Opinion statement
The heart has historically been considered to be a non-regenerative organ. Recent insights have suggested that cardiomyocytes have a small but measurable ability to regenerate. Moreover, recent work has also shown that manipulating the expression of specific genetic pathways can improve the ability of the heart to repair itself. These new insights set the stage for the development of new treatments for heart failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
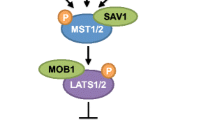
Initially defined in Drosophila melanogaster, the Hippo signaling pathway controls organ size by regulating cellular proliferation, apoptosis, and cell fate decisions. The major components of the Hippo signaling pathway are evolutionally conserved. The core components of the pathway are serine threonine kinases that function in a phosphorylation cascade. In mice, Mst1/2 kinases (orthologous to Drosophila Ste20-like kinase Hippo, Hpo) form a complex with the adaptor protein Salvador family WW domain-containing protein 1 (Salv) to phosphorylate Lats 1/2 kinases (orthologous to Drosophila warts). In turn, Lats 1/2 kinases then phosphorylate the Hippo signaling pathway effectors Yap (orthologous to Drosophila Yki) and Taz (also known as Wwtr1), which inhibits them from shuttling into the nucleus and promotes their 14-3-3 binding and cytoplasmic degradation. In the absence of Hippo-mediated repression, Yap/Taz move into the nucleus and form a nuclear complex with partner transcription factors to regulate their downstream target genes. In most studies, Yap/Taz promote cell survival, proliferation, and tissue growth, whereas Hippo signaling inhibits cell proliferation and promotes apoptosis primarily by inhibiting Yap/Taz [1, 2••, 3•, 4•, 5].
Previous findings have shown that Yap and Taz are also involved in the nuclear transduction of mechanical and cytoskeletal signals [6–9]. Yap localization is affected by the rigidity of the extracellular matrix, a key element of architectural signals that informs cell decisions. When cells were cultured on a stiff substrate, filamentous actin (F-actin) tended to be polymerized and opposed Yap phosphorylation through Lats-dependent and independent pathways [6–9].Yap activation may form a positive feedback loop to enhance actomyosin contractility by upregulating myosin regulatory light chain (MRLC) expression in cancer-associated fibroblasts [10].
The Hippo signaling pathway functions as a central mediator of key signals during both normal and pathologic development in multiple tissues and organs. For example, Hippo signaling is involved in cross-talk with Wnt signaling, transforming growth factor-beta (TGF-β) signaling, and G protein-coupled receptor (GPCR) signaling. The many important functions of the Hippo signaling pathway therefore make it crucial to understand the underlying mechanisms that mediate Hippo function in development and regeneration. Although mammalian hearts do not have the same regenerative capacity as those of amphibians and fish [11, 12], multiple studies have indicated that mammalian hearts have a limited capacity to regenerate. However, their regenerative ability is insufficient for promoting repair after cardiac injury [13–16]. We have recently shown that the deletion of Hippo kinase Salv or Lats1/2 in mouse cardiomyocytes increases the proliferation of adult cardiomyocytes and extends the regenerative window [2••]. Other groups have shown that Yap gain of function is critical for promoting mouse heart regeneration [3•, 4•, 17••]. However, the molecular mechanisms underlying the Hippo signaling pathway in cardiac regeneration remain poorly understood and require further investigation.
Hippo signaling in cardiac development
Unlike in the adult heart, cardiomyocytes actively proliferate in the developing mammalian heart. Recent studies indicate that the mouse heart retains its regenerative capacity until postnatal day 7 after cardiac apex resection. In the newly formed cardiac apex, lineage tracing studies showed that cardiomyocytes in the regenerated apex were derived from pre-existing cardiomyocytes [18••]. This exciting finding connects heart developmental studies with cardiac regeneration and supports the idea that cell cycle withdrawal prevents repair of the adult mammalian heart after injury.
