.2) were below the detection limit (=1 CFU/mL) after HPP (400 MPa/3 min) and thermal processing (85 °C/45 s). Timmermans et al. (2011) also reported that the TPC and Y & M of orange juice (pH 3.3) were detected below 10 CFU/mL after HPP (600 MPa/1 min) and mild heat pasteurization (72 °C/20 s). Mckay et al. (2011) claimed that the TPC and Y & M of apple juice were reduced to be below the detection limit (=1 CFU/mL) immediately after HPP (500 MPa/1 min/20 °C).
During refrigeration, no TPC in HPP-treated samples was detected before 25 days of storage, and the TPC was 1.60 log10 CFU/mL at day 30, while the TPC in HTST-treated samples was detected as 1.55 log10 CFU/mL at day 9 and showed no significant increase in the following 16 days. The Y & M in HPP-treated samples was below the detection limit during 30 days of refrigerated storage, while it was detected in HTST-treated samples after 25 days of refrigerated storage.
These results indicated that the TPC and Y & M in HPP-treated samples during refrigeration showed a lower level compared with HTST-treated samples in this study. The microbial inactivation was attributed to the killing effect of HPP. Low pH (3.47) in HPP- and HTST-treated grapefruit juices and low temperature of 4 °C were conducive to control microbial growth during 30 days of storage. The Y & M is more sensitive to HPP and was inactivated in orange juice (Timmermans et al. 2011), pomegranate juice (Varela-Santos et al. 2012), pear juice (Rendueles et al. 2011), and cranberry juice (Lavinas et al. 2008). Uckoo et al. (2013) reported that the count of TPC and Y & M in grapefruit juice was always under detection limit after HPP (400 MPa/3 min) and thermal processing (85 °C/45 s) during 28 days of storage at 4 °C. Mckay et al. (2011) also reported that the TPC and Y & M in apple juice (pH 3.3–3.7) after HPP (500 MPa/1 min/20 °C) did not increase during 35 days of storage at 4 °C. Landl et al. (2010) observed that the TPC and Y & M in apple puree (pH 3.2) after HPP (400–600 MPa/5 min) was always under the detection limit during 21 days of storage at 5 °C.
The recovery of the TPC and Y & M in two grapefruit juices in this study was possibly attributed to the occurrence of sublethal bacteria (Bull et al. 2005) and/or viable but non-culturable state (VBNC) (San Martin et al. 2002), which were not detected by total plating method. Picouet et al. (2014) also claimed that the counts of TPC and Y & M were under the detection limit in HPP-treated (600 MPa/5 min) carrot juice (pH 6.4), while they were detected as 1.4 and 2.3 log10 CFU/mL after 21 days of storage at 5 °C.
Total Phenols, Ascorbic Acid, and Antioxidant Capacity
As shown in Fig. 1a, total phenols in HPP-treated samples showed no significant difference (P > 0.05) compared with the control, but those in HTST-treated samples decreased by 7.7 %, which was attributed to thermal decomposition. Similarly, Landl et al. (2010) and Queiroz et al. (2014) claimed that no change of total phenols in apple puree and apple juice was caused by HPP at 400 MPa/20 °C/5 min and 400 MPa/7 min, respectively. However, Wang et al. (2012), Varela-Santos et al. (2012), and Barba et al. (2012) reported that the content of total phenols in purple sweet potato juice, pomegranate juice, and blueberry juice was slightly but not significantly higher (P > 0.05) than that in the control after HPP at 400–600 MPa/2.5–10 min, 350–550 MPa/0.5–2.5 min, and 200 MPa/5–15 min, respectively. The difference could be probably due to the different food system (e.g., the residual enzyme activity PPO) and processing conditions (e.g., boosting/unloading rate and temperature change within the autoclave) (Keenan et al. 2012). The content of total phenols treated by both HPP and HTST showed no significant decline during 30 days of refrigerated storage at 4 °C, and the content of total phenols in HPP-treated samples was always higher than that in HTST-treated samples. Landl et al. (2010) observed that the level of total phenols remained unchanged during 21 days of storage at 4 °C after HPP (400 MPa/5 min). Barba et al. (2012) indicated that the content of total phenols slightly decreased by 9.3 % during 56 days of refrigerated storage after HPP (600 MPa/5 min).
Changes in total phenols (a) and ascorbic acid (b) in grapefruit juice treated by control (■), high pressure processing (□, HPP, 550 MPa/10 min), and high temperature short time (⊠, HTST, 110 °C/8.6 s) during 30 days of storage at 4 °C. All data were the means ± SD, n = 3. The capital letter within one column are significantly different (P < 0.05). Values with different letters within one row are significantly different (P < 0.05).
