Abstract
Freezing is a very well-established food preservation process that produces high quality nutritious foods with a long storage life. However, freezing is not suitable for all foods, and freezing can cause physical and chemical changes in some foods that are perceived as reducing the quality of either the thawed material or the final product. This paper reviews the many innovative freezing processes that are currently being researched and developed throughout the world to improve freezing conditions and product quality. Some innovative freezing processes (impingement and hydrofluidisation) are essentially improvements of existing methods (air blast and immersion, respectively) to produce far higher surface heat transfer rates than previous systems and thus improve product quality through rapid freezing. In these cases, the advantages may depend on the size of the product, since the poor thermal conductivity of many foods limits the rate of cooling in large objects rather than the heat transfer between the heat transfer medium and the product. Other processes (pressure shift, magnetic resonance, electrostatic, microwave, radiofrequency, and ultrasound) are adjuncts to existing freezing systems that aim to improve product quality through controlling the way that ice is formed in the food during freezing. Another alternative is to change the properties of the food itself to control how ice is formed during freezing (such as in dehydrofreezing and the use of antifreeze and ice-nucleation proteins).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
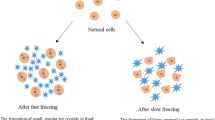
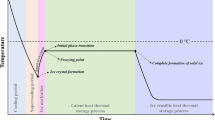
Freezing is a long established food preservation process that produces high quality nutritious foods with a long storage life. In general, the term freezing refers to the process in which the temperature of the food is reduced to a temperature below its freezing point, while the term frozen is used to describe the subsequent state the food is kept in, i.e., the maintenance of the food below that temperature during the rest of the cold-chain. The formation of ice crystals during freezing and frozen storage causes physical changes to the structure of foods. In most cases, these changes are perceived as reducing the quality of the thawed material. In extreme cases, such as cucumbers or salad vegetables, freezing completely destroys the structure of the food. In meat and fish, the main result is increased drip on thawing. There is a general view that fast freezing and the formation of small ice crystals offers some quality advantages, although not true for all foods (Poulsen 1977; Spiess 1979; Jul 1984). However, minimising freezing times can be advantageous in reducing energy consumption, increasing throughput, and improving yield.
Established food freezing technologies encompass air blast, contact/plate, immersion, and spray freezing systems. Although often considered a separate technology, cryogenics can be considered to be a sub field of blast, immersion, or spray, depending on how the cryogen is used and the method of application. Hybrid systems such as cryomechanical also exist. Cryomechanical systems initially crust freeze the product using cryogens before completing the freezing process with a conventional mechanical air blast or belt freezer (Agnelli and Mascheroni 2002). The rapid crust freezing reduces weight loss during freezing and prevents clumping. Most of these technologies have been with us since the 1960s, and although there have been improvements these technologies have essentially remained the same since then. This paper reviews new developments in alternative methods of freezing and discusses their potential applications in the food industry.
Innovative Freezing Technologies
In the past few decades, there has been considerable scientific interest in the development of novel systems that can improve the organoleptic quality or economics of production of frozen foods. Some of these novel freezing technologies are aimed at increasing the rate of heat removal from the food while others change the physical/chemical structure of the product. However, no critical comparisons of novel freezing technologies have been published in the past decade. The last reviews being those of Li and Sun (2002a) and Fikiin (2003). Li and Sun (2002a) reviewed and compared four novel freezing technologies: high-pressure freezing, dehydrofreezing, and applications of antifreeze protein and ice nucleation protein. Fikiin (2003) reviewed hydrofluidisation, ice-slurries, high pressure, and magnetic resonance freezing technologies. Since then, further studies on these technologies and studies on a number of new freezing methods (i.e., electrostatic, ultrasound, microwave, and radiofrequency-assisted freezing) have been published.
In the following sub-sections, 13 novel freezing technologies are reviewed separately. The relative state of the technologies, their current industrial maturity, and their future potential in food refrigeration are then discussed in Discussion section.
Impingement Freezing
The use of impingement technology to increase the surface heat transfer in air and cryogenic freezing systems has been one of the few recent innovations to be truly commercially realised (Newman 2001; Sarkar et al. 2004; Sarkar and Singh 2004; Winney 2012). Impingement is the process of directing a jet or jets of fluid at a solid surface to effect a change. The very high velocity (up to 50 m s−1) impingement jets are used to “break up” the static surface boundary layer of gas that surrounds a food product (Newman 2001). The resulting medium around the product is more turbulent, and the heat exchange through this zone becomes much more effective and can produce substantially faster freezing than conventional blast systems (Newman 2001; Sarkar et al. 2004; Sarkar and Singh 2004).
Impingement freezing (IF) is best suited to products with a high surface area to weight ratio (i.e., products with one small dimension, such as burgers or fish fillets). Testing has shown that products less than 20-mm-thick freeze most effectively in an impingement heat transfer environment (Newman 2001). The process is also very attractive for products that require very rapid surface freezing, i.e., crust freezing.
Perhaps because of the commercial success of impingement technologies, there has been relatively little published on this technology. Of those papers that have been published, the emphasis has generally been on measuring heat transfer coefficients and measuring/modeling freezing rates (Newman 2001; Ho 2004; Sarkar et al. 2004; Sarkar and Singh 2004; Jafari and Alavi 2008) rather than analysing whether there is any quantifiable difference in food quality between conventional and IF.
In contrast to the accepted belief that increasing the air velocity will improve frozen quality (due to speeding up the rate of freezing), Soto and Bórquez (2001) reported that in their studies a decrease in air velocity (for velocities ranging from 45 to 62 m s−1) and an increase in particle diameter improved texture (as measured using a puncture test with an Instron) and decreased drip in relation to freezing cylindrical carrot samples. This finding may be because the system used was more akin to a fluidised bed freezer than an impingement tunnel, thus the high velocity increased mechanical damage to the particles by causing them to crash into each other and the walls of the vessel during freezing.
Sundsten et al. (2001) appear to have published the only practical comparison of IF to other methods. Their study compared three different freezing systems (all manufactured by Frigoscandia): spiral freezing (SF), cryogenic freezing (liquid nitrogen, LN), and IF. The times required to freeze a 10-mm-thick 80 g hamburger from +4 to −18 °C in the SF, LN, and IF systems were 22 min, 5 min 30 s, and 2 min 40 s, respectively. The study reported that weight losses were significantly higher for hamburgers frozen in SF (1.2 %) compared to LN (0.4 %) and IF (0.4 %). However, no significant difference could be seen in cooking losses, even after storage for 2 months. While ice crystals were significantly larger in hamburgers frozen in SF compared to LN and IF, sensory analysis revealed no difference in eating quality between the three freezing methods after 2 months frozen storage.
A detailed assessment of IF by Salvadori and Mascheroni (2002), based on measured and supplied data on burger freezing (using air at −32 to −47.2 °C and 33.0 to 35.6 m s−1), concluded that IF offered the following advantages:
-
1.
Freezing times were 62 to 79 % faster, depending on product thickness, than conventional (at the same air temperatures).
-
2.
Weight losses were 36 to 72 % lower, depending on product thickness, than for conventional (at the same air temperatures).
-
3.
Overall “Freezing times and weight losses for impingement freezing are similar to those of cryogenic freezing at a noticeably lower operating cost”.
-
4.
The authors concluded that “the results obtained with an impingement freezer are equivalent to those of a blast tunnel operating with a much lower air temperature. This means that the high cost of the additional refrigeration of the air can be significantly reduced using impingement technology”.
A series of studies have been published by Góral and Kluza (2006, 2009, 2012) on “reverse fluidisation” IF of vegetable samples (i.e., the impingement jets directed from above are designed to fluidise the vegetables). The system used is pilot scale and has been designed by the authors. The capability and design of the equipment appear to have changed over the years according to the details provided in the various papers. The current configuration is capable of supplying air at −40 °C at air velocities ranging from 3.2 to 9.1 m s−1 providing measured heat transfer coefficients (h) of 62 to 200 Wm−2 K−1, respectively (Góral and Kluza 2012). Relatively little data is provided in these papers on what effect freezing in this system has on vegetable quality. Freezing times were reported to be faster than “conventional” (usually a cabinet freezer).
As already stated, IF systems are widely available as complete commercial systems and can be purchased from many equipment companies. Most the systems are modular in design and will therefore accommodate a wide range of throughputs. The capital cost of an impingement freezer is greater than that of a conventional air blast freezer, and energy costs can be greater due to greater fan power consumption. However, these higher costs are reported to be justified at throughputs of 500 kg h−1 and greater (Winney 2012).
Hydrofluidisation Freezing
Hydrofluidization (HF) freezing is essentially a form of immersion freezing. It may be considered analogous to liquid IF. HF freezing uses a circulating system that pumps the refrigerating liquid upwards, through orifices or nozzles, in a refrigerating vessel, thereby creating agitating jets. These form a fluidised bed of highly turbulent liquid and moving products, and thus provide extremely high surface heat transfer coefficients that enable rapid freezing (Fikiin and Fikiin 1998; Fikiin 2003).
There is some research literature related to the design of HF systems (Peralta et al. 2009, 2012) and particularly surface heat transfer characteristics (Peralta et al. 2010; Verboven et al. 2003), but little published data on foods (Table 1) and little evidence of it being attempted commercially, as yet.
Freezing rates for fish, vegetables, and fruits have been shown to exceed those for Individually Quick Frozen (IQF) products in conventional blast freezers, with h exceeding 900 W m−2 K−1 (Fikiin 1992; Fikiin and Pham 1985; Fikiin et al. 2003). Using ice slurry as a fluidisation media gives an h of 1000–2000 W m−2 K−1 (Fikiin and Fikiin 1998; Fikiin et al. 2003). By comparison in non-agitated liquid, the h is about 100 W m−2 K−1, and about 400 W m−2 K−1 in an agitated liquid (Pham 2008). Ice slurries, brines, and sugar-ethanol cooling media have been employed depending on the product and conditions required. The high freezing rates are claimed to improve product quality, but no detailed study appears to have been published that has actually measured quality parameters. It appears to be generally assumed that quality will be better because the rate of freezing is faster.
No refrigeration equipment manufacturers appear to be currently marketing HF freezing systems for food use; however, with the design information available, there would appear to be nothing stopping the manufacture of commercial systems of any required size.
Pressure-assisted Freezing
Freezing foods while applying high pressures (typically of 200 to 400 MPa), and in particular “pressure-shift” freezing (PSF), have attracted considerable scientific interest in recent years. Well over 50 papers on aspects of pressure-assisted freezing have been published in international journals since 2000. Despite this surprisingly few academic reviews of this technology have been published (Cheftel et al. 2000; Le Bail et al. 2002; Li and Sun 2002a; Cheftel et al. 2002), although a comprehensive book chapter has been written recently on this subject by Otero and Sanz (2012). Due to the wealth of published data on pressure-assisted freezing, it is beyond the scope of our paper to review this subject in as much detail as we would wish, but we would like to draw attention to some of the conclusions reached by researchers who have considered this subject. There are three distinct forms of freezing using high pressure, high pressure-assisted freezing (HPF), pressure shift-assisted freezing (PSF), and high pressure-induced crystallisation (HPIC) or pressure-induced solid-solid phase transition. All are adjuncts to existing freezing systems that aim to improve product quality through controlling the way that ice is formed in the food during freezing, or after in the case of HPIC. The term “High Pressure–Low Temperature” (HPLT) processing has been used by some to describe all such processes (Volkert et al. 2012).