In mouse embryonic hearts, Heallen et al. reported that the cardiac-restricted knockout of the Hippo components Mst1/2, Lats2, or Salv led to cardiomegaly with increased proliferation of cardiomyocytes [2••]. In addition, the role of the downstream effector of the Hippo pathway Yap has also been characterized in fetal and neonatal hearts. Yap is active during development, but its activity dramatically decreases with age [3•]. The cardiac-restricted inactivation of Yap during development resulted in embryonic death and the presence of a thin myocardium. However, overexpressing Yap in fetal cardiomyocytes promoted their proliferation, which is consistent with the report by Heallen et al. in which the loss of Hippo kinase increased cardiomyocyte proliferation [4•]. Interestingly, inactivating the upstream kinase of the Hippo pathway or overexpressing the active form of Yap (YapS127A) promoted proliferation in postnatal cardiomyocytes, which typically have exited the cell cycle [3•, 19]. Moreover, Yap does not appear to be involved with physiologic hypertrophy or increased cell size after birth [3•].
In fetal and neonatal hearts, some molecular mechanisms of Hippo/Yap-controlled cardiomyocyte proliferation have been identified. Since Yap is a transcriptional co-factor, its activity requires another DNA-binding transcription factor. Tead factors are known Yap DNA-binding partners [20]. The disruption of the Tead1-Yap1 interaction during cardiac development was shown to attenuate cardiomyocyte proliferation and the expression of positive cell cycle regulators, such as Cdk1 and Cyclin A2 [3•].
In addition, Wnt/β-catenin signaling has been shown to be essential in Hippo/Yap-regulated cardiac overgrowth. Transcriptional profiling of Salv conditional knockout or Yap gain-of-function hearts showed that canonical Wnt signaling was elevated [2••, 4•]. In Hippo-deficient hearts, the loss of Wnt/β-catenin function rescued the phenotypes of cardiomegaly, trabecular expansion, and increased cardiomyocyte proliferation. On the basis of these findings, Heallen et al. [2••] proposed a model of Hippo-Wnt interaction in heart development in which Yap and β-catenin form a complex in the nucleus and act through each respective DNA-binding partner, Tead and Tcf/Lef, to activate downstream genes. Therefore, inhibiting Hippo kinase or activating Yap could potentially enhance the Hippo-Wnt interaction to promote cell proliferation and heart overgrowth.
Insulin-like growth factor (IGF) signaling has also been proposed to mediate the Hippo-Wnt interaction [4•]. Yap activates the IGF-phosphoinositide 3-kinase (PI3K)-Akt pathway and promotes the phosphorylation of Akt and glycogen synthase kinase-3 beta (GSK-3β). Phosphorylated GSK-3β is rendered inactive, and its increased levels enhance the stability of β-catenin, which is necessary for Yap-induced cardiomyocyte proliferation. In this interaction model, Yap transcriptional activity is required for activating β-catenin, which adds a layer of complexity to the Hippo-Wnt interaction in promoting cardiomyocyte proliferation during development.
The studies described above strongly link Hippo signaling and Yap to cardiomyocyte proliferation. Hippo signaling was shown to prevent cardiac overgrowth and continuous postnatal cardiomyocyte proliferation, suggesting the therapeutic promise of manipulating Hippo signaling and Yap to drive postnatal cardiomyocytes to re-enter the cell cycle. In the second part of this review, we will discuss how these developmental findings may be applied to studies of cardiac regeneration.
Hippo signaling in cardiac regeneration
Hippo in adult cardiomyocyte regeneration
Adult cardiomyocytes have a limited capacity to proliferate, preventing the heart from repairing itself. A nexus for cell survival and proliferation, the Hippo pathway is of primary interest to investigators in the cardiac regeneration field as a means to activate the proliferation of cardiomyocytes and their integration into the region of injury. Hippo signaling attenuation or Yap activation provides a unique target for promoting cardiac regeneration in adult hearts. A growing body of evidence supports the idea that the Hippo signaling pathway is a critical barrier to cardiac regeneration.
Whereas initial studies focused on the detrimental effects of Hippo signaling or Yap loss of function, subsequent reports have described the beneficial effects of attenuating upstream Hippo signaling or activating Yap activity in the damaged heart. Early reports indicated that the activation of Mst1 or Lats2 promoted apoptosis and cardