Abstract
Purpose of Review
Targeted temperature management (TTM) has been the mainstay of secondary brain injury prevention for unconscious cardiac arrest survivors. In this ever-changing landscape of post-cardiac arrest care, TTM practices are evolving with emergence of new evidence. We discuss the pre-clinical data paving the scientific premise for temperature control in hypoxic-ischemic brain injury and dissect through landmark TTM trials. We then describe how the practice of TTM has changed in response to the most pivotal trials and discuss exciting topics that are under investigation.
Recent Findings
The advent of TTM2 has challenged the use of lower temperature targets in out-of-hospital cardiac arrest of presumed cardiac etiology by finding similar survival and neurological function at 6 months between cooling post-arrest patients to 33 °C for 24 h (followed by gradual rewarming and targeted normothermia) and actively preventing fever (< 37.8 °C) for 72 h.
Summary
Temperature control remains the cornerstone in secondary brain injury prevention post-cardiac arrest, and practices surrounding temperature targets are evolving over time as new evidence emerges. Future studies on tailored temperature control to individualized factors, including depth and duration, as well as rate of rewarming will be crucial to address prevailing knowledge gaps.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiac arrest is a leading cause of morbidity and mortality worldwide. In the USA, the incidence of out-of-hospital cardiac arrest (OHCA) assisted by Emergency Medical Services (EMS) was estimated at 92.3 per 100,000 persons in 2021 [1, 2]. Survival to hospital discharge remains alarmingly low at 9.1% in OHCA [1]. This figure was even lower (7.2%) for adults surviving with a favorable neurological outcome (based on Cerebral Performance Category score of 1 or 2) [1]. Regardless of etiology, one of the primary roles of the neurologist is the recognition and prevention of secondary brain injury—one of the main determinants of outcome following hypoxic-ischemic brain injury. No other neuroprotective therapy has been as extensively studied and debated over than targeted temperature management (TTM). Promising preclinical data laid the groundwork for TTM nearly a century ago—and while initial landmark trials suggested a benefit in humans decades later—emerging conflicting evidence has since challenged the use of hypothermia in post-arrest care.
We will discuss the preclinical evidence that paved the way for the pivotal trials of TTM in humans, analyze the conflicting evidence that has challenged the scientific premise of its neuroprotective effects, and discuss the direction of future research by outlining prevailing knowledge gaps.
Why should we Care About Temperature?
Hypoxic-ischemic brain injury often leads to devastating neurological injury. Neurological damage inflicted by cardiac arrest is twofold, including both the initial ischemic event and the multifactorial process of secondary brain injury that occurs despite restoration of systemic circulation (ROSC). Primary injury is thought to be mediated by anoxic depolarizations—a massive influx of cations disrupting the transmembrane ionic gradient leading to spreading depolarization, cytotoxic edema, and release of glutamate [3]. Components of secondary brain injury include microcirculatory dysfunction, oxygen and nitrogen free radical production, loss of cerebral autoregulation, excitotoxicity, activation of protease cascades, and cerebral edema [4]. All of these processes may be exacerbated by further insults such as hypotension, hypoglycemia, and hyperthermia. Higher body temperatures lead to increased permeability of the blood–brain barrier, increased cerebral metabolism—in tissue that is already subjected to imbalances in bioenergetic supply and demand—and promotes release of inflammatory and pro-apoptotic cytokines [4]. The goal of TTM is to halt ongoing secondary injury pathways that are temperature sensitive.
Early case reports published in the 1950s document the first attempts of using temperature control following cardiac arrest in an array of etiologies [5]. All patients received hypothermia from 30 to 34 °C between 24 and 72 h and were found to have minimal to no neurological deficits days after rewarming [5]. There were no controls who did not receive temperature modulation, all cases were treated at a single center, and the study had a high risk of selection bias. A few years later, another case series documented the use of temperature control in 19 patients following cardiac arrest occurring either intraoperatively, during a procedure, or spontaneously in the “accident room” [6]. Neurological deficits were assessed at time of resuscitation and classified either as “none” (answering questions and moving all extremities), “moderate” (awake and responding “some” to verbal stimuli), or “severe” (comatose or convulsing). Only patients with moderate or severe neurological injury were included in the study. Goal temperatures ranged from 30 to 32 °C, achieved by use of blankets with circulating coolant, and meperidine or promethazine was used to treat shivering, if necessary. Similarly, various etiologies of arrest were included, and the duration of hypothermia ranged between 3 h and 8 days in the experimental group. Survival to hospital discharge was 14% in the control group (7 patients) and 50% in the group that received hypothermia (12 patients) [6].
