Opinion statement
The bicuspid aortic valve (BAV) phenotype is becoming increasingly recognized as a complex and heterogeneous clinical entity, with some but not all patients developing accelerated degrees of both aortic insufficiency (AI) and aortic stenosis (AS) in comparison to patients with tricuspid aortic valves (TAV). In addition, there remains a well-established association between the BAV phenotype and aortic enlargement independent of valve function as well as progression among some to ascending aortic aneurysm and the attendant concern over risk of aortic dissection. Because the understanding of the complexity of the BAV phenotype is evolving as quickly as are the options for medical, surgical, and interventional therapy, this review aims to provide an update on the most clinically relevant recent advances in the realm of BAV and associated aortopathy from a genetic, morphologic, and clinical outcomes perspective in order to give the practicing clinician a deeper understanding of how to approach both medical and surgical decision-making in the patient with BAV. The following major principles have emerged in recent years including (1) the importance of cusp anatomy and its implications on the long-term risk of AI, aortic dilation, and aortic dissection, (2) the role of post-valvular flow dynamics in the pathogenesis of aortic dilation in BAV patients, (3) the ability of aortic valve replacement to halt accelerated dilation rates, and (4) the finding that the risk of aortic dissection, while still overall intermediate is much more akin to the baseline risk present in TAV patients rather than the much higher rates observed in patients with Marfan’s disease. Together, these data support the less aggressive approach to aortic replacement in BAV patients as reflected in the most recent ACC/AHA guidelines and provide a stronger basis upon which future studies, including those aimed at medical and transcatheter therapies, stand to make further impact on our ability to optimally treat this epidemiologically important and complex population of patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Overview and demographics
The BAV phenotype is the most common congenital heart defect in the general population, with a prevalence estimated between 0.5 and 2% and a male to female predominance of about 3:1 [1]. Among these patients, the majority will require surgery during their lifetime to treat aortic valvular or aortic complications [2]. Accordingly, the epidemiological implications of this condition are massive. Based on the 2010 United States Census Bureau data demonstrating over 186 million people under the age of 45, given a reasonably conservative estimate of 0.50% incidence of eventual requirement for cardiac surgery for BAV-related pathology as this population ages, one can project over 930,000 procedures will be required over the next half century and beyond. Small adjustments to the thresholds for operating on BAV-associated disease will therefore have a significant absolute epidemiological impact; the authors feel that the degree of attention and controversy paid to these questions in the literature recently are accordingly appropriate to the magnitude of the problem at hand.
Genetics
Recent studies have revealed increasing complexity in the genetics that determine the BAV phenotype but the clinical relevance remains unclear. It has long been recognized that BAV can exhibit a pattern of sporadic or autosomal dominant inheritance with incomplete penetrance. Determining a single gene that is responsible, however, has proven a challenge. While there is increasing appreciation for the heterogeneous spectrum of BAV phenotypes, there appears to be an underlying heterogeneity in the genotype as well [3]. So far, two main genetic pathways involved in the normal development of TAV have been identified, namely the NOTCH signaling pathway and the nitric oxide synthase (NOS) pathway. Mutations in the NOTCH1 genes which normally encode a receptor protein expressed in endothelial cells of the cardiac outflow tract during development have been linked directly to the development of BAV in humans with a wide variety of leaflet fusion phenotypes [4]. Meanwhile, the NOS3 gene in the NOS pathway, when absent, has been shown to lead to fusion of the right and non-coronary leaflets preferentially [5]. Despite the identification of additional genes including GATA5 and NKX2.5 that have also been linked to development of BAV, there currently exist no point-of-care means to identify these mutations and interpret their significance for clinical decision-making. Additional work is necessary to establish meaningful connections between genotype and clinical risk phenotypes that would impact clinical management.
Phenotypes
The term BAV, at its most basic level, refers simply to the condition in which there are functionally two aortic valve cusps instead of the usual three. Beyond this, however, there is marked heterogeneity on several interrelated phenotypic spectra including (1) the anatomic phenotype, i.e., the specific anatomic variant of the valve including but not limited to the presence, absence, and specific position of a ridge, or raphe that joins two of the three normally existing cusps and (2) the pathophysiologic phenotype, i.e., the variable propensity of a patient with BAV to develop early aortic stenosis versus insufficiency or both and additionally, the propensity to develop associated ascending aortic aneurysmal disease either independent of or in conjunction with the actual aortic valvulopathy.
