Abstract
Purpose of Review
This comprehensive review explores the increasing prevalence of obesity among inflammatory bowel disease (IBD) patients and investigates its impact on disease pathophysiology, response to therapy, and overall clinical outcomes. As obesity rates rise globally, it is important to recognize the complex interplay between obesity and IBD.
Recent Findings
Contrary to the misconception that IBD patients are predominantly underweight, current evidence suggests that 15–40% of IBD patients have obesity. The review delves into recent epidemiological data indicating a parallel increase in obesity rates and IBD incidence. Moreover, it highlights the significance of visceral adiposity, over traditional measures such as body mass index (BMI), in disease severity, surgical outcomes, and response to therapies in patients with IBD.
Summary
This review highlights importance of addressing the growing prevalence of obesity among IBD patients. The intricate relationship between obesity, visceral adiposity, and IBD outcomes necessitates a shift from BMI-centric evaluations to more nuanced assessments such as visceral fat measurements. Understanding the impact of these parameters on response to therapy, especially biologics, is crucial for optimizing treatment strategies in this high-risk population. Additionally, the review emphasizes the bidirectional relationship between obesity and IBD, and the unmet needs to guide future research to elucidate these complex interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence and prevalence of both obesity and inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis UC), have been rising globally [1]. It is estimated that by 2030, roughly 50% of the United States (US) population will be obese and nearly 1 in 4 people will have severe obesity with a body mass index (BMI) over 40 [2]. This rising obesity epidemic is associated with poor health and lost productivity, absenteeism, presenteeism and disability; in 2014, being overweight or obese was associated with over 26 million cases of hypertension, 16 million cases of type 2 diabetes and 15 million cases of chronic back pain in the US, leading to an estimated 210 billion dollars per year of medical costs attributable to the obesity epidemic [3].
Despite the classic misconception that the majority of patients with IBD are underweight or undernourished, approximately 15–40% of IBD patients today are obese [1, 4, 5]. Given this rise in the prevalence of obesity among patients with IBD, there is significant interest in understanding the impact of obesity and visceral adiposity on the etiology and pathophysiology of IBD and the efficacy of current IBD therapeutics. Epidemiologically, the rising obesity rates have paralleled the rising incidence of IBD, especially in Westernized countries, suggesting that obesity may be associated with the development of IBD. This is supported by studies in other chronic inflammatory conditions, demonstrating that obesity is a significant risk factor in their development [4, 6]. Not only does obesity have an impact on the epidemiology of chronic illness, recent studies have suggested that obesity and more specifically visceral adiposity have a negative impact on IBD related outcomes, leading to impaired response to biologics, increased inflammatory burden, impaired quality of life, increased surgical complications and more frequent disease flares [7, 8]. Therefore, the aim of this review is to provide an up to date and comprehensive discussion on the pathophysiology of obesity in patients with IBD and the impact of obesity and visceral adiposity on the disease course, response to biologics (including the pharmacokinetics of traditional therapies and novel therapeutic agents) and the development of IBD-related complications.
The Epidemiology and Pathophysiology of Obesity
While the precise incidence and prevalence of obesity in patients with IBD are not well documented, the rising rates of obesity are clear throughout the literature. To date, it is estimated that roughly 20–40% of IBD patients are overweight, 15–40% have class I obesity obese (( i.e. BMI 30–34.9 kg/m2) and 2–3% of patients have class III obesity, (i.e. BMI > 40 kg/m2) [1]. In a systematic review pooling clinical trial data from 40 studies and over 10,000 patients with CD, there was a significant increase in the mean BMI from the early 1990s to the early 2000s [9]. Furthermore, while rising prevalence of obesity is very evident in the US, this rise in obesity among patients with IBD has been demonstrated across the globe, most recently with studies from France and Korea [10, 11]. Unfortunately, the majority of these epidemiologic studies on obesity in IBD use BMI to define obesity; however, in patients with CD and UC, the presence of intraperitoneal fat (defined as visceral or mesenteric adiposity) may be more clinically relevant. Evaluating the prevalence of increased visceral fat in patients with IBD is an area of increasing interest. With the projected rise in obesity rates both in the US and internationally over the next 5 years, it is anticipated that the number of patients with IBD and comorbid obesity will similarly continue to rise, highlighting the importance of understanding both the impact of obesity and the ideal treatment options in this high-risk population. This rise in obesity is thought to be independent of disease activity or treatment and instead parallels the global obesity pandemic.
Obesity is considered a state of low-grade chronic inflammation [12]. Interestingly, visceral fat (VAT), which includes fat surrounding the small bowel in the abdominal cavity, has been defined in the literature as a hallmark of CD and is metabolically active [13]. VAT has been associated with adipocyte hyperplasia and increased T cell and macrophage infiltration [13,14,15,16]. VAT differs anatomically and metabolically from subcutaneous or non-visceral fat, and this distinction is clinically significant. In contrast to subcutaneous adipose tissue and total body fat, VAT is associated with increased risk of metabolic and inflammatory diseases [17]. VAT is implicated in the regulation of the innate immune system. Adipokines and hormones (adiponectin, leptin, resistin) secreted by the VAT have metabolic functions and with both pro-inflammatory (TNFα, IL‐6, IL‐1β, IL‐18) and anti-inflammatory (TGFαβ, IL‐1RA) properties [15, 18]. In a basic science study, preadipocytes isolated from individuals with CD and UC released higher levels of IL-1-beta and IL-17 in contrast to those from healthy subjects, which did not release pro-inflammatory cytokines and instead released IL-10, an anti-inflammatory cytokine [18].