HPF is the process of cooling a sample under pressure up to its phase change temperature at the applied pressure, i.e., the freezing takes place under a constant pressure (Le Bail et al. 2002). When water is frozen at atmospheric pressure, its volume increases which causes tissue damage in foods. However, theoretically freezing under high pressure results in ice with a different structure being formed that has a greater density than water, does not expand in volume during formation and exists in a “vitreous” non-crystaline state, which may reduce tissue damage. At a pressure of 200 MPa, the freezing point drops to about −22 °C, enabling a depth of vitrification of about 200 μm, so that objects with a thickness of up to 0.4–0.6 mm could be well frozen (Fikiin 2003).
With PSF the food is cooled under high pressure to sub-zero temperatures but does not undergo a phase change and freeze until the pressure is released. When the pressure is released, theoretically, rapid nucleation takes place throughout the food resulting in small, uniform, homogenously distributed ice crystals. This should result in a reduced duration of the phase transition, less mechanical stress during formation of the ice crystals, and smaller ice crystals with a uniform distribution throughout the food. In theory, this would be especially useful when freezing foods with large dimensions in which the poor thermal conductivity of many foods results in thermal gradients that make it difficult to achieve fast freezing rates within the center of such foods.
A novel form of PSF is “high pressure carbonic immersion (HPCI)” recently described and demonstrated by Xu et al. (2014). In this process, the food is processed in a pressurised chamber into which high pressure carbon dioxide is introduced. This process has been shown to freeze carrot slices to below −18 °C within 5 min under a pressure treatment of 6 MPa (substantially lower than the pressures used in most other studies using high pressures). The samples had less tissue damage, drip loss, and better nutrient retention than samples frozen by immersion in LN or in a −80 °C freezer.
High pressure induced crystallisation (HPIC) differs from HPF and PSF in that it is applied after conventional freezing. The purpose of HPIC is to change ice I to ice III and back to ice I (van Buggenhout et al. 2007). This phase shift treatment has been particularly studied as a possible low temperature method for reducing microbial populations on foods.
The majority of published studies have been on PSF rather than HPF. A few studies, such as Fernandez et al. (2006a), have compared both approaches. Fernandez et al. (2006a) concluded that PSF was more advantageous than HPF, since phase transition times were shorter and produced a more homogeneous distribution of small ice crystals.
High pressure is used commercially to preserve foods, and some studies have investigated the effects of pressurisation at sub-zero temperatures on microbial inactivation (Picart et al. 2004). Some of these studies have used PSF (Picart et al. 2004) while others have utilised HPIC processes (Yuste et al. 2002; van Buggenhout et al. 2007). Picart et al. (2004) demonstrated that PSF from a level of 207 MPa and −21 °C was able to inactivate various microorganisms (Listeria innocua, Micrococcus luteus, and Pseudomonas fluorescens) introduced in smoked salmon mince. It has also been speculated that PSF and HPF could inactivate enzymes thus removing the need for blanching. However, studies by Préstamo et al. (2004, 2005) failed to show sufficient inactivation of peroxidase (POD) and/or polyphenoloxidase (PPO) enzymes in broccoli or potatoes subjected to such treatments.
While studies have shown pressure-assisted freezing to produce smaller more widely distributed ice crystals in many products (Table 2), it remains to be established whether this results in a commercially better product. For example, studies on pork and beef (Fernández-Martín et al. 2000; Zhu et al. 2004b) have failed to show any real commercial quality advantage with these foods. Some studies have also shown PSF to damage tissue structures in carrots (van Buggenhout et al. 2006b).
The capital cost of equipment is one obstacle holding back further development of pressure-assisted freezing, as are the inherent batch nature of the current process and the long pre-cooling step required before nucleation; however, the penetration of other high pressure processes into the food industry may begin to solve these problems. Alternatively, it may be possible to achieve pressure freezing without an external source. The preservation of rapidly frozen uncryoprotected medical specimens in confined containers has been found to be “comparable to that achieved with high-pressure freezing in the presence of cryoprotectant” according to a study by Leunissen and Yi (2009). According to these researchers, “the results are explained by the fact that cooling of water in a confined space below the melting point gives rise to pressure build-up, which may originate from the conversion of a fraction of the water content into low-density hexagonal ice and/or expansion of water during supercooling”. Because the pressure is generated inside the specimen holders as a result of the cooling rather than applied from an external source as in high-pressure freezing, the researchers suggest that the technique be referred to as self-pressurised rapid freezing.
We would agree with Otero and Sanz’s (2012) recent conclusion that the development of suitable continuous and semi-continuous industrial scale pressure-assisted freezing equipment is required before adoption of this technology can be contemplated by the food industry. Otero and Sanz (2012) recommended that such developments require optimising “the efficiency in the heat removal under pressure since the long pre-cooling step, before nucleation, is the limiting factor which must be reduced to make the process competitive”.
Ultrasound-assisted Freezing (UAF)
While ultrasound-assisted thawing has been a proven process for a relatively long time, its use to assist freezing is more recent. Research has mainly utilised “Power ultrasound” (Zheng and Sun 2005; Delgado and Sun 2012), a form of ultrasound wave with low frequency (18–20 kHz to 100 kHz) and high intensity (generally higher than 1 W/cm2). Reviews on the principles of ultrasonically-assisted freezing have been published by Zheng and Sun (2005), Zheng and Sun (2006), and Delgado and Sun (2012).
In theory, the ultrasound creates cavitation bubbles throughout the product, which promotes more even ice nucleation and fragments ice crystals already present into smaller crystals. It may also accelerate convective heat transfer in the cooling media thus speeding up the freezing process. In addition, ultrasound can inactivate some enzymes (Islam et al. 2014), thus, potentially negating the need for blanching prior to freezing for some products. A number of studies have demonstrated that ultrasound reduces the degree of supercooling prior to nucleation in frozen foods (Comandini et al. 2013; Kiani et al. 2013b; Cheng et al. 2014). Both Comandini et al. (2013) and Cheng et al. (2014) found that ultrasound reduced the overall freezing time of immersion frozen potato or strawberry samples, respectively, by reducing the degree of supercooling, thus shortening the time before nucleation took place, however, ultrasound increased the phase transition times in both studies. In contrast, Hu et al. (2013) demonstrated that ultrasound can reduce the phase transition time (as well as the time of all other freezing stages) in a study using dough.
Studies have published on the application of ultrasonic-assisted freezing of a range of foods (Table 3). Pretreatment of ice cream prior to manufacture has been shown to decrease the freezing time, increase overrun (degree of aeration), and improve sensory properties (Mortazavi and Tabatabaie 2008). Zheng and Sun (2006) cite the work by Gareth (1992) that experimental investigation showed that during the production of ice lollipops the application of power ultrasound resulted in product that had much smaller ice crystals and uniform crystal size distribution. Although the small crystals made the product difficult to bite, it strongly improved the adhesion of the lollipop to the supporting wooden stick (Gareth 1992).
Laboratory scale experiments (Li and Sun 2002b) on potato sticks have shown that ultrasound-assisted immersion freezing (15.85 W) significantly reduced the freezing time (defined in this study as the time from −1 to −7 °C) by up to 14 %, compared to immersion freezing without ultrasound. An analyses conducted on the microstructure of potato tissue using cryo-scanning electron microscopy technique (Sun and Li 2003) showed that the potato tissue exhibited a more intact cellular structure after ultrasonic-assisted freezing, with less intercellular void and cell disruption, in comparison to immersion freezing without ultrasonic assistance. This was attributed to the high freezing rate obtained under a high ultrasonic level and thus the domination of intracellular small ice crystals. Similar results were obtained on apple cylinders (Delgado et al. 2009). In this study, the freezing time was reduced by up to 8 %, compared to immersion freezing without ultrasound. Some evidence of the influence of ultrasound to induce primary nucleation was also observed from analysis of the freezing curves. However, no direct ice crystal analysis was carried out or sensory/chemical/texture analysis.
Studies by Xin et al. (2014a, b) show that the acoustic intensity used in UAF may be critical. In their initial studies (Xin et al. 2014a), the application of UAF during immersion freezing of broccoli in calcium chloride brine at selected acoustic intensity with a range of 0.250–0.412 W/cm2 decreased the freezing time (by around 14 %) and the loss of cell-wall bound calcium content. Compared to unassisted immersion freezing in calcium chloride brine, textural properties, color, and l-ascorbic acid content were better preserved, and drip loss was significantly minimized by the application of UAF. However, when outside that range of acoustic intensity, the freezing time of the UAF broccoli was longer and the quality inferior to that of the unassisted immersion frozen samples. Further work (Xin et al. 2014b) established that applying different process conditions at different stages (pre-cooling, phase change, and sub-cooling stages) during the freezing of broccoli samples produced the fastest freezing times and best quality attributes.
The application of ultrasound during immersion (in glycol) freezing (Islam et al. 2014) was shown to inactivate polyphenol oxidase (PPO) and POD enzymes in three mushroom varieties (Lentinula edodes, Agaricus bisporus, and Pleorotus eryngii). UAF also reduced nucleation and freezing time significantly, as well as thawing time. Drip losses were reduced noticeably and thawed texture improved.
All published studies on the effects of UAF have studied its application during immersion freezing and at a bench top scale. While these individual studies have investigated the effect of different ultrasound parameters, they do not appear to have looked at what effect changing the freezing conditions (coolant circulation/agitation rate or temperature) may have on freezing rate or quality properties, or compared the freezing of different products under the same conditions. A number of studies have utilised calcium chloride brines, a known texturing agent, while others have used ethylene glycol. This may or may not have a synergistic effect on the freeze-thaw quality of foods. While Zheng and Sun (2006) suggested possible methods for integrating ultrasonic devices into some commercial freezers (such as air blast and plate freezers), as yet we are not aware of any commercial-scale ultrasonic-assisted freezing installations, though laboratory level research is ongoing.
Magnetic Resonance-assisted Freezing (MRAF)
The effects of electric and magnetic fields on freezing have been reviewed by Woo and Mujumdar (2010). There is evidence that water, being a diamagnetic material, can be magnetised in a magnetic field. Woo and Mujumdar (2010) cite a number of independent studies, similar to those carried out to investigate electrostatic-assisted freezing, that have demonstrated that freezing water under the influence of a magnetic field can change the freezing characteristics. However, in a recent critique, Wowk (2012) questioned the claims made in recent papers using a Cells Alive System (CAS), given the very small (<1 mT) field strengths used. In response, Kobayashi and Kirschvink (2013), while agreeing that the “papers and patents published by the ABI group postulate mechanisms of action that do not agree with basic biophysics”, offered a plausible mechanism for the disruption of ice-crystal nucleation in super-cooled water by magnetically-induced mechanical oscillation. This was based on the action of magnetic fields on ferromagnetic materials in biological tissues.