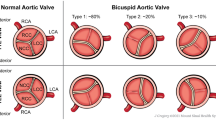
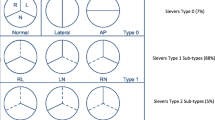
With respect to anatomic phenotype, the most common arrangement is that of two asymmetric cusps in which the larger cusp represents fusion of two smaller cusps at a raphe, while the smaller cusp has a base larger than one third the circumference of the annulus. Less commonly (7%), the valve is truly bicuspid with two nearly equal-sized cusps devoid of a raphe (Fig. 1) [7, 8]. Among BAV patients in whom a raphe is present, the most common arrangement is fusion of the left and right coronary cusps (RL fusion pattern which results in anteroposterior (AP) arrangement of the two cusps; 59% of BAV), followed by fusion of the right coronary and non-coronary cusps (RN fusion pattern which results in a lateral arrangement of the cusps; 37% of BAV) [9]. Meanwhile, the LN fusion pattern is rarely seen.
Bicuspid aortic valve types. a Type 0 phenotype with no raphe, b type 1 phenotype with a single raphe attached at the base of the conjoint cusps (pseudocommissure) with asymmetry of the aortic sinuses, and c type 2 phenotype with two raphes and asymmetry of the aortic sinuses. Used with permission [6].
Several anatomic classification systems, including those by Sabet et al. [10] and Sievers et al. [8], have been created, but their practical usage has primarily been limited to facilitating communication and research among authors, and as such their significance is more academic than clinical until recently. The goal of these classification systems has been to correlate the anatomic phenotype with pathophysiologic phenotypes. Chief among these is the finding that the AP orientation (i.e., RL cusp fusion, the most common variant) is more common among those presenting with aortic regurgitation than lateral orientation (RN or LN cusp fusion), but that the prevalence of AS was either nearly equivalent to the prevalence of AI (AP group) or significantly higher (lateral group) [11, 12]. In general, however, AS is by far more common than AI in the entire BAV population (7 vs. 15%) [10]. Mixed AI and AS is also common (22–25%), as is isolated AS (27%) [11, 13], if there is RL fusion. Among those with lateral orientation of the cusps 66 to 86% of patients have aortic stenosis as the dominant lesion even if mixed AS/AI is present [11, 13]. The most important principle gleaned from these studies is the existence of a clear link between the anatomic “sub-phenotype” and the functional phenotype in general, giving additional force to the idea that cusp fusion patterns also have bearing on the more pressing clinical problem, the relationship to aortic dilation.
Multiple surgical studies of BAV patients show that those with the RL fusion phenotype have a higher incidence of ascending aortic dilation as well as faster rates of dilation over time [5, 11, 14•, 15]. The main criticism of these studies is selection bias, but Page et al. recently reported a study of non-surgical BAV patients (i.e., those without AI or AS sufficient to warrant surgery) and showed again that the rate of aortic dilatation is higher by a striking degree among those with RL fusion compared to those with RN fusion (0.41 vs. 0.01 mm/year) [12]. Taken together, these studies suggest that the size and clinical thresholds for aortic replacement among BAV patients should perhaps take valve phenotype into consideration, with the RL phenotype considered to be higher risk not only for the presence of aortic dilation to begin with but also for faster rates of dilation on an annual basis and therefore lower the size threshold for replacing the aorta.
Medical therapy
The mainstays of medical treatment in patients with AI or AS remain unchanged in the BAV population in comparison to the TAV population. For those with AS, cautious use of angiotensin-converting enzyme inhibitors and diuretics is beneficial for those who have developed heart failure symptoms while awaiting definitive intervention for the diseased valve by reducing afterload. Care must be taken to not reduce preload too acutely in the setting of the diastolic dysfunction that so often accompanies the left ventricular hypertrophy present in patients with chronic significant AS [16]. Meanwhile, beta-blockers are helpful in patients with angina related to AS by decreasing myocardial oxygen demand and wall stress and sub-endocardial ischemia. For those with severe AI, the ACC/AHA guidelines recommend the use of vasodilators for most patients with symptomatic severe left ventricular dysfunction; reduction of afterload using vasodilators is indicated to improve symptoms either chronically or while awaiting surgery in those who are reasonable operative candidates [16]. There remains no evidence that these guidelines should be any different in patients with BAV. Furthermore, it remains unknown whether any medical therapy can slow the progression of AI or AS development in BAV patients.