Further evidence supporting neuroprotective effects of temperature control was demonstrated in animal models of hypoxic-ischemic brain injury. In a gerbil model of global ischemia, hypothermia to 32 °C for 12 or 24 h was associated with greater preservation of hippocampal CA1 neurons and improved memory function compared to controls [7]. A follow-up study found that this protective effect persisted at 6 months and was more pronounced when initiation of hypothermia was not delayed [8]. A canine model of ventricular fibrillation showed improved neurological function in dogs cooled to 30 °C and 34 °C for 1 h after induced cardiac arrest, compared to those cooled to 15 °C or maintained at 37.5 °C [9]. In addition to suggesting a potential benefit of hypothermia following cardiac arrest, this experiment demonstrated that a limit may exist at which lower temperatures become detrimental and lead to worse outcomes.
These early experiences with temperature modulation in humans and animal models paved the scientific premise for two pivotal, randomized controlled trials which led to a paradigm shift in temperature control following cardiac arrest in the early 2000s.
Landmark Clinical Trials
Several randomized clinical trials have studied temperature modulation in the post-cardiac arrest period for unconscious patients following ROSC with varying degrees of rigor. A comprehensive comparison of the details of eligibility criteria is summarized in Table 1.
The Hypothermia After Cardiac Arrest (HACA) trial enrolled patients across five European countries with out-of-hospital cardiac arrest (OHCA) and a shockable rhythm (i.e., ventricular fibrillation and/or pulseless ventricular tachycardia). A total of 138 subjects were randomized to receive no temperature intervention, and 137 had TTM at 32–34 °C for 24 h followed by 8 h of passive rewarming. Lower mortality (41% versus 55%) and higher proportion of favorable neurological outcome (i.e., Glasgow-Pittsburgh Cerebral Performance Categories Scale—CPC 1–2) (55% versus 39%) were found at 6 months in the TTM group compared to controls [10]. Also published in 2002, the Australian trial led by Bernard enrolled 77 adults with OHCA with ventricular fibrillation, who were allocated to either TTM to 33 °C for 12 h or standard care with no temperature control. Good neurological outcome (i.e., discharge to home or acute rehabilitation facility) was achieved in 49% of subjects in the TTM group versus 26% in the control group [11]. Notably, in both trials, normothermia was not actively maintained during the intervention period for the control groups, or after the intervention period for both groups.
These findings supported that adult patients who remained unable to follow verbal commands following OHCA should be cooled for 12 to 24 h to improve neurological outcomes, provided they had a shockable initial rhythm.
What is the Optimal Temperature?
Likely the subject of the most fervent debate regarding use of temperature control is the optimal temperature target during the post-arrest period. In the HACA trial, many patients in the control group had temperatures that were recorded ≥ 38 °C. The investigators of the TTM1 trial suspected that the benefit of temperature modulation could be accomplished with milder hypothermia. The TTM1 trial randomized 950 adult OHCA patients with any non-perfusing rhythm (except for asystole in unwitnessed arrests) to receive TTM to either 33 °C or 36 °C for 24 h, followed by slow rewarming and strict normothermia (37 °C) with active prevention of fever until 72 h post-arrest. There were no significant differences in all-cause mortality or poor neurological outcome (CPC 3 to 5 or modified Rankin scale [mRS] 4 to 6) between the two groups at 6 months. These findings suggested that following OHCA with a cardiac or presumed cardiac etiology, there was no strong indication for benefit of 33 °C over 36 °C, so long as a bundle of care with controlled slow rewarming, standardized post-cardiac arrest care and neuroprognostication was provided [12].
The findings of the TTM1 trial published in 2013 prompted a shift in target temperatures in many centers. The largest cohort documenting a single center experience transitioning TTM practices assessed survival and neurological outcomes of 453 unconscious OHCA patients from 2010 to 2017 [13]. In 2014, the standardized TTM target at this center was changed from 33 °C (258 patients) to 36 °C (195 patients). Patients in the 33 °C group were slightly older (mean 56.5 years versus 51.6 years; p < 0.05), more commonly had cardiac etiology of arrest (45.0% versus 35.4%; p < 0.05), and had faster time from 911 call to initiation of TTM (1.5 h versus 3.5 h; p < 0.001) compared to patients in the 36 °C group; otherwise, the groups were balanced. Forty percent of patients cooled to 33 °C had a favorable neurological outcome at discharge (CPC 1–2), compared to 30% of patients cooled to 36 °C (p < 0.05), but no significant difference between groups regarding survival to hospital discharge [13].