After processing, HPP caused 8.82 % loss of ascorbic acid in grapefruit juice, while HTST resulted in 27.9 % loss of ascorbic acid in grapefruit juice, indicating that thermal processing induced greater loss of ascorbic acid. As shown in Fig. 1b, HPP-treated grapefruit juice had a significant higher content of ascorbic acid than HTST-treated grapefruit juice, which could be attributed to ascorbic acid degradation by high temperature. Uckoo et al. (2013) reported 4.8 and 11.9 % loss of ascorbic acid in grapefruit juices by HPP at 400 MPa/31 °C/3 min and thermal processing at 85 °C/45 s, showing greater loss of ascorbic acid in grapefruit juice by thermal processing; however, these loss percentages of ascorbic acid were different from this study, which resulted from different processing parameters. During 30 days of refrigeration storage, ascorbic acid in HPP- and HTST-treated samples showed a reduction by 21.1 and 22.4 %. This significant loss in both HPP- and HTST-treated samples were associated with chemical degradation, especially associated with non-enzymatic browning reaction, as well as the aerobic oxidation which reacted with the content of ascorbic acid (Davidek et al. 1990; Oey et al. 2008), respectively. This result was in accordance with earlier studies about orange juice (Wicklund et al. 2005), strawberry juice (Li et al. 2010), apple juice (Guerrero-Beltran et al. 2005), and pomegranate juice (Keenan et al. 2012). Uckoo et al. (2013) reported that the content of ascorbic acid in grapefruit juice by HPP at 400 MPa/31 °C/3 min and thermal processing at 85 °C/45 s declined by 32.5 and 18.9 % during refrigerated storage. Landl et al. (2010) also reported a greater loss of ascorbic acid content about 75.2 and 81.8 % after HPP (400–600 MPa/31 °C/3 min) and mild conventional pasteurization (75 °C/10 min) during 21 days of storage at 5 °C, respectively.
As shown in Fig. 2, compared with control sample, the antioxidant capacity of grapefruit juice right after HPP using ·DPPH and FRAP methods had no significant difference, while it was decreased by 1.4 and 8.5 % after HTST, respectively, indicating that HPP helped preserve the antioxidant capacity of grapefruit juice. Similarly, Butz et al. (2003) reported that the antioxidant capacity was not obviously influenced by HPP at 600–800 MPa in orange juice, apple juice, and mixed citrus juice. Basu (2012) found that the antioxidant capacity using ·DPPH method was decreased by 25 % by thermal processing. Cao et al. (2012) also claimed similar result in clarified and cloudy strawberry juice by HPP (550 MPa/5 min), HTST (110 °C/8.6 s), and thermal processing (90 °C/1 min). Figure 2 showed that the antioxidant capacity using ·DPPH and FRAP was decreased by 5.0 and 14.3 % in HPP-treated grapefruit juice and 5.3 and 12.8 % in HTST-treated grapefruit juice during 30 days of storage at 4 °C, respectively. During refrigerated storage, the antioxidant capacity of HPP-treated samples was always higher than that of HTST-treated samples, which was in accordance with the losses of total phenols and ascorbic acid by treatments of HPP and HTST (Fig. 2). This result was similar to previous reports for strawberry juice during 6 months of storage at 4 °C (Oszmiański and Wojdyło 2009) and for cashew apple juice during 45 days of storage at 4 °C (Queiroz et al. 2014).
Changes in antioxidant capacity of ·DPPH (a) and FRAP (b) in grapefruit juice treated by control (■), high pressure processing (□, HPP, 550 MPa/10 min), and high temperature short time (⊠, HTST, 110 °C/8.6 s) during 30 days of storage at 4 °C. All data were the means ± SD, n = 3. The capital letter within one column are significantly different (P < 0.05). Values with different letters within one row are significantly different (P < 0.05).
Color Analysis
Color differences of grapefruit juices after HPP and HTST during 30 days of storage at 4 °C were shown in Table 1 by measuring Hunter L*, a*, and b*. A higher L* value was exhibited in HTST-treated samples compared with the control, while the L* value was not changed in HPP-treated samples. The increase of the L* value was probably due to the damage of levels of anthocyanin content (Cortes et al. 2007). These results were in accordance with earlier findings in grapefruit juice at 4 °C (Uckoo et al. 2013), in apricot juice at 4 °C (Huang et al. 2013), in orange juice at 4 and 10 °C (Cortes et al. 2007), and in orange-carrot compound juice at normal temperature (Rivas et al. 2006). The L* value in HPP- and HTST-treated samples did not significantly change during refrigerated storage. Uckoo et al. (2013) also reported similar result in grapefruit juice during 21 days of refrigerated storage.