The more interesting question that has been studied of late is whether or not medical therapy can slow the progression of aortic aneurysmal disease in BAV patients. Three classes of drugs have been studied primarily, including statins, beta-blockers, and ACE-Is/angiotensin receptor blockers (ARBs). There is mounting evidence that increased expression of matrix metalloproteinases (namely MMP-2 and MMP-9) is implicated in the pathogenesis of aortic aneurysmal disease in patients with BAV [17, 18]. Statins may reduce the expression of MMPs [19] and have been shown to reduce aneurysmal dilation rates in animal models [20]. Several retrospective studies have also suggested reduced aortic diameters in BAV patients presenting for surgery for AI or AS that have been on a preoperative statin [21,22,23]. Further work, namely a randomized controlled trial, would be useful in demonstrating a clear causative effect.
Extrapolating from the experience with Marfan’s disease, the effect of beta-blockers and ACE-Is/ARBs in BAV patients has been explored. The beta blockers and ARBs in bicuspid aortic valve (BAV) disease aortopathy study is a Canadian multi-institutional randomized controlled trial of atenolol and telmisartan that has recently completed data collection for the primary endpoint of slowing aortic dilation (ClinicalTrials.gov number, NCT01202721). The results will have implications for the routine administration of these drugs to those with pre-surgical BAV, and whether a randomized controlled trial of medical versus surgical treatment would be best-advised in those with moderately dilated ascending aortas (e.g., 40–50 mm).
For patients with BAV, the lifetime risk of developing infective endocarditis is approximately 2–3% [24]. Historically, antibiotic prophylaxis against infective endocarditis had been recommended for patients with predisposing cardiac conditions (including cyanotic congenital heart disease and BAV disease) to decrease this incidence. However, based in part on more recent studies demonstrating that an extremely large number of patients would require antibiotic prophylaxis to prevent a very small number of cases of endocarditis [25], the AHA, in a significant departure from prior guidelines, recommended in 2007 that antibiotic prophylaxis is only indicated at the time of dental procedures if (1) the planned dental procedure involves perforation of the dental mucosa or gingival/periapical tissues and (2) the predisposing cardiac condition is one of several that confer the highest risk of lifetime development of infective endocarditis. This list includes unrepaired cyanotic congenital heart disease, corrected congenital heart disease involving prosthetic materials or devices, cardiac transplant recipients that develop valvulopathy, and those with a personal history of native or prosthetic valve infective endocarditis, but excludes more common conditions such as mitral valve prolapse and BAV [24].
Bicuspid aortic valve-associated aortopathy
Although the association between the BAV phenotype and dissection has been appreciated since the late 1970s when Edwards et al. reported the significantly higher prevalence of BAV in autopsy specimens of those who had succumbed to this condition [26], four decades later, there remains significant controversy over which individual BAV patients should receive an aortic replacement. In light of the evidence that those with the BAV phenotype have a high prevalence of aortic aneurysmal dilation (ranging from 56 to 88% depending on patient age and study demographics) [27, 28••] and that the rate of growth of these aneurysms is accelerated in comparison to tricuspid aortic valve (TAV) patients [1, 29], an aggressive approach to surgical prophylaxis against aortic dissection for smaller aortic diameters (less than the traditional 5.5-cm threshold) was espoused by many groups. Fueled in part by a study showing that 12.5% of patients with BAV who developed type A aortic dissections had aortic diameters smaller than 5.0 cm [30], the 2010 ACC/AHA guidelines issued a class I recommendation (level of evidence C) to perform isolated elective replacement of the ascending aorta and/or root for maximal diameters of between 4.0 and 5.0 cm depending on the clinical condition even in patients not otherwise requiring an aortic valve replacement (AVR) [31].
Subsequently, however, there has been increasing evidence to challenge this position. Despite general agreement that there is an inherent abnormality in the ascending aortic wall of the BAV patient, several studies have recently shown that the mean diameter of the ascending aorta or root in patients who present with dissection may actually be higher in BAV patients than in TAV patients [32, 33], suggesting that the abnormal biologic properties of the aorta in BAV that lead to aneurysmal formation do not necessarily confer a greater risk of aortic dissection in comparison to TAV patients for a given aortic diameter. These and other data have led some to recommend a more conservative approach recognizing that the actual overall survival impact of replacing a moderately dilated ascending aorta in BAV patients is unknown given the inherent practical difficulty in defining the true risk of aortic dissection without surgery in this population [34, 35•]. The AHA/ACC guidelines for valvular heart disease were updated in 2014 [36] and further clarified in 2016 [37], recommending that the threshold for replacement of the ascending aorta and/or root should be replaced when the maximum diameter is 5.5 cm or greater (class I, LOE B) and 4.5 cm if the patient is already undergoing AVR (class IIa, LOE C), in essence equivalent to those with a TAV.