CAS is a novel patented (Owada and Kurita 2001; Owada 2007) food processing process marketed by the Japanese company ABI (ABI Corporation Ltd, Chiba, Japan) and claimed to provide a process where a product can be frozen “in such a manner as to approximate the quality of a fresh product once thawed”. The technique uses permanent magnets and induction coils to produce a weak oscillating magnetic field within the freezing chamber. CAS is not a refrigeration process itself, but is claimed to assist in improving existing freezing processes (both in terms of process speeds and product quality). It is claimed in the patent literature for CAS (Owada and Kurita 2001; Owada 2007) that the oscillating magnetic field acts on polarised water molecules to delay formation of ice crystals. Whether this is due to engendering of super-cooling or movement of the molecules during the ice formation process is unclear from any of the ABI literature or patents. However, the claimed consequence is that because of the delay, most of the ice crystals form at the same time thus restricting their size. Small ice crystals will result in less cellular damage during freezing, and therefore a less structurally damaged product once thawed. In the work, we have carried out using ABI pilot scale CAS rigs installed at the Grimsby Institute, UK, the authors have not seen enhanced super-cooling (James et al. 2015). In our own conversation with the inventor, he claimed that the effect was on initial ice crystal structure with less emphasis on super-cooling.
While widely marketed for food applications for a number of years, there is very little scientific published data on CAS freezing of foodstuffs (Fikiin 2003; James and James 2012), although a number of papers have been published on CAS-assisted freezing of biological samples (such as teeth) using ABI equipment (Kaku et al. 2010; Abedini et al. 2011; Kaku et al. 2012) and even on whole-organism survival of frozen small animals like drosophila (Naito et al. 2012). A two-stage process was reported to have the best effect on retaining cell viability, samples were placed in the CAS freezer with a 60 Hz alternating magnetic field of 0.1 mT at −5 °C for 15 min (holding-time) and then cooled to −30 °C (plunging temperature) at a rate of −0.5 °C/min (very similar to standard commercial food freezing rates). After the freezing procedure, the teeth were transferred to a −150 °C deep freezer for long-term storage. One of the only published analysis on freezing of foodstuffs exposed to oscillating weak magnetic fields are those of Suzuki et al. (2009) and Watanabe et al. (2011), who’s results were negative. Using a “copy” of the ABI, CAS system reported that they had found that oscillating weak magnetic fields had no affect on the freezing curves of sweet potato, spinach, fish, agar gel, or water and had no visible effect on cellular microstructures of the tissues they examined. Kim et al. (2013) have reported faster freezing and lower drip losses in some meats when using magnetic resonance freezing but increased cooking losses. However, they do not appear to have compared like with like. The air blast freezer used for the magnetic resonance freezing was different to the air blast freezer used for the conventional comparison trials. The operating temperatures were also different. The magnetic resonance freezing was carried out in air at −55 °C, while the conventional air blast freezing was carried out at operating temperatures of −45 and −20 °C (although the published temperature curves indicate that actual temperatures in the conventional air blast were nearer −40 and −15 °C). Thus, some of the differences in freezing time and product quality claimed can be attributed to the difference in operating conditions.
Sato and Fujita (2006), as reviewed by Woo and Mujumdar (2010), have also patented a magnetic-assisted freezing system. In this patent, they claim that the magnetic field actually vibrates the molecules, thus breaking the hydrogen bonds between the water molecules, impeding freezing. Additionally, they claim, such crystallization can be induced by progressively reducing the magnetic field strength. In this manner, it is claimed that in regions of low magnetic field strength, small un-clustered ice nucleation occurs and growth of these nucleation occurs uniformly due to the rotation effect. The importance of using cool, dehumidified air in their convective cooling system is emphasized in the patent. It is claimed that frosting on the surface of the material to be frozen greatly impedes the overall freezing quality.
As yet, although full scale commercial ABI CAS food freezing installations have been reported in Japan, there is very little peer-reviewed published work on MRAF, and only a few published studies have demonstrated it for food (Table 4). Our own experience of CAS is that although it has been marketed as a “proven” technology there is still a need for scientific validation of any quality or process improvements with real foods.
Electrostatic-assisted Freezing
Electrostatic-assisted freezing has only been investigated, so far, at a laboratory scale in model systems (Petersen et al. 2006; Le Bail et al. 2010; Woo and Mujumdar 2010; Le Bail et al. 2012a). However, only one study (Table 5) on the application of an external static electric field to freezing of real food has been published (Xanthakis et al. 2013). Their results showed that during the freezing of pork tenderloin in the presence of high voltage static electric field (electrofreezing) the damage to the meat microstructure was significantly reduced, as a result of controlled nucleation, the authors postulated that such a reduction may “improve the final quality of the frozen product” (although no texture or drip analysis appears to have been carried out).
The principle is that applying an electric field to a food will orientate the polar molecules, such as water, thus controlling super-cooling and ice crystallisation. The capability of a high voltage to induce nucleation in super-cooled water was reported in the 1950s, and this effect used to explain the freezing of water droplets in the atmosphere as far back as 1861 (Petersen et al. 2006). Its main application has been to induce nucleation in super-cooled liquids (Woo and Mujumdar 2010; Le Bail et al. 2012a).
Currently, electrostatic-assisted freezing can be considered to be at an early research and development stage. Proof of principle has been demonstrated in model food systems but it is only starting to be applied to real foods at a laboratory scale. It is not yet clear how advantageous this technology will be compared to current freezing technologies and how easy it will be to apply in commercial applications.
Microwave-assisted Freezing
The potential of microwaves to assist freezing has been studied by a few researchers (Hanyu et al. 1992; Jackson et al. 1997; Xanthakis et al. 2013).
The proposed principle behind microwave-assisted freezing is to exploit water dipole rotation induced by microwaves to disrupt ice nucleation and formation during freezing (Jackson et al. 1997). The application may enhance vitrification at ultra low temperatures (Jackson et al. 1997).
Two published studies in the 1990s showed some potential for microwave-assisted freezing, but the applications were for the cryopreservation (immersion in LN) of small biomaterials (Hanyu et al. 1992; Jackson et al. 1997), and one used a cryoprotectant (ethylene glycol). Samsung Electronics have also patented a device utilising microwaves to prevent spontaneous nucleation of super-cooled liquid during storage (Woo and Mujumdar 2010).
A study based on the same principle as microwave-assisted freezing was recently published on the application of radiofrequency to assist freezing of pork (Anese et al. 2012). This work prompted Xanthakis et al. (2013) to study the effect of microwave-assisted freezing on the same food system, namely pork tenderloin (Table 5). The application of microwaves (using an adapted domestic microwave oven) during cooling the samples caused an “oscillated decrease of temperature” (i.e., temperatures fluctuated during freezing), this resulted in a longer freezing time and a decrease in the degree of supercooling (circa 92 %) under the tested conditions, in comparison with freezing without applying microwaves. As admitted by the authors, such conditions would normally be expected to result in the formation of larger ice crystals; however, an evaluation of the meat microstructure showed a 62 % decrease in the average ice crystal size when samples were frozen under a microwave field as compared to the conventional freezing process. The authors postulated that “the limited oscillation of the temperature during the genesis of the ice nuclei and crystal growth may have been responsible for instantaneous recurring melting and regeneration of ice crystals which in turn prohibited the crystal growth and led to the numerous and smaller ice crystals which were observed”. These results are similar to those reported for radiofrequency-assisted freezing (Anese et al. 2012) and indicate that the application of microwave or radiofrequency radiation during freezing may reduce damage of the meat tissue and consequently retain a better texture in the frozen meat.
At present, microwave-assisted freezing (in common with radiofrequency-assisted freezing) is at an early research and development stage. Only one published study has demonstrated proof of principle in a real food at a laboratory scale. It is not yet clear how advantageous this technology will be compared to current freezing technologies, or how easy it will be to apply in commercial applications. Further studies are clearly needed before commercial applications can be contemplated.
Radiofrequency-assisted Freezing
Work (Table 5) has recently been published on the potential of radiofrequency (RF) to assist food freezing (Anese et al. 2012). The proposed principle behind radiofrequency-assisted freezing is similar to that microwave-assisted freezing (see above), i.e., to exploit water dipole rotation induced by RF waves to disrupt ice nucleation and formation during freezing (Anese et al. 2012). It may also depress the freezing point thus producing more nucleation sites (Anese et al. 2012).
Anese et al. (2012) demonstrated that cryogenic (LN) direct spray freezing of pork loin (45 mm cubes) assisted by low voltage RF pulses (2 kV) produced fewer intercellular voids and less cell disruption to the microstructure of meat when RF was applied. Drip losses were similar for air and cryogenically frozen samples, but much lower for RF-assisted frozen samples. The authors postulated that this may have been because the cryogenic freezing caused fracturing due to rapid surface freezing which did not occur during RF application. In comparison to the conventionally frozen (−40 °C, 2 m s−1) samples, RF frozen meat had smaller ice crystals that were mainly intracellular.
At present, RF-assisted freezing (in common with microwave-assisted freezing) is again at an early research and development stage. Only one published study has demonstrated proof of principle in a real food at a laboratory scale. It is not yet clear how advantageous this technology will be compared to current freezing technologies, or how easy it will be to apply in commercial applications. Further research and development is clearly needed before commercial applications can be contemplated.
Dehydrofreezing
Dehydrofreezing has been recently reviewed by the authors (James et al. 2014), so we will not dwell on this topic in this review. Despite being often cited as a new, novel, or emerging technology, the concept of dehydrofreezing is not new, with published studies carried out since the 1940s. The process was developed, and term coined, at the Western Regional Research Laboratory of the US Department of Agriculture and first described by Howard and Campbell 1946, with full details of the process presented in Tressler and Evers’ Third Edition of The Freezing Preservation of Foods (1957). Dehydrofreezing is an adjunct to freezing in which a food is first dehydrated to a desirable moisture and then frozen (Howard and Campbell 1946). If the drying/dehydrating pretreatment is specifically osmotic, the whole process may also be termed osmodehydrofreezing.
The principle behind this process is that since fresh fruits and vegetables contain more water than meat and their cellular structure is less elastic they are prone to more damage during freezing than meat. Removing some of the water prior to freezing theoretically allows the ice crystals to form and ice to expand without damaging the cellular structure.
Dehydration pretreatments may produce potentially advantageous changes to the thermal properties of the treated produce (Tocci and Mascheroni 2008). The water reduction reduces the enthalpy and heat capacity (Tocci and Mascheroni 2008), and, combined with absorption of sugars and salts in the case of osmotic dehydration pretreatments, leads to a lowering of the freezing point temperature (Tocci and Mascheroni 2008) and an increase in the value of the glass transition temperature (Forni et al. 1997; del Valle et al. 1998; Torreggiani et al. 1999; Giannakourou and Taoukis 2003).
Freezing time is also less as there is less water to freeze (Pham 2008; Shizuka et al. 2008; Wu et al. 2009; Ramallo and Mascheroni 2010). Freezing time may be less than half that for untreated products (Ramallo and Mascheroni 2010). Reducing the water load also reduces the amount of energy required to freeze by reducing the heat load (Huxsoll 1982). Crivelli et al. (1987a, b) estimated energy savings of 27 % for dehydrofreezing as compared with conventional freezing. Reducing mass and volume may also reduce distribution costs (Tressler and Evers 1957). Dehydrofreezing may also promote supercooling during freezing (Pham 2008). There is also evidence that dehydrofreezing retains and improves color, texture, and nutrient retention and may also decrease enzymic browning in some produce (James et al. 2014).