The a* value was decreased by both HPP and HTST, while it showed a slightly higher level in HPP-treated samples. This result was in accordance with the report of González-Cebrino et al. (2013) that a* value exhibited a higher level after HPP (400–600 MP/5 min) in plum juice. Picouet et al. (2014) reported that a* value was higher by HPP (600 MPa/5 min) compared with mild heating (80 °C/7 min) in acidified carrot juice. At the end of refrigerated storage, the a* value showed a significant increase in HPP-treated samples, while it showed no significant change in HTST-treated samples. It has been considered that the redder color could be more attractive to consumers for grapefruit juice. However, Uckoo et al. (2013), Perera et al. (2010), and Barba et al. (2012) noticed no significant change of a* in grapefruit juice, pineapple juice, and blueberry juice during refrigerated storage, which were different from this study, which were most probably attributed to different processing parameters and various food system.
The b* value, which represents the yellowness, showed higher in HTST-treated samples compared with HPP-treated samples. Similarly, Uckoo et al. (2013) reported that thermal processing (85 °C/45 s) achieved higher b* value than HPP (450 MPa/3 min) in grapefruit juice. The b* value increased slightly in HTST-treated samples during storage. It also showed a significant increase in HPP-treated samples during the storage, and the color shift toward positive b* directions became significantly different after the ninth day of storage. The increase of b* values after HPP during storage was also reported by González-Cebrino et al. (2013) in plum juice, Picouet et al. (2014) in carrot juice, and Mert et al. (2013) in grape juice.
It has been considered that a total color change (ΔE, which indicates the magnitude of the color difference of 3.00) could be a noticeable visual difference in a number of situations (Tangwongchai et al. 2000; Terefe et al. 2009). The ΔE value showed that there was no obvious change in grapefruit juice after HPP (ΔE between 0.14 and 0.63) and HTST (ΔE between 1.75 and 2.48) during refrigerated storage. This result was in accord with the report of Uckoo et al. (2013) in grapefruit juice during the 21 days of storage at 4 °C. Tangwongchai et al. (2000) and Torres et al. (2011) also observed similar results in blueberry juice, strawberry juice, and blackberry purees.
As shown in Table 1, the BD value was not changed after HPP but significantly increased 9.4 % after HTST (P < 0.05); this greater BD value in HTST-treated grapefruit juice, which was attributed to thermal destruction, was in accordance with previous investigations in strawberry juice (Cao et al. 2012) and in grape juice (Mert et al. 2013). During refrigerated storage, BD value increased by 17.2 and 46.7 % in HPP- and HTST-treated samples, respectively, showing a higher BD value in HTST-treated samples. The increase of BD value may be attributed to combined effect inducing enzymatic browning, non-enzymatic browning, degradation of pigments, and the growth of microorganisms (Wang et al. 2006; Landl et al. 2010). In this study, the PPO activity was below the detection limit, and POD had no significant difference during the storage, indicating that enzymatic browning had little effect on the BD value. According to the report of Huang et al. (2013), amino-carbonyl reaction was not the main reason attributing to browning of apricot juice in storage at 4 °C. It was not studied and cannot be decided that the increase of BD owning to non-enzymatic browning in this paper. At the end of storage, 1.92 log10 CFU/mL TPC and 1.17 log10 CFU/mL Y & M was observed in HTST-treated samples and 1.6 log10 CFU/mL TPC in HPP-treated samples, which is probably the reason of BD increase of both treatments. The content of ascorbic acid was decreased by 21.1 and 22.4 % in HPP and HTST-treated samples during cold storage, respectively, which was probably the reason of BD decreasing.
PME and POD Activity
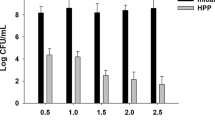
The activity of PPO, which causes browning in fruit and vegetable juices, was below the detection limit in all samples. The activities of PME and POD were completely inactivated by HTST in this study, and no activity was detected during storage.
As shown in Fig. 3, the activity of PME after HPP lost about 22.5 % compared with that of the control, showing its barotolerancy. Similarly, Liu et al. (2012) reported that the activity of PME was only reduced by 18.1 % after HPP (600 MPa/1 min) in mango pulp. However, Igual et al. (2013) achieved the 80 % loss of PME activity after HPP (700 MPa/75 °C/20 min) in grapefruit jam, which was probably due to high temperature during HPP processing. During refrigerated storage, the activity of PME showed a significant 24.1 % reduction. Similar results were also reported in avocado pulp (Jacobo-Velázquez and Hernández-Brenes 2010), in avocado juice (Gomes and Ledward 1996), in mango puree (Ramos-Parra 2006), in green bean (Krebbers et al. 2002), and in greek navel orange juice (Polydera et al. 2005).
Changes in PME (□) and POD (⊠) activities in grapefruit juice treated by control (■), high pressure processing (HPP, 550 MPa/10 min) during 30 days of storage at 4 °C. All data were the means ± SD, n = 3. The capital letter within one column are significantly different (P < 0.05). Values with different letters within one row are significantly different (P < 0.05). PME and POD were completely inactivated by high temperature short time (HTST, 110 °C/8.6 s)
Furthermore, HPP increased the activity of POD by 10.4 % in comparison with the control (Fig. 3). The increased activity was probably due to greater extractability of enzymes attributed to pressure on membrane permeability (Soysal et al. 2004; Igual et al. 2013; Rastogi et al. 2007). Hsu (2008) also reported that the activity of POD was increased by HPP (350 MPa/30 min) in tomato, lettuce, and onion. Garcia-Palazon et al. (2004) observed that the activity of POD was increased by 13 and 1 % in HPP-treated strawberry at 400 MPa applied for 5 and 10 min, respectively. However, more than 600 MPa of high pressure and more than 10 min of processing time seemed to reduce the activity of POD, but not completely, to inactivate it in different food systems, such as reduced by 35 % in strawberry juice at 600 and 800 MPa/15 min (Garcia-Palazon et al. 2004), by 71 % in kiwifruit juice at 600 MPa/50 °C/30 min (Liang et al. 2008), and by 91 % in carrot juice at 600 MPa/45 °C/15 min (Soysal et al. 2004). During the refrigerated storage, the activity of POD in HPP-treated grapefruit juice was not significantly changed (P > 0.05). Yen and Lin (1996) also reported that the activity of POD in HPP-treated (600 MPa/15 min) guava puree indicated no significant difference during 30 days of storage at 4 °C.
PSD and Cloud Stability
Figure 4 showed the change in PSD against the volume fraction of HPP- and HTST-treated samples at days 0 and 30. As shown in Tables 2 and 3, the PSD of controlled grapefruit juice was between 0.95 and 76.42 μm, and the D [4,3] and D [3,2] were 11.37 ± 1.30 and 4.85 ± 0.32 μm, respectively. In HTST-treated samples, the volume fraction of larger particles reduced from 1.16 to 0.13 % and smaller particles increased from 3.88 to 4.75 %, indicating higher cloud stability (Fig. 4). However, the PSD in HPP-treated samples had no significant difference from the control. Ahmed et al. (2005) also reported the similar result in mango pulp after HPP (100–400 MPa/15 min). Zhou et al. (2010) claimed that the PSD of raspberry juice was not affected by HPP (400–600 MPa/20 min) except increasing the particle number at peak. At day 30 of refrigerated storage, the PSD of larger particles (10–100 μm) significantly decreased and that of smaller particles (0.1–1 μm) relatively increased in both HPP- and HTST-treated samples. Baron et al. (2006) also reported this result in apple juice by HPP (425 MPa/5.5 min) and thermal processing (65 °C/90 min), respectively.
Changes in particle size distribution in grapefruit juices treated by control, high pressure processing (HPP, 550 MPa/10 min), and high temperature short time (HTST, 110 °C/8.6 s). Black line represented the control, red and purple lines represented HPP at day 0 and day 30, and blue and green lines represented HTST at day 0 and day 30. Data of other days were not shown
The cloud stability of HPP- and HTST-treated samples was indicated in Fig. 5. HPP did not change the cloud stability of grapefruit juice, while HTST caused an obvious increase by 28.6 %, which was in accordance with the result of PSD. This result was probably due to the centrifugal separation of macromolecular material caused by thermal processing in HTST-treated samples (Beveridge 2002). Timmermans et al. (2011) also reported that mild heat pasteurization (72 °C/20 s) was found to result in a more stable orange juice compared with HPP (600 MPa/1 min). Krapfenbauer et al. (2006) observed that cloud stability achieved slightly higher residual values by mild heating (70 °C/100 s). The cloud stability of both HPP- and HTST-treated samples significantly declined by 70.35 and 70.77 % during refrigerated storage, respectively. The cloud stability of HTST-treated samples was always higher than that of HPP-treated samples during refrigerated storage; this result was in agreement with earlier reports in orange juice (Goodner et al. 1999) and in cloudy apple juice (Krapfenbauer et al. 2006).
Changes in cloud stability in grapefruit juice treated by control (■), high pressure processing (□, HPP, 550 MPa/10 min), and high temperature short time (⊠, HTST, 110 °C/8.6 s) during 30 days of storage at 4 °C. All data were the means ± SD, n = 3. The capital letter within one column are significantly different (P<0.05). Values with different letters within one row are significantly different (P < 0.05).