Although patients with BAV do have faster rates of aortic dilation than those with TAV and in general, the size of the aorta is related to the risk of dissection in all-comers and especially those with Marfan’s disease, it has been an assumption that BAV patients therefore have higher rates of dissection for a given aortic diameter compared to TAV patients. In a study by Girdauskas et al., the outcomes of BAV patients were compared to TAV patients undergoing surgery for aortic valvulopathy over a total of 3566 patient-years and found only three dissections, all of which occurred in the TAV group, with a rate of reoperation that was paradoxically lower in the BAV group than in the TAV group [38]. One could argue that a more aggressive approach to replacing aortas of moderate diameters in retrospective studies such as this could have accounted for the unexpected difference seen here, but this was a study of patients who had isolated AVR performed in the setting of moderate aortic dilation (4.0 to 5.5 cm). Several recent studies have also shown that the rates of aortic dilation for BAV after AVR return to the very low baseline rates seen in TAV patients after AVR [39, 40•], suggesting that repairing the aortic valve somehow stabilizes the rate of aortic dilation by altering its flow dynamics. These clinical observations come on the heels of a series of studies using 4D flow MRI demonstrating that the flow dynamics in the proximal aorta are significantly altered in the setting of BAV such that for patients with RL fusion, there is a marked increase in eccentric flow and wall stress directed at the greater curve of the proximal ascending aorta, while the RN fusion phenotype experiences marked increases in eccentric flow near the proximal arch, in contrast to the uniform flow dynamics and wall stress in the aortas of the TAV phenotype (Fig. 2) [41,42,43]. Animal studies have shown that BAV-mediated aberrancies in wall stress create adverse aortic wall remodeling at the cellular and molecular level including increases in MMP-2 activity [44, 45]. Taken together, these studies endorse a multi-factorial pathogenesis of aortopathy in BAV patients including the altered flow dynamics seen in BAV in addition to the underlying genetics as the impetus for the development of the changes in the aortic wall remodeling seen in BAV associated with the development of aneurysms. Elimination of these altered flow dynamics after AVR may be responsible for the post-operative reduction in the rate of aortic dilation seen in clinical series.
The flow dynamics associated with a the RL fusion pattern create greater shear stress on the greater curve of the ascending aorta leading to aneurysmal formation that favors the ascending aorta, while those of b the RN fusion pattern lead to posterior directed jets that favor dilation of the proximal arch. Used with permission [28••].
What about BAV patients in whom AVR is not warranted nor performed? Even in the absence of aortic valvulopathy requiring AVR (i.e., not correcting for the abnormal hemodynamics that might exist in the BAV population), Davies et al. found that the medium-term risk (5 years) of dissection, rupture, and death was actually lower in the BAV population despite faster baseline growth rates of the ascending aorta in BAV compared to TAV patients [46]. This may, as the authors acquiesce, be related to an overall younger population with less comorbidities, but a meta-analysis of these and similar studies showed that overall risk of aortic dissection and overall aortic events in BAV is low [47], and on the order of 0.03 [48] to 0.1% [49] per patient year, i.e., on par with rates experienced by TAV patients.
Given the genetic contribution to BAV-associated aortic aneurysms, they were once likened more to that of the Marfan’s patient. However, Itakagi et al. reported in a direct comparison of Marfan’s and BAV patients and non-Marfan’s non-BAV controls undergoing AVR without aortic replacement that in long-term follow-up (15 years), Marfan’s patients were significantly more likely to develop thoracic aortic dissections than BAV patients and control patients (5.5 vs. 0.55 and 0.41%, respectively) [50••]. They were also more likely than both of these groups to develop thoracic aortic aneurysms and undergo reoperation for aneurysmal disease [50••]. These findings support the view that the biology and long-term clinical risk of thoracic aortic aneurysms and dissections in BAV patients, although unquestionably in part due to an inherent abnormality of the aorta, are intermediate in behavior compared to TAV and Marfan’s and are likely much more similar to the former than the latter. Accordingly, the recent enthusiasm for aggressively replacing moderately dilated aortas in patients with BAV has been criticized. An aggressive approach without clear benefit may unnecessarily expose patients with moderately dilated aortas to increased risk, which according to a recent Society of Thoracic Surgeons database review estimates the unadjusted mortality risk of elective aortic replacement to be 3.4% even at high-volume centers [51].
