Abstract
Purpose of review
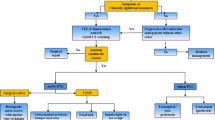
As the number of surgical and transcatheter valve replacements continue to increase in the aging population, so does the incidence of paravalvular leak (PVL). Given its impact on morbidity and mortality, this article will focus on the epidemiology, clinical presentation, diagnostic assessment, and available treatments for PVL.
Recent findings
Despite being performed on inoperable and typically higher risk patients, short-term complication rates of transcatheter PVL closure appear relatively low (< 10%). When indirectly compared with surgical PVL closure, long-term mortality, reoperation rates and degree of symptom improvement are similar. Nonetheless, current transcatheter closure devices are off-label and repurposed from other indications. Further development of percutaneous closure devices is an essential next step in order to improve and optimize outcomes.
Summary
In patients with surgical and especially transcatheter-replaced heart valves, clinicians need to maintain vigilance for the presence of PVL, particularly in those with new-onset heart failure or hemolysis. Multimodality imaging is essential to detect and quantify PVL. Echocardiography (both transthoracic and transesophageal) is the backbone of diagnosis and quantification, and cardiac computed tomography and cardiac magnetic resonance imaging play an important role in defect characterization and in periprocedural planning. For those patients who are unable to undergo surgery, transcatheter PVL closure is an appropriate next step in management as it has similar outcomes to surgical intervention when performed in a center of expertise.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Valvular heart disease affects approximately 2.5% of people in the United States (U.S) and accounts for 1.9% of all U.S mortality [1]. The vast majority of cases involve the aortic valve (AV) and/or the mitral valve (MV). Prevalence is increasing across all ages but is most common in those patients ≥ 75 years old [2]. As a result, the number of valve surgeries continues to grow with over 120,000 surgical valve procedures performed annually in the U.S. [1] Since its approval in 2011 by the Food and Drug Administration (FDA), the number of transcatheter aortic valve replacements (TAVR) has also been rising, with over 50,000 procedures performed in 2017. [3] With its recent approval for use in low-risk surgical patients and the expectation that it will soon be available for those with bicuspid aortic valves, this number is expected to continue to increase significantly.
Paravalvular prosthetic regurgitation (i.e., paravalvular leak [PVL]) has been demonstrated in 5–18% of all implanted surgical valves, with an incidence of 2–10% in the aortic position and 7–17% in the mitral position [4,5,6,7,8]. PVL may result from tissue friability, infection, or annular calcification. Pre-disposing surgical factors include the use of a mechanical MV prosthesis and supra-annular AV prosthesis, the use of sutures without pledgets, or the use of continuous sutures in the mitral position [9, 10]. Although PVL is significantly more prevalent in patients undergoing TAVR, with an estimated incidence of 50–85%, the majority is trace to mild in degree [11]. Annular calcification, valve prosthesis malpositioning, underexpansion, or undersizing can result in poor apposition of the TAVR valve to the aortic annulus and result in PVL.
While the majority of patients have subclinical PVL, those with more significant regurgitation can develop hemolysis, severe heart failure, or both. Previously this was managed exclusively by repeat operation or, in cases where repeat surgery was thought to be unsuitable, by medical therapy. In the last two decades, transcatheter closure of PVL has evolved as a viable option for these patients. Indeed, according to the 2017 American College of Cardiology/American Heart Association Focused Guidelines Update for the management of valvular heart disease, transcatheter repair of PVL is a class IIa recommendation in patients with prosthetic heart valves and intractable hemolysis or New York Heart Association (NYHA) class III/IV heart failure who are at high surgical risk and have suitable anatomic features for transcatheter closure in an experienced center [12].
Given the scope of this issue and the clinical ramifications, this review will focus on the clinical presentation, diagnosis, quantification, and management of PVL.
Clinical presentation
PVL is clinically significant in 2–5% of patients with surgical valve replacements [8]. The majority of clinical presentations are due to heart failure [13]. Over time, chronic regurgitation can result in left atrial and left ventricular pressure and volume overload with pulmonary edema and symptoms of heart failure. However, even less severe regurgitation can cause heart failure if the receiving chamber is non-compliant, making the degree of regurgitation alone insufficient to judge its contribution to patient symptoms [9]. Less commonly, patients present with signs and symptoms of hemolytic anemia, which develops as a result of increased shear stress on red blood cells. This occurs more frequently in patients with smaller paravalvular defects whereas larger defects are typically associated with heart failure. It has been estimated that between 33 and 75% of patients with symptomatic PVL will have clinically significant hemolysis [8, 14]. Hemolytic anemia often presents insidiously with new-onset fatigue but can progress to include conjunctival and mucosal pallor, petechiae, and even overt jaundice. Patients may require blood or platelet transfusions in light of significant anemia. Importantly, it is estimated that 18–51% and 5–10% of patients will have subclinical hemolysis with contemporary mechanical and tissue prostheses, respectively (i.e., patients have laboratory evidence of hemolysis without an associated anemia) [15]. Since patients with PVL have an associated paravalvular defect, they are also at risk for the development of endocarditis in the presence of bacteremia [16].
