Abstract
Purpose of review
The purpose of this review article is to summarize and discuss the recent advances in the treatment of restless legs syndrome (RLS), as well as REM sleep behavior disorder (RBD), and periodic leg movement disorder (PLMD).
Recent findings
Traditionally, dopaminergic therapy has been considered the sole option for first-line treatment of RLS due to their impressive acute efficacy. Dopamine agonists such as oral pramipexole and ropinirole, as well as transdermal rotigotine are all effective treatment options. However, augmentation of the RLS symptoms is a major limitation of oral dopaminergic therapy. Recently, gabapentinoid agents such as gabapentin enacarbil and pregabalin have shown comparable short-term efficacy to dopaminergics with lower risk of augmentation of the RLS symptoms. Recent evidence on the efficacy of oxycodone-naloxone in treatment-resistant RLS provides an additional therapeutic avenue. The increasing understanding of the role of iron in RLS pathophysiology has led to new options in iron supplementation therapy in RLS, including treatment with ferric carboxymaltose.
Summary
With emerging evidence of augmentation being a side effect specific to dopaminergic treatment, gabapentinoids are considered a safer option as initial treatment. In severe refractory RLS, oxycodone-naloxone can be used. If iron stores are low, IV iron formulations should be the initial treatment choice. New treatment options are needed to address issues with current therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Restless legs syndrome (RLS) is the most common sleep-related movement disorder. It typically manifests as an urge to move together with uncomfortable feeling in the calves and thighs. The feeling is precipitated by evening- and nighttime inactivity and relieved by movement of the legs, preventing rest and sleep. The diagnosis is based on a physician diagnostic interview addressing these core symptoms [1]. Clinically significant RLS, requiring daily treatment, has a prevalence of 2.7% in European and North American populations [2], while being much less frequent in Africa and Asia [3, 4]. The pathological mechanism of RLS is largely unknown, but different hypotheses suggest the involvement of the embryonal development of the basal ganglia [5], brain iron intake, [6] or spinal circuitry [7].
RLS is a highly hereditary disorder, associated with several genetic loci in genome-wide association studies [8]. In addition, the disease appearance and phenotype is also heavily affected by changes in metabolism and the internal environment, such as renal failure or pregnancy [9]. The susceptibility of an individual to RLS is therefore dependent on both environmental and genetic influences, in a way where early onset familial cases have a high genetic but lower environmental burden. RLS in patients with multiple comorbidities, on the other hand, have a low genetic burden but require higher environmental burden to trigger the disease [9].
Patients suffering from RLS may experience severe discomfort, decreased sleep quality and quality of life as well as psychiatric symptoms including anxiety and depression [10]. In addition, inadequate treatment of RLS contributes a large financial burden to the society [11]. Therefore, the development of new, effective therapy for RLS is of great importance. In this review, we summarize the existing evidence for different therapeutic approaches in the treatment of RLS.
Methods
We conducted a comprehensive search of literature on PubMed from 1982 to June 2018 with the following search terms: [“restless legs syndrome” AND treatment], [(“REM sleep behavior disorder” OR “Rapid eye movement sleep behavior disorder”) AND treatment], and [“periodic leg movement disorder” AND treatment]. Reports discussing therapeutic interventions on any of these disorders, including case reports for emerging therapies, were included in the review process. Only English language reports were included in the review.
RLS: Pharmacological treatment
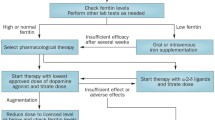
The main pharmacological treatment options for RLS are summarized in Table 1.
Dopaminergic treatment
Historically, levodopa was the first effective treatment to be discovered for RLS [12], establishing the efficiency of dopaminergic agents in RLS. While the discovery of levodopa was of great importance, leading the way in RLS research, the use of levodopa/carbidopa formulations has reduced over time. Currently, levodopa is only recommended to be used intermittently in the treatment of RLS [13••]. Instead, longer-acting dopaminergic agents are used. After levodopa, both ergot and non-ergot-derived dopamine agonists were studied in the context of RLS. Due to the serious cardiac risks associated with the ergot-derived substances pergolide and cabergoline [14], only non-ergot dopamine agonists are currently recommended. Therefore, pramipexole, ropinirole, and rotigotine are currently the most commonly used dopamine agonists for the treatment of RLS.