Finally, what about the patient who presents with BAV-associated valvulopathy requiring AVR in whom the proximal ascending aorta is dilated to greater than 5.5 cm but in whom the aortic root and/or the transverse arch are only moderately dilated? What is the fate of the root and the transverse arch if left unreplaced? Is the added incremental risk of replacing the root and/or transverse arch in these situations justifiable? Several recent studies have shed light on these questions which by and large are not specifically addressed in even the most recent guidelines from the ACC/AHA [52]. Park, et al. found in a study of 218 BAV patients undergoing AVR and ascending aortic replacement without root replacement at a median follow-up period of 17 years only one patient that underwent late reoperation for replacement of a significantly dilated aortic root, and no patients that developed aortic dissection [53]. Unfortunately, the baseline diameter of the aortic root is unknown in this cohort of patients, but Regeer, et al. recently performed a similar study in which they specifically measured rates of aortic root dilation with serial echocardiograms in patients receiving AVR for BAV disease (but without aortic replacement of any kind) and found that paradoxically, there was actually a trend towards faster rates of root dilation in TAV patients (0.15 vs. −0.01 mm/year, p = 0.096) [40]. Taken together, there is a strong suggestion that aortic roots that are not expressly dilated beyond the well-established threshold of 5.5 cm for general indications unrelated to BAV or connective tissue disease should in most instances be left unreplaced regardless of whether the ascending aorta requires replacement for the same indication. The same appears to hold true for the arch as well. Chan et al. demonstrated in a separate study that in BAV patients undergoing surgical aortic valve intervention (repair or replacement) with either aortic root or ascending replacement and sparing of the proximal arch, progressive dilation of the proximal arch was uncommon after surgery, with a mean preoperative arch diameter of 33.3 mm preoperatively (with 18% of patients having arch diameters of over 40 mm) and 31.9 mm post-operatively at a mean follow-up of 4 years [54].
In summary, the thoracic aorta in patients with BAV behaves much more like those in patients with TAV than with Marfan’s disease, and the return to a more conservative approach to replacement is appropriate in our view, i.e., a threshold size of 5.5 cm for patients not requiring AVR (5.0 cm if there is a family history of aortic dissection, the growth rate is >0.5 cm/year, or if the patient is low-risk and the procedure can be performed at a center of expertise) and 4.5 cm for patients undergoing AVR (Table 1). For those undergoing AVR without ascending aortic replacement, the AVR itself appears to exert a protective effect on subsequent aortic dilation, perhaps by virtue of correcting the altered flow dynamics associated with cusp fusion that played a role in creating the aortic dilation in the first place. For those undergoing AVR with a clear indication to replace a segment of the thoracic aorta (usually the ascending), the remaining segments can likely be left safely unreplaced (namely the root and proximal arch) even if moderately dilated. For BAV patients who are asymptomatic and do not require AVR but in whom the aorta is moderately dilated, traditional criteria for lowering the size threshold to 5.0 cm for aortic replacement (family history, accelerated growth rates of >5 mm/year) are also reasonable. Further studies may validate the addition of the RL fusion phenotype to this list of reasons to consider slightly earlier aortic replacement, since this phenotype is associated with accelerated rates of growth, especially in the setting of a non-replaced aortic valve.
Transcatheter aortic valve replacement
Transcatheter aortic valve replacement (TAVR) has recently been validated as non-inferior for patients who are deemed high risk for surgical AVR (inoperable patients or those with an estimated operative mortality risk greater than 8%) [55], an indication which more recently has even expanded to include moderate risk patients for whom the estimated operative mortality is 4–8% [56]. These large randomized trials excluded patients with BAV over concerns of valve seating given the results of early small studies of TAVR in this population using early generation devices showing relatively high rates of paravalvular leaks on the order of 25–28% (as defined by moderate or greater AI) [57,58,59]. More recently, the Edwards SAPIEN 3 transcatheter aortic valve prosthesis, which incorporates an outer fabric seal designed to adapt to and interface better with irregular annular shapes and dimensions, was found in a multi-institutional study of 51 patients to decrease the mean aortic gradient from 49 to 11 mmHg with a rate of post-procedural mild AI of 37%, and a 0% rate of moderate or severe AI [60]. The long-term development of mild leaks into more severe leaks, a phenomenon noted in similar studies [60, 61] and their impact on overall morbidity and mortality, remains unknown. The impact of these modifications on the applicability of the technology to BAV remains as yet unknown. Several studies have shown that the RL fusion phenotype with the presence of a raphe had better outcomes than in those without a raphe and than in those with RN fusion phenotypes, but the mechanistic reasons for this also remain unclear 59, 60].