The first randomized clinical trial to include in-hospital cardiac arrest (IHCA), the HYPERION study, focused exclusively on non-shockable rhythms. In this study, 584 adults who remained unconscious following resuscitation were randomized to either 33 °C or 37 °C (+ / − 0.5 °C) for 24 h, followed by controlled slow rewarming over 24 h and normothermia for additional 48 h. In the hypothermic arm, 10.2% of subjects achieved independence at 90 days versus 5.7% in the normothermia group (p < 0.04); however, both survival to hospital discharge and mortality at 90 days did not differ between groups. There was a high fragility index to this finding, indicating that the benefit only very narrowly achieved statistical significance [14•].
A prospective observational study assessed 270 patients with OHCA that were cooled to 33 °C for 24 h followed by gradual rewarming to 36.5 °C and no further use of temperature control other than antipyretics. Mortality and neurological deficit based on CPC was assessed at discharge and at 6-month follow-up, and patients were determined to have post-hypothermia fever if they had recorded temperature of 38.5 °C or higher. Compared with patients that had good neurological outcomes (CPC 1–2), patients with poor neurological outcomes (CPC 3–5) had higher maximum temperatures post-cooling (38.0–38.7 °C versus 38.1–39.2 °C, p = 0.001). Additionally, rebound hyperthermia was more prevalent (61% versus 45%, p = 0.02) and of longer duration (median 8 h versus 5 h, p = 0.002) in patients with poor versus good neurological outcomes, respectively [15].
To further test whether active prevention of fever was non-inferior to cooling to 33 °C following OHCA, the largest international multicenter trial in cardiac arrest, the TTM2 trial, randomized 1861 unconscious adult OHCA patients with any initial non-perfusing rhythm (except for unwitnessed arrests with asystole) and a presumed cardiac etiology to either 33 °C or early treatment of fever (i.e., ≥ 37.8 °C). This study was unprecedented in the field, both in number of subjects and in rigor of study design. Neuroprognostication was standardized and performed no earlier than 96 h after randomization by a physician blinded to treatment allocation. Patients in the 33 °C group were cooled for 28 h, followed by rewarming from 0.3 °C per hour, and controlled normothermia at 36.5 to 37.7 °C for 72 h. The normothermia group targeted < 37.8 °C, with active cooling via surface or endovascular cooling if conservative measures, such as antipyretics, were insufficient—these were required in 46% of patients to maintain target temperature. At 6 months, there were no significant differences in all-cause mortality or neurological outcome between groups. Patients in the 33 °C group experienced more clinically significant bradycardia (24% vs. 17%; p < 0.001), but no other differences in adverse events between the groups were found. As in the TTM1 study, over 70% of patients in both groups had a shockable rhythm [16••]. These data suggested that for patients with OHCA of presumed cardiac etiology, there is no additional neuroprotection benefit from lowering temperatures to 33 °C if a standardized bundle of care, including sedation, delayed neuroprognostication and active fever control is in place.
A meta-analysis published in 2021 assessed 10 randomized clinical trials studying the use of TTM after cardiac arrest of any initial rhythm or etiology [17]. Studies were included if subjects were randomized to TTM of at least two different temperatures, one of which lower than 37 °C; temperature targets included 31–32 °C (6.5% of total subjects), 33–34 °C (49.5%), 35–36 °C (11.0%), and 37–37.8 °C (33%). The primary outcome was survival with good functional outcome at discharge (CPC 1–2, mRS 0–3, or blind clinical evaluation demonstrating mild, moderate or no disability) or latest time point recorded up to 6 months. There was no strong evidence of improvement with mild (35–36 °C; OR 1.44 [95% CI 0.74–2.80]), moderate (33–34 °C; OR 1.34 [95% CI 0.92–1.94]), or deep (31–32 °C; OR 1.30 [95% CI 0.73–2.30]) hypothermia on survival or good functional outcome at discharge compared with normothermia (37–37.8 °C) [17]. No additional benefit on survival or functional outcome between moderate and deep hypothermia was seen, though arrhythmias were significantly more common in subjects receiving deep hypothermia compared to those receiving moderate hypothermia (OR 2.47 [95% CI 1.25–4.88]). There were no significant differences in bleeding events or pneumonia between any groups. Subjects were not stratified by initial rhythm in this analysis and comprehensive details on presenting rhythm were not reported, therefore, the application of these findings to specific cardiac arrest subgroups is limited.