Using cross sectional imaging, several observational studies have shown that patients with CD have increased VAT as well as higher ratios of VAT: BMI and VAT: total abdominal fat compared to healthy controls [19,20,21,22]. Of interest, such association has not been found in patients with UC [23]. VAT can be measured using cross sectional abdominal imaging, including computed tomography (CT), dual x-ray absorptiometry (DEXA) scan and magnetic resonance imaging (MRI) [24]. Novel techniques to assess VAT include the use of ultrasound (US) to measure the distance from the linea alba to the superior edge of the aorta and bioelectrical impedance analysis (BIA) to estimate VAT. In the literature, there is significant variability in how VAT is measured; on cross sectional imaging, VAT is often measured on axial images of the lumbar vertebra, however, it is important to recognize that estimates of VAT from a single 2D slice on cross sectional imaging may not be indicative of total VAT due to variations of fat distribution among individuals [25]. While measurements of VAT are increasingly being done for research, these tools are time consuming and require special knowledge and software for segmentation, making this inaccessible to most clinicians. Therefore, surrogate markers of VAT that are both accurate and easy to obtain are important. Early studies have suggested that waist circumference as well as waist-to-hip ratio may be used as a surrogate marker of VAT in clinical practice [26]. While VAT on imaging and waist circumference will likely be highly associated in studies, it is important to recognize that waist circumference alone is unable to differentiate subcutaneous fat from VAT, which in IBD may be clinically relevant. Ultimately, the heterogeneity in the metrics to assess VAT in the current literature limits the generalizability of the results and larger, more standardized studies are needed to understand the ideal tool to assess VAT in patients with IBD.
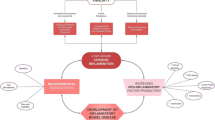
In addition to VAT, the prevalence and clinical relevance of creeping fat is an area of great interest. Creeping fat refers to the mesenteric or visceral fat that specifically wraps around the intestinal wall and is metabolically more active than other types of fat [4]. Creeping fat is difficult to measure, as differentiating the VAT from the fat specifically in contact with the bowel wall can be challenging on cross sectional imaging. Differentiating visceral fat from creeping fat is important; while the two occupy the visceral abdominal cavity, many believe that these two types of fat are, in fact, distinct, both in anatomical location (with creeping fat wrapping around the bowel wall) and in hormonal activity [27]. It is thought that there is a bi-directional relationship between the creeping fat and intestinal inflammation; local adipokine signaling from the fat may increase systemic inflammation while translocation of bacteria and other cytokines from the bowel may stimulate adipocyte growth. In fact, creeping fat is known to be a rich source of pro inflammatory cytokines including TNF, IL-10 and IL-6 among others. In addition, the creeping fat has plentiful Th1 cells and M2 macrophages, not only supporting the production of pro-inflammatory cytokines but also promoting fibrosis of the affected, inflamed bowel [28] (Fig. 1). Unfortunately, there is limited primary data quantifying creeping fat in patients with IBD and correlating this with IBD related outcomes including stricture formation, fistulization and other disease complications. Notable, surgeons have recognized creeping fat for decades as a hallmark operative finding in patients with CD [29, 30].
Impact of Obesity on IBD Activity and Severity
Obesity has been associated with both IBD activity and disease severity in numerous studies. Of interest, early studies in patients with CD suggested that obesity, as measured by BMI, was not complications in patients with IBD. In a multivariable analysis, visceral obesity, defined as consistently associated with disease phenotype and perhaps was protective against some complications. For example, in a prospective, cross-sectional study with 846 prospectively enrolled CD patients, there was no association between BMI and disease phenotype (risk of perianal disease, stricturing disease or the need for surgery). Those patients with a BMI < 25 kg/m2 had a lower risk of penetrating disease (odds ratio [OR] 0.56, 95% confidence interval [CI] 0.31–0.99) [31]. While the authors concluded that obesity does not negatively impact the disease course in patients with CD, further studies looking beyond BMI have suggested that there is in fact a deleterious relationship. In a subsequent study from the same research group, patients with CD who had the highest quartile of VAT (as measured on CT) had a significantly higher risk of requiring surgery (OR 2.02, 95%CI 1.09–3.76, p 0.006) [32]. Similarly, those with greater VAT had a higher risk of developing penetrating disease (but not stricturing or perianal disease) [32]. These two studies suggest that VAT is a better measure of obesity as compared to BMI when using this as a predictor of disease complications. This is supported by a subsequent retrospective study including patients with both CD and UC in which a higher VAT to subcutaneous fat (SAT) ratio (and not BMI) was independently associated with a shorter time to flare in patients with IBD, particularly those with CD [8]. Several other studies have similarly shown an independent association of VAT (as measured with variable techniques/metrics) with complicated disease phenotype, activity and CD-related hospitalizations [22, 33, 34]. Finally, Bryant et al. found that VAT:SAT ratio was independently associated with stricturing disease (OR, 1.70; 95% CI, 0.30 to 3.00) and fecal calprotectin in patients with ileocolonic CD (P = 0.003) [35]. Overall, while the studies evaluating an association between BMI with IBD outcomes are quite variable, the more recent studies looking at the impact of VAT (instead of BMI) suggest that adiposity (in the abdominal cavity) is in fact deleterious for patients with IBD and represents an important target for weight management strategies. Furthermore, while traditional screenings for obesity are usually limited to calculation of the BMI, surrogate metrics of VAT, such as waist circumference, may be more useful as a risk stratification tool in clinical practice.