Pramipexole is a D3 receptor preferential dopamine agonist whose efficacy in the treatment of RLS has been established in numerous trials [15, 16•, 17]. Like most dopamine agonists in low doses, pramipexole is well tolerated, the most common adverse effects reported in clinical trials being headache, nausea, and fatigue [16•]. The efficacy of pramipexole in RLS has been demonstrated by large randomized clinical trials, showing the superiority of pramipexole at doses ranging from 0.125 to 0.75 mg/day over placebo for up to 12 weeks [18, 19]. In addition to the subjective RLS symptoms, pramipexole also effectively suppresses PLM [20], often associated with RLS, but may not improve the fragmentation of sleep [21]. Pramipexole may also be beneficial when targeting the quality of life of the patients [19], or the comorbid psychiatric symptoms such as depression or anxiety [22] although scarce data exists.
The receptor profile of ropinirole is very similar to that of pramipexole with slightly lower affinity to the D3 receptor, but higher binding affinity to the 5-HT1A receptor. The efficacy of ropinirole in the treatment of RLS and its side effect profile are therefore also comparable to those of pramipexole [23], although no direct comparative trials have been performed. In clinical trials, ropinirole at doses of 0.25–4.0 mg/day have been demonstrated to be effective in suppressing RLS symptoms in both North American and European populations [24]. Similar to pramipexole, ropinirole is effective when targeting the quality of life of the RLS patients [25] and suppressed PLM [26]. In addition, ropinirole has been shown to improve sleep parameters in polysomnographic studies [27].
Rotigotine is another dopamine agonist with demonstrated efficacy in the treatment of RLS [28, 29]. The receptor profile of rotigotine is wider than those of pramipexole and ropinirole, with a higher affinity to D1 and D5 dopamine receptors, in addition to the D2 and D3 receptors [30]. Rotigotine is delivered by transdermal patches, which provides a more stable release of the substance over time. Due to the delivery method, skin reactions on the site of application are a common reason for discontinuation of therapy [29]. In a 6-month double-blind randomized trial, rotigotine was shown to be effective in treating moderate to severe RLS at doses 1–3 mg/day [31]. Rotigotine was also shown to restore the quality-of-life of the RLS patients in the same trial. Another 6-month trial only showed efficacy superior to placebo at doses of 2–3 mg/day [29]. In an open-label follow-up study, the efficacy of rotigotine was shown to persist for up to 5 years, providing the longest-term evidence of RLS treatment to date [32].
The major limitation of dopaminergic therapy in RLS is a specific, iatrogenic side effect called augmentation. Augmentation can be identified during dopaminergic treatment by the reappearance of the subjective RLS symptoms, manifesting with more intensity, appearing earlier during the day, or spreading to further body parts as compared to the symptoms before starting the treatment [33]. The highest risk is seen during treatment with levodopa, where an estimate of 27.1% of patients develops augmentation [34]. With oral dopamine agonists, the rate is lower but still significant, 6% [34]. During treatment with rotigotine, the augmentation rate seems to be slightly lower (4%) than with oral dopamine agonists [32], but the lack of prospective studies with equally long follow-up period on pramipexole and ropinirole make direct comparisons difficult. Due to the risk of augmentation, special monitoring is advised when prescribing dopamine agonists and levodopa for RLS [35]. To prevent the development of augmentation, other first line treatment options, such as alpha-2-delta ligands may be considered at the start of treatment in countries where they are approved for the treatment of RLS (see Table 1).
Gabapentinoids
The gabapentinoid drugs are ligands of the alpha-2-delta subunit of a subset of voltage-gated calcium channels. They are primarily anticonvulsants, also frequently used in the treatment of neuropathic pain. Gabapentin was the first alpha-2-delta ligand to be studied in clinical trials for treatment of RLS, and the efficacy has been demonstrated in multiple reports since then. In a small double-blind RCT, it was shown to be efficacious for up to 6 weeks with a mean effective dose of 1855 mg/day [36]. However, due to the limited and unpredictable bioavailability of gabapentin, especially at higher doses, a prodrug gabapentin enacarbil was developed, and research focus shifted to the new formulation. In controlled trials, gabapentin enacarbil has proven to be efficacious in the treatment of RLS in both North American and Japanese populations for up to 12 weeks at doses of 600–1200 mg/day [37, 38].