Most studies have removed water from the fruits and vegetables prior to freezing by osmotic dehydration in sugar (for fruits) or sodium chloride (for vegetables) solutions or have used air drying. Osmotic dehydration methods have generally been preferred over air drying. Such a process may be particularly suited to products destined for use as ingredients in processed foods.
Two innovations in commercial dehydrofreezing have recently been announced. According to reports (Anon 2013; Durance 2013), Bonduelle, a Canada-based frozen vegetable producer, has been granted the exclusive global rights to develop and launch dehydrofrozen vegetables using EnWave Corporation’s Radiant Energy Vacuum (“REV™”) technology. This technology is a form of vacuum-microwave drying, and it is claimed that this method has advantages over conventional air drying in terms of speed and operating at low temperatures (Anon 2014). The principles of combined vacuum-microwave drying have been described by Scaman and Durance (2005). In Spain, the “Nice Cold System” has been developed by the Universitat Politecnica de Catalunya (Anon 2012), resulting in the spin-off “Nice Fruit”. This patented system (Anmella 2010) subjects the food to a slow and slight (only a 2 to 10 % loss by weight) dehydration pretreatment. The patent claims that it is important for the pre-treated foods to rest for between 1 and 24 h before freezing to allow for redistribution of the free water within the product. According to the patent, dehydration is accomplished using cold semi-humid air at 5 to 10 °C and 60 to 70 % RH.
Ice Structuring Proteins (Antifreeze Proteins)
Early interest in “antifreeze” proteins was famously caused by the observation of the survival of fish inhabiting polar and northern coastal waters, whose freezing point is below the plasma freezing point of the other fish. These proteins have since been identified also in many invertebrates, fungi, bacteria, and plants (Li and Sun 2002a; Venketesh and Dayananda 2008; Hassas-Roudsari and Goff 2012). However, the term “antifreeze” does not always accurately describe their natural function or their application in frozen systems, where they do not prevent freezing, but control the size, shape, and aggregation of ice crystals. Thus, the term “ice structuring proteins” (ISPs) is becoming more common (Clarke et al. 2002).
The properties and potential use of ISPs in frozen foods have been extensively reviewed and discussed by Griffith and Ewart (1995), Feeney and Yeh (1998), Li and Sun (2002a), Clarke et al. (2002), Venketesh and Dayananda (2008), Hassas-Roudsari and Goff (2012), and Wang and Sun (2012). The function of ISPs is in lowering the freezing temperature and suppressing the growth of ice nuclei, thus inhibiting ice formation, altering ice crystal growth, and retarding recrystallisation on frozen storage (Li and Sun 2002a; Clarke et al. 2002). ISPs are essentially food ingredients, thus need to declared and approved for food use.
Some plants, such as carrots, have been shown to produce and accumulate ISPs as a method of protecting the plant from tissue damage during cold weather (Gómez and Sjöholm 2004). A small study by Gómez and Sjöholm (2004) indicates that cold-acclimatised carrots (with high levels of natural ISPs) are more resistant to damage during freezing than non-cold-acclimatised carrots. However, they note that blanching may compromise this effect by damaging the cellular structure and denaturing the ISPs. They recommended that studies be carried out to identify mild blanching processes that would inactivate the enzymes responsible for off flavor and color changes but not denature ISPs. ISPs isolated from carrots have been studied to improve the freezing of dough (Zhang et al. 2007a, 2007b, 2008).
It has been suggested that the use of antifreeze proteins in meat may reduce drip loss (Payne et al. 1994; Payne and Young 1995; Yeh et al. 2009). Antifreeze protein can be used to soak the meat or injected intravenously before slaughter (0.01 μg/kg AFGP injected 24 h before slaughter). Antifreeze proteins have also been shown to improve the fermentation capacity and final quality of breads baked from frozen dough (Zhang et al. 2007a, 2007b, 2008; Yeh et al. 2009). A number of studies have shown that the vacuum infiltration of delicate vegetables or fruits with ISPs may enable such products to be successfully frozen. Vacuum infiltration of watercress with ISPs has been shown to maintain a degree of turgidly in the leaves after freezing and thawing due to the suppression of large ice crystals (Cruz et al. 2009), while the vacuum infiltration of strawberries with ISPs or ISPs in combination with trehalose can improve freezing tolerance (Velickova et al. 2013).
One application of antifreeze proteins that is already finding use in the food industry is in low fat ice creams and frozen yogurts to retard recrystallisation during frozen storage (Soukoulis and Fisk 2014). However, this is not without controversy due to some of these proteins being genetically modified.
While ISPs have found a successful commercial application in ice cream production, it is disappointing that there have been far more published reviews on the potential food application of ISPs than actual published studies on food applications. As yet published studies have been carried out for a relatively small range of foodstuffs (Table 6), the wider use of ISPs will depend on cost, safety, and consumer acceptance (Li and Sun 2002a; Wang and Sun 2012).
Ice Nucleation Proteins
Ice-nucleation proteins (INPs) are a functionally distinct and opposite class of protein to antifreeze proteins. They are produced by some gram-negative bacteria, such as Pseudomonas syringae, Erwinia, and Xanthomonas (Li and Lee 1995; Li and Sun 2002a; Petzold and Aguilera 2009; Wang and Sun 2012; Soukoulis and Fisk 2014). The function of INPs is to raise the temperature of ice nucleation and reduce the degree of supercooling, thus shortening the freezing time and aiding the nucleation of ice throughout the product. In theory, this may lead to beneficial changes in the texture of frozen foods (Li and Sun 2002a; Wang and Sun 2012; Soukoulis and Fisk 2014).
According to Petzold and Aguilera (2009), at least seven species of ice nucleation bacteria have been studied (P. fluorescens, P. syringae, Pseudomonas viridiflava, Erwinia herbicola, Erwinia ananas, Erwinia uredovora, and Xanthomonas campestris), of which P. syringae has been the most widely used. A number of applications for the freezing of fish, surimi, egg, juice, agar gels, and ice cream were reviewed by Li and Lee (1995). Most studies have been on model solutions or liquid foods and at a laboratory scale. Zhang et al. (2010) recently studied the addition of ice nucleation bacteria (P. syringae) in a solid model food (Tylose). Although overall freezing time (under the conditions studied) was not found to change, the addition of the bacteria did increase the nucleation temperature and reduce ice crystal size.
As yet INPs appear to have received less interest than ISPs. While ISPs have found commercial applications, we have found no evidence of INPs having yet been used for any commercial products and current developments appear to remain at a laboratory stage. As is the case with ISPs, it can be concluded that the use of INPs will depend on cost, safety, and consumer acceptance. Since such proteins are bacterial in origin, concerns regarding the safety of such bacteria and how to make sure that inedible microorganisms are killed completely, or removed, before the food consumed remains a major barrier in the take up of INPs (Li and Sun 2002a).
Cryoprotectant Agents
Cryoprotectants are substances that have the ability to protect foods from freezing damage. In its broadest sense ISPs, INPs and texturing agents may be considered cryoprotectants, but are treated separately in this review. Cryoprotectant agents include sugars, amino acids, polyols, methyl amines, carbohydrates, some proteins, and inorganic salts such as potassium phosphate and ammonium sulfate (Jaczynski et al. 2012; Le Bail et al. 2012b). The use of cryoprotectants is not particularly novel; however, some particular cryoprotectants (such as trehalose) are more novel than others and there have been recent innovations in how to apply cryoprotectants (such as PEF and vacuum impregnation).
In recent years, trehalose has been added to the list of cryoprotectant agents (Table 7). Trehalose (α-d-glucopyranosyl-α-d-glucopyranoside) is a non-reducing diglucose sugar. It is widespread in nature, being found in bacteria, yeasts, fungi, insects, invertebrates, and plants (Le Bail et al. 2012b). The preservation mechanisms of trehalose in food systems were described by Patist and Zoerb (2005). Trehalose has 45 % the sweetness of sucrose (Patist and Zoerb 2005).
Cryoprotectants are important freezing stabilisers in surimi. Sugar and sorbitol mixtures are the standard cryoprotectant in surimi and are responsible for its sweet taste. In recent years, there has been a focus on reducing the amounts of these sugars due to health awareness. In addition, while the sweetness imparted by these cryoprotectants complements fish surimi, they are often perceived as too sweet for use in surimi-like material produced from red or poultry meat. Trehalose has been demonstrated to be an effective cryoprotective alternative to sucrose in fish surimi (Osako et al. 2005; Zhou et al. 2006; Pan et al. 2010) and surimi-like beef (Kovačević and Mastanjević 2011) and chicken (Kovačević and Mastanjević 2014).
A series of studies have been carried out by Gómez at Lund University on infusing cryoprotectants into vegetables and fruits to improve the freeze tolerance of these foods. These studies have shown that trehalose either on its own or in combination with ISPs can improve the freezing tolerance of delicate foods, such as leafy vegetables. In order to improve the distribution of the cryoprotectant throughout the product, they have investigated vacuum infiltration (VI) and PEF pretreatments. The PEF pretreatment has been shown to improve the uptake of cryoprotectants and is discussed in detail in a separate section of this review. PEF pretreatment in combination with VI with trehalose has been shown to potentially improve the freezing tolerance of spinach leaves (Phoon et al. 2008). The preliminary results presented showed that sections of spinach leaves subjected to the combined process and then frozen in LN for 7 s followed by immediate thawing in water at room temperature remained turgid after thawing. The VI of strawberries with trehalose in combination with ISPs was also shown to improve freezing tolerance (Velickova et al. 2013). After VI, the fruits in this study were frozen in LN for 25 s then stored at −18 °C for 10 min before thawing at room temperature for 2 h. The pretreatment reduced drip and improved texture when compared with frozen/thawed untreated fruit.
Shayanfar et al. (2013, 2014) studied the effect of combined PEF and a cryoprotectant (glycerol and trehalose) and texturizing agent (calcium chloride) pretreatments on the quality of frozen potato (Shayanfar et al. 2013) and carrot (Shayanfar et al. 2014). The application of trehalose and calcium chloride in combination with PEF resulted in significantly firmer products with less weight loss after thawing.
Trehalose is commercially available and was approved in the EU as a novel food or novel food ingredient in 2001 (Decision 2001/721/EC). Trehalose is already used in several food products, such as bakery, beverages, confectionary, cereals, and meat products, to reduce sweetness and moisture absorption, prevent browning reactions and starch retrogradation, and reduce freeze-thaw damage (Le Bail et al. 2012b; Watrous 2014).
Pulsed Electric Field Pretreatment
Pulsed electric fields (PEF; Table 8) have been shown to accelerate mass transfer by affecting membrane permeability properties (Rastogi et al. 1999; Weaver and Chizmadzhev 1996). Thus, it has been evaluated as a method of improving the uptake of cryoprotectant and texturizing agents (Phoon et al. 2008; Shayanfar et al. 2013; Shayanfar et al. 2014) prior to freezing and in improving osmotic dehydration of dehydrofrozen products (Rastogi et al. 1999; Ben Ammar et al. 2010). One study has suggested that increasing the permeability properties by PEF alone may lead to better accessibility of intracellular materials to freezing and thus reducing the freezing time (Jalté et al. 2009). It has also been reported to reduce shrinkage and maintain color and uniformity in shape (Jalté et al. 2009; Shayanfar et al. 2013).