Like surgical valve replacement, PVL after TAVR can also result in heart failure, hemolytic anemia, or endocarditis. Most recent estimates suggest that moderate to severe PVL occurs in approximately 2.5% of patients post-procedurally [3]. Studies evaluating the overall impact of mild PVL after TAVR have yielded conflicting results [17]. While it was generally believed that only moderate or severe regurgitation would affect long-term outcomes, analysis from the PARTNER trial showed that even mild PVL after TAVR is associated with increased mortality [18]. Conversely, other large registries have only demonstrated adverse effects of moderate or greater PVL [19, 20]. Interestingly, it has been suggested that PVL only impacts post-TAVR outcomes in patients without pre-existing aortic regurgitation. Specifically, patients with small, hypertrophied ventricles are unable to tolerate any increase in volume loading from any degree of PVL, even if only mild in severity [20, 21].
New murmurs that could be consistent with paravalvular leak (i.e., a holosystolic murmur in a patient with a mitral prosthesis or a diastolic murmur of aortic regurgitation in a patient with an aortic prosthesis) should be investigated with further imaging. Conversely, however, clinically significant PVL may only be associated with a soft murmur, so a high index of suspicion should be maintained when evaluating a patient for possible PVL [9].
Diagnosis and evaluation of PVL
Echocardiography
Transthoracic echocardiography (TTE) is the initial diagnostic test of choice for all patients with suspected PVL [22, 23]. Echocardiography is widely and readily available, is non-invasive, and provides a direct evaluation of prosthetic valve function. Two-dimensional (2D), three-dimensional (3D), and Doppler assessment of the prosthetic valve should be performed, in addition to a comprehensive cardiac evaluation of atrial and ventricular size and function, pulmonary artery systolic pressure, and concomitant native valvular disease. Additionally, given the association of PVL with endocarditis, careful investigation should be pursued for independently mobile masses on either the prosthetic or native valves.
While TTE is a superior method for the assessment of valvular gradients and overall cardiac function, it is often limited by acoustic shadowing from mechanical components of prosthetic valves (e.g., bileaflet tilting discs), accrued bioprosthetic leaflet or annular calcification, prosthetic valve sewing rings and struts, or the TAVR prosthesis skirt. Acoustic shadowing not only precludes visualization of prosthetic valve components but may also result in absence of color Doppler signal with potential underestimation of the degree of PVL and difficulty in delineating valvular vs. paravalvular regurgitation [24]. This is especially true for the assessment of MV prostheses where PVL may only be detected in off-axis imaging (e.g., subcostal views) or not seen at all. For aortic prosthetic valves, posterior PVL may be missed due to anterior acoustic shadowing. However, with careful assessment utilizing multiple imaging windows (including parasternal long-axis, parasternal short-axis, apical 3-chamber, and apical 5-chamber views), aortic prosthesis PVL can be interrogated completely with TTE [25]. For all prosthetic valves, Doppler evaluation can be a critical clue to the presence of significant or underestimated PVL (see Tables 1 and 2). This includes elevated Doppler velocities across prosthetic valves, dense continuous wave Doppler tracings, rapid continuous wave Doppler deceleration times, holodiastolic flow reversal in the descending aorta for aortic surgical prosthesis and TAVR, or pulmonary vein flow reversal for mitral surgical prostheses [9, 26]. These Doppler findings should prompt further diagnostic evaluation.
Transesophageal echocardiography (TEE) is usually the subsequent diagnostic test pursued in patients with suspected clinically significant or confirmed PVL. TEE plays a major role in the delineation of the degree and mechanism of PVL and is important in clarifying whether there is eccentric valvular vs. paravalvular regurgitation. For patients with MV prostheses, TEE is the method of choice for assessment of PVL given the degree of acoustic shadowing that is present. Conversely, however, aortic prosthesis PVL (especially with a TAVR valve) is generally well evaluated by TTE imaging. TEE imaging has the advantage of imaging the posterior aspects of aortic prostheses but is limited by anterior prosthesis acoustic shadowing. Thorough evaluation with midesophageal and deep transgastric views is essential for complete quantification of PVL [25]. 3D TEE can be invaluable in localizing PVL, particularly intraoperatively, but suffers from the same shadowing limitations as 2D echocardiography and generally has a lower spatial and temporal resolution [27]. Moreover, drop-out artifacts may occur leading to inappropriate diagnoses of PVL when no such defect exists. Therefore, integrating 2D and 3D imaging is essential in accurate PVL diagnosis.