In addition to the impact on the disease course, obese patients with IBD have been shown to be more likely to require surgery for disease control. In two different cohorts of CD patients VAT volume and VAT area were associated with up to a two-fold increase in the risk of first-time bowel resection [32, 36]. Similarly, CD patients who had VAT volumes ≥ 3,000cm3 and a visceral to subcutaneous adipose tissue ratio ≥ 0.67 were more likely to require surgery within 6 and 12 months following the initiation of anti-TNF treatment [37]. These findings suggest the potential role of VAT indices in predicting need for surgery in CD patients. Additional studies have suggested that obesity is similarly associated with higher rates of disease recurrence after surgery. In a Chinese cohort of CD patients, both VAT total area and the VAT:SAT ratio were predictors of endoscopic recurrence within 6 months of ileocolonic resection (ICR) [38]. In addition, analysis of data from the POCER study identified that a ratio of VAT: height2 > 1.5 times the gender specific mean had higher rates of endoscopic recurrence at 18 months (relative risk 2.1, 95%CI 1.5–2.0, p = 0.012) [39]. This relationship has not been reproduced in all of the literature; in a retrospective study of 347 CD patients who underwent ICR over a 10 year period, body composition was assessed using MRI and none of the body composition metrics including VAT were associated with CD recurrence [40]. However, the ratio of VAT: total fat area approached significance (p = 0.059) [40]. Given the estimates that roughly 20% of patients with UC and approximately 80% of patients with CD will have surgery during their lifetime, the significant impact of obesity on surgical outcomes and disease recurrence after surgery highlights the need for successful weight management strategies in these high-risk patients [41].
While disease severity is often associated with unintentional weight loss, malabsorption and overall, under nutrition, some studies have shown that relationship is more complex. Studies have assessed BMI in patients with IBD and attempted to associate this with disease activity, severity and even phenotype. Patients with CD have been shown to have a lower BMI compared to a healthy control (age and sex matched). However, this does not hold true for patients with UC [42]. In a retrospective study of 470 patients with IBD (of varying disease activity), 41% were normal weight, 9% were underweight, 33% were overweight and 17% were obese. Neither disease extent nor disease complications (including need for surgery or medication initiation) correlated with BMI [43]. Future studies are necessary to understand the impact of disease phenotype and severity on both weight and more specifically VAT. Moreover, there is very limited data on the changes in weight that occur with the natural progression of the disease course (both disease flares and periods of remission). In a study assessing weight changes after biologic initiation of 294 patients, the authors identified statistically significant weight gain in those treated with infliximab and vedolizumab for 12 months and this was higher in men, those with a high c-reactive protein and low albumin at baseline [44]. While we suspect that weight gain from biologic initiation is in part associated with mucosal healing and improved absorption, future studies are needed to better assess this phenomenon.
Obesity not only increases the risk of hospitalization in patients with IBD, but it also associated with an increased length of stay as well as other hospitalization complications. A large national inpatient database evaluated 282,005 hospitalizations among patients with IBD from 2016 to 2018 [45]. In this study, IBD patients who were obese who required hospitalization had significantly higher total hospitalization charges ($50,126 vs. $45,001, P < 0.001), increased length of stay (5.5 days vs. 4.9 days, P < 0.001) and increased overall complications as compared to the IBD patients who were not obese [45]. The prolonged hospitalization in this study was attributed to increased rates of sepsis, acute kidney failure, acute respiratory failure, and pulmonary embolism [45]. However, this study did not specifically investigate the prevalence of IBD related complications. A subsequent study using the Nationwide Readmissions Database similarly showed increased length of stay (median, 8 days vs 5 days) (p < 0.01), higher hospitalization related costs (median, $17,277 vs $11,847, Pp < 0.01) and increased likelihood of experiencing a thromboembolic event (14% vs 9%; p < 0.01) among obese individuals with IBD in contrast to those without obesity. Further studies are needed to identify additional IBD complications associated with obesity in order to better educate and guide patients going forward.
Impact of Obesity on Response to IBD Therapies
The impact of obesity on a patient’s response to biologics and other therapies is an area of current research. Monoclonal antibodies and other biologics used to treat IBD have variable drug concentrations based on the bioavailability and the pharmacokinetics of each drug [46]. Studies have shown that the pharmacokinetics of these agents is influenced by hypoalbuminemia, systemic and/or luminal inflammation, immunogenicity, concomitant immunosuppression, and adipose tissue [46]. In fact, obesity is associated with accelerated drug clearance and increased systemic volume of distribution, both of which lead to impaired efficacy [46]. Obesity is thought to impact the efficacy of these drugs regardless of the dosing (fixed dose vs. weight based dosing regimens) and this effect is most pronounced with the anti-tumor necrosis factor (TNF) agents. Not only are the pharmokinetics of the drug altered by obesity, but obese patients have a higher burden of systemic inflammation, making it more challenging to induce and maintain remission [47].
While the pharmokinetics of these agents suggests that they should be less effective in obese patients, results from real world data are somewhat variable. For example, using a pooled analysis from individual participant data in 4 Infliximab (IFX) clinical trials (1207 patients), obesity (defined by BMI) was not associated with achieving clinical remission, clinical response or mucosal healing [48]. These results were consistent across IBD types and trial design (induction and maintenance studies) [48]. In contrast, in a retrospective cohort study of 124 patients with IBD treated with IFX, a loss of response to IFX was seen in CD patients who were obese (defined as BMI > 30 kg/m2), (HR: 3.03, P < 0.001), with a similar, although non-statistically significant, trend in patients with UC (HR: 9.68, P = 0.06) compared to those with lower BMIs [49]. The authors of this study concluded that increased adiposity was associated with a faster loss of response to IFX in patients with IBD. In contrast, in a similarly sized cohort study evaluating the impact of BMI on loss of response in patients bionaive to both IFX and adalimumab (ADA) demonstrated that a BMI ≥ 30 kg/m2 was only associated with loss of response to ADA but not IFX [50]. A subsequent Danish cohort of IBD patients treated with IFX or ADA showed no statistically significant association between BMI and treatment failure [51]. Furthermore, Kurnool et al. conducted a retrospective study in UC patients that received weight-based (infliximab) or fixed-dose biologics (adalimumab, golimumab, vedolizumab, certolizumab pegol) and showed that for each 1 kg/m2 increase in BMI there was a 4% and 8% increased risk of treatment failure and surgery, respectively [52] (Table 1).
While the above studies focused on the data associating BMI with response to biologics, subsequent studies have similarly looked at the impact of VAT on biologic efficacy. The most recent of these studies, from Yarur et al. evaluated the impact of VAT on the efficacy of 3 biologics: IFX, vedolizumab (VDZ) and ustekinumab (UST) [7]. In this study, patients with higher amounts of VAT were less likely to achieve corticosteroid free deep remission (p < 0.001) or endoscopic remission (p = 0.02) compared to those with less VAT [7]. While those in this study with higher VAT and no response to the biologic had higher serum levels of IL-6 and TNF-a compared to those with lower VAT and a good response to the biologic, interestingly, the drug pharmacokinetics and microbiome diversity did not differ based on VAT [7]. Similarly, in 2 other studies, higher amount of VAT as measured on cross sectional imaging was independently associated with lower IFX trough levels but not with secondary loss of response [37, 53]. Interestingly, in one of these studies, the VAT:SAT ratio was a predictor for therapy failure in patients treated with ADA but it was not associated with trough levels [53].
The majority of the data on the association between obesity and impaired response to biologics comes from studies on anti-TNFs medications; however, this association may also exist with newer biologics and small molecule therapies. In a post hoc analysis of the IM-UNITI trial, Wong et al. demonstrated that rates of clinical remission did not differ based on BMI categories (underweight, normal, overweight and obese) and multivariate logistic analysis did not identify BMI as a significant predictor of clinical response [54]. Of interest, as compared to patients who were not obese, the UST serum level at week 44 was significantly lower in those patients who were obese, however, this was not clinically significant [54]. Similar findings were reported in a cohort of patients treated with UST for psoriasis [55]. Although there is no specific data looking at the impact of other IL23 inhibitors recently approved for the treatment of UC and CD including risankizumab and mirikizumab, an early multicenter retrospective study in 113 patients with overweight or obese patients with psoriasis treated with guselkumab, risankizumab and tildrakizumab showed that there was no impact of weight on therapeutic response and no increased safety risk in the overweight and obese cohort [56]. Further data is needed to understand the impact of obesity on the efficacy of these novel agents in patients with IBD.
To date, there is very limited data on the impact of obesity on small molecule therapies including janus kinase inhibitors (JAKi). A post-hoc analysis of the randomized clinical trials assessing the efficacy of tofacitinib in the induction and maintenance of remission in patients with UC, showed no difference in remission rates or safety when patients were stratified by BMI [57]. Similarly, in a systematic review assessing the impact of BMI on treatment response with biologics including JAKi for rheumatoid arthritis, psoriatic arthritis and axial spondylarthritis, the authors identified 5 studies that included JAKi and concluded that while more data is needed specifically assessing the impact of obesity on the pharmacokinetics and efficacy of these medications, BMI does not seem to have a significant effect [58].
Together, these findings demonstrate the need for additional research to gain a deeper understanding of how obesity and VAT may affect the effectiveness of treatment for IBD and the potential implications of different dosing regimens. This is particularly important, as life-style modification and therapeutic efforts directed to weight loss may be associated with improved response to therapy, particularly TNF-alpha antagonists.
Impact of Obesity on Surgical Outcomes in Patients with IBD
In the surgical community, it is well-known that obesity makes intra-abdominal surgery more technically difficult, with higher rates of post operative complications [4]. In the general population, higher BMIs have been associated with prolonged length of surgery, increased risk of wound infections and higher volumes of blood loss in the general population [59]. Intra-abdominal surgery in patients with obesity is technically more challenging to perform. First, as the amount of subcutaneous tissue increases, it becomes more difficult to get adequate retraction and therefore exposure and visualization may be impaired [60]. Therefore, surgery times are generally longer in patients who are obese as compared to those who are not obese [4]. In addition, it is technically more challenging to create a stoma in patients who are obese [4]. A few studies have demonstrated that obese patients who require a stoma have higher rates of stoma complications including retraction, parastomal hernias, prolapse and mucocutaneous separation [4, 61, 62]. In addition, mesenteric fat can lead to a foreshortened mesentery, making it difficult to mobilize the bowel.Patients with UC and obesity who require subtotal colectomy with ileoanal pouch anastomosis (IPAA) are more likely to require an open procedure, have longer operation times, greater complications (including anastomotic leaks, blood loss, and incisional hernia etc.) and longer hospital stays compared to patients with a normal BMI [63]. Finally, in a retrospective study looking at the impact of VAT in patients with UC who have undergone a subtotal colectomy with IPAA, an increase in VAT after surgery was associated with adverse pouch outcomes including chronic pouch inflammation, new pouch sinus, and pouch failure (OR 12.6, 95%CI 1.19–133.5, p = 0.035) [64]. Given that the creation of the ileoanal pouch and takedown of the ileostomy (stages 2 and 3 of a traditional IPAA) are generally elective, many providers are encouraging patients to lose weight in anticipation of the procedure to improve outcomes [65].
Looking at the association of obesity (defined by BMI) with surgical complications in patients with IBD, a nationwide US study including more than 382,637 patients with IBD demonstrated that obese patients undergoing surgery had significantly higher rates of post operative complications including wound infection (OR 1.35, p = 0.01), pulmonary complications (OR 1.21 p = 0.02), infections (OR 1.16, p = 0.02), and shock (OR 1.3, p = 0.02) [66]. Conversely, in this study, there was no difference in the rate of perforations (OR 1.04, P = 0.71), venous thromboembolism (OR 1.18, P = 0.40), cardiovascular complications (OR 1.09, P = 0.52) or death (OR 0.73, P = 0.07) between those who were obese and non-obese [66].
In addition, there is growing evidence supporting an association between VAT and post-operative VAT area > 130 cm2, was associated with longer operative time, longer bowel resection length and more blood loss in CD patients undergoing first time ICR [67]. In addition, Stidham et al. demonstrated that patients with CD with a high VAT:SAT ratio had a significantly increased risk of infectious complications after surgical resection (OR 2.01, 95%CI 1.2–3.2, p = 0.006), while the authors did not see a similar association with BMI (p = 0.193) [68]. Similarly, in a single center retrospective study of patients with CD undergoing elective ICR, patients with a high VAT:SAT ratio had increased complications including ileus, wound infections, anastomotic leaks, intra-abdominal abscess and hemorrage [69].
Is There a Bi-Directional Relationship Between Obesity and IBD?
While it is clear that obesity and more specifically visceral adiposity as well as creeping fat have a significant clinical impact on the IBD course, some have proposed that IBD may similarly predispose to worsened obesity (Fig. 1). Although IBD is commonly associated with under nutrition and malabsorption, pre-clinical studies have suggested that systemic inflammation and the dysbiosis seen in IBD may in fact stimulate adipocytes [70]. Recent studies have suggested that the gut microbiome plays a significant role in regulating homeostasis, insulin resistance and the development of obesity [71]. Of interest, the obesity associated patterns of dysbiosis overlap with the dysbiosis seen in patients with IBD, suggesting this bi-directional relationship. This is supported by numerous pre-clinical studies in which transplanting stool from lean, healthy donors to obese recipients has been shown to improve obesity and overall metabolic syndrome in the obese recipients [72]. While this has anecdotally been reported in humans who have undergone FMT, there are no clear studies demonstrating an improvement in BMI or metabolic syndrome in obese patients who receive stool from a lower BMI donor. Nonetheless, these pre-clinical studies suggest there is a signal connecting the microbiome, short chain fatty acid production and metabolic syndrome, necessitating further studies to better elucidate this relationship [73].
Future Directions and Clinical Considerations for Practice
The current literature certainly highlights the deleterious effects of obesity and excess visceral adiposity on IBD related outcomes. From reduced biologic efficacy to the development of disease complications including strictures and impaired post operative outcomes after intestinal resection, the hormonally active nature of the VAT is evident in patients with IBD. Given this, clinicians need to identify patients who increased VAT early in the disease course in order to provide a timely and effective weight management strategy to reduce disease complications and improve overall well-being. While traditionally weight and BMI has been used as the standard to assess for adiposity, the associations between IBD outcomes and adiposity are significantly stronger when looking at VAT as compared to BMI. Therefore, there is an unmet need to identify accurate surrogate markers of VAT that are technically easy to obtain in a busy clinical practice in order to identify patients at risk of disease complications. In an era when intestinal ultrasound is more widely utilized for luminal disease assessment, measurement of VAT at the same time adds less than 5 min to the exam and requires minimal additional training. Therefore, point of care bedside US may be a logistically feasible technique to assess for excess VAT in a busy clinical practice. Furthermore, measurements of waist circumference may also be valuable surrogate markers of VAT for patients with IBD.
Once the patients at risk for disease complications secondary to obesity are identified, effective weight management strategies should be put into place. This can include a combination of lifestyle modifications featuring dietary modifications (i.e. hypocaloric diet, intermittent fasting, and time restricting eating) and increased physical activity, anti-obesity medications including glucagon-like peptide-1 receptor agonists (GLP-1RAs), as well as endobariatric and bariatric surgical procedures to help reduce adipokine signaling and potentially improve overall clinical outcomes [74]. Finally, further studies to better understand the pathophysiology of obesity in patients with IBD are needed; for example, the studies on VAT and creeping fat have demonstrated that not all fat has the same cytokine activity or impact on clinical outcomes. Adipose tissue can be differentiated not only by location but also by type of fat; while white adipose tissue is the predominant form of fat in the body and mediates energy hemostasis, brown adipose tissue dissipates stored energy as heat. Most recently, an intermediate type of fat, known as ‘beige adipose tissue’, has been described, which occurs when white adipocytes undergo a ‘browning’ process [75]. While visceral fat is thought to be mostly white fat, the inflammatory properties of brown fat and/or beige fat are unclear and the impact of novel obesity pharmacotherapies or lifestyle modifications on each type of fat is an area of great interest. Future studies to help identify the predominant type of fat and the impact of weight management strategies on the cytokine production of the remaining fat will be crucial in furthering our understanding of the impact of obesity in patients with IBD.
Data Availability
No datasets were generated or analysed during the current study.
References
Kim JH, Oh CM, Yoo JH. Obesity and novel management of inflammatory bowel disease. World J Gastroenterol. 2023;29(12):1779–94.
Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. State-Level Prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381(25):2440–50.
Fast Facts Costs of Obesity: Stop Obesity Alliance. Strategies to Overcome & Prevent (STOP) Obesity Alliance at the Sumner M. Redstone Global Center for Prevention & Wellness.
Singh S, Dulai PS, Zarrinpar A, Ramamoorthy S, Sandborn WJ. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017;14(2):110–21.
Johnson AM, Loftus EV. Impact of Obesity on the Management of Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y). 2020;16(7):350–9.