Conclusion
The treatment of HPP achieved better quality properties of grapefruit juice compared with HTST. During the 30 days of storage at 4 °C, HPP-treated samples showed to be microbiologically safe. HPP-treated juice preserved a higher content of total phenols and better antioxidant capacity in storage. Especially the color of grapefruit can be better protected by HPP treatment and got redder in the storage showing more attraction. The rheological feature can be achieved by the HPP treatment, which was closer to the fresh grapefruit juice, while PME and POD enzymes were completely inactivated by the processing of HTST instead of HPP. Hence, HPP treatment was an innovative and commercialized way to process fresh grapefruit juice.
References
Ahmed, J., Ramaswamy, H. S., & Hiremath, N. (2005). The effect of high pressure treatment on rheological characteristics and colour of mango pulp. International Journal of Food Science and Technology, 40, 885–895.
Augusto, P. E. D., Ibarz, A., & Cristianini, M. (2012). Effect of high pressure homogenization (HPH) on the rheological properties of tomato juice: Time-dependent and steady-state shear. Journal of Food Engineering, 111(4), 570–579.
Barba, F., Jäger, H., Meneses, N., Esteve, M., Frígola, A., & Knorr, D. (2012). Evaluation of quality changes of blueberry juice during refrigerated storage after high-pressure and pulsed electric fields processing. Innovative Food Science & Emerging Technologies, 14, 18–24.
Baron, A., Dénes, J.-M., & Durier, C. (2006). High-pressure treatment of cloudy apple juice. LWT-Food Science and Technology, 39(9), 1005–1013.
Basu, S. S. (2012). U.S. rheological, textural, microstructural, and sensory properties of sorbitol-substituted mango jam. Food and Bioprocess Technology. doi:10.1007/s11947-012-0795-8.
Benzie, I. F. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry, 239, 70–76.
Beveridge, T. (2002). Opalescent and cloudy fruit juices: formation and particle stability. Critical Reviews in Food Science and Nutrition, 42(4), 317–337.
Bhimangouda, S. P., Jayaprakasha, G. K., Murthy, K. N. C., & Navindra, P. S. (2012). Emerging trends in dietary components for preventing and combating disease. American Chemical Society, 6, 1093.
Brand-William, W., Cuvelier, M. E., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology, 28, 25–30.
Bull, M. K., Hayman, M. M., Stewart, C. M., et al. (2005). Effect of prior growth temperature, type of enrichment medium, and temperature and time of storage on recovery of Listeria monocytogenes following high pressure processing of milk. International Journal of Food Microbiology, 101, 53–61.
Burana-osota, J., Soonthornchareonnonb, N., Chaidedgumjorna, A., Hosoyamac, S., & Toida, T. (2010). Determination of galacturonic acid from pomelo pectin in term of galactose by HPAEC with fluorescence detection. Carbohydrate Polymers, 81(2), 461–465.
Butz, P., Fernandez-Garcia, A., Lindauer, R., et al. (2003). Influence of ultra high pressure processing on fruit and vegetable products. Journal of Food Engineering, 56(2–3), 233–236.
Cao, X., Xu, W., Liao, X., Hu, X., & Zhang, Y. (2011). Effect of high pressure processing steps on antioxidants and antioxidant activity in strawberry juice drinks. Food and Nutrition in China, 17(10), 30–35.
Cao, X. M., Zhang, Y., Zhang, F. S., et al. (2012). Effects of high hydrostatic pressure on enzymes, phenolic compounds, anthocyanins, polymeric color and color of strawberry pulps. Journal of the Science of Food and Agriculture, 91(5), 877–885.
Cortes, C., Esteve, M. J., & Frigola, A. (2007). Color of orange juice treated by high intensity pulsed electric fields during refrigerated storage and comparison with pasteurized juice. Food Control, 19, 151–158.
Clydesdale, F. M., & Francis, F. J. Pigments. (1976). Food Chemistry, 385–426.
Davidek, J., Velisek, J., & Pokorny, J. (1990). Chemical changes during food processes. Oxford:Elsevier.
Feng, C. (2008). Study on the processing technic of high transmittance shaddock juice. Fuzhou, Fujian. doi:10.7666/d.y1515119.
Garcia-Palazon, A., Suthanthangjai, W., Kajda, P., & Zabetakis, I. (2004). The effects of high hydrostatic pressure on b-glucosidase, peroxidase and polyphenoloxidase in red raspberry (Rubus idaeus) and strawberry (Fragaria ‘ ananassa). Food Chemistry, 88, 7–10.