Pregabalin, another gabapentinoid drug, is also used off-label to treat RLS. In one of the only comparative trials conducted in RLS, a 300-mg daily dose of pregabalin was compared to 0.25-mg and 0.5-mg daily doses of pramipexole, as well as to placebo [16•]. Pregabalin was shown to be superior to placebo and comparable to both doses of pramipexole in efficacy at 12 weeks of treatment. A 52-week follow-up demonstrated a significantly lower rate of augmentation compared to pramipexole 0.5 mg (2.1% vs. 7.7%, p = 0.001). In addition, pregabalin has showed superior improvement of sleep quality, measured as wakefulness after sleep onset, compared to pramipexole [39]. Small dose-finding trials suggest that pregabalin may be beneficial for RLS already at lower doses [40, 41]. The most common adverse events with pregabalin were dizziness, somnolence, and fatigue, the total occurrence of adverse events being comparable to pramipexole [16•].
Due to the similar efficacy and lower risk of augmentation, recent treatment guidelines recommend gabapentinoids as first-line treatment as an option to dopamine agonists [13••]. However, although the augmentation rates have been demonstrated to be lower than those of dopamine agonists for 1 year after treatment [16•], the lack of longer-term prospective studies with both oral dopamine agonists and gabapentinoids limits our ability to draw final conclusions yet. In addition, the off label status of gabapentinoids in the treatment of RLS in most countries (see Table 1) limits their usage as first-line therapy.
Opioids
The only opioid formulation studied in a large-scale trial for RLS is oxycodone-naloxone. A randomized, double-blind, controlled trial of 12 weeks with a 40-week open label extension was performed across several European countries [42•]. At doses ranging from 5.0 mg/2.5 mg to 40 mg/20 mg (oxycodone/naloxone) twice a day, the formulation was shown to reduce IRLS severity significantly better than placebo. The trial included patients with severe RLS, inadequately treated with previous treatment. Due to the risks of addiction and abuse, oxycodone-naloxone is only recommended as a therapeutical option in patients with severe RLS whose treatment with dopamine agonists and gabapentinoids has already failed.
The evidence for other opioids is more limited. Oxycodone has been studied in a small controlled trial, with promising results [43]. In addition, two open-label studies using methadone to treat RLS [44, 45] have been published. A retrospective analysis of the medical records of three large hospitals identified 113 patients who had received opioid therapy either alone or in combination with another formulation [46]. Six different opioids were observed, including oxycodone, codeine, propoxyphene, and methadone. Opioids were observed to have a long-term efficacy in treating RLS, but monitoring was warranted for the development of sleep apnea, based on polysomnographic findings.
Iron preparations
Iron is an essential element of a healthy diet, an integral part of a number of biological processes, including dopamine synthesis. The prevalence of RLS in patients suffering from iron deficiency anemia is high, up to 23.9% of the patients [47], leading to the hypothesis that iron plays a major role in the pathophysiology of RLS [6]. Indeed, the transport of iron from the blood circulation, through the blood–brain barrier into the central nervous system could be impaired in RLS, leading to low iron levels in the substrantia nigra [48]. This is hypothesized to generate the RLS symptoms through activation of hypoxic response pathways in the central nervous system.
The correlation between iron parameters and RLS symptoms leads to testing oral iron supplements as RLS treatment. In a double-blind controlled study, however, no significant amelioration of RLS symptoms was observed after treatment with 325 mg oral ferrous sulfate for 12 weeks [49]. Although previous anecdotal findings suggested opposing results [50], it is likely that oral iron does not provide sufficient benefit in RLS, at least in patients that are already iron-sufficient. This could be due to the very low absorption of iron in the healthy, iron-sufficient gut.
Due to this issue, intravenous iron supplements have emerged as an alternative way of iron delivery. Intravenous iron sucrose has been studied in two different trials. One randomized, double-blind study in idiopathic RLS was interrupted due to inadequate power and lack of clinically significant treatment effect [51]. Another study was finished and showed a positive treatment effect in the acute phase as well as in long-term follow-up [52]. This study was performed in patients with low ferritin, indicating iron deficiency. This may indicate that iron sucrose could be beneficial in RLS patients with low iron stores, but not in those that have sufficient levels of peripheral iron. However, more evidence is required to confirm this. In addition, high molecular weight iron dextran has been studied as treatment for RLS, but due to a considerable incidence of anaphylactic shock, it is not considered safe enough to justify the risk.
An alternative iron formulation, ferric carboxymaltose (FCM), designed to deliver iron in a more controlled manner without releasing extensive ionic iron into the serum, has been the most recent development in iron therapy for RLS. In controlled trials, FCM (1000 mg or 2 × 500 mg infusions) was shown to reduce RLS severity for up to 6 weeks after the infusion [53, 54]. However, a recent trial with a similar dose in patients with non-anemic iron deficiency suggested that the efficacy of FCM may take up to 12 weeks to be detectable in clinical trials [55]. A lower dosage of 500 mg, on the other hand, showed a lack of efficacy [56], suggesting that lowering the dose from 1000 mg is not possible.
RLS: non-pharmacological treatment
Although not studied as thoroughly as pharmacological therapy, some evidence exists for the efficacy of non-pharmacological treatment of RLS. A double-blind randomized trial was able to demonstrate the superiority of pneumatic compression devices against sham devices in treating RLS [57]. However, this being a small single trial, the evidence should not be considered sufficient to conclude on the efficacy of the devices. In addition to compression, near-infrared light therapy [58], acupuncture [59], and vibration [60] pads have been studied in smaller trials.
Another potential non-pharmacological treatment strategy for RLS is exercise. Although the mechanism of exercise in RLS is not known, it has been studied as a treatment option in idiopathic RLS as well as RLS patients on hemodialysis. In idiopathic RLS patients, exercise was shown to significantly improve RLS symptoms in a small study [61]. However, the severity of the symptoms was lower than most treatment trials, and some of the patients were on concomitant medication. In hemodialysis patients, exercise may be beneficial both as the only therapy [62] and together with a small dose of a dopamine agonist [63]. In this subgroup of patients, often displaying severe symptoms, exercise is one of the only treatment strategies studied extensively, and should be always considered as a potential therapy.
RLS: emerging treatment
The potential of adenosine-targeting agents has arisen from the recent findings that the adenosinergic system in the brain may play a major role in the pathogenesis of RLS [64]. Indeed, in a small open-label study, dipyridamole improved the IRLS severity in moderate to severe RLS patients by 12.7 units at a dose of 100–400 mg [65]. In addition, dipyridamole was effective in suppressing PLM and was well tolerated. Despite the promising results, further double-blind trials are needed to determine if the adenosinergic system can be targeted effectively and safely in the treatment of RLS.
In a recent genome wide association meta-analysis, an association was discovered between RLS and a genetic locus near the gene CRBN [8]. CRBN encodes the protein cereblon, part of a ubiquitin ligase complex also binding to MEIS2, another candidate gene for RLS. Thalidomide, an old drug previously indicated for sleeplessness among other conditions, and used nowadays as an anti-cancer drug, targets cereblon. Therefore, thalidomide may be considered another potentially emerging treatment option for RLS.
REM sleep behavior disorder
REM sleep behavior disorder (RBD), although classified as a parasomnia, affects sleep through abnormal movement behavior, and is therefore briefly commented on here. Typically, RBD manifests as a lack of muscle atonia during REM sleep together with complex nocturnal motor behavior. The anomalous and sometimes violent behavior is often interpreted as dream enactment. The prevalence of RBD is higher in men and in elderly populations. RBD is diagnosed with a polysomnography (PSG) performed most often in a sleep laboratory. In most cases, the diagnosis of RBD precedes the later development of α-synucleinopathies, neurogenerative diseases resulting from the noxious aggregation of alpha-synuclein in neurons. These diseases include Parkinson’s disease and multiple system atrophy and dementia with Lewy bodies.
The published research on the treatment of RBD has recently been reviewed in this journal [66]. In that review, it was determined that the two treatment options that have been studied in detail, melatonin and clonazepam, are equally effective in the treatment of RBD. Clonazepam, a benzodiazepine, appears a good candidate for the treatment of RBD since it reduces motor activity during sleep, but does not restore REM sleep atonia, in a disease mouse model. While it was indeed able to reduce potentially dangerous motor activity in an open-label study in human patients, further evidence is needed to determine its efficacy. Melatonin reduced the epochs of REM sleep without atonia by 39% in a randomized crossover study [67]. In a survey study, melatonin and clonazepam were equally effective in the treatment of RBD, although melatonin was better tolerated than clonazepam [68].
Since the publication of the previous review, no new treatment trials in RBD have been published.
Periodic limb movement disorder
Periodic leg movement disorder (PLMD) is a sleep disorder characterized by excessive PLM during sleep. The leg movements, often associated with both cortical and autonomic arousals [69], may reduce sleep quality by not allowing sufficient amounts of slow-wave sleep during the night. This can lead to daytime sleepiness and reduced work capacity. PLMD is diagnosed with a polysomnography, where EMG is recorded on one or both tibialis anterior muscles. Leg movements are then detected according to established guidelines [70], and an index of the number of leg movements per hour of sleep is calculated. The disorder should not be confused with RLS, which is characterized by subjective daytime symptoms.