The Therapeutic Hypothermia Following Out-of-Hospital Cardiac Arrest (CAPITAL CHILL) trial randomized 367 adult unconscious OHCA patients of any non-perfusing rhythm to 31 °C or 34 °C for 24 h followed by gradual rewarming to 37 °C maintained for 48 h [18]. At 6 months, there were no significant differences in all-cause mortality or neurological function as determined by both mRS and Disability Rating Scale. There were also no significant differences in rate of pneumonia, seizures, or thrombosis in either group, though median length of ICU stay was significantly longer in the 31 °C group (10 versus 7 days in the 34 °C group; p = 0.004). Most patients in both groups had shockable initial rhythm (85.9% in the 31 °C and 86.3% in the 34 °C group), thus, the study likely was underpowered to assess the effect of 31 °C in the non-shockable subgroup [18].
A post hoc analysis of a prospective cohort assessed differences in neurological outcome at 33 °C vs. 36 °C based on the degree of encephalopathy determined by EEG in unconscious patients following OHCA or IHCA [19]. In this before (2010–2014, target 33 °C) and after (2014–2017, target 36 °C) study that captured a transition in temperature targets following TTM1 publication, patients were sub-divided into three groups—mild, moderate or severe encephalopathy—assessed at 12- and 24-h EEG post-arrest by blinded encephalographers. Following 24 h of TTM to either 33 °C or 36 °C and passive rewarming at 0.25–0.5 °C, temperature control targeting 36.5–37.5 °C was restarted for any unconscious patient who developed a temperature > 38 °C for 48 h. Patients were excluded if EEG data were missing at 12- or 24-h post-arrest, which was more common in the 36 °C group. Therefore, the severity of encephalopathy was only able to be determined in 48% of patients in the 36 °C group, compared with 67% in the 33 °C group. Ultimately, 479 patients were included in the analysis and neurological outcomes were similar between groups in the overall cohort, and among mild and severe encephalopathy subgroups. Nonetheless, in the subgroup with moderate encephalopathy, cooling to 33 °C was associated with increased likelihood of achieving independence at 6 months compared with 36 °C group (66% versus 45%; OR 2.38 [95% CI = 1.32–4.30]; p = 0.004) [19]. One remarkable aspect of this study is the use of a tool that provides individualized information on the degree of hypoxic-ischemic injury by factoring in, albeit indirectly, the cerebral resilience of patients. The stratification of injury severity solely based on characteristics of the arrest misses this key component that is unique to each patient. The use of EEG in differentiating these populations to potentially guide treatment is an exciting prospect; however, follow-up randomized clinical trials are necessary to validate these findings.
How Early should we Start Temperature Control?
Preclinical data suggested that shorter time to target temperature improves neurological outcome [20].
In 2014, 1359 OHCA patients were randomized to receive either 2 l of normal saline at 4 °C as soon as possible after ROSC or initiation of cooling upon hospital arrival. The intervention arm also included neuromuscular blockade and 1–2 mg of diazepam. Both groups had a target temperature of 32–34 °C maintained for 24 h, which was achieved approximately 1 h faster in the prehospital cooling group. No significant differences were found in either mortality or neurological status by Glasgow Outcome Scale at discharge, or rates of hypotension requiring vasopressors; however, rearrest rates (26% versus 21%; p = 0.008) and early clinically significant pulmonary edema (41% versus 30%; p < 0.001) were more prevalent in the prehospital cooling group. While outcomes were assessed at discharge by evaluation of hospital charts, and long-term outcomes were not studied, these findings suggested that prehospital cooling with infusion of cold saline did not confer benefit to this population and may expose to unnecessary harm [21].
What if the goal temperature were achieved as close to the time of primary neurological injury as possible? The RINSE trial randomized 1198 subjects with OHCA to receive 30 cc/kg (maximum of 2 l) of normal saline at 3 °C during cardiac arrest or standard of care. After arrival at hospital, both groups received TTM to 33 °C for 24 h. Patients who received intra-arrest cooling had lower body temperatures at hospital arrival (34.7 °C versus 35.4 °C; p < 0.001); however, no difference in survival to hospital discharge between groups were found (10.2% intra-arrest cooling vs 11.4% control; p = 0.71). Compared to standard care, subjects with shockable initial rhythm who received intra-arrest cooling had decreased rates of ROSC (41.2% vs. 50.6%; p = 0.03) and a higher likelihood of death prior to hospital arrival (44.3% vs. 34.1%; p = 0.01). The intra-arrest group also had higher rates of acute pulmonary edema (10.0% vs. 4.5%; p < 0.001). Importantly, neurological function at discharge or long-term follow-up was not assessed [22]. These findings were confirmed by a meta-analysis comprising 10 randomized controlled trials which compared 4220 OHCA patients of any rhythm who received either prehospital cooling (either intra-arrest or after ROSC) or initiation of TTM upon hospital arrival. Of note, the assessment of neurological status was not uniform across each study, and some utilized fairly crude measures of neurological function at discharge [23].