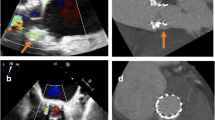
TEE is also an essential component of pre-procedural planning, particularly if transcatheter closure will be performed. As such, during the interrogation of prosthetic valves for the presence of PVL, it is also critical to localize the defect in relation to the sewing ring, prosthetic valve leaflets or discs, and subvalvular structures and to determine its size including the width and percent circumference of the entire sewing ring. It is also crucial to rule out any active endocarditis, excessive rocking motion of the prosthesis (i.e. dehiscence), or significant valvular regurgitation, all of which preclude percutaneous closure. Finally, it should be emphasized that PVL jets are commonly multiple, eccentric, and follow serpiginous tracks, making them challenging to image and quantify by echocardiography. Thorough prosthetic valve interrogation should be pursued with multiple views and off-axis imaging (if necessary) in order to best quantify the degree of PVL and differentiate between valvular and paravalvular regurgitation (see Fig. 1a–c).
Transesophageal echocardiography images of a patient with PVL of a mechanical aortic valve (AoV) prosthesis showing a long-axis view of an aortic valve with aortic regurgitation. From this image, it is not clear whether the regurgitation is valvular or paravalvular. b, c Deep transgastric views demonstrate a visible tract around the aortic valve prosthesis with mild but highly eccentric paravalvular aortic regurgitation. d Cineangiography with contrast injection during PVL closure shows a serpiginous and eccentric course of the paravalvular defect. e In a different patient with mechanical mitral prosthesis paravalvular leak, the image shows a multiplanar reconstruction of real-time 3D TEE data, which allows for accurate measurements of defect size and its vena contracta. f 3D image of the mechanical prosthesis from a “surgeon’s view” with the left atrial appendage positioned laterally, at the 9 o’clock position. The aortic valve is not seen in this image but should be located anteriorly at 12 o’clock. The paravalvular defect in this case is located laterally, at the 9–10 o’clock position (red arrows). g 3D echocardiography imaging after percutaneous closure with two Amplatzer muscular ventricular septal defect occluder devices (AMVSDO, red arrows). The aforementioned lateral defect is no longer visualized.
To ensure effective and consistent communication between the echocardiographer, interventional cardiologist, and cardiac surgeon, a standardized orientation and nomenclature are used to define paravalvular defects. On 3D imaging, mitral valve prostheses are oriented such that the aortic valve is located anteriorly at the 12 o’clock position, the interatrial septum medially at the 3 o’clock position, and the left atrial appendage laterally at the 9 o’clock position (see Fig. 1f). This is also known as the “surgeon’s view.” Like mitral valve prostheses, aortic valve prostheses are oriented in the short-axis view such that the location of PVL can be reported in terms of a clock face. Alternatively, the origin of PVL can be identified with respect to the native aortic valve cusp location (right, left, or non-coronary cusps).
Cardiac CT
In situations where TTE and TEE are unable to clearly delineate the extent and location of PVL, cardiac computed tomography (CT) has emerged as a useful adjunct imaging technique. Cardiac CT is already an important part of pre-operative planning in patients undergoing TAVR. Pre-operative assessment with cardiac CT to assess the size and shape of the aortic annulus has been shown to decrease the degree of post-TAVR PVL compared with 2D echocardiography [28]. Moreover, it can evaluate the location and degree of annular calcification, another risk factor for PVL. Studies have mostly suggested that cardiac CT has no advantage of PVL detection in comparison with 2D TEE, although a recent study did suggest similar efficacy between the two in evaluation of prosthetic mitral PVL [28,29,30]. Limitations of cardiac CT include its requirement for intravenous contrast, the exposure to ionizing radiation, and difficult cardiac gating for rapid or irregular heart rates. However, cardiac CT can be invaluable in the anatomical characterization of PVL in patients with significantly limited echocardiographic images and can help define optimal fluoroscopic angles of the prosthetic annular plane to be used for PVL closure [31].
Cardiac MRI
Like cardiac CT, cardiac magnetic resonance imaging (CMR) can be utilized to diagnose and quantify the degree of regurgitation in patients with AV and MV prostheses where TTE and TEE have failed to adequately do so. Importantly, virtually all prosthetic valves (including mechanical valves) can be imaged by CMR [29]. Phase-contrast velocity mapping is performed in the short-axis plane just distal to the prosthetic valve, with subsequent quantitation of regurgitant volume and regurgitant fraction [32]. Given that total regurgitant volumes are measured, prior imaging should be used to delineate the degree of valvular vs. paravalvular regurgitation. Important limitations of CMR include its tendency to overestimate the degree of PVL compared with echocardiography due to incorporation of coronary flow and decreased accuracy in the setting of arrhythmia. Nonetheless, CMR can play a major role in quantifying PVL in the presence of multiple, eccentric, and irregularly directed jets and when there is significant acoustic shadowing on echocardiography [25, 33].