Because most RLS medication is also effective in suppressing PLM, the treatment of PLMD follows the treatment or RLS. Dopaminergic therapy has an impressive acute effect on PLM already after the first administration, both ropinirole and pramipexole suppressing most PLM [26]. In addition, pregabalin has been shown to reduce the elevated PLM arousal index in RLS patients with comparable effect to pramipexole [39].
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Allen RP, Picchietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–11.
Allen RP, Walters AS, Montplaisir J, Hening W, Myers A, Bell TJ, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165:1286–92.
Chen N-H, Chuang L-P, Yang C-T, Kushida CA, Hsu SC, Wang PC, et al. The prevalence of restless legs syndrome in Taiwanese adults. Psychiatry Clin Neurosci. 2010;64:170–8.
Winkler AS, Trendafilova A, Meindl M, Kaaya J, Schmutzhard E, Kassubek J. Restless legs syndrome in a population of northern Tanzania: a community-based study. Mov Disord. 2010;25:596–601.
Spieler D, Kaffe M, Knauf F, Bessa J, Tena JJ, Giesert F, et al. Restless legs syndrome-associated intronic common variant in Meis1 alters enhancer function in the developing telencephalon. Genome Res. 2014;24:592–603.
Earley CJ, Connor J, Garcia-Borreguero D, Jenner P, Winkelman J, Zee PC, et al. Altered brain iron homeostasis and dopaminergic function in restless legs syndrome (Willis–Ekbom disease). Sleep Med. 2014;15:1288–301.
Clemens S, Rye D, Hochman S. Restless legs syndrome: revisiting the dopamine hypothesis from the spinal cord perspective. Neurology. 2006;67:125–30.
Schormair B, Zhao C, Bell S, Tilch E, Salminen AV, Pütz B, et al. Identification of novel risk loci for restless legs syndrome in genome-wide association studies in individuals of European ancestry: a meta-analysis. Lancet Neurol. 2017;16:898–907.
Trenkwalder C, Allen R, Högl B, Paulus W, Winkelmann J. Restless legs syndrome associated with major diseases: a systematic review and new concept. Neurology. 2016;86:1336–43.
Picchietti DL, Van Den Eeden SK, Inoue Y, Berger K. Achievements, challenges, and future perspectives of epidemiologic research in restless legs syndrome (RLS). Sleep Med. 2017;31:3–9.
Durgin T, Witt EA, Fishman J. The Humanistic and Economic Burden of Restless Legs Syndrome. PLoS One. 2015;10:e0140632.
Akpinar S. Treatment of restless legs syndrome with levodopa plus benserazide. Arch Neurol. 1982;39:739.
•• Garcia-Borreguero D, Silber MH, Winkelman JW, et al. Guidelines for the first-line treatment of restless legs syndrome/Willis–Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-foundation. Sleep Med. 2016;21:1–11 This recent treatment guideline paper, written by a task force and commissioned by the leading RLS study groups, provides state-of-the art recommendations on therapeutical avenues for RLS.
Tran T, Brophy JM, Suissa S, Renoux C. Risks of cardiac valve regurgitation and heart failure associated with ergot- and non-ergot-derived dopamine agonist use in patients with Parkinson’s disease: a systematic review of observational studies. CNS Drugs. 2015;29:985–98.
Ma J-F, Wan Q, Hu X-Y, Sun SG, Wang WZ, Zhao ZX, et al. Efficacy and safety of pramipexole in Chinese patients with restless legs syndrome: results from a multi-center, randomized, double-blind, placebo-controlled trial. Sleep Med. 2012;13:58–63.
• Allen RP, Chen C, Garcia-Borreguero D, Polo O, DuBrava S, Miceli J, et al. Comparison of pregabalin with pramipexole for restless legs syndrome. N Engl J Med. 2014;370:621–31 This is the largest comparative trial performed in RLS, demonstrating the comparable efficacy but lower rate of augmentation of pregabalin, compared to pramipexole.
Högl B, Garcia-Borreguero D, Trenkwalder C, Ferini-Strambi L, Hening W, Poewe W, et al. Efficacy and augmentation during 6 months of double-blind pramipexole for restless legs syndrome. Sleep Med. 2011;12:351–60.
Oertel WH, Stiasny-Kolster K, Bergtholdt B, Hallström Y, Albo J, Leissner L, et al. Efficacy of pramipexole in restless legs syndrome: a six-week, multicenter, randomized, double-blind study (effect-RLS study). Mov Disord. 2007;22:213–9.