Could the adverse effect of the cooling method used in these trials—inherently associated with the risk of volume overload and pulmonary edema from infusion of chilled saline—have mitigated the potential benefit of ultra-early hypothermia? The PRINCESS trial randomized 671 patients with OHCA of cardiac etiology to receive either intra-arrest intranasal evaporative cooling or standard care alone, which included 24 h of TTM from 32 to 34 °C for 24 h followed by gradual rewarming and active fever prevention for 72 h. The primary outcome was survival with a good neurological outcome (CPC 1 or 2) at 30 days. Similar to the RINSE trial, patients who received intranasal cooling reached goal temperature faster (34.6 °C versus 35.8 °C on arrival; p < 0.001); however, there were no significant differences in survival or neurological outcome [24•].
Intriguingly, shorter duration between induction time to achieve 32–34 °C was associated with lower CPC score at discharge in a retrospective study of 321 subjects [25]. Patients in the poor neurological outcome group (CPC 3 to 5) also had significantly longer downtimes, were older and had lower rates of shockable rhythm than the group with CPC 1 to 2. The authors hypothesized that the relationship between poor neurological outcomes and shorter induction time to target temperature could be due to loss of thermoregulatory control in patients who suffered more severe neurological injury during their arrest.
How Long should Hypothermia be Maintained?
In the Bernard study, hypothermia was maintained for 12 h, and in HACA, patients were cooled for 24 h. The duration of 24 h for hypothermia phases was also used in TTM1, HYPERION, TTM2, and CAPITAL CHILL trials. In the TH48 trial, 355 unconscious patients following OHCA of any rhythm were randomized to receive either 24 or 48 h of temperature control at 33 °C followed by gradual rewarming [26]. No differences were found in CPC scores between groups at 6 months, though the 48-h group had premature rewarming in 6% of cases compared with 2% in the 24-h group; per study protocol, life-threatening arrhythmia or uncontrolled hemorrhage would trigger raising the TTM target to 36 °C. Patients in the 48-h group had significantly more hypotension (62% versus 49%; p = 0.013) and a higher number of adverse events (97% versus 91%; p = 0.04), though the 24-h group had more severe bleeding events (requiring more than 2 units of blood in 24 h, 4% versus 1%; p = 0.013) [26]. This trial provided evidence that 24 h of temperature control was non-inferior to 48 h and was associated with fewer adverse events. The ongoing ICECAP (Influence of Cooling Duration on Efficacy in Cardiac Arrest Patients) study is evaluating various durations of hypothermia on neurological outcomes following cardiac arrest, which will help broaden our understanding of this important variable in TTM [27].
How should we Control Temperature?
Most landmark trials have been agnostic to temperature control methods due to their pragmatic nature. A comparison of the various temperature control methods used in these trials is provided in Table 2.
A meta-analysis comprised of 22 studies including 8027 patients compared at least two temperature control methods for differences in either neurological outcome or mortality at any time point. Methods were classified either as “core” (e.g., cold intravenous saline infusion, extracorporeal membrane oxygenation, endovascular catheters, trans-nasal) or “surface” (e.g., ice packs, cooling blankets, adhesive cooling pads) and further subdivided into temperature feedback devices (TFD) and non-TFD. “Core cooling” devices and TFD were associated with a lower likelihood of unfavorable neurological outcome (CPC 3–5) than surface cooling (OR 0.85 [95% CI 0.75–0.96]; p = 0.008) or non-TFD (OR 0.64 [95% CI 0.56–0.74]; p = 0.003, respectively. There was no significant difference between core and surface cooling on survival. Importantly, statistical significance for these endpoints was obtained largely from prospective or retrospective cohorts, both of which are more susceptible to confounding effect. Time to goal temperature was significantly shorter with core and invasive methods in some studies, though invasive methods (such as endovascular cooling) were associated with less fluctuation in temperature during the cooling period and lower rates of unexpected rewarming [28]. Similar conclusions were reached in a separate meta-analysis, comprised of 12 studies and 5581 patients [29].
In the TTM2 trial, 46% of patients in the normothermia arm required active cooling with a device to maintain goal temperature. This rate is comparable to findings from a retrospective cohort study evaluating temperature trends in patients following cardiac arrest who received either no temperature intervention, antipyretics only, endovascular cooling only, or antipyretics plus endovascular cooling. The study was limited due to much lower number of patients receiving active cooling with a device and lack of standardization of antipyretic medications; however, normothermia (< 38 °C) over 48 h was achieved in only 57.7% of patients receiving antipyretics alone, compared with 82.1% in the endovascular cooling groups. This provides further evidence that cooling devices are often needed to maintain even physiologic temperatures following cardiac arrest [30].