Jalté et al. (2009) found that although the PEF pretreatment of potato decreased the freezing time this was accompanied by significant structural damage to the freeze-thawed samples. The effective freezing time was found to be directly related to the degree of damage to the sample (estimated from the electrical conductivity disintegration index). Shayanfar and colleagues studied the effect of combined PEF and cryoprotectant (glycerol and trehalose) and texturizing (calcium chloride) agent pretreatments on the quality of frozen potato (Shayanfar et al. 2013) and carrot (Shayanfar et al. 2014). The study using potato indicated that PEF treatment by itself did not prevent softening after defrosting; however, the study using carrots did show some advantages of a PEF treatment alone, in terms of reduced weight loss and improved firmness, in comparison with freezing without a pretreatment.
At present, studies on PEF pretreatment have been at a laboratory scale. It appears to be a useful method of improving the uptake of cryoprotectant and texturizing agents. However, it is not yet clear how advantageous this technology will be compared to current freezing technologies and how effective it will be to apply in commercial applications. In our opinion, further research and development is needed to evaluate this technology at a commercial scale. Such development will also depend on the effectiveness and interest in cryoprotectants and texturizing agents.
Discussion
Li and Sun (2002a) expressed the hope that their review in 2002 would encourage more investigations into novel freezing technologies. In some ways that can be said to have happened, they reviewed four different freezing technologies in 2002 while we have covered 13 in this review. However, it is disappointing that in other respects things have not advanced that far in the last 10 years, or so. Many of the innovative freezing technologies discussed in this review have yet to be shown to be truly practical, and fewer yet have been commercially realised.
Overall, current innovative freezing technologies generally fall into three different approaches:
-
1.
Some innovative freezing processes (impingement and HF) are essentially improvements of existing methods (air blast and immersion, respectively) that by providing far higher surface heat transfer rates than previous systems aim to improve product quality through rapid freezing. In these cases, the advantages depend on the size of the product. The poor thermal conductivity of many foods limits the rate of cooling in large objects (with low surface to mass ratios) rather than the heat transfer between the cooling medium and the product. Thus, increasing the surface heat transfer will not significantly increase the rate of freezing in thick foods with low surface to mass ratios, such as joints of meat, but should increase the rate of freezing in thin foods with high surface to mass ratios, such as burgers and pizzas.
-
2.
Other processes (such as pressure shift, magnetic resonance, electrostatic, microwave, radiofrequency, and ultrasound) are adjuncts to existing freezing systems (such as air blast, plate, cryogenic, or immersion) that aim to improve product quality through controlling the way that ice is formed in the food during freezing. Some claim to achieve this by inducing extensive super-cooling throughout the product prior to ice crystal nucleation. By super-cooling (taking the product below its initial freezing point without ice formation), the food prior to nucleation it is hoped that when nucleation occurs it will occur instantaneously and homogenously throughout the food. Thus, resulting in a reduced duration of the phase transition, less mechanical stress during formation of the ice crystals and smaller ice crystals with a uniform distribution throughout the food. In theory, this would be especially useful when freezing foods with large dimensions in which the poor thermal conductivity of many foods results in thermal gradients that make it difficult to reach fast freezing rates within the centre of such foods. Others claim to disrupt ice nucleation and prevent the formation of large ice crystals or clumping of smaller crystals. Several freezing methods attempt to improve product quality by eliminating ice crystal formation and vitrifying the food (i.e., taking the ice into a glassy state).
-
3.
An alternative is to change the properties of the food itself to control how ice is formed during freezing (such as in dehydrofreezing or the use of antifreeze and ice-nucleation proteins). Antifreeze proteins and ice-nucleation proteins are two functionally distinct and opposite classes of proteins that offer two different approaches, one depressing the nucleation point while the other induces nucleation. Other established pre-freezing treatments include electrical stimulation of meat and blanching.
Of the innovative freezing technologies described in this review, impingement, dehydrofreezing (particularly given that it was first developed in the 1940s), and ISPs may be considered the only mature technologies. While it has been widely reported in general articles in the food trade press that there are magnetic resonance-assisted CAS freezers being used by some companies, we have been unable to independently confirm this (despite direct talks with ABI, the manufacturers of CAS). Other freezing technologies have yet to be applied at a commercial scale.
The premise behind many of these innovative technologies is that the quality of frozen foods can be improved by controlling how ice crystals are formed and where they are formed, and that the speed of freezing offers some quality advantage. There is some evidence that fast freezing rates will promote more nucleation and a greater number of crystals of smaller size which should result in less physical damage to the structure of the food. While there are foods where the rate of freezing and form of ice crystallisation has been shown to be important, there are many foods where this has not been shown to be important, particularly when considering the final eating quality of the cooked product. We would recommend that readers should read Poulsen (1977), Spiess (1979), and particularly Jul (1984) regarding the relative sensitivity of different foods to freezing damage. In addition, few of these studies have investigated the effect of subsequent frozen storage on these ice crystal structures. It is not clear how well small ice crystal structures are maintained during subsequent storage, as ice crystals over time will agglomerate, the phenomenon know as Ostwald ripening. Also, many frozen foods (fruits and vegetables) require blanching in order to deactivate enzymes that would cause undesirable quality deterioration during frozen storage. The interaction of different blanching parameters with freezing methods is often not investigated. That said, although rapid freezing may not always offer advantages in terms of product quality, it often does in terms of improved throughput and efficiency, which is of considerable importance to most frozen food manufacturers.
Since a number of innovative freezing technologies are adjuncts to existing freezing systems, it is a little disappointing that relatively few studies into these technologies have looked at the importance of rate, or different methods, of freezing when applying the technology in question. For example, few dehydrofreezing studies have utilised cryogenic freezing methods, or other more novel rapid freezing methods such as impingement, HF, high pressure assisted, ultrasonic assisted, etc. A number of these technologies are potentially complementary, and thus combined or sequential applications could offer advantages not seen when applied on their own. In addition, the “conventional” freezing regime used as a control comparison by some studies is often perfunctory and non-representative of standard commercial freezing methods (such as the use of static storage freezers operating at −18 to −20 °C). It is to be hoped that such issues will be addressed in future studies.
One issue that can hold back the adoption of novel technologies by the food industry are worries of consumer acceptance. Few studies have been carried out on what consumers’ may think of the innovative freezing technologies discussed in this article. However, one study has addressed high pressure freezing (Lampila and Lahteenmaki 2007). This survey found that their respondents generally had neutral attitudes towards this process and found that price was the most important factor influencing their purchasing decisions.
Conclusions
This review has discussed many innovative freezing processes. Some of these innovative freezing processes (impingement and HF) are essentially improvements of existing methods (air blast and immersion, respectively) that by providing far higher surface heat transfer rates than previous systems, aim to improve product quality through rapid freezing. In these cases, the advantages may depend on the size of the product, since the poor thermal conductivity of many foods limits the rate of cooling in large objects rather than the heat transfer between the heat transfer medium and the product. Other processes reviewed (pressure shift, magnetic resonance, electrostatic, microwave, radio frequency, and ultrasound) are adjuncts to existing freezing systems that aim to improve product quality through controlling the way that ice is formed in the food during freezing. An alternative is to change the properties of the food itself to control how ice is formed during freezing (such as in dehydrofreezing and the use of antifreeze and ice-nucleation proteins). Many of these innovative technologies are still in the development stage, while for others the biggest obstacle to adoption by the food industry is high capital cost. Some of these technologies potentially offer energy savings, primarily through reducing the freezing time. Other technologies may offer quality advantages at the cost of greater energy consumption. In such cases, it remains to be established how important quality versus cost is to the consumer and food processor when it comes to frozen foods.
References
Abedini, S., Kaku, M., Kawata, T., Koseki, H., Kojima, S., Sumi, H., Motokawa, M., Fujita, T., Ohtani, J., Ohwada, N., & Tanne, K. (2011). Effects of cryopreservation with a newly-developed magnetic field programmed freezer on periodontal ligament cells and pulp tissues. Cryobiology, 62(3), 181–187.
Agnelli, M. E., & Mascheroni, R. H. (2002). Quality evaluation of foodstuffs frozen in a cryomechanical freezer. Journal of Food Engineering, 52(3), 257–263.
Alizadeh, E., Chapleau, N., de Lamballerie, M., & Le-Bail, A. (2007a). Effect of different freezing processes on the microstructure of Atlantic salmon (Salmo salar) fillets. Innovative Food Science & Emerging Technologies, 8(4), 493–499.
Alizadeh, E., Chapleau, N., de Lamballerie, M., & Le-Bail, A. (2007b). Effect of freezing and thawing processes on the quality of Atlantic salmon (Salmo salar) fillets. Journal of Food Science, 72(5), E279–E284.
Alizadeh, E., Chapleau, N., de-Lamballerie, M., & Le-Bail, A. (2009). Impact of freezing process on salt diffusivity of seafood: Application to salmon (Salmo salar) using conventional and pressure shift freezing. Food and Bioprocess Technology, 2(3), 257–262.
Anese, M., Manzocco, L., Panozzo, A., Beraldo, P., Foschia, M., & Nicoli, M. C. (2012). Effect of radiofrequency assisted freezing on meat microstructure and quality. Food Research International, 46(1), 50–54.
Anmella, J. M. N. (2010). Process for the production of frozen foods, particularly vegetables or fruits. US Patent 2010/0143564A1.
Anon. (2012). Nice Fruit, frozen fruit and vegetables. http://www.upc.edu/saladepremsa/al-dia/mes-noticies/nice-fruit-frozen-fruit-and-vegetables?set_language=en. Accessed 6 November 2014.
Anon. (2013). Bonduelle signs commercial royalty-bearing license with EnWave. Refrigerated & Frozen Foods. http://www.refrigeratedfrozenfood.com/articles/87491. Accessed 6 February 2014.
Anon. (2014). Dehydration technology for discrete food pieces. EnWave Corporation. http://www.enwave.net/nutrarev.php. Accessed 5 February 2014.
Ben Ammar, J., Lanoisellé, J.-L., Lebovka, N. I., Hecke, E., & Vorobiev, E. (2010). Effect of a pulsed electric field and osmotic treatment on freezing of potato tissue. Food Biophysics, 5(3), 247–254.
Castro, S. M., Van Loey, A., Saraiva, J. A., Smout, C., & Hendrickx, M. (2007). Effect of temperature, pressure and calcium soaking pre-treatments and pressure shift freezing on the texture and texture evolution of frozen green bell peppers (Capsicum annuum). European Food Research and Technology, 226(1–2), 33–43.
Cheftel, J. C., Levy, J., & Dumay, E. (2000). Pressure-assisted freezing and thawing: principles and potential applications. Food Reviews International, 16(4), 453–483.
Cheftel, J. C., Thiebaud, M., & Dumay, E. (2002). Pressure-assisted freezing and thawing of foods: a review of recent studies. International Journal of High Pressure Research, 22(3–4), 601–611.
Cheng, X., Zhang, M., Adhikari, B., Islam, M. N., & Xu, B. (2014). Effect of ultrasound irradiation on some freezing parameters of ultrasound-assisted immersion freezing of strawberries. International Journal of Refrigeration. doi:10.1016/j.ijrefrig.2014.04.017.