As the indications for TAVR expand, it remains to be seen if the stabilization of aortic dilation seen in AVR patients with both normal-sized and moderately dilated aortas is also exhibited after TAVR. In other words, if TAVR is demonstrated to impart the same benefit in this regard, it would add strength to the contention that the abnormally elevated eccentric flow dynamics and wall stress of the ascending aorta in BAV disease are primary determinants of aortic dilation and as a corollary, increase the level of comfort in patient, cardiologist, and surgeon in leaving a moderately dilated aorta alone after addressing the aortic valve. Further studies in this regard may become feasible as the role of TAVR expands.
Conclusions
BAV disease is a complex entity for which sound medical and surgical decision-making relies on an understanding of the complex relationship that exists between the BAV phenotype and the long-term risk of not only the valvulopathy itself (AS and AI) but also the associated aortopathy and the question of what the true risk of aortic dissection is at a given aortic diameter. Although the trend has been towards a less aggressive approach towards replacing the aorta in BAV patients as borne out in the most recent guidelines, future research aimed at identifying factors other than just the absolute aortic diameter will be important in identifying which patients with moderately dilated at aortas are at significantly higher risk of early dissection and therefore mortality.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol. 2010;55(25):2789–800.
Ward C. Clinical significance of the bicuspid aortic valve. Heart. 2000;83(1):81–5.
Longobardo L, Jain R, Carerj S, Zito C, Khandheria BK. Bicuspid aortic valve: unlocking the morphogenetic puzzle. Am J Med. 2016;129(8):796–805.
Koenig SN, Bosse K, Majumdar U, Bonachea EM, Radtke F, Garg V. Endothelial Notch1 is required for proper development of the semilunar valves and cardiac outflow tract. J Am Heart Assoc. 2016;5(4).
Fernandez B, Duran AC, Fernandez-Gallego T, et al. Bicuspid aortic valves with different spatial orientations of the leaflets are distinct etiological entities. J Am Coll Cardiol. 2009;54(24):2312–8.
Prodromo J, D’Ancona G, Amaducci A, Pilato M. Aortic valve repair for aortic insufficiency: a review. J Cardiothorac Vasc Anesth. 2012;26(5):923–32.
Mathieu P, Bosse Y, Huggins GS, et al. The pathology and pathobiology of bicuspid aortic valve: state of the art and novel research perspectives. J Pathol Clin Res. 2015;1(4):195–206.
Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg. 2007;133(5):1226–33.
Friedman T, Mani A, Elefteriades JA. Bicuspid aortic valve: clinical approach and scientific review of a common clinical entity. Expert Rev Cardiovasc Ther. 2008;6(2):235–48.
Sabet HY, Edwards WD, Tazelaar HD, Daly RC. Congenitally bicuspid aortic valves: a surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2715 additional cases. Mayo Clin Proc. 1999;74(1):14–26.
Shin HJ, Shin JK, Chee HK, Kim JS, Ko SM. Characteristics of aortic valve dysfunction and ascending aorta dimensions according to bicuspid aortic valve morphology. Eur Radiol. 2015;25(7):2103–14.
Page M, Mongeon FP, Stevens LM, Souliere V, Khairy P, El-Hamamsy I. Aortic dilation rates in patients with biscuspid aortic valve: correlations with cusp fusion phenotype. J Heart Valve Dis. 2014;23(4):450–7.
Kim JS, Ko SM, Chee HK, Shin JK, Song MG, Shin HJ. Relationship between bicuspid aortic valve phenotype, valvular function, and ascending aortic dimensions. J Heart Valve Dis. 2014;23(4):406–13.
• Khoo C, Cheung C, Jue J. Patterns of aortic dilatation in bicuspid aortic valve-associated aortopathy. J Am Soc Echocardiogr: Off Publ Am Soc Echocardiogr. 2013;26(6):600–5. This study demonstrates that the RL cusp fusion pattern is associated with faster rates of aortic root and ascending aortic dilation.
Thanassoulis G, Yip JW, Filion K, et al. Retrospective study to identify predictors of the presence and rapid progression of aortic dilatation in patients with bicuspid aortic valves. Nat Clin Pract Cardiovasc Med. 2008;5(12):821–8.
Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(22):2438–88.
Nataatmadja M, West M, West J, et al. Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in Marfan syndrome and bicuspid aortic valve thoracic aortic aneurysm. Circulation. 2003;108(Suppl 1):II329–34.
Fedak PW, de Sa MP, Verma S, et al. Vascular matrix remodeling in patients with bicuspid aortic valve malformations: implications for aortic dilatation. J Thorac Cardiovasc Surg. 2003;126(3):797–806.
Schmoker JD, McPartland KJ, Fellinger EK, et al. Matrix metalloproteinase and tissue inhibitor expression in atherosclerotic and nonatherosclerotic thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 2007;133(1):155–61.
Shiraya S, Miyake T, Aoki M, et al. Inhibition of development of experimental aortic abdominal aneurysm in rat model by atorvastatin through inhibition of macrophage migration. Atherosclerosis. 2009;202(1):34–40.
Taylor AP, Yadlapati A, Andrei AC, et al. Statin use and aneurysm risk in patients with bicuspid aortic valve disease. Clin Cardiol. 2016;39(1):41–7.
Goel SS, Tuzcu EM, Agarwal S, et al. Comparison of ascending aortic size in patients with severe bicuspid aortic valve stenosis treated with versus without a statin drug. Am J Cardiol. 2011;108(10):1458–62.
Yuan SM, Jing H, Lavee J. The bicuspid aortic valve and its relation to aortic dilation. Clinics. 2010;65(5):497–505.
Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736–54.
Duval X, Alla F, Hoen B, et al. Estimated risk of endocarditis in adults with predisposing cardiac conditions undergoing dental procedures with or without antibiotic prophylaxis. Clin Inf Dis: Off Publ Inf Dis Soc Am. Jun 15 2006;42(12):e102–e107.
Edwards WD, Leaf DS, Edwards JE. Dissecting aortic aneurysm associated with congenital bicuspid aortic valve. Circulation. 1978;57(5):1022–5.
Della Corte A, Bancone C, Quarto C, et al. Predictors of ascending aortic dilatation with bicuspid aortic valve: a wide spectrum of disease expression. Eur J Cardiothorac Surg: Off J Eur Assoc Cardiothor Surg. 2007;31(3):397–404. discussion 404–395
•• Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. The New England Journal of Medicine. 2014;370(20):1920–9. An excellent review of BAV that describes cogently the link between post-valvular flow dynamics and their effect on patterns of aortic dilation as well the associated changes in the microstructural composition of the aortic wall in BAV patients.
Ferencik M, Pape LA. Changes in size of ascending aorta and aortic valve function with time in patients with congenitally bicuspid aortic valves. Am J Cardiol. 2003;92(1):43–46.
Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection in patients with bicuspid aortic valves. J Thorac Cardiovasc Surg. 2003;126(3):892–3.
Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121(13):e266–369.
Eleid MF, Forde I, Edwards WD, et al. Type A aortic dissection in patients with bicuspid aortic valves: clinical and pathological comparison with tricuspid aortic valves. Heart. 2013;99(22):1668–74.
Etz CD, von Aspern K, Hoyer A, et al. Acute type A aortic dissection: characteristics and outcomes comparing patients with bicuspid versus tricuspid aortic valve. Eur J Cardiothorac Surg: Off J Eur Assoc Cardiothorac Surg. 2015;48(1):142–50.
Wasfy JH, Armstrong K, Milford CE, Sundt TM. Bicuspid aortic disease and decision making under uncertainty—the limitations of clinical guidelines. Int J Cardiol. 2015;181:169–71.
• Sundt TM. Aortic replacement in the setting of bicuspid aortic valve: how big? How much? The Journal of Thoracic and Cardiovascular Surgery. 2015;149(2 Suppl):S6–9. A short editorial that describes the concept of “denominator neglect” and the associated pitfalls inherent in assuming that just because some BAV patients present with aortic dissection at moderate diameters, all patients with moderately dilated aortas should be replaced.
Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2014;148(1):e1–e132.
ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the management of patients with thoracic aortic disease representative M, Hiratzka LF, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2016;133(7):680–6.
Girdauskas E, Disha K, Borger MA, Kuntze T. Long-term prognosis of ascending aortic aneurysm after aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Thorac Cardiovasc Surg. 2014;147(1):276–82.
Charitos EI, Stierle U, Petersen M, et al. The fate of the bicuspid valve aortopathy after aortic valve replacement. Eur J Cardiothorac Surg: Off J Eur Assoc Cardiothorac Surg. May 2014;45(5):e128–35.
• Regeer MV, Versteegh MI, Klautz RJ, et al. Effect of aortic valve replacement on aortic root dilatation rate in patients with bicuspid and tricuspid aortic valves. Ann Thorac Surg. 2016. This study demonstrates the ability of AVR to stabilize the rate of aortic dilation in BAV patients to that seen in the general population, suggesting that the correction of flow dynamics accompanying AVR may be responsible for this effect.