Sterry W, Strober BE, Menter A, International Psoriasis C. Obesity in psoriasis: the metabolic, clinical and therapeutic implications. Report of an interdisciplinary conference and review. Br J Dermatol. 2007;157(4):649–655.
Yarur AJ, Bruss A, Moosreiner A, et al. Higher Intra-Abdominal Visceral Adipose Tissue Mass Is Associated With Lower Rates of Clinical and Endoscopic Remission in Patients With Inflammatory Bowel Diseases Initiating Biologic Therapy: Results of the Constellation Study. Gastroenterology. 2023;165(4):963-975.e5.
Sehgal P, Su S, Zech J, et al. Visceral adiposity independently predicts time to flare in inflammatory bowel disease but body mass index does not. Inflamm Bowel Dis. 2023;30(4):594–601.
Moran GW, Dubeau MF, Kaplan GG, Panaccione R, Ghosh S. The increasing weight of Crohn’s disease subjects in clinical trials: a hypothesis-generatings time-trend analysis. Inflamm Bowel Dis. 2013;19(13):2949–56.
Kim SK, Lee HS, Kim BJ, et al. The Clinical Features of Inflammatory Bowel Disease in Patients with Obesity. Can J Gastroenterol Hepatol. 2021;2021:9981482.
Blain A, Cattan S, Beaugerie L, Carbonnel F, Gendre JP, Cosnes J. Crohn’s disease clinical course and severity in obese patients. Clin Nutr. 2002;21(1):51–7.
Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–84.
Bilski J, Mazur-Bialy A, Wojcik D, et al. Role of obesity, mesenteric adipose tissue, and adipokines in inflammatory bowel diseases. Biomolecules. 2019;9(12):780.
Kaazan P, Seow W, Yong S, Heilbronn LK, Segal JP. The Impact of Obesity on Inflammatory Bowel Disease. Biomedicines. 2023;11(12):3256.
Peyrin-Biroulet L, Chamaillard M, Gonzalez F, et al. Mesenteric fat in Crohn’s disease: a pathogenetic hallmark or an innocent bystander? Gut. 2007;56(4):577–83.
Desreumaux P, Ernst O, Geboes K, et al. Inflammatory alterations in mesenteric adipose tissue in Crohn’s disease. Gastroenterology. 1999;117(1):73–81.
Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17(4):319–26.
Sideri A, Bakirtzi K, Shih DQ, et al. Substance P mediates pro-inflammatory cytokine release form mesenteric adipocytes in Inflammatory Bowel Disease patients. Cell Mol Gastroenterol Hepatol. 2015;1(4):420–32.
Bryant RV, Schultz CG, Ooi S, et al. Obesity in Inflammatory Bowel Disease: Gains in Adiposity despite High Prevalence of Myopenia and Osteopenia. Nutrients. 2018;10(9):1192.
Rowan CR, McManus J, Boland K, O’Toole A. Visceral adiposity and inflammatory bowel disease. Int J Colorectal Dis. 2021;36(11):2305–19.
Magro DO, Barreto MRL, Cazzo E, Camargo MG, Kotze PG, Coy CSR. Visceral fat is increased in individuals with crohn’s disease: a comparative analysis with healthy controls. Arq Gastroenterol. 2018;55(2):142–7.
Büning C, von Kraft C, Hermsdorf M, et al. Visceral adipose tissue in patients with Crohn’s disease correlates with disease activity, inflammatory markers, and outcome. Inflamm Bowel Dis. 2015;21(11):2590–7.
Gu P, Dube S, McGovern DPB. Medical and Surgical Implications of Mesenteric Adipose Tissue in Crohn’s Disease: A Review of the Literature. Inflamm Bowel Dis. 2023;29(3):458–69.
Klopfenstein BJ, Kim MS, Krisky CM, Szumowski J, Rooney WD, Purnell JQ. Comparison of 3 T MRI and CT for the measurement of visceral and subcutaneous adipose tissue in humans. Br J Radiol. 2012;85(1018):e826-830.
Shen W, Punyanitya M, Wang Z, et al. Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr. 2004;80(2):271–8.
Borruel S, Molto JF, Alpanes M, et al. Surrogate markers of visceral adiposity in young adults: waist circumference and body mass index are more accurate than waist hip ratio, model of adipose distribution and visceral adiposity index. PLoS ONE. 2014;9(12):e114112.
Kredel LI, Siegmund B. Adipose-tissue and intestinal inflammation - visceral obesity and creeping fat. Front Immunol. 2014;5:462.
Yin Y, Xie Y, Ge W, Li Y. Creeping fat formation and interaction with intestinal disease in Crohn’s disease. United European Gastroenterol J. 2022;10(10):1077–84.
Sheehan AL, Warren BF, Gear MW, Shepherd NA. Fat-wrapping in Crohn’s disease: pathological basis and relevance to surgical practice. Br J Surg. 1992;79(9):955–8.
Weakley FL, Turnbull RB. Recognition of regional ileitis in the operating room. Dis Colon Rectum. 1971;14(1):17–23.
Pringle PL, Stewart KO, Peloquin JM, et al. Body Mass Index, Genetic Susceptibility, and Risk of Complications Among Individuals with Crohn’s Disease. Inflamm Bowel Dis. 2015;21(10):2304–10.
Van Der Sloot KW, Joshi AD, Bellavance DR, et al. Visceral Adiposity, Genetic Susceptibility, and Risk of Complications Among Individuals with Crohn’s Disease. Inflamm Bowel Dis. 2017;23(1):82–8.