Clarke, C. J., Buckley, S. L., & Lindner, N. (2002). Ice structuring proteins—a new name for antifreeze proteins. CryoLetters, 23, 89–92.
Comandini, P., Blanda, G., Soto-Caballero, M. C., Sala, V., Tylewicz, U., Mujica-Paz, H., Valdez Fragoso, A., & Gallina Toschi, T. (2013). Effects of power ultrasound on immersion freezing parameters of potatoes. Innovative Food Science & Emerging Technologies, 18, 120–125.
Crivelli, G., Torregiani, D., Bertolo, G., Forni, E., & Maestrelli, A. (1987a). Research on dehydrofreezing of fruit. Part 2: Utilization for the preparation of fruit salad. XVIIth International Congress of Refrigeration, IIR, Paris, C, 468–471.
Crivelli, G., Torregiani, D., Bertolo, G., Forni, E., & Maestrelli, A. (1987b). Research on dehydrofreezing of fruit. Part 1: Utilization for the preparation of fruit salad. Annales Istituto Sperimentale Valorizzazione Tecnologica dei Prodotti Agricoli, 18, 63–67.
Cruz, R. M. S., Vieira, M. C., & Silva, C. L. M. (2009). The response of watercress (Nasturtium officinale) to vacuum impregnation: Effect of an antifreeze protein type I. Journal of Food Engineering, 95, 339–345.
Del Valle, J. M., Cuadros, T. R. M., & Aguilera, J. M. (1998). Glass transitions and shrinkage during drying and storage of osmosed apple pieces. Food Research International, 31, 191–204.
Delgado, A., & Sun, D. W. (2012). Ultrasound-accelerated freezing. In D. W. Sun (Ed.), Handbook of Frozen Food Processing and Packaging (2nd ed., pp. 645–666). Boca Raton: CRC Press, Taylor & Francis Group.
Delgado, A. E., Zheng, L., & Sun, D. W. (2009). Influence of ultrasound on freezing rate of immersion-frozen apples. Food and Bioprocess Technology, 2(3), 263–270.
Durance, T. (2013). EnWave signs commercial royalty-bearing license with Bonduelle. EnWave Corporation. http://www.enwave.net/news.php?id=478. Accessed 5 February 2014.
Feeney, R. E., & Yeh, Y. (1998). Antifreeze proteins: Current status and possible food uses. Trends in Food Science & Technology, 9(3), 102–106.
Fernandez, P. P., Otero, L., Guignon, B., & Sanz, P. D. (2006a). High-pressure shift freezing versus high-pressure assisted freezing: Effects on the microstructure of a food model. Food Hydrocolloids, 20(4), 510–522.
Fernandez, P. P., Prestamo, G., Otero, L., & Sanz, P. D. (2006b). Assessment of cell damage in high-pressure-shift frozen broccoli: Comparison with market samples. European Food Research and Technology, 224(1), 101–107.
Fernández-Martín, F., Otero, L., Solas, M. T., & Sanz, P. D. (2000). Protein denaturation and structural damage during high-pressure-shift freezing of porcine and bovine muscle. Journal of Food Science, 65, 1002–1008.
Fikiin, A. G. (1992). New method and fluidized water system for intensive chilling and freezing of fish. Food Control (Oxford), 3(3), 153–160.
Fikiin, A. G. (1994). Quick freezing of vegetables by hydrofluidisation. In New Applications of Refrigeration to Fruit and Vegetables Processing, Proceedings of IIR Conference, Istanbul (Turkey), Refrigeration Science and Technology, International Institute of Refrigeration, 1994–3, 85–91.
Fikiin, K. (2003). Novelties of Food Freezing Research in Europe and Beyond. Flair-Flow 4 Synthesis Report. SMEs No. 10, Project No: QLK1-CT-2000-00040.
Fikiin K. A., & Fikiin A. G. (1998). Individual quick freezing of foods by hydrofluidisation and pumpable ice slurries. In Advances in the Refrigeration Systems, Food Technologies and Cold Chain, Ed.: K. Fikiin, Proceedings of IIR Conference, Sofia (Bulgaria), Refrigeration Science and Technology, International Institute of Refrigeration, 1998–6, 319–326.
Fikiin, A. G., & Pham, V. H. (1985). System for examination of heat transfer regimes during hydrorefrigeration of foodstuffs. Invention Certificate No. 39749, Bulgarian Patent Agency INRA.
Fikiin, K., Tsvetkov, O., Laptev, Yu., Fikiin, A., & Kolodyaznaya, V. (2003). Thermophysical and engineering issues of the immersion freezing of fruits in ice slurries based on sugar-ethanol aqueous solution. Ecolibrium, August, 10–15.
Forni, E., Sormani, A., Scalise, S., & Torreggiani, D. (1997). The influence of sugar composition on the colour stability of osmodehydrofrozen intermediate moisture apricots. Food Research International, 30, 87–94.
Gareth, J. (Ed.). (1992). Current trends in sonochemistry. Cambridge: The Royal Society of Chemistry.
Giannakourou, M. C., & Taoukis, P. S. (2003). Stability of dehydrofrozen green peas pretreated with nonconventonal osmotic agents. Journal of Food Science, 68, 2002–2010.
Gómez, F., & Sjöholm, I. (2004). Applying biochemical and physiological principles in the industrial freezing of vegetables: a case study on carrots. Trends in Food Science & Technology, 15, 39–43.
Góral, D., & Kluza, F. (2006). Physical changes of vegetables during freezing by conventional and impingement methods. Acta Agrophysica, 7(1), 59–71.
Góral, D., & Kluza, F. (2009). Cutting test application to general assessment of vegetable texture changes caused by freezing. Journal of Food Engineering, 95(2), 346–351.
Góral, D., & Kluza, F. (2012). Heat transfer coefficient in impingement fluidization freezing of vegetables and its prediction. International Journal of Refrigeration, 35, 871–879.
Griffith, M., & Ewart, K. V. (1995). Antifreeze proteins and their potential use in frozen foods. Biotechnology Advances, 13(3), 375–402.
Hansen, E., Trinderup, R. A., Hviid, M., Darre, M., & Skibsted, L. H. (2003). Thaw drip loss and protein characterization of drip from air-frozen, cryogen-frozen, and pressure-shift-frozen pork longissimus dorsi in relation to ice crystal size. European Food Research and Technology, 218(1), 2–6.
Hanyu, Y., Ichikawa, M., & Matsumoto, G. (1992). An improved cryofixation method—Cryoquenching of small tissue blocks during microwave irradiation. Journal of Microscopy (Oxford), 165, 255–271.
Hassas-Roudsari, M., & Goff, H. D. (2012). Ice structuring proteins from plants: Mechanism of action and food application. Food Research International, 46, 425–436.
Ho, S. Y. (2004). A turbulent conjugate heat-transfer model for freezing of food products. Journal of Food Science, 69(5), E224–E231.
Howard, L. B., & Campbell, H. (1946). Dehydrofreezing—a new way of preserving food. Food Industries, 18, 674–676.
Hu, S.-Q., Liu, G., Li, L., Li, Z.-X., & Hou, Y. (2013). An improvement in the immersion freezing process for frozen dough via ultrasound irradiation. Journal of Food Engineering, 114, 22–28.
Huxsoll, C. C. (1982). Reducing the refrigeration load by partial concentration of foods prior to freezing. Food Technology, 36(5), 98–102.
Islam, M. N., Zhang, M., Adhikari, B., Xinfeng, C., & Xu, B.-G. (2014). The effect of ultrasound-assisted immersion freezing on selected physicochemical properties of mushrooms. International Journal of Refrigeration. doi:10.1016/j.ijrefrig.2014.02.012.
Jackson, T. H., Ungan, A., Critser, J. K., & Gao, D. Y. (1997). Novel microwave technology for cryopreservation of biomaterials by suppression of apparent ice formation. Cryobiology, 34(4), 363–372.
Jaczynski, J., Tahergorabi, R., Hunt, A. L., & Park, J. W. (2012). Safety and quality of frozen aquatic food products. In D. W. Sun (Ed.), Handbook of Frozen Food Processing and Packaging (2nd ed., pp. 343–385). Boca Raton: CRC Press, Taylor & Francis Group.
Jafari, M., & Alavi, P. (2008). Analysis of food freezing by slot jet impingement. Journal of Applied Sciences, 8(7), 1188–1196.
Jalté, M., Lanoiselle, J. L., Lebovka, N. I., & Vorobiev, E. (2009). Freezing of potato tissue pre-treated by pulsed electric fields. LWT- Food Science and Technology, 42, 576–580.
James, S. J., & James, C. (2012). Innovative freezing technologies for foods. New Food, 15(4), 21–24.
James, C., Purnell, G., & James, S. J. (2014). A critical review of dehydrofreezing of fruits and vegetables. Food and Bioprocess Technology, 7, 1219–1234.
James, C., Reitz, B. G., & James, S. J. (2015). The freezing characteristics of garlic bulbs (Allium sativum L.) frozen conventionally or with the assistance of an oscillating weak magnetic field. Food and Bioprocess Technology, 8(3), 702–708.
Jul, M. (1984). The Quality of Frozen Foods. Orlando: Academic.
Kaku, M., Kamada, H., Kawata, T., Koseki, H., Abedini, S., Kojima, S., Motokawa, M., Fujita, T., Ohtani, J., Tsuka, N., Matsuda, Y., Sunagawa, H., Hernandes, R. A. M., Ohwada, N., & Tanne, K. (2010). Cryopreservation of periodontal ligament cells with magnetic field for tooth banking. Cryobiology, 61(1), 73–78.
Kaku, M., Kawata, T., Abedini, S., Koseki, H., Kojima, S., Sumi, H., Shikata, H., Motokawa, M., Fujita, T., Ohtani, J., Ohwada, N., Kurita, M., & Tanne, K. (2012). Electric and magnetic fields in cryopreservation: a response. Cryobiology, 64(3), 304–305.
Kiani, H., Zhang, Z., Delgado, A., & Sun, D.-W. (2011). Ultrasound assisted nucleation of some liquid and solid model foods during freezing. Food Research International, 44(9), 2915–2921.
Kiani, H., Sun, D.-W., & Zhang, Z. (2012a). The effect of ultrasound irradiation on the convective heat transfer rate during immersion cooling of a stationary sphere. Ultrasonics Sonochemistry, 19, 1238–1245.
Kiani, H., Sun, D. W., Zhang, Z. H., Al-Rubeai, M., & Naciri, M. (2013a). Ultrasound-assisted freezing of Lactobacillus plantarum subsp. plantarum: The freezing process and cell viability. Innovative Food Science & Emerging Technologies, 18, 138–144.
Kiani, H., Zhang, Z., & Sun, D.-W. (2013b). Effect of ultrasound irradiation on ice crystal size distribution in frozen agar gel samples. Innovative Food Science & Emerging Technologies, 18, 126–131.
Kim, Y. B., Woo, S. M., Jeong, J. Y., Ku, S. K., Jeong, J. W., Kum, J. S., & Kim, E. M. (2013). Temperature changes during freezing and effect of physicochemical properties after thawing on meat by air blast and magnetic resonance quick freezing. Korean Journal of Food Science of Animal Resources, 33, 763–771.