Cineangiography
While cineangiography with an aortic root injection can be used for the determination of the total degree of aortic regurgitation, this is typically relegated to evaluation during TAVR given its invasive nature and contrast-associated risks. Moreover, angiographic grading of aortic regurgitation correlates poorly with other assessments of regurgitant severity and cannot reliably delineate central and paravalvular aortic regurgitation [26, 33].
Quantitation of PVL
Once it has been established that there is paravalvular (rather than valvular) regurgitation, quantification is essential. Estimating the severity of aortic PVL can be very challenging due to acoustic shadowing and the “garden hose” effect, wherein color Doppler may occupy the entire left ventricular outflow tract despite blood only traveling through a small (albeit serpiginous) tract (see Fig. 1a–d). Conversely, mitral PVL is easier to evaluate on TEE, with excellent 3D reconstruction for optimal localization and sizing of the defect prior to any intervention (see Fig. 1f, g).
As alluded to in the previous section, quantification of PVL is typically accomplished through 2D and 3D echocardiography but may also be assessed by cardiac CT, CMR, and cineangiography. Multiple grading systems have been created for the evaluation of PVL, including a 3-class, an angiographic 4-class, and a unifying 5-class grading scheme [8, 25, 26, 34,35,36]. All echocardiographic grading systems, however, integrate 2D assessment of the prosthetic valve (e.g., sewing ring motion, stent and leaflet morphology), color Doppler (e.g., vena contracta area, circumferential extent of PVL, effective regurgitant orifice area), pulsed and continuous wave Doppler (e.g., density of regurgitant waveforms, pressure half time), ventricular size and function, and pulmonary artery systolic pressure. Importantly, regurgitant fraction from CMR has also been included in these schemata. See Tables 1 and 2 for examples of a 3-class grading scheme for PVL of mitral and aortic prosthetic valves, respectively.
Treatment
Medical therapy
While medical therapy is an important component of symptom management for patients with hemolysis or heart failure due to PVL, there are no known medical therapies to prevent or reverse PVL or its underlying cause [22, 23]. Only surgical repair or transcatheter intervention provides definitive treatment.
For patients with severe heart failure, medical management should mimic the recommendations for patients with symptomatic mitral regurgitation and aortic insufficiency. This includes diuretic therapy for volume overload and afterload reduction in patients with hypertension [22, 23]. Similarly, in patients with endocarditis, appropriate antibiotic therapy should be instituted.
For patients with hemolytic anemia, red blood cell destruction results in accelerated erythropoiesis. As a result, patients should receive folic acid and iron supplementation (either oral or intravenous) [15]. In some cases, hemolytic anemia may be so severe to warrant transfusion, although there is no agreed upon threshold to transfuse to, which is also influenced by the patient comorbidities. In limited retrospective studies, beta-blockers have demonstrated improvement in hemolytic anemia, postulated to occur due to a decrease in red blood cell shear stress [37,38,39]. Similarly, erythropoietin has been used with some success in patients with PVL who were unable to undergo surgical replacement but in small patient series [40].
Surgical replacement
Based on the 2017 AHA/ACC Focused Guidelines Update for the management of valvular heart disease, surgical valve replacement is a class I indication for operable patients with mechanical valves with intractable hemolysis or heart failure due to severe PVL [12]. For many patients, surgical replacement may carry moderate or high operative risk. This is a result of the necessity for a re-do operation and the fact that most patients are typically older with a greater number of comorbidities. Moreover, until recently, the majority of patients who underwent TAVR did so due to their intermediate or higher surgical risk at the time of valve implantation.
Transcatheter repair
For patients with high surgical risk and severe, symptomatic PVL, transcatheter repair carries a class IIa indication according to the 2017 AHA/ACC Focused Guidelines Update for the management of valvular heart disease [12]. Critically, it should be noted that transcatheter repair of PVL is contraindicated in patients with active endocarditis, rocking motion or instability of the prosthesis (i.e., valvular dehiscence) PVL involving > 30% of the sewing ring (relative contraindication) [8].
Careful review of available images and discussion among team members are key pre-procedural steps for optimal outcomes. Pre-procedural planning should be performed using 2D and 3D TTE and TEE, in addition to cardiac CT scanning which can help better define the extent and shape of PVL and can find the optimal fluoroscopic angle. The chosen device(s) should be larger than the defects to avoid embolization, but not so large so as to interfere with valve function, particularly when intervening on mechanical valves. Interventional cardiologists should have skill and facility with complex catheter techniques, including transseptal puncture, wire snaring, rail creation, delivery of vascular plug devices, and most importantly the integration of fluoroscopic and echocardiographic imaging. Intraprocedurally, communication of cardiac imaging findings between the echocardiographer and the interventional cardiologist is essential for defect localization, crossing of the interatrial septum, device maneuvering, wire crossing of the paravalvular defect, device positioning, assessing the degree of residual regurgitation, evaluation of possible interference with valve function, and deciding on the need for further intervention.