The most recent ERC-ESICM guidelines do not make any recommendation regarding rate of rewarming following cardiac arrest due to lack of good evidence, as there have been no randomized trials comparing outcomes at various rewarming rates. In most trials discussed here, rewarming is accomplished by actively or passively increasing body temperature between 0.25 and 0.5 °C per hour.
Potential Complications
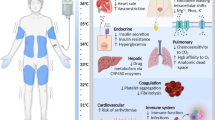
When caring for patients receiving TTM, it is important to be aware of the adverse effects that can be expected in this population; these are summarized in Fig. 1. Specific attention to electrolyte shifts, insulin requirements and metabolism of drugs is key when targeting lower temperatures, as the magnitude of the impact of TTM in each system hinges upon depth of hypothermia. Similarly, the effect of hypothermia on coagulation and the cardiovascular system is transient and temperature dependent. These complications have been covered in detail in other studies [31]. To maximize the potential benefit of TTM post-cardiac arrest, and minimize its deleterious effect, a standardized bundle of care focused on early achievement of target temperature during induction, stability of temperature during maintenance, aggressive shivering control across all phases, slow rewarming and controlled normothermia has been proposed [32].
Special Considerations in Pregnancy
Pregnancy has been among the most common exclusion criteria in trials on temperature control for cardiac arrest. Data supporting its use in this population is limited to case reports [33–35], which demonstrate feasibility of temperature control in pregnant patients, but do not provide sufficient evidence towards efficacy and safety in this population. The decision to initiate temperature control in pregnant patients should be made by transdisciplinary consensus between maternal fetal medicine, obstetrics, and neurology on a case-by-case basis.
Summary and Current Recommendations
In January of 2022, the European Resuscitation Council released an updated set of guidelines regarding temperature control in patients who remain unconscious following cardiac arrest. These recommendations include continuous monitoring of core temperature and prevention of fever (defined as > 37.7 °C) for at least 72 h, with the use of antipyretic medications or a cooling device, if necessary [36••]. This recommendation is largely based on the TTM2 trial. The guidelines acknowledge the insufficient evidence to recommend for or against actively cooling patients to 32–36 °C (as in prior guidelines) or the use of early cooling post-arrest. Additionally, they recommended against the active rewarming of comatose patients following cardiac arrest who are hypothermic and recommended against using large volume infusions of cold fluid to cool patients immediately after achieving ROSC [36••]. A focused update from the American Heart Association on temperature control is in process and will consider new emerging evidence that has become available since its previous guidelines on this topic were published in 2020 [37].
Direction for Future Research
Most randomized controlled trials and meta-analyses utilize CPC or mRS scores to assess neurological status at discharge or long-term follow-up. These are relatively crude measures of neurological function and may miss important granular differences in outcome. This is important, as patients with a CPC of 1 or 2 may have new neurological deficits at one year follow-up, including mood disorders, emotional lability, or memory deficits, among others [38]—all of which could be missed by these outcome scales, especially if performed by someone who is not well trained in neurology or psychology as has often been the case in prior studies. Furthermore, many studies use even more rudimentary assessments of neurological status, such as hospital discharge disposition, and follow-up is often performed via telephone which significantly limits the accuracy of neurological assessments.
The ideal duration of hypothermia remains unclear, as only one trial has studied this variable [26]. The ICECAP study is currently enrolling patients and will assess the effect of different durations (from 6 to 72 h) of hypothermia targeting 33 °C in unconscious patients following OHCA using an adaptive design [27]. The PRECICECAP (Precision Care in Cardiac Arrest) is an ancillary study to ICECAP and represents the first rigorous initiative in precision care for TTM post-cardiac arrest aiming at identifying signatures based on individualized multimodal physiologic data to predict the optimal duration of cooling [39].
Unwitnessed arrest with asystole as the non-perfusing rhythm was excluded from both TTM1 and TTM2. These studies were likely underpowered to make a definitive recommendation on target temperature for non-shockable rhythms, and though the HYPERION study supported use of hypothermia at 33 °C, its low fragility index challenges this recommendation. The optimal temperature target for patients that were not heavily represented in robust trials of cardiac arrest, in particular IHCA and non-cardiac etiology, remains an important knowledge gap.