Gomes, M. R. A., & Ledward, D. A. (1996). Effect of high-pressure treatment on the activity of some polyphenoloxidases. Food Chemistry, 56(1), 1–5.
González-Cebrino, F., Durán, R., Delgado-Adámez, J., Contador, R., & Ramírez, R. (2013). Changes after high-pressure processing on physicochemical parameters, bioactive compounds, and polyphenol oxidase activity of red flesh and peel plum purée. Innovative Food Science and Emerging Technologies, 20, 34–41.
Goodner, J. K., Braddock, R. J., Parish, M. E., & Sims, C. A. (1999). Cloud stabilization of orange juice by high pressure processing. Journal of Food Science, 64(4), 699–700.
Guerrero-Beltran, J. A., Barbosa-Canovas, G., & Swanson, B. G. (2005). High hydrostatic pressure processing of fruit and vegetable products. Food Reviews International, 21, 411–425.
Hernández-Carrión, M., Hernando, I., & Quiles, A. (2014). High hydrostatic pressure treatment as an alternative to pasteurization to maintain bioactive compound content and texture in red sweet pepper. Innovative Food Science and Emerging Technologies, 7(22), 1–10.
Hsu, K. C. (2008). Evaluation of processing qualities of tomato juice induced by thermal and pressure processing. LWT-Food Science and Technology, 41(3), 450–459.
Huang, W. S., Bi, X. F., Zhang, X., et al. (2013). Comparative study of enzymes, phenolics, carotenoids and color of apricot nectars treated by high hydrostatic pressure and high temperature short time. Innovative Food Science & Emerging Technologies, 18, 74–82.
Igual, M., Sampedro, F., Martínez-Navarrete, N., & Fan, X. (2013). Combined osmodehydration and high pressure processing on the enzyme stability and antioxidant capacity of a grapefruit jam. Journal of Food Engineering, 114, 514–521.
Jacobo-Velázquez, D. A., & Hernández-Brenes, C. (2010). Biochemical changes during the storage of high hydrostatic pressure processed avocado paste. Journal of Food Science, 75(6), S264–S270.
Keenan, D. F., Brunton, N. P., Mitchell, M., et al. (2012). Flavour profiling of fresh and processed fruit smoothies by instrumental and sensory analysis. Food Research International, 45(1), 17–25.
Knorr, D. (1993). Effects of high hydrostatic pressure processes on food safety and quality. Food Technology, 47(6), 56–161.
Krapfenbauer, G., Kinner, M., Gossinger, M., Nlechner, R. S., & Berghofer, E. (2006). Effect of thermal treatment on the quality of cloudy apple juice. Agricultural and Food Chemistry, 54, 5453–5460.
Krebbers, B., Matser, A. M., Koets, M., & Van den Berg, R. W. (2002). Quality and storage-stability of high-pressure preserved green beans. Journal of Food Engineering, 54, 27–33.
Krebbers, B., Matser, A. M., Hoogerwerf, S. W., et al. (2003). Combined high-pressure and thermal treatments for processing of tomato puree: evaluation of microbial inactivation and quality parameters. Innovative Food Science and Emerging Technologies, 4(4), 377–385.
Labell, F. (1993). Pink grapefruit beverages: mainstream refreshment. Food Processing, 54(5), 66.
Landl, A., Abadias, M., Sarraga, C., et al. (2010). Effect of high pressure processing on the quality of acidified Granny Smith apple puree product. Innovative Food Science and Emerging Technologies, 11, 557–564.
Lavinas, F. C., Miguel, M. A. L., Lopes, M. L. M., et al. (2008). Effect of high hydrostatic pressure on cashew apple (Anacardium occidentale L.) juice preservation. Journal of Food Science, 73, 273–277.
Li, L., Feng, L., Yi, J., et al. (2010). High hydrostatic pressure inactivation of total aerobic bacteria, lactic acid bacteria, yeasts in sour Chinese cabbage. International Journal of Food Microbiology, 142, 180–184.
Liang, F., Jiang, B., & Zhang, T. (2008). Effect of combined high pressure and thermal treatment on kiwifruit peroxidase. Food Chemistry, 109(4), 802–807.
Liu, F., Wang, Y., Bi, X., et al. (2012). Comparison of microbial inactivation and rheological characteristics of mango pulp after high hydrostatic pressure treatment and high temperature short time treatment. Food and Bioprocess Technology. doi:10.1007/s11947-012-0953-z.
McKay, A. M., Linton, M., Stirling, J., Mackle, A., & Patterson, M. F. (2011). A comparative study of changes in the microbiota of apple juice treated by high hydrostatic pressure (HHP) or high pressure homogenisation (HPH). Food Microbiology, 28, 1426–1431.