Kobayashi, A., & Kirschvink, J. L. (2013). A ferromagnetic model for the action of electric and magnetic fields in cryopreservation. Cryobiology, 68(2), 163–165.
Kovačević, D., & Mastanjević, K. (2011). Cryoprotective effect of trehalose and maltose on washed and frozen stored beef meat. Czech Journal of Food Science, 29, 15–23.
Kovačević, D., & Mastanjević, K. (2014). Cryoprotective effect of trehalose on washed chicken meat. Journal of Food Science and Technology, 51, 1006–1010.
Lampila, P., & Lahteenmaki, L. (2007). Consumers’ attitudes towards high pressure freezing of food. British Food Journal, 109(10), 838–851.
Le Bail, A., Chevaliera, D., Mussaa, D. M., & Ghoul, M. (2002). High pressure freezing and thawing of foods: a review. International Journal of Refrigeration, 25(5), 504–513.
Le Bail, A., Orlowska, M., & Havet, M. (2010). Possible interest of electric field during food freezing; A review on electrofreezing. Sustainability & the Cold Chain, Meeting of IIR Commissions B1, B2, C2, D1 and D2, Cambridge, UK.
Le Bail, A., Orlowska, M., & Havet, M. (2012a). Electrostatic field-assisted food freezing. In D. W. Sun (Ed.), Handbook of Frozen Food Processing and Packaging (2nd ed., pp. 685–691). Boca Raton: CRC Press, Taylor & Francis Group.
Le Bail, A., Tzia, C., & Giannou, V. (2012b). Quality and safety of frozen bakery products. In D. W. Sun (Ed.), Handbook of Frozen Food Processing and Packaging (2nd ed., pp. 501–528). Boca Raton: CRC Press, Taylor & Francis Group.
Leunissen, J. L. M., & Yi, H. (2009). Self-pressurized rapid freezing (SPRF): a novel cryofixation method for specimen preparation in electron microscopy. Journal of Microscopy, 235, 25–35.
Li, J., & Lee, T. C. (1995). Bacterial ice nucleation and its potential application in the food industry. Trends in Food Science & Technology, 6, 259–265.
Li, B., & Sun, D. W. (2002a). Novel methods for rapid freezing and thawing of foods—a review. Journal of Food Engineering, 54, 175–182.
Li, B., & Sun, D. W. (2002b). Effect of power ultrasound on freezing rate during immersion freezing of potatoes. Journal of Food Engineering, 55(3), 277–282.
Luscher, C., Schluter, O., & Knorr, D. (2005). High pressure-low temperature processing of foods: Impact on cell membranes, texture, color and visual appearance of potato tissue. Innovative Food Science & Emerging Technologies, 6(1), 59–71.
Mortazavi, A., & Tabatabaie, F. (2008). Study of ice cream freezing process after treatment with ultrasound. World Applied Sciences Journal, 4, 188–190.
Naito, M., Hirai, S., Mihara, M., Terayama, H., Hatayama, N., Hayashi, S., Matsushita, M., & Itoh, M. (2012). Effect of a magnetic field on Drosophila under supercooled conditions. PLoS ONE, 7.
Newman, M. (2001). Cryogenic impingement freezing utilizing atomized liquid nitrogen for the rapid freezing of food products. Rapid Cooling of food, Meeting of IIR Commission C2, Bristol (UK), Section 2, 145–151.
Osako, K., Hossain, M. A., Kuwahara, K., & Nozaki, Y. (2005). Effect of trehalose on the gel-forming ability, state of water and myofibril denaturation of horse mackerel Trachurus japonicus surimi during frozen storage. Fisheries Science, 71, 367–373.
Otero, L., & Sanz, P. D. (2012). High-pressure shift freezing. In D. W. Sun (Ed.), Handbook of Frozen Food Processing and Packaging (2nd ed., pp. 667–683). Boca Raton: CRC Press, Taylor & Francis Group.
Owada, N. (2007). Highly-efficient freezing apparatus and highly-efficient freezing method. US Patent 7237400 B2.
Owada, N., & Kurita, S. (2001). Super-quick freezing method and apparatus therefore. US Patent US 6250087 B1.
Pan, J., Shen, H., & Luo, Y. (2010). Cryoprotective effects of trehalose on grass carp (Ctenopharyngodon idellus) surimi during frozen storage. Journal of Food Processing and Preservation, 34, 715–727.
Patist, A., & Zoerb, H. (2005). Preservation mechanisms of trehalose in food and biosystems. Colloids and Surfaces B: Biointerfaces, 40(2), 107–113.
Payne, S. R., & Young, O. A. (1995). Effects of pre-slaughter administration of antifreeze proteins on frozen meat quality. Meat Science, 41(2), 147–155.
Payne, S. R., Sandford, D., Harris, A., & Young, O. A. (1994). The effects of antifreeze proteins on chilled and frozen meat. Meat Science, 37(3), 429–438.
Peralta, J. M., Rubiolo, A. C., & Zorrilla, S. E. (2009). Design and construction of a hydrofluidization system. Study of the heat transfer on a stationary sphere. Journal of Food Engineering, 90(3), 358–364.
Peralta, J. M., Rubiolo, A. C., & Zorrilla, S. E. (2010). Mathematical modeling of the heat transfer and flow field of liquid refrigerants in a hydrofluidization system with a stationary sphere. Journal of Food Engineering, 99(3), 303–313.
Peralta, J. M., Rubiolo, A. C., & Zorrilla, S. E. (2012). Mathematical modeling of the heat and mass transfer in a stationary potato sphere impinged by a single round liquid jet in a hydrofluidization system. Journal of Food Engineering, 109(3), 501–512.
Petersen, A., Schneider, H., Rau, G., & Glasmacher, B. (2006). A new approach for freezing of aqueous solutions under active control of the nucleation temperature. Cryobiology, 53(2), 248–257.
Petzold, G., & Aguilera, J. M. (2009). Ice morphology: Fundamentals and technological applications in foods. Food Biophysics, 4(4), 378–396.
Pham, Q. T. (2008). Advances in food freezing/thawing/freeze concentration modelling and techniques. Japan Journal of Food Engineering, 9(1), 21–32.
Phoon, P. Y., Gomez, F., Vicente, A., & Dejmek, P. (2008). Pulsed electric field in combination with vacuum impregnation with trehalose improves the freezing tolerance of spinach leaves. Journal of Food Engineering, 88, 144–148.
Picart, L., Dumay, E., Guiraud, J. P., & Cheftel, J. C. (2004). Microbial inactivation by pressure-shift freezing: effects on smoked salmon mince inoculated with Pseudomonas fluorescens, Micrococcus luteus and Listeria innocua. LWT--Food Science and Technology, 37(2), 227–238.
Poulsen, K. P. (1977). The freezing process under industrial conditions. Freezing, frozen storage and Freeze drying, Meeting of IIR Commissions C1, C2, Karlsruhe (GDR), Section 6, 347–353.
Préstamo, G., Palomares, L., & Sanz, P. (2004). Broccoli (Brasica oleracea) treated under pressure-shift freezing process. European Food Research and Technology, 219(6), 598–604.
Préstamo, G., Palomares, L., & Sanz, P. (2005). Frozen foods treated by pressure shift freezing: Proteins and enzymes. Journal of Food Science, 70(1), S22–S27.
Ramallo, L. A., & Mascheroni, R. H. (2010). Dehydrofreezing of pineapple. Journal of Food Engineering, 99(3), 269–275.
Rastogi, N. K., Eshtiagi, M. N., & Knorr, D. (1999). Accelerated mass transfer during osmotic dehydration of high intensity electrical field pulse pretreated carrots. Journal of Food Science, 64, 1020–1023.
Salvadori, V. O., & Mascheroni, R. H. (2002). Analysis of impingement freezers performance. Journal of Food Engineering, 54(2), 133–140.
Sarkar, A., & Singh, R. P. (2004). Modeling flow and heat transfer during freezing of foods in forced airstreams. Journal of Food Science, 69(9), E488–E496.
Sarkar, A., Nitin, N., Karwe, M., & Singh, R. P. (2004). Fluid flow and heat transfer in air jet impingement in food processing. Journal of Food Science, 69(4), R113–R122.
Sato, M., & Fujita, K. (2006). Refrigerating device, refrigerating method and refrigerated object. US Patent US 2006/0112699 A1.
Scaman, C. H., & Durance, T. D. (2005). Combined microwave vacuum drying. In D. W. Sun (Ed.), Emerging technologies for food processing (pp. 507–533). Oxford: Elsevier Ltd.
Schluter, O., Benet, G. U., Heinz, V., & Knorr, D. (2004). Metastable states of water and ice during pressure-supported freezing of potato tissue. Biotechnology Progress, 20(3), 799–810.
Sequeira-Munoz, A., Chevalier, D., Simpson, B. K., Le Bail, A., & Ramaswamy, H. S. (2005). Effect of pressure-shift freezing versus air-blast freezing of carp (cyprinus carpio) fillets: a storage study. Journal of Food Biochemistry, 29(5), 504–516.
Shayanfar, S., Chauhan, O. P., Toepfl, S., & Volker, H. (2013). The interaction of pulsed electric fields and texturizing—Antifreezing agents in quality retention of defrosted potato strips. International Journal of Food Science and Technology, 48, 1289–1295.
Shayanfar, S., Chauhan, O. P., Toepfl, S., & Heinz, V. (2014). Pulsed electric field treatment prior to freezing carrot discs significantly maintains their initial quality parameters after thawing. International Journal of Food Science and Technology, 49, 1224–1230.
Shim, K.-B., Hong, G.-P., Choi, M. J., & Min, S.-G. (2009). Effect of high pressure freezing and thawing process on the physical properties of pork. Korean Journal for Food Science of Animal Resources, 29(6), 736–742.
Shizuka, J., Ogawa, Y., & Tagawa, A. (2008). Effects of freezing and thawing on the physical and electrical properties of dehydrated radish. Journal of the Japanese Society for Food Science and Technology, 55, 158–163.
Soto, V., & Bórquez, R. (2001). Impingement jet freezing of biomaterials. Food Control, 12, 515–522.
Soukoulis, C., & Fisk, I. (2014). Innovative ingredients and emerging technologies for controlling ice recrystallisation, texture and structure stability in frozen dairy desserts: a review. Critical Reviews in Food Science and Nutrition. doi:10.1080/10408398.2013.876385.
Spiess, W. E. L. (1979). Impact of freezing rates on product quality of deep-frozen foods. Food Process Engineering, 8th European Food Symposium, Espo, Finland, 689–694.
Su, G., Ramaswamy, H. S., Zhu, S., Yu, Y., Hu, F., & Xu, M. (2014). Thermal characterization and ice crystal analysis in pressure shift freezing of different muscle (shrimp and porcine liver) versus conventional freezing method. Innovative Food Science & Emerging Technologies, 26, 40–50.
Sun, D. W., & Li, B. (2003). Microstructural change of potato tissues frozen by ultrasound-assisted immersion freezing. Journal of Food Engineering, 57(4), 337–345.
Sundsten, S., Andersson, A., Tornberg, E. (2001). The effect of the freezing rate on the quality of hamburger. Rapid Cooling of Food, Meeting of IIR Commission C2, Bristol, UK, 2001, Section 2, pp181-186.