Conclusion
Despite ongoing controversies surrounding specific aspects of targeted temperature management, the use of a bundle of care including strict temperature control is associated with improved outcomes after cardiac arrest and should not be abandoned. It is crucial to recall that 46% of patients in the control arm of the TTM2 trial required active temperature control with a device to maintain strict normothermia. Temperature control is associated with improved outcomes following cardiac arrest and should remain an integral part of post-arrest care.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cardiac Arrest Registry to Enhance Survival. 2021 CARES Annual Report. [Internet]. 2021. [updated 2021; cited 2022 July 18]. Available from: https://mycares.net/sitepages/uploads/2022/2021_flipbook/index.html?page=1.
Odom E, Nakajima Y, Vellano K, Al-Araji R, Coleman King S, Zhang Z, et al. Trends in EMS-attended out-of-hospital cardiac arrest survival, United States 2015–2019. Resuscitation. 2022.
Kaminogo M, Suyama K, Ichikura A, Onizuka M, Shibata S. Anoxic depolarization determines ischemic brain injury. Neurol Res. 1998;20(4):343–8.
Sekhon MS, Ainslie PN, Griesdale DE. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: a “two-hit” model. Crit Care. 2017;21(1):90.
Williams GR Jr, Spencer FC. The clinical use of hypothermia following cardiac arrest. Ann Surg. 1958;148(3):462–8.
Benson DW, Williams GR Jr, Spencer FC, Yates AJ. The use of hypothermia after cardiac arrest. Anesth Analg. 1959;38:423–8.
Colbourne F, Corbett D. Delayed and prolonged post-ischemic hypothermia is neuroprotective in the gerbil. Brain Res. 1994;654(2):265–72.
Colbourne F, Corbett D. Delayed postischemic hypothermia: a six month survival study using behavioral and histological assessments of neuroprotection. J Neurosci. 1995;15(11):7250–60.
Weinrauch V, Safar P, Tisherman S, Kuboyama K, Radovsky A. Beneficial effect of mild hypothermia and detrimental effect of deep hypothermia after cardiac arrest in dogs. Stroke. 1992;23(10):1454–62.
Hypothermia after Cardiac Arrest Study G. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–56.
Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–63.
Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med. 2013;369(23):2197–206.
Johnson NJ, Danielson KR, Counts CR, Ruark K, Scruggs S, Hough CL, et al. Targeted temperature management at 33 versus 36 degrees: a retrospective cohort study. Crit Care Med. 2020;48(3):362–9.
• Lascarrou JB, Merdji H, Le Gouge A, Colin G, Grillet G, Girardie P, et al. Targeted temperature management for cardiac arrest with nonshockable rhythm. N Engl J Med. 2019;381(24):2327–37. This study enrolled only cardiac arrest patients with non-shockable rhythms, a population either missing or in small numbers in most major TTM trials, and found better neurological outcomes in patients cooled to 33°C compared to 37 °C.
Bro-Jeppesen J, Hassager C, Wanscher M, Soholm H, Thomsen JH, Lippert FK, et al. Post-hypothermia fever is associated with increased mortality after out-of-hospital cardiac arrest. Resuscitation. 2013;84(12):1734–40.
•• Dankiewicz J, Cronberg T, Lilja G, Jakobsen JC, Levin H, Ullen S, et al. Hypothermia versus normothermia after out-of-hospital cardiac arrest. N Engl J Med. 2021;384(24):2283–94. The largest randomized controlled trial in the field of TTM to date; this study showed no difference in mortality or long-term neurological outcome between cooling to 33 °C or controlled normothermia.
Fernando SM, Di Santo P, Sadeghirad B, Lascarrou JB, Rochwerg B, Mathew R, et al. Targeted temperature management following out-of-hospital cardiac arrest: a systematic review and network meta-analysis of temperature targets. Intensive Care Med. 2021;47(10):1078–88.
Le May M, Osborne C, Russo J, So D, Chong AY, Dick A, et al. Effect of moderate vs mild therapeutic hypothermia on mortality and neurologic outcomes in comatose survivors of out-of-hospital cardiac arrest: the CAPITAL CHILL randomized clinical trial. JAMA. 2021;326(15):1494–503.
Nutma S, Tjepkema-Cloostermans MC, Ruijter BJ, Tromp SC, van den Bergh WM, Foudraine NA, et al. Effects of targeted temperature management at 33 degrees C vs. 36 degrees C on comatose patients after cardiac arrest stratified by the severity of encephalopathy. Resuscitation. 2022;173:147–53.
Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med. 1993;21(9):1348–58.
Kim F, Nichol G, Maynard C, Hallstrom A, Kudenchuk PJ, Rea T, et al. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest: a randomized clinical trial. JAMA. 2014;311(1):45–52.
Bernard SA, Smith K, Finn J, Hein C, Grantham H, Bray JE, et al. Induction of therapeutic hypothermia during out-of-hospital cardiac arrest using a rapid infusion of cold saline: the RINSE trial (Rapid Infusion of Cold Normal Saline). Circulation. 2016;134(11):797–805.
Lindsay PJ, Buell D, Scales DC. The efficacy and safety of pre-hospital cooling after out-of-hospital cardiac arrest: a systematic review and meta-analysis. Crit Care. 2018;22(1):66.
• Nordberg P, Taccone FS, Truhlar A, Forsberg S, Hollenberg J, Jonsson M, et al. Effect of trans-nasal evaporative intra-arrest cooling on functional neurologic outcome in out-of-hospital cardiac arrest: the PRINCESS randomized clinical trial. JAMA. 2019;321(17):1677–85. This trial suggested that trans-nasal evaporative cooling prior to hopsital arrival, despite leading to faster goal temperatures, did not significantly improve survival or neurological outcome.
Perman SM, Ellenberg JH, Grossestreuer AV, Gaieski DF, Leary M, Abella BS, et al. Shorter time to target temperature is associated with poor neurologic outcome in post-arrest patients treated with targeted temperature management. Resuscitation. 2015;88:114–9.
Kirkegaard H, Soreide E, de Haas I, Pettila V, Taccone FS, Arus U, et al. Targeted temperature management for 48 vs 24 hours and neurologic outcome after out-of-hospital cardiac arrest: a randomized clinical trial. JAMA. 2017;318(4):341–50.
Muerer WJ, Silbergleit R, Geocadin R. Influence of Cooling Duration on Efficacy in Cardiac Arrest Patients. 2020.
Calabro L, Bougouin W, Cariou A, De Fazio C, Skrifvars M, Soreide E, et al. Effect of different methods of cooling for targeted temperature management on outcome after cardiac arrest: a systematic review and meta-analysis. Crit Care. 2019;23(1):285.
Bartlett ES, Valenzuela T, Idris A, Deye N, Glover G, Gillies MA, et al. Systematic review and meta-analysis of intravascular temperature management vs. surface cooling in comatose patients resuscitated from cardiac arrest. Resuscitation. 2020;146:82–95.
Alnabelsi TS, Faulkner SP, Cook M, Freeman K, Shelton J, Paranzino M, et al. Passive antipyretic therapy is not as effective as invasive hypothermia for maintaining normothermia after cardiac arrest. Am J Emerg Med. 2021;50:202–6.
Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med. 2009;37(3):1101–20.
Taccone FS, Picetti E, Vincent JL. High quality targeted temperature management (TTM) after cardiac arrest. Crit Care. 2020;24(1):6.
Rittenberger JC, Kelly E, Jang D, Greer K, Heffner A. Successful outcome utilizing hypothermia after cardiac arrest in pregnancy: a case report. Crit Care Med. 2008;36(4):1354–6.
Chauhan A, Musunuru H, Donnino M, McCurdy MT, Chauhan V, Walsh M. The use of therapeutic hypothermia after cardiac arrest in a pregnant patient. Ann Emerg Med. 2012;60(6):786–9.
Wible EF, Kass JS, Lopez GA. A report of fetal demise during therapeutic hypothermia after cardiac arrest. Neurocrit Care. 2010;13(2):239–42.
•• Nolan JP, Sandroni C, Andersen LW, Bottiger BW, Cariou A, Cronberg T, et al. ERC-ESICM guidelines on temperature control after cardiac arrest in adults. Resuscitation. 2022;172:229–36. Focused and updated guidelines regarding TTM after cardiac arrest, based on the most recent and higest quality evidence available in the field.
Berg KM, Soar J, Andersen LW, Bottiger BW, Cacciola S, Callaway CW, et al. Adult Advanced Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2020;142(16_suppl_1):S92-S139.
Caro-Codon J, Rey JR, Lopez-de-Sa E, Gonzalez Fernandez O, Rosillo SO, Armada E, et al. Long-term neurological outcomes in out-of-hospital cardiac arrest patients treated with targeted-temperature management. Resuscitation. 2018;133:33–9.
Elmer J, He Z, May T, Osborn E, Moberg R, Kemp S, et al. Precision care in cardiac arrest: ICECAP (PRECICECAP) study protocol and informatics approach. Neurocrit Care. 2022.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Nicholas Nelson declares that he has no conflict of interest. Briana Wasserstrom declares that she has no conflict of interest. Carolina Maciel declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Critical Care Neurology
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nelson, N.J., Wasserstrom, B.E. & Maciel, C.B. Temperature Control in Hypoxic-Ischemic Brain Injury—a Focused Update. Curr Treat Options Neurol 24, 551–572 (2022). https://doi.org/10.1007/s11940-022-00738-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11940-022-00738-z