Importantly, no device has been specifically approved for PVL closure by the FDA [31]. Rather, self-expander occluder devices are used in an off-label fashion depending on the size and shape of the PVL (see Fig. 2). The majority are Amplatzer (St. Jude Medical [now Abbott], St. Paul, MN) devices and include the Amplatzer vascular plug (AVP) family of occluder devices (AVP II, AVP III, and AVP IV), the Amplatzer duct occluder (ADO I and ADO II), the Amplatzer atrial septal occluder (ASO), and the Amplatzer muscular ventricular septal defect occluder (AMVSDO) [41]. The AVP II and AVP IV devices are most commonly used in the U.S and have the benefit of being re-capturable after deployment. The AVP III device, unique due to its oblong shape, is only available in Europe. The Occlutech (Occlutech, GmbH, Jena, Germany) paravalvular leak device is the first device to be designed specifically for PVL closure, and while it has demonstrated initial success, it is also only available in Europe [42].
Paravalvular leak closure devices. These include the Amplatzer vascular plug (AVP) family of devices, Amplatzer duct occluder (ADO), Amplatzer atrial septal occluder (ASO), Amplatzer muscular ventricular septal defect occluder (AMVSDO), and Occlutech devices (rectangular and square shaped). *Not available in the U.S.
Transcatheter closure of mitral valve prosthesis paravalvular leak
Transcatheter closure of MV PVL is typically performed under general anesthesia given the importance of concomitant TEE imaging. There are three main approaches to closure of mitral PVL and include the antegrade transseptal, retrograde transaortic, or retrograde transapical approaches [9]. The antegrade transseptal approach is most commonly used and involves a transseptal puncture with subsequent wire crossing of the paravalvular defect under TEE guidance. Importantly, a tip deflectable left atrial sheath is inserted that can be steered in three dimensions, allowing for defect crossing and device placement. For added support when deploying multiple devices, the “anchor wire” technique can be used (wherein a parallel “buddy wire” is maintained across the paravalvular defect during the deployment of the first device allowing defect re-access for the deployment of additional devices, if needed) or creation of a “stable A-V rail” can be created (wherein the wire is snared in the descending aorta via arterial puncture, or in the left ventricular cavity via transapical access).
Other techniques for transcatheter closure include the retrograde transaortic approach, wherein arterial access is obtained, the AV is traversed, and the paravalvular defect is crossed from the left ventricle into the left atrium. Subsequently, the wire is snared (by an independently performed transseptal puncture), forming an arteriovenous rail for device delivery. Finally, for patients with prior interatrial septal closure or those with medially located defects, transapical puncture should be considered. Although reported rates have varied widely, complications of transapical access for percutaneous interventions include hemothorax, pericardial effusion, and coronary laceration [43,44,45]. These are generally associated with 6F sheath size delivery catheters and are more common when transapical closure devices are not used [43, 45].
Transcatheter closure of aortic valve prosthesis paravalvular leak
Since AV PVL can often be visualized with TTE imaging, transcatheter PVL closure does not always necessitate the use of concomitant TEE (and therefore general anesthesia). However, TEE is used in the majority of cases (especially those with mechanical AV prostheses) for intraprocedural guidance.
For patients with AV PVL, closure is most commonly performed by the retroaortic approach using femoral access [46]. Closure device delivery can occur using one of three techniques to enhance support and maintain position across the defect. The catheter-only technique is typically used for small defects that require only one closure device. In this approach, there is no remaining guidewire in the left ventricle after deployment, and as such, it would create a challenge in crossing any residual paravalvular defect. The anchor wire technique preserves access across the defect by advancing a second wire into the left ventricle adjacent to the closure device delivery system. Finally, if a more stable rail is needed for deployment, a wire can be advanced across the paravalvular defect, retrograde through the AV, and then snared in the descending aorta and exteriorized to the contralateral femoral artery. This arterio-arterial rail technique is not recommended for patients with mechanical aortic valves due to the risk of immobilized leaflets and rapid hemodynamic deterioration from aortic regurgitation. In these situations, the rail wire may also be snared through a transseptal or transapical approach.