Mahadevia R, Barker AJ, Schnell S, et al. Bicuspid aortic cusp fusion morphology alters aortic three-dimensional outflow patterns, wall shear stress, and expression of aortopathy. Circulation. 2014;129(6):673–82.
Barker AJ, Markl M, Burk J, et al. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging. 2012;5(4):457–66.
Fedak PW, Barker AJ, Verma S. Year in review: bicuspid aortopathy. Curr Opin Cardiol. 2016;31(2):132–8.
Atkins SK, Cao K, Rajamannan NM, Sucosky P. Bicuspid aortic valve hemodynamics induces abnormal medial remodeling in the convexity of porcine ascending aortas. Biomech Model Mechanobiol. 2014;13(6):1209–25.
Atkins SK, Sucosky P. Etiology of bicuspid aortic valve disease: focus on hemodynamics. World J Cardiol. 2014;6(12):1227–33.
Davies RR, Kaple RK, Mandapati D, et al. Natural history of ascending aortic aneurysms in the setting of an unreplaced bicuspid aortic valve. Ann Thorac Surg. 2007;83(4):1338–44.
Hardikar AA, Marwick TH. Surgical thresholds for bicuspid aortic valve associated aortopathy. JACC Cardiovasc Imaging. 2013;6(12):1311–20.
Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306(10):1104–12.
Tzemos N, Therrien J, Yip J, et al. Outcomes in adults with bicuspid aortic valves. JAMA. 2008;300(11):1317–25.
•• Itagaki S, Chikwe JP, Chiang YP, Egorova NN, Adams DH. Long-term risk for aortic complications after aortic valve replacement in patients with bicuspid aortic valve versus Marfan syndrome. J Am Coll Cardiol. 2015;65(22):2363–9. A timely clinical study that examines the post-AVR natural history of aortopathy in patients with BAV, TAV, and Marfan’s syndrome and demonstrates a significantly higher rate of aortic complications in Marfan’s patients compared to both other groups. This argues strongly in favor of an approach to aortic replacement in BAV patients that does not simply extrapolate from Marfan’s disease.
Hughes GC, Zhao Y, Rankin JS, et al. Effects of institutional volumes on operative outcomes for aortic root replacement in North America. J Thorac Cardiovasc Surg. 2013;145(1):166–70.
Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;67(6):724–31.
Park CB, Greason KL, Suri RM, Michelena HI, Schaff HV, Sundt TM 3rd. Fate of nonreplaced sinuses of Valsalva in bicuspid aortic valve disease. J Thorac Cardiovasc Surg. 2011;142(2):278–84.
Park CB, Greason KL, Suri RM, Michelena HI, Schaff HV, Sundt TM 3rd. Should the proximal arch be routinely replaced in patients with bicuspid aortic valve disease and ascending aortic aneurysm? J Thorac Cardiovasc Surg. 2011;142(3):602–7.
Mack MJ, Leon MB, Smith CR, et al. 5-Year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385(9986):2477–84.
Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. The New England Journal of Medicine. 2016;374(17):1609–20.
Bauer T, Linke A, Sievert H, et al. Comparison of the effectiveness of transcatheter aortic valve implantation in patients with stenotic bicuspid versus tricuspid aortic valves (from the German TAVI Registry). The American Journal of Cardiology. 2014;113(3):518–21.
Zhao ZG, Jilaihawi H, Feng Y, Chen M. Transcatheter aortic valve implantation in bicuspid anatomy. Nat Rev Cardiol. 2015;12(2):123–8.
Mylotte D, Lefevre T, Sondergaard L, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. Journal of the American College of Cardiology. 2014;64(22):2330–9.
Perlman GY, Blanke P, Dvir D, et al. Bicuspid aortic valve stenosis: favorable early outcomes with a next-generation transcatheter heart valve in a multicenter study. JACC. Cardiovascular Interventions. 2016;9(8):817–24.
Yousef A, Simard T, Webb J, et al. Transcatheter aortic valve implantation in patients with bicuspid aortic valve: a patient level multi-center analysis. Int J Cardiol. 2015;189:282–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Valvular Heart Disease
Rights and permissions
About this article
Cite this article
Kwon, M.H., Sundt, T.M. Bicuspid Aortic Valvulopathy and Associated Aortopathy: a Review of Contemporary Studies Relevant to Clinical Decision-Making. Curr Treat Options Cardio Med 19, 68 (2017). https://doi.org/10.1007/s11936-017-0569-8
Published:
DOI: https://doi.org/10.1007/s11936-017-0569-8