Winkelman JW, Sethi KD, Kushida CA, Becker PM, Koester J, Cappola JJ, et al. Efficacy and safety of pramipexole in restless legs syndrome. Neurology. 2006;67:1034–9.
Manconi M, Ferri R, Zucconi M, Oldani A, Fantini ML, Castronovo V, et al. First night efficacy of pramipexole in restless legs syndrome and periodic leg movements. Sleep Med. 2007;8:491–7.
Manconi M, Ferri R, Zucconi M, Bassetti CL, Fulda S, Aricò D, et al. Dissociation of periodic leg movements from arousals in restless legs syndrome. Ann Neurol. 2012;71:834–44.
Saletu M, Anderer P, Saletu-Zyhlarz G, Hauer C, Saletu B. Acute placebo-controlled sleep laboratory studies and clinical follow-up with pramipexole in restless legs syndrome. Eur Arch Psychiatry Clin Neurosci. 2002;252:185–94.
Quilici S, Abrams KR, Nicolas A, Martin M, Petit C, LLeu PL, et al. Meta-analysis of the efficacy and tolerability of pramipexole versus ropinirole in the treatment of restless legs syndrome. Sleep Med. 2008;9:715–26.
Walters AS, Ondo WG, Dreykluft T, Grunstein R, Lee D, Sethi K, et al. Ropinirole is effective in the treatment of restless legs syndrome. TREAT RLS 2: a 12-week, double-blind, randomized, parallel-group, placebo-controlled study. Mov Disord. 2004;19:1414–23.
Trenkwalder C, Garcia-Borreguero D, Montagna P, Lainey E, de Weerd AW, Tidswell P, et al. Ropinirole in the treatment of restless legs syndrome: results from the TREAT RLS 1 study, a 12 week, randomised, placebo controlled study in 10 European countries. J Neurol Neurosurg Psychiatry. 2004;75:92–7.
Manconi M, Ferri R, Zucconi M, Oldani A, Giarolli L, Bottasini V, et al. Pramipexole versus ropinirole: polysomnographic acute effects in restless legs syndrome. Mov Disord. 2011;26:892–5.
Allen R, Becker PM, Bogan R, Schmidt M, Kushida CA, Fry JM, et al. Ropinirole decreases periodic leg movements and improves sleep parameters in patients with restless legs syndrome. Sleep. 2004;27:907–14.
Inoue Y, Shimizu T, Hirata K, Uchimura N, Ishigooka J, Oka Y, et al. Efficacy and safety of rotigotine in Japanese patients with restless legs syndrome: a phase 3, multicenter, randomized, placebo-controlled, double-blind, parallel-group study. Sleep Med. 2013;14:1085–91.
Hening WA, Allen RP, Ondo WG, Walters AS, Winkelman JW, Becker P, et al. Rotigotine improves restless legs syndrome: a 6-month randomized, double-blind, placebo-controlled trial in the United States. Mov Disord. 2010;25:1675–83.
Wood M, Dubois V, Scheller D, Gillard M. Rotigotine is a potent agonist at dopamine D1 receptors as well as at dopamine D2 and D3 receptors. Br J Pharmacol. 2015;172:1124–35.
Trenkwalder C, Beneš H, Poewe W, et al. Efficacy of rotigotine for treatment of moderate-to-severe restless legs syndrome: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2008;7:595–604.
Oertel W, Trenkwalder C, Beneš H, Ferini-Strambi L, Högl B, Poewe W, et al. Long-term safety and efficacy of rotigotine transdermal patch for moderate-to-severe idiopathic restless legs syndrome: a 5-year open-label extension study. Lancet Neurol. 2011;10:710–20.
García-Borreguero D. Dopaminergic augmentation in restless legs syndrome/Willis-Ekbom disease. Sleep Med Clin. 2015;10:287–92.
Liu GJ, Wu L, Wang SL, Ding L, Xu LL, Wang YF, et al. Incidence of augmentation in primary restless legs syndrome patients may not be that high: evidence from a systematic review and meta-analysis. Medicine (Baltimore). 2016;95:e2504.
Winkelmann J, Allen RP, Högl B, Inoue Y, Oertel W, Salminen AV, et al. Treatment of restless legs syndrome: evidence-based review and implications for clinical practice (revised 2017). Mov Disord. 2018;33:1077–91. https://doi.org/10.1002/mds.27260.