One would expect that treatment of TAVR-related PVL is similar in principle to treatment of PVL for surgically implanted aortic prostheses. However, there are some important distinctions. First, for PVL that is due to TAVR underexpansion or undersizing, primary therapy would be repetition of balloon dilation. This would be suggested by PVL that is circumferential with multiple small jets in a previously placed balloon-expandable valve [47]. For TAVR valves that are positioned too high or too low relative to the AV annulus, valve-in-valve TAVR may be a more appropriate therapy. The remainder of PVL closures, however, is technically similar to that of surgically implanted prostheses. Importantly, these procedures may be complicated in TAVR patients owing to significant annular calcification, the presence of sealing skirts, and the (generally) smaller sized defects.
Outcomes and complications of transcatheter repair
Defined as mild or less residual regurgitation in the absence of death or major complications, successful PVL closure is achieved in approximately 70–85% of centers with procedural expertise [23, 48,49,50,51]. Successful reduction in residual PVL has been associated with decreases in cardiac mortality, less cardiac reoperations, and an improvement in NYHA functional class or hemolytic anemia [52, 53]. Similarly, residual leak after PVL closure has been correlated with increased all-cause mortality and major adverse cardiac events (MACE) [48].
Despite its success, however, complications still occur in approximately 9% of patients. In one study evaluating 115 patients, 30-day adverse event rates were 1.7% for sudden or unexplained death, 2.6% for stroke, 0.9% for emergency surgery, 5.2% for periprocedural bleeding, and 1.7% for device embolization with retrieval [50]. Another study examined 200 patients undergoing PVL closure, noting a 7% rate of MACE at 30 days [54]. Interestingly, this same study emphasized the importance of center experience, with greater experience correlating with decreases in fluoroscopy time, procedural time, length of hospital stay, and MACE. Finally, 259 patients were evaluated in a multicenter series of PVL closures in which the majority were in surgically implanted mitral and aortic valves [48]. After a median follow-up of 110 days, adverse event rates included 16.2% for death, 6% for recurrent valve surgery, 1.6% for significant hemolysis, 0.4% for device embolization, and 0.4% for leaflet interference.
There have been no prospective comparisons of surgical vs. transcatheter PVL repair, especially considering many patients are referred for transcatheter repair because of their high surgical risk. Indirect comparisons suggest that outcomes are similar. Surgical correction is associated with 30-day mortality of 8.8–11.5% and long-term survival of 30–57.8% [48, 53, 55]. One meta-analysis examined 2373 patients who underwent surgical and transcatheter PVL closure [56]. Interestingly, despite greater technical success in reducing PVL (96.7% vs. 72.1%), surgical intervention appears to bring with it an upfront cost, including higher 30-day mortality (8.6% vs. 6.8%), stroke (3.3% vs. 1.4%), and hospitalization duration. However, by 1 year, there were no differences in mortality (17.3% vs. 17.2%), reoperation rates (9.1% vs. 9.9%), NYHA class, or heart failure readmissions.
Future directions
While prospective comparisons between surgical and transcatheter closure for PVL would be valuable, they are unlikely to occur in this population (many of whom are declined for surgery due to their high surgical risk). Given the poor prognosis associated with significant PVL and the inherent morbidity and mortality associated with repeat surgical intervention, transcatheter devices for PVL closure are poised to play a more important role in future treatment. Importantly, the development and testing of devices specifically dedicated to transcatheter PVL repair will be essential, particularly in light of the often irregularly and elliptically shaped defects. Improvements in hybrid imaging (including echocardiography, cardiac CT, and fluoroscopy) may also yield tangible results in terms of percutaneous PVL management.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Matiasz R, Rigolin VH. 2017 focused update for management of patients with valvular heart disease: summary of new recommendations. J Am Heart Assoc. 2018;7:e007596.
Writing Group M, Mozaffarian D, Benjamin EJ, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–360.
Vemulapalli S, Carroll JD, Mack MJ, et al. Procedural volume and outcomes for transcatheter aortic-valve replacement. N Engl J Med. 2019;380:2541–50.
Davila-Roman VG, Waggoner AD, Kennard ED, et al. Prevalence and severity of paravalvular regurgitation in the Artificial Valve Endocarditis Reduction Trial (AVERT) echocardiography study. J Am Coll Cardiol. 2004;44:1467–72.
Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian C, Rahimtoola SH. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol. 2000;36:1152–8.
Ionescu A, Fraser AG, Butchart EG. Prevalence and clinical significance of incidental paraprosthetic valvar regurgitation: a prospective study using transoesophageal echocardiography. Heart. 2003;89:1316–21.
O’Rourke DJ, Palac RT, Malenka DJ, Marrin CA, Arbuckle BE, Plehn JF. Outcome of mild periprosthetic regurgitation detected by intraoperative transesophageal echocardiography. J Am Coll Cardiol. 2001;38:163–6.