Mert, M., Buzrul, S., & Alpas, H. (2013). Effects of high hydrostatic pressure on microflora and some quality attributes of grape juice. High Pressure Research: An International Journal, 33(1), 55–63.
Oey, I., Lille, M., Van Loey, A., & Hendrickx, M. (2008). Effect of high-pressure processing on colour, texture and flavour of fruit- and vegetable-based food products: a review. Trends in Food Science & Technology, 19(6), 320–328.
Oszmiański, J., & Wojdyło, A. (2009). Comparative study of phenolic content and antioxidant activity of strawberry puree, clear, and cloudy juices. European Food Research and Technology, 228(4), 623–631.
Perera, N., Gamage, T. V., Wakeling, L., Gamlath, G. G. S., & Versteeg, C. (2010). Color and texture of apples high pressure processed in pineapple juice. Innovative Food Science and Emerging Technologies, 11(1), 39–46.
Picouet, P. A., Sarraga, C., Cofan, S., Nicoletta, B., & Dolors Guardia, M. (2014). Effects of thermal and high-pressure treatments on the carotene content, microbiological safety and sensory properties of acidified and of non-acidified carrot juice. LWT - Food Science and Technology, 7, 1–7.
Polydera, A. C., Stoforos, N. G., & Taoukis, P. S. (2005). Effect of high hydrostatic pressure treatment on post processing antioxidant activity of fresh navel orange juice. Food Chemistry, 91(3), 495–503.
Queiroz, C., Moreira, C. F. F., Lavinas, F. C., Lopes, M. L. M., Fialho, E., & Valente-Mesquita, V. L. (2014). Effect of high hydrostatic pressure on phenolic compounds, ascorbic acid and antioxidant activity in cashew apple juice. High Pressure Research: An International Journal, 30(4), 507–513.
Ramos-Parra, P. A. (2006). Factores que afectan la estabilidad de mango (Mangifera indica L.) procesado por alta presión hidrostática. MSc Thesis, Tecnológico de Monterrey, Nuevo León, México.
Rastogi, N. K., Raghavarao, K. S. M. S., Balasubramaniam, V. M., et al. (2007). Opportunities and challenges in high pressure processing of foods. Critical Reviews in Food Science and Nutrition, 47: 69-112.
Rendueles, E., Omer, M. K., Alvseike, O., et al. (2011). Microbiological food safety assessment of high hydrostatic pressure processing, a review. Food Science and Technology, 44, 1251–1260.
Rivas, A., Rodrigo, D., Martinez, A., et al. (2006). Effect of PEF and heat pasteurization on the physical-chemical characteristics of blended orange and carrot juice. Lwt-Food Science and Technology, 39, 1163–1170.
Rodrigo, D., Arranz, J. I., Koch, S., Frigola, M. C., Rodrigo, M. J., Esteve, M. J., Calvo, C., & Rodrigo, M. (2003). Physicochemical characteristics and quality of refrigerated Spanish orange-carrot juices and influence of storage conditions. Journal of Food Science, 68, 2111–2116.
Roig, M. G., Bello, J. F., Riera, Z. S., & Kennedy, J. F. (1999). Studies on the occurrence of non-enzymatic browning during storage of citrus juice. Food Research International, 32, 609–619.
Sampedroa, F., Rodrigob, D., & Hendrickxc, M. (2008). Inactivation kinetics of pectin methyl esterase under combined thermal–high pressure treatment in an orange juice–milk beverage. Journal of Food Engineering, 86(1), 133–169.
Sánchez-Moreno, C., De Ancos, B., Plaza, L., Elez-Martínez, P., & Cano, M. P. (2010). Effects of high-pressure processing and pulsed electric fields on nutritional quality and health-related compounds of fruit and vegetable products. Nonthermal Processing Technologies for Food. Wiley-Blackwell, 50, 502–536.
San Martin, M. F., Barbosa-Canovas, G. V., & Swanson, B. G. (2002). Food processing by high hydrostatic pressure. Critical Reviews in Food Science and Nutrition, 42, 627–645.
Singleton, V. L., Orthofer, R., & Lamuela-Raventors, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymolog, 299, 152–178.
Soysal, Ç., Söylemez, Z., & Bozoğlu, F. (2004). Effect of high hydrostatic pressure and temperature on carrot peroxidase inactivation. European Food Research and Technology, 218, 152–156.
Tangwongchai, R., Ledward, D. A., & Ames, J. M. (2000). Effect of high-pressure treatment on the texture of cherry tomato. Journal of Agricultural and Food Chemistry, 48(5), 1434–1441.