Suzuki, T., Takeuchi, Y., Masuda, K., Watanabe, M., Shirakashi, R., Fukuda, Y., Tsuruta, T., Yamamoto, K., Koga, N., Hiruma, N., Ichioka, J., & Takai, K. (2009). Experimental investigation of effectiveness of magnetic field on food freezing process. Transactions of the Japan Society of Refrigerating and Air Conditioning Engineers, 26(4), 371–386.
Tironi, V., Le-Bail, A., & de Lamballerie, M. (2007). Effects of pressure-shift freezing and pressure-assisted thawing on sea bass (Dicentrarchus labrax) quality. Journal of Food Science, 72(7), C381–C387.
Tironi, V., de Lamballerie-Anton, M., & Le-Bail, A. (2009). DSC determination of glass transition temperature on sea bass (Dicentrarchus labrax) muscle: Effect of high-pressure processing. Food and Bioprocess Technology, 2(4), 374–382.
Tironi, V., de Lamballerie, M., & Le-Bail, A. (2010). Quality changes during the frozen storage of sea bass (Dicentrarchus labrax) muscle after pressure shift freezing and pressure assisted thawing. Innovative Food Science & Emerging Technologies, 11(4), 565–573.
Tocci, A. M., & Mascheroni, R. H. (2008). Some thermal properties of fresh and osmotically dehydrated Kiwifruit above and below the initial freezing temperature. Journal of Food Engineering, 88, 20–27.
Torreggiani, D., Forni, E., Guercilena, I., Maestrelli, A., Bertolo, G., Archer, G. P., Kennedy, C. J., Bone, G., Blond, G., Contreras-Lopez, E., & Champion, D. (1999). Modifcation of glass transition temperature through carbohydrates additions: Effect upon colour and anthocyanin pigment stability in frozen strawberry juices. Food Research International, 32, 441–446.
Tressler, D. K., & Evers, C. F. (1957). The Freezing Preservation of Foods: Volume 1 – Freezing of Fresh Foods. The AVI Publishing Company, Inc.
Urrutia Benet, G., Chapleau, N., Lille, M., Le Bail, A., Autio, K., & Knorr, D. (2006). Quality related aspects of high pressure low temperature processed whole potatoes. Innovative Food Science & Emerging Technologies, 7(1), 32–39.
Van Buggenhout, S., Messagie, I., Van Loey, A., & Hendrickx, M. (2005). Influence of low-temperature blanching combined with high-pressure shift freezing on the texture of frozen carrots. Journal of Food Science, 70(4), S304–S308.
van Buggenhout, S., Lille, M., Messagie, I., Van Loey, A., Autio, K., & Hendrickx, M. (2006a). Impact of pretreatment and freezing conditions on the microstructure of frozen carrots: Quantification and relation to texture loss. European Food Research and Technology, 222, 543–553.
Van Buggenhout, S., Messagie, I., Maes, V., Duvetter, T., Van Loey, A., & Hendrickx, M. (2006b). Minimizing texture loss of frozen strawberries: effect of infusion with pectinmethylesterase and calcium combined with different freezing conditions and effect of subsequent storage/thawing conditions. European Food Research and Technology, 223(3), 395–404.
Van Buggenhout, S., Grauwet, T., Van Loey, A., & Hendrickx, M. (2007). Effect of high-pressure induced ice i/ice iii-transition on the texture and microstructure of fresh and pretreated carrots and strawberries. Food Research International, 40(10), 1276–1285.
Van Buggenhout, S., Grauwet, T., Van Loey, A., & Hendrickx, M. (2008). Structure/processing relation of vacuum infused strawberry tissue frozen under different conditions. European Food Research and Technology, 226, 437–448.
Velickova, E., Tylewicz, U., Rosa, M. D., Winkelhausen, E., Kuzmanova, S., & Galindo, F. G. (2013). Effect of vacuum infused cryoprotectants on the freezing tolerance of strawberry tissues. LWT - Food Science and Technology, 52, 146–150.
Venketesh, S., & Dayananda, C. (2008). Properties, potentials, and prospects of antifreeze proteins. Critical Reviews in Biotechnology, 28, 57–82.
Verboven, P., Scheerlinck, N., & Nicolai, B. M. (2003). Surface heat transfer coefficients to stationary spherical particles in an experimental unit for hydrofluidisation freezing of individual foods. International Journal of Refrigeration, 26(3), 328–336.
Volkert, M., Puaud, M., Wille, H. J., & Knorr, D. (2012). Effects of High Pressure–Low Temperature treatment on freezing behavior, sensorial properties and air cell distribution in sugar rich dairy based frozen food foam and emulsions. Innovative Food Science & Emerging Technologies, 13, 75–85.
Wang, S., & Sun, D. W. (2012). Antifreeze proteins. In D. W. Sun & R. Boca (Eds.), Handbook of Frozen Food Processing and Packaging (2nd ed., pp. 693–708). Boca Raton: CRC Press, Taylor & Francis Group.
Watanabe, M., Kanesaka, N., Masuda, K., & Suzuki, T. (2011). Effect of oscillating magnetic field on supercooling in food freezing. Proceedings of the 23rd IIR International Congress of Refrigeration; refrigeration for sustainable development, August 21–26, Prague, Czech Republic. 1, 2892–2899.
Watrous, M. (2014). Taco Bell, trehalose and the trend of transparency. New Business News. http://www.foodbusinessnews.net/articles/news_home/Supplier-Innovations/2014/05/Taco_Bell_trehalose_and_the_tr.aspx?ID=%7BBD0024D9-9546-4D4E-B04A-5DB17EE1A039%7D. Accessed 18 March 2015.
Weaver, J. C., & Chizmadzhev, Y. A. (1996). Theory of electroporation: a review. Bioelectrochemistry and Bioenergetics, 41, 135–160.
Winney, N. (2012). From the field to the supermarket – post harvest cooling, part 2 of 4. Cold Chain, April – June 2012, C20-C26.
Woo, M. W., & Mujumdar, A. S. (2010). Effects of electric and magnetic field on freezing and possible relevance in freeze drying. Drying Technology, 28(4), 433–443.
Wowk, B. (2012). Electric and magnetic fields in cryopreservation. Cryobiology, 64, 301–303.
Wu, L., Orikasa, T., Tokuyasu, K., Shiina, T., & Tagawa, A. (2009). Applicability of vacuum-dehydrofreezing technique for the long-term preservation of fresh-cut eggplant: Effects of process conditions on the quality attributes of the samples. Journal of Food Engineering, 91(4), 560–565.
Xanthakis, E., Havet, M., Chevallier, S., Abadie, J., & Le-Bail, A. (2013). Effect of static electric field on ice crystal size reduction during freezing of pork meat. Innovative Food Science and Emerging Technologies, 20, 115–120.
Xanthakis, E., Le-Bail, A., & Ramaswamy, H. (2014). Development of an innovative microwave assisted food freezing process. Innovative Food Science and Emerging Technologies, 26, 176–181.
Xin, Y., Zhang, M., & Adhikari, B. (2014a). Ultrasound assisted immersion freezing of broccoli (Brassica oleracea L. var. botrytis L.). Ultrasonics Sonochemistry, 21, 1728–1735.
Xin, Y., Zhang, M., & Adhikari, B. (2014b). The effects of ultrasound-assisted freezing on the freezing time and quality of broccoli (Brassica oleracea L. var. botrytis L.) during immersion freezing. International Journal of Refrigeration, 41, 82–91.
Xu, H. N., Huang, W., Jia, C., Kim, Y., & Liu, H. (2009). Evaluation of water holding capacity and breadmaking properties for frozen dough containing ice structuring proteins from winter wheat. Journal of Cereal Science, 49, 250–253.
Xu, Z., Guo, Y., Ding, S., An, K., & Wang, Z. (2014). Freezing by immersion in liquid CO2 at variable pressure: Response surface analysis of the application to carrot slices freezing. Innovative Food Science & Emerging Technologies, 22, 167–174.
Yeh, C.-M., Kao, B.-Y., & Peng, H.-J. (2009). Production of a recombinant type 1 antifreeze protein analogue by l. Lactis and its applications on frozen meat and frozen dough. Journal of Agricultural and Food Chemistry, 57(14), 6216–6223.
Yu, D., Liu, B., & Wang, B. (2012). The effect of ultrasonic waves on the nucleation of pure water and degassed water. Ultrasonics Sonochemistry, 19, 459–463.
Yuste, J., Pla, R., Beltram, E., & Mor-Mur, M. (2002). High pressure processing at subzero temperature: Effect on spoilage microbiota of poultry. High Pressure Research, 22, 673–676.
Zhang, C., Zhang, H., & Wang, L. (2007a). Effect of carrot (Daucus carota) antifreeze proteins on the fermentation capacity of frozen dough. Food Research International, 40(6), 763–769.
Zhang, C., Zhang, H., Wang, L., Gao, H., Guo, X. N., & Yao, H. Y. (2007b). Improvement of texture properties and flavor of frozen dough by carrot (Daucus carota) antifreeze protein supplementation. Journal of Agricultural and Food Chemistry, 55(23), 9620–9626.
Zhang, C., Zhang, H., Wang, L., & Guo, X. (2008). Effect of carrot (Daucus carota) antifreeze proteins on texture properties of frozen dough and volatile compounds of crumb. LWT--Food Science and Technology, 41(6), 1029–1036.
Zhang, S., Wang, H., & Chen, G. (2010). Addition of ice-nucleation active bacteria: Pseudomonas syringae pv. Panici on freezing of solid model food. LWT--Food Science and Technology, 43(9), 1414–1418.
Zheng, L., & Sun, D. W. (2005). Ultrasonic assistance of food freezing. In D. W. Sun (Ed.), Emerging Technologies for Food Processing (pp. 603–626). London: Elsevier Academic Press.
Zheng, L., & Sun, D. W. (2006). Innovative applications of power ultrasound during food freezing processes - a review. Trends in Food Science & Technology, 17(1), 16–23.
Zhou, A., Benjakul, S., Pan, K., Gong, J., & Liu, X. (2006). Cryoprotective effects of trehalose and sodium lactate on tilapia (Sarotherodon nilotica) surimi during frozen storage. Food Chemistry, 96, 96–103.
Zhu, S. M., Le Bai, A., & Ramaswamy, H. S. (2003). Ice crystal formation in pressure shift freezing of atlantic salmon (Salmo salar) as compared to classical freezing methods. Journal of Food Processing and Preservation, 27(6), 427–444.
Zhu, S. M., Le Bail, A., Chapleau, N., Ramaswamy, H. S., & de Lamballerie-Anton, M. (2004a). Pressure shift freezing of pork muscle: Effect on color, drip loss, texture, and protein stability. Biotechnology Progress, 20(3), 939–945.
Zhu, S. M., Le Bail, A., Ramaswamy, H. S., & Chapleau, N. (2004b). Characterization of ice crystals in pork muscle formed by pressure-shift freezing as compared with classical freezing methods. Journal of Food Science, 69(4), 190–197.
Acknowledgments
The authors would like to thank Air Products for funding the work required to carry out this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
James, C., Purnell, G. & James, S.J. A Review of Novel and Innovative Food Freezing Technologies. Food Bioprocess Technol 8, 1616–1634 (2015). https://doi.org/10.1007/s11947-015-1542-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-015-1542-8