Garcia-Borreguero D, Larrosa O, de la Llave Y, Verger K, Masramon X, Hernandez G. Treatment of restless legs syndrome with gabapentin: a double-blind, cross-over study. Neurology. 2002;59:1573–9.
Inoue Y, Hirata K, Uchimura N, Kuroda K, Hattori N, Takeuchi M. Gabapentin enacarbil in Japanese patients with restless legs syndrome: a 12-week, randomized, double-blind, placebo-controlled, parallel-group study. Curr Med Res Opin. 2013;29:13–21.
Lee DO, Ziman RB, Perkins AT, Ph D, Poceta JS, Walters AS, et al. A randomized, double-blind, placebo-controlled study to assess the efficacy and tolerability of gabapentin enacarbil in subjects with restless legs syndrome. J Clin Sleep Med. 2011. https://doi.org/10.5664/jcsm.1074.
Garcia-Borreguero D, Patrick J, DuBrava S, Becker PM, Lankford A, Chen C, et al. Pregabalin versus pramipexole: effects on sleep disturbance in restless legs syndrome. Sleep. 2014;37:635–43. https://doi.org/10.5665/sleep.3558.
Allen RP, Chen C, Soaita A, Wohlberg C, Knapp L, Peterson BT, et al. A randomized, double-blind, 6-week, dose-ranging study of pregabalin in patients with restless legs syndrome. Sleep Med. 2010;11:512–9.
Garcia-Borreguero D, Larrosa O, Williams AM, Albares J, Pascual M, Palacios JC, et al. Treatment of restless legs syndrome with pregabalin: a double-blind, placebo-controlled study. Neurology. 2010;74:1897–904.
• Trenkwalder C, Beneš H, Grote L, et al. Prolonged release oxycodone-naloxone for treatment of severe restless legs syndrome after failure of previous treatment: a double-blind, randomised, placebo-controlled trial with an open-label extension. Lancet Neurol. 2013;12:1141–50 This randomized controlled trial is the only large-scale study assessing the efficacy and safety of an opioid in the treatment of RLS.
Walters AS, Wagner ML, Hening WA, Grasing K, Mills R, Chokroverty S, et al. Successful treatment of the idiopathic restless legs syndrome in a randomized double-blind trial of oxycodone versus placebo. Sleep. 1993;16:327–32.
Silver N, Allen RP, Senerth J, Earley CJ. A 10-year, longitudinal assessment of dopamine agonists and methadone in the treatment of restless legs syndrome. Sleep Med. 2011;12:440–4.
Ondo WG. Methadone for refractory restless legs syndrome. Mov Disord. 2005;20:345–8.
Walters AS, Winkelmann J, Trenkwalder C, Fry JM, Kataria V, Wagner M, et al. Long-term follow-up on restless legs syndrome patients treated with opioids. Mov Disord. 2001;16:1105–9.
Allen RP, Auerbach S, Bahrain H, Auerbach M, Earley CJ. The prevalence and impact of restless legs syndrome on patients with iron deficiency anemia. Am J Hematol. 2013;88:261–4.
Connor JR, Ponnuru P, Wang X-S, Patton SM, Allen RP, Earley CJ. Profile of altered brain iron acquisition in restless legs syndrome. Brain. 2011;134:959–68.
Davis BJ, Rajput A, Rajput ML, Aul EA, Eichhorn GR. A randomized, double-blind placebo-controlled trial of iron in restless legs syndrome. Eur Neurol. 2000;43:70–5.
O’Keeffe ST, Noel J, Lavan JN. Restless legs syndrome in the elderly. Postgrad Med J. 1993;69:701–3.
Earley CJ, Horská A, Mohamed MA, Barker PB, Beard JL, Allen RP. A randomized, double-blind, placebo-controlled trial of intravenous iron sucrose in restless legs syndrome. Sleep Med. 2009;10:206–11.
Grote L, Leissner L, Hedner J, Ulfberg J. A randomized, double-blind, placebo controlled, multi-center study of intravenous iron sucrose and placebo in the treatment of restless legs syndrome. Mov Disord. 2009;24:1445–52.
Allen RP, Adler CH, Du W, Butcher A, Bregman DB, Earley CJ. Clinical efficacy and safety of IV ferric carboxymaltose (FCM) treatment of RLS: a multi-centred, placebo-controlled preliminary clinical trial. Sleep Med. 2011;12:906–13.
Cho YW, Allen RP, Earley CJ. Clinical efficacy of ferric carboxymaltose treatment in patients with restless legs syndrome. Sleep Med. 2016;25:16–23.