Ruiz CE, Hahn RT, Berrebi A, et al. Clinical trial principles and endpoint definitions for paravalvular leaks in surgical prosthesis: an expert statement. J Am Coll Cardiol. 2017;69:2067–87.
Eleid MF, Cabalka AK, Malouf JF, Sanon S, Hagler DJ, Rihal CS. Techniques and outcomes for the treatment of paravalvular leak. Circ Cardiovasc Interv. 2015;8:e001945.
Englberger L, Schaff HV, Jamieson WR, et al. Importance of implant technique on risk of major paravalvular leak (PVL) after St. Jude mechanical heart valve replacement: a report from the Artificial Valve Endocarditis Reduction Trial (AVERT). Eur J Cardiothorac Surg. 2005;28:838–43.
Lerakis S, Hayek SS, Douglas PS. Paravalvular aortic leak after transcatheter aortic valve replacement: current knowledge. Circulation. 2013;127:397–407.
Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2017;70:252–89.
Kliger C, Eiros R, Isasti G, et al. Review of surgical prosthetic paravalvular leaks: diagnosis and catheter-based closure. Eur Heart J. 2013;34:638–49.
Concistre G, Chiaramonti F, Bianchi G, et al. Aortic valve replacement with perceval bioprosthesis: single-center experience with 617 implants. Ann Thorac Surg. 2018;105:40–6.
Alkhouli M, Farooq A, Go RS, Balla S, Berzingi C. Cardiac prostheses-related hemolytic anemia. Clin Cardiol. 2019;42:692–700.
Nietlispach F, Maisano F, Sorajja P, Leon MB, Rihal C, Feldman T. Percutaneous paravalvular leak closure: chasing the chameleon. Eur Heart J. 2016;37:3495–502.
Dahou A, Ribeiro HB, Rodes-Cabau J, Pibarot P. Impact and management of paravalvular regurgitation after transcatheter aortic valve replacement. Interv Cardiol Clin. 2015;4:67–82.
Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366:1686–95.
Gilard M, Eltchaninoff H, Iung B, et al. Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med. 2012;366:1705–15.
Van Belle E, Juthier F, Susen S, et al. Postprocedural aortic regurgitation in balloon-expandable and self-expandable transcatheter aortic valve replacement procedures: analysis of predictors and impact on long-term mortality: insights from the FRANCE2 Registry. Circulation. 2014;129:1415–27.
Jerez-Valero M, Urena M, Webb JG, et al. Clinical impact of aortic regurgitation after transcatheter aortic valve replacement: insights into the degree and acuteness of presentation. JACC Cardiovasc Interv. 2014;7:1022–32.
Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–91.
Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;129:2440–92.
Bertrand PB, Levine RA, Isselbacher EM, Vandervoort PM. Fact or artifact in two-dimensional echocardiography: avoiding misdiagnosis and missed diagnosis. J Am Soc Echocardiogr. 2016;29:381–91.
Zoghbi WA, Asch FM, Bruce C, et al. Guidelines for the evaluation of valvular regurgitation after percutaneous valve repair or replacement: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2019;32:431–75.
Pibarot P, Hahn RT, Weissman NJ, Monaghan MJ. Assessment of paravalvular regurgitation following TAVR: a proposal of unifying grading scheme. JACC Cardiovasc Imaging. 2015;8:340–60.
Lang RM, Badano LP, Tsang W, et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr. 2012;25:3–46.
Binder RK, Webb JG, Willson AB, et al. The impact of integration of a multidetector computed tomography annulus area sizing algorithm on outcomes of transcatheter aortic valve replacement: a prospective, multicenter, controlled trial. J Am Coll Cardiol. 2013;62:431–8.
Sucha D, Symersky P, Tanis W, et al. Multimodality imaging assessment of prosthetic heart valves. Circ Cardiovasc Imaging. 2015;8:e003703.
Suh YJ, Hong GR, Han K, et al. Assessment of mitral paravalvular leakage after mitral valve replacement using cardiac computed tomography: comparison with surgical findings. Circ Cardiovasc Imaging. 2016;9.
Transcatheter management of paravalvular leaks. 2017. at https://www.acc.org/latest-in-cardiology/articles/2017/02/02/08/25/transcatheter-management-of-paravalvular-leaks. Accessed 16 Sep 2019.
Orwat S, Diller GP, Kaleschke G, et al. Aortic regurgitation severity after transcatheter aortic valve implantation is underestimated by echocardiography compared with MRI. Heart. 2014;100:1933–8.
Croft CH, Lipscomb K, Mathis K, et al. Limitations of qualitative angiographic grading in aortic or mitral regurgitation. Am J Cardiol. 1984;53:1593–8.
Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg. 2013;145:6–23.
Michel PL, Vahanian A, Besnainou F, Acar J. Value of qualitative angiographic grading in aortic regurgitation. Eur Heart J. 1987;8(Suppl C):11–4.