Erhayiem B, Dhingsa R, Hawkey CJ, Subramanian V. Ratio of visceral to subcutaneous fat area is a biomarker of complicated Crohn’s disease. Clin Gastroenterol Hepatol. 2011;9(8):684-687.e681.
Cravo ML, Velho S, Torres J, et al. Lower skeletal muscle attenuation and high visceral fat index are associated with complicated disease in patients with Crohn’s disease: An exploratory study. Clin Nutr ESPEN. 2017;21:79–85.
Bryant RV, Schultz CG, Ooi S, et al. Visceral Adipose Tissue Is Associated With Stricturing Crohn’s Disease Behavior, Fecal Calprotectin, and Quality of Life. Inflamm Bowel Dis. 2019;25(3):592–600.
Grillot J, D’Engremont C, Parmentier AL, et al. Sarcopenia and visceral obesity assessed by computed tomography are associated with adverse outcomes in patients with Crohn’s disease. Clin Nutr. 2020;39(10):3024–30.
Gu P, Chhabra A, Chittajallu P, et al. Visceral Adipose Tissue Volumetrics Inform Odds of Treatment Response and Risk of Subsequent Surgery in IBD Patients Starting Antitumor Necrosis Factor Therapy. Inflamm Bowel Dis. 2022;28(5):657–66.
Li Y, Zhu W, Gong J, et al. Visceral fat area is associated with a high risk for early postoperative recurrence in Crohn’s disease. Colorectal Dis. 2015;17(3):225–34.
Holt DQ, Moore GT, Strauss BJ, Hamilton AL, De Cruz P, Kamm MA. Visceral adiposity predicts post-operative Crohn’s disease recurrence. Aliment Pharmacol Ther. 2017;45(9):1255–64.
Schineis CHW, Pozios I, Boubaris K, et al. Role of visceral fat on postoperative complications and relapse in patients with Crohn’s disease after ileocecal resection: Is it overrated? Int J Colorectal Dis. 2024;39(1):20.
Sica GS, Biancone L. Surgery for inflammatory bowel disease in the era of laparoscopy. World J Gastroenterol. 2013;19(16):2445–8.
Dong J, Chen Y, Tang Y, et al. Body Mass Index Is Associated with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. PLoS ONE. 2015;10(12):e0144872.
Lima JS, de Brito CAA, Celani LMS, et al. Body Mass Index Profile of Adult Patients with Inflammatory Bowel Disease in a Multicenter Study in Northeastern Brazil. Clin Exp Gastroenterol. 2023;16:213–24.
Kaazan P, Tan Z, Maiyani P, et al. Weight and BMI Patterns in a Biologicals-Treated IBD Cohort. Dig Dis Sci. 2022;67(12):5628–36.
Dahiya DS, Kichloo A, Wani F, Singh J, Solanki D, Shaka H. A nationwide analysis on the influence of obesity in inflammatory bowel disease hospitalizations. Intest Res. 2022;20(3):342–9.
Bassi M, Singh S. Impact of Obesity on Response to Biologic Therapies in Patients with Inflammatory Bowel Diseases. BioDrugs. 2022;36(2):197–203.
Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13(4):851–63.
Singh S, Proudfoot J, Xu R, Sandborn WJ. Obesity and Response to Infliximab in Patients with Inflammatory Bowel Diseases: Pooled Analysis of Individual Participant Data from Clinical Trials. Am J Gastroenterol. 2018;113(6):883–9.
Harper JW, Sinanan MN, Zisman TL. Increased body mass index is associated with earlier time to loss of response to infliximab in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(10):2118–24.
Chuck W, Shadbolt BF, Nordin F, Subramaniam K. BMI is important in predicting the loss of response in inflammatory bowel disease patients on tumour necrosis factor-α inhibitors. Eur J Gastroenterol Hepatol. 2022;34(6):622–9.
Madsen KG, Pottegård A, Hallas J, Kjeldsen J. Treatment Failure of TNF-α Inhibitors in Obese Patients With Inflammatory Bowel Disease-A Cohort Study. Inflamm Bowel Dis. 2018;24(12):2628–33.
Kurnool S, Nguyen NH, Proudfoot J, et al. High body mass index is associated with increased risk of treatment failure and surgery in biologic-treated patients with ulcerative colitis. Aliment Pharmacol Ther. 2018;47(11):1472–9.
Lim Z, Welman CJ, Raymond W, Thin L. The Effect of Adiposity on Anti-Tumor Necrosis Factor-Alpha Levels and Loss of Response in Crohn’s Disease Patients. Clin Transl Gastroenterol. 2020;11(9):e00233.
Wong ECL, Marshall JK, Reinisch W, Narula N. Body Mass Index Does Not Impact Clinical Efficacy of Ustekinumab in Crohn’s Disease: A Post Hoc Analysis of the IM-UNITI Trial. Inflamm Bowel Dis. 2021;27(6):848–54.
Lebwohl M, Yeilding N, Szapary P, et al. Impact of weight on the efficacy and safety of ustekinumab in patients with moderate to severe psoriasis: rationale for dosing recommendations. J Am Acad Dermatol. 2010;63(4):571–9.
Ricceri F, Chiricozzi A, Peris K, Prignano F. Successful use of anti-IL-23 molecules in overweight-to-obese psoriatic patients: A multicentric retrospective study. Dermatol Ther. 2022;35(11):e15793.
Farraye FA, Qazi T, Kotze PG, et al. The impact of body mass index on efficacy and safety in the tofacitinib OCTAVE ulcerative colitis clinical programme. Aliment Pharmacol Ther. 2021;54(4):429–40.
Gialouri CG, Pappa M, Evangelatos G, Nikiphorou E, Fragoulis GE. Effect of body mass index on treatment response of biologic/targeted-synthetic DMARDs in patients with rheumatoid arthritis, psoriatic arthritis or axial spondyloarthritis. Syst Rev Autoimmun Rev. 2023;22(7):103357.
Tjeertes EK, Hoeks SE, Beks SB, Valentijn TM, Hoofwijk AG, Stolker RJ. Obesity–a risk factor for postoperative complications in general surgery? BMC Anesthesiol. 2015;15:112.
Wang Y, Lou Z, Zhang W. Surgical strategy for stoma creation in the challenging patients. Zhonghua Wei Chang Wai Ke Za Zhi. 2022;25(11):961–4.
Duchesne JC, Wang YZ, Weintraub SL, Boyle M, Hunt JP. Stoma complications: a multivariate analysis. Am Surg. 2002;68(11):961–6.
Beck SJ. Stoma issues in the obese patient. Clin Colon Rectal Surg. 2011;24(4):259–62.
Emile SH, Khan SM, Wexner SD. A systematic review and meta-analysis of the outcome of ileal pouch anal anastomosis in patients with obesity. Surgery. 2021;170(6):1629–36.
Liu G, Wu X, Li Y, et al. Postoperative excessive gain in visceral adipose tissue as well as body mass index are associated with adverse outcomes of an ileal pouch. Gastroenterol Rep (Oxf). 2017;5(1):29–35.
Chang S, Shen B, Remzi F. When Not to Pouch: Important Considerations for Patient Selection for Ileal Pouch-Anal Anastomosis. Gastroenterol Hepatol (N Y). 2017;13(8):466–75.
Jain A, Limketkai B, Hutfless S. The Effect of Obesity on Post-Surgical Complications During Hospitalizations for Inflammatory Bowel Disease: A Nationwide Analysis. Gastroenterology. 2014;146:595–6.
Ding Z, Wu XR, Remer EM, et al. Association between high visceral fat area and postoperative complications in patients with Crohn’s disease following primary surgery. Colorectal Dis. 2016;18(2):163–72.
Stidham RW, Waljee AK, Day NM, et al. Body fat composition assessment using analytic morphomics predicts infectious complications after bowel resection in Crohn’s disease. Inflamm Bowel Dis. 2015;21(6):1306–13.
Connelly TM, Juza RM, Sangster W, Sehgal R, Tappouni RF, Messaris E. Volumetric fat ratio and not body mass index is predictive of ileocolectomy outcomes in Crohn’s disease patients. Dig Surg. 2014;31(3):219–24.
Eder P, Adler M, Dobrowolska A, Kamhieh-Milz J, Witowski J. The Role of Adipose Tissue in the Pathogenesis and Therapeutic Outcomes of Inflammatory Bowel Disease. Cells. 2019;8(6):628.
Jang HR, Lee HY. Mechanisms linking gut microbial metabolites to insulin resistance. World J Diabetes. 2021;12(6):730–44.
Kulecka M, Paziewska A, Zeber-Lubecka N, et al. Prolonged transfer of feces from the lean mice modulates gut microbiota in obese mice. Nutr Metab (Lond). 2016;13(1):57.
Islam MR, Arthur S, Haynes J, Butts MR, Nepal N, Sundaram U. The Role of Gut Microbiota and Metabolites in Obesity-Associated Chronic Gastrointestinal Disorders. Nutrients. 2022;14(3):624.
Lavallee CM, Bruno A, Ma C, Raman M. A review of the role of intermittent fasting in the management of inflammatory bowel disease. Therap Adv Gastroenterol. 2023;16:17562848231171756.
Tschurtschenthaler M, Verstockt B. Targeting a Hallmark of Crohn’s Disease: Browning of the Hypertrophic Mesenteric Adipose Tissue as a Novel Strategy to Reduce Inflammation? J Crohns Colitis. 2023;17(8):1177–8.
Funding
-AK: Research Support: Abbvie, Janssen, Landos, Pfizer, Salix, Osiris, Merck. Advisor/Consultant: Abbvie, Janssen, Takeda, Prometheus, Merck, Pfizer, Amgen, Celltrion, Shire, Salix, Ferring, Boehringer-Ingelheim, Bristol Myers-Squibb, Stock/Stock options: Spatz Medical, Trellus Health, Innervate Radiopharmaceuticals.
-SG: Nestle Nutrition Institute Fellow 2023, Supported by a Crohn’s and Colitis Foundation Career Development Award, Medical Board Members of Nutritional Therapy for IBD.
-MS: None.
-EA: None.
-JP: None.
-MR: Takeda, Pfizer, Janssen, Abbvie, Bausch: Unrestricted Educational Grants.
Lupin, Abbvie, Takeda: Speaker Fees.
LyfeMD Director: Shareholder.
Author information
Authors and Affiliations
Contributions
EA: Contributed to writing of the main manuscript, reviewed the initial draft & final draft of the manuscript JP: Contributed to writing of the main manuscript, prepared the table, reviewed the initial draft & final draft of the manuscript MS: Contributed to writing of the main manuscript, reviewed the initial draft & final draft of the manuscript SG: Contributed to writing of the main manuscript, prepared the figure, reviewed the initial draft & final draft of the manuscript MR: Contributed to manuscript study planning, reviewed the initial draft & final draft of the manuscript AK: Contributed to manuscript study planning, reviewed the initial draft & final draft of the manuscript All authors reviewed the initial draft and final draft of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human or animal subjects.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peraza, J., Abbott, E., Shneyderman, M. et al. The Rising Epidemic of Obesity in Patients with Inflammatory Bowel Disease. Curr Treat Options Gastro 22, 134–144 (2024). https://doi.org/10.1007/s11938-024-00453-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11938-024-00453-5