Trenkwalder C, Winkelmann J, Oertel W, Virgin G, Roubert B, Mezzacasa A. Ferric carboxymaltose in patients with restless legs syndrome and nonanemic iron deficiency: a randomized trial. Mov Disord. 2017;32:1478–82.
Cho YW, Allen RP, Earley CJ. Efficacy of ferric carboxymaltose (FCM) 500 mg dose for the treatment of restless legs syndrome. Sleep Med. 2018;42:7–12.
Lettieri CJ, Eliasson AH. Pneumatic compression devices are an effective therapy for restless legs syndrome: a prospective, randomized, double-blinded, sham-controlled trial. Chest. 2009;135:74–80.
Mohammadi MM, Raygani AAV, Ghobadi A, Samadzadeh S, Salari N. Effect of near-infrared light therapy based on acupoints on the severity of restless legs syndrome in patients undergoing hemodialysis: a single-blind, randomized controlled trial. Clin Med Res. 2018;16:1–8.
Raissi GR, Forogh B, Ahadi T, Ghahramanpoori S, Ghaboussi P, Sajadi S. Evaluation of acupuncture in the treatment of restless legs syndrome: a randomized controlled trial. J Acupunct Meridian Stud. 2017;10:346–50.
Mitchell UH, Hilton SC, Hunsaker E, Ulfberg J. Decreased symptoms without augmented skin blood flow in subjects with RLS/WED after vibration treatment. J Clin Sleep Med. 2016;12:947–52.
Aukerman MM, Aukerman D, Bayard M, Tudiver F, Thorp L, Bailey B. Exercise and restless legs syndrome: a randomized controlled trial. J Am Board Fam Med. 2006;19:487–93.
Giannaki CD, Hadjigeorgiou GM, Karatzaferi C, Maridaki MD, Koutedakis Y, Founta P, et al. A single-blind randomized controlled trial to evaluate the effect of 6 months of progressive aerobic exercise training in patients with uraemic restless legs syndrome. Nephrol Dial Transplant. 2013;28:2834–40.
Giannaki CD, Sakkas GK, Karatzaferi C, Maridaki MD, Koutedakis Y, Hadjigeorgiou GM, et al. Combination of exercise training and dopamine agonists in patients with RLS on dialysis. ASAIO J. 2015;61:738–41.
Ferré S, García-Borreguero D, Allen RP, Earley CJ. New insights into the neurobiology of restless legs syndrome. Neuroscientist. 2018;107385841879176. https://doi.org/10.1177/1073858418791763.
Garcia-Borreguero D, Guitart X, Garcia Malo C, Cano-Pumarega I, Granizo JJ, Ferré S. Treatment of restless legs syndrome/Willis-Ekbom disease with the non-selective ENT1/ENT2 inhibitor dipyridamole: testing the adenosine hypothesis. Sleep Med. 2018;45:94–7.
Jung Y, St. Louis EK. Treatment of REM sleep behavior disorder. Curr Treat Options Neurol. 2016;18:50.
Kunz D, Mahlberg R. A two-part, double-blind, placebo-controlled trial of exogenous melatonin in REM sleep behaviour disorder. J Sleep Res. 2010;19:591–6.
McCarter SJ, Boswell CL, St. Louis EK, Dueffert LG, Slocumb N, Boeve BF, et al. Treatment outcomes in REM sleep behavior disorder. Sleep Med. 2013;14:237–42.
Ferri R, Zucconi M, Rundo F, Spruyt K, Manconi M, Ferini-Strambi L. Heart rate and spectral EEG changes accompanying periodic and non-periodic leg movements during sleep. Clin Neurophysiol. 2007;118:438–48.
Zucconi M, Ferri R, Allen R, Baier PC, Bruni O, Chokroverty S, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the international restless legs syndrome study Grou. Sleep Med. 2006;7:175–83.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Aaro V. Salminen declares no potential conflict of interest. Juliane Winkelmann has received speaker honoraria from Bayer and UCB, as well as research grants from Else Kröner Foundation and German Research Foundation (DFG). Juliane Winkelmann also has a patent filed related to Thalidomide in RLS pending.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Movement Disorders
Rights and permissions
About this article
Cite this article
Salminen, A.V., Winkelmann, J. Restless Legs Syndrome and Other Movement Disorders of Sleep—Treatment Update. Curr Treat Options Neurol 20, 55 (2018). https://doi.org/10.1007/s11940-018-0540-3
Published:
DOI: https://doi.org/10.1007/s11940-018-0540-3