Terefe, N. S., Matthies, K., Simons, L., & Versteeg, C. (2009). Combined high pressure mild temperature processing for optimal retention of physical and nutritional quality of strawberries (Fragaria × ananassa). Innovative Food Science and Emerging Technologies, 10(3), 297–307.
Timmermans, R. A. H., Mastwijk, H. C., Knol, J. J., et al. (2011). Comparing equivalent thermal, high pressure and pulsed electric field processes for mild pasteurization of orange juice. Part I: impact on overall quality attributes. Innovative Food Science and Emerging Technologies, 12, 235–243.
Torres, B., Tiwari, B. K., Patras, A., et al. (2011). Stability of anthocyanins and ascorbic acid of high pressure processed blood orange juice during storage. Innovative Food Science and Emerging Technologies, 12, 93–97.
Uckoo, R. M., Jayaprakasha, G. K., Nelson, S. D., & Patil, B. S. (2011). Rapid simultaneous determination of amines and organic acids in citrus using high-performance liquid chromatography. Talanta, 83(3), 948–954.
Uckoo, R. M., Jayaprakasha, G. K., & Bhimanagouda, S. P. (2012). Chromatographic techniques for the separation of polymethoxyflavones from citrus. Emerging trends in dietary components for preventing and combating disease. American Chemical Society, 1093, 3–15.
Uckoo, R. M., Jayaprakasha, G. K., & Somerville, J. A. (2013). High pressure processing controls microbial growth and minimally alters the levels of health promoting compounds in grapefruit (Citus paradisi Macfad) juice. Innovative Food Science and Emerging Technologies, 4(18), 7–14.
Uemura, K., & Inoue, T. (2010). Inactivation of Bacillus subtilis spores in orange juice and the quality change by high electric field alternating current. Japan Agricultural Research., 44(1), 61–66.
Varela-Santos, E., Ochoa-Martinez, A., Tabilo-Munizaga, G., et al (2012). Effect of high hydrostatic pressure (HHP) processing on physicochemical properties, bioactive compounds and shelf-life of pomegranate juice. Innovative Food Science and Emerging Technologies, 13, 13–22.
Wang, Y. T., Liu, F. X., Cao, X. M., et al. (2012). Comparison of high hydrostatic pressure and high temperature short time processing on quality of purple sweet potato nectar. Innovative Food Science and Emerging Technologies, 16, 326–334.
Wang, R., Zhou, X., & Chen, Z. X. (2008). High pressure inactivation of lipoxygenase in soy milk and crude soybean extract. Food Chemistry, 106, 603–611.
Wang, HY., Hu, XS., Chen, F., Wu, JH., Liao, XJ., & Wang, ZF. (2006). Kinetic analysis of non-enzymatic browning in carrot juice concentrate during storage. European Food Research and Technology, 223(2): 282-289.
Wicklund, T., Rosenfeld, H. J., Martinsen, B. K., Sundfør, M. W., Lea, P., Bruun, T., Blomhoff, R., & Haffner, K. (2005). Antioxidant capacity and colour of strawberry jam as influenced by cultivar and storage conditions. LWT - Food Science and Technology, 38, 387–391.
Xiao, Q., Tong, Q., & Lim, L. T. (2012). Pullulan-sodium alginate based edible films: rheological properties of film forming solutions.
Yen, G.-C., & Lin, H.-T. (1996). Comparison of high pressure treatment and thermal pasteurization effects on the quality and shelf life of guava puree. International Journal of Food Science and Technology, 31, 205–213.
Zhang, Y. (2009). Study on production and comprehensive utilization technology of special shaddock in Fujian. Fujian, Fuzhou. doi:10.7666/d.y1515196
Zhao, L., Wang, S., Liu, F., Dong, P., Huang, W., Xiong, L., & Liao, X. (2013). Comparing the effects of high hydrostatic pressure and thermal pasteurization combined with nisin on the quality of cucumber juice drinks. Innovative Food Science & Emerging Technologies, 17, 27–36.
Zhou, L. Y., Zhang, Y., Leng, X. J., Liao, X. J., & Hu, X. S. (2010). Acceleration of precipitation formation in peach juice induced by high-pressure carbon dioxide. Agricultural and Food Chemistry, 58, 9605–9610.
Acknowledgments
This work was supported by the project “Recommend International Advanced Agricultural Science and Technology Plan” (no. 2011-G20 (5)) of the “948” project of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, G., Zhao, L., Ma, Y. et al. Microorganisms and Some Quality of Red Grapefruit Juice Affected by High Pressure Processing and High Temperature Short Time. Food Bioprocess Technol 8, 2096–2108 (2015). https://doi.org/10.1007/s11947-015-1556-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-015-1556-2