Zoghbi WA, Chambers JB, Dumesnil JG, et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2009;22:975–1014; quiz 82–4.
Aoyagi S, Fukunaga S, Tayama E, Nakamura E, Egawa N, Hosokawa Y. Benefits of a beta-blocker for intractable hemolysis due to paraprosthetic leakage. Asian Cardiovasc Thorac Ann. 2007;15:441–3.
Okita Y, Miki S, Kusuhara K, Ueda Y, Tahata T, Yamanaka K. Propranolol for intractable hemolysis after open heart operation. Ann Thorac Surg. 1991;52:1158–60.
Santinga JT, Flora JD, Rush JB, Penner JA, Willis PW. The effect of propranolol on hemolysis in patients with an aortic prosthetic valve. Am Heart J. 1977;93:197–201.
Hirawat S, Lichtman SM, Allen SL. Recombinant human erythropoietin use in hemolytic anemia due to prosthetic heart valves: a promising treatment. Am J Hematol. 2001;66:224–6.
Kim MS, Casserly IP, Garcia JA, Klein AJ, Salcedo EE, Carroll JD. Percutaneous transcatheter closure of prosthetic mitral paravalvular leaks: are we there yet? JACC Cardiovasc Interv. 2009;2:81–90.
Goktekin O, Vatankulu MA, Ozhan H, et al. Early experience of percutaneous paravalvular leak closure using a novel Occlutech occluder. EuroIntervention. 2016;11:1195–200.
Jelnin V, Dudiy Y, Einhorn BN, Kronzon I, Cohen HA, Ruiz CE. Clinical experience with percutaneous left ventricular transapical access for interventions in structural heart defects a safe access and secure exit. JACC Cardiovasc Interv. 2011;4:868–74.
Kliger C, Jelnin V, Sharma S, et al. CT angiography-fluoroscopy fusion imaging for percutaneous transapical access. JACC Cardiovasc Imaging. 2014;7:169–77.
Pitta SR, Cabalka AK, Rihal CS. Complications associated with left ventricular puncture. Catheter Cardiovasc Interv. 2010;76:993–7.
Eleid M. Interventional management of paravalvular leak. Heart. 2018;104:1797–802.
Waterbury TM, Reeder GS, Pislaru SV, Cabalka AK, Rihal CS, Eleid MF. Techniques and outcomes of paravalvular leak repair after transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2017;90:870–7.
Calvert PA, Northridge DB, Malik IS, et al. Percutaneous device closure of paravalvular leak: combined experience from the United Kingdom and Ireland. Circulation. 2016;134:934–44.
Ruiz CE, Jelnin V, Kronzon I, et al. Clinical outcomes in patients undergoing percutaneous closure of periprosthetic paravalvular leaks. J Am Coll Cardiol. 2011;58:2210–7.
Sorajja P, Bae R, Lesser JA, Pedersen WA. Percutaneous repair of paravalvular prosthetic regurgitation: patient selection, techniques and outcomes. Heart. 2015;101:665–73.
Sorajja P, Cabalka AK, Hagler DJ, Rihal CS. Percutaneous repair of paravalvular prosthetic regurgitation: acute and 30-day outcomes in 115 patients. Circ Cardiovasc Interv. 2011;4:314–21.
Millan X, Skaf S, Joseph L, et al. Transcatheter reduction of paravalvular leaks: a systematic review and meta-analysis. Can J Cardiol. 2015;31:260–9.
Alkhouli M, Zack CJ, Sarraf M, et al. Successful percutaneous mitral paravalvular leak closure is associated with improved midterm survival. Circ Cardiovasc Interv. 2017;10.
Sorajja P, Cabalka AK, Hagler DJ, Rihal CS. The learning curve in percutaneous repair of paravalvular prosthetic regurgitation: an analysis of 200 cases. JACC Cardiovasc Interv. 2014;7:521–9.
Akins CW, Bitondo JM, Hilgenberg AD, Vlahakes GJ, Madsen JC, MacGillivray TE. Early and late results of the surgical correction of cardiac prosthetic paravalvular leaks. J Heart Valve Dis. 2005;14:792–9; 800.
Busu T, Alqahtani F, Badhwar V, Cook CC, Rihal CS, Alkhouli M. Meta-analysis comparing transcatheter and surgical treatments of paravalvular leaks. Am J Cardiol. 2018;122:302–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Valvular Heart Disease
Rights and permissions
About this article
Cite this article
Bernard, S., Yucel, E. Paravalvular Leaks—From Diagnosis to Management. Curr Treat Options Cardio Med 21, 67 (2019). https://doi.org/10.1007/s11936-019-0776-6
Published:
DOI: https://doi.org/10.1007/s11936-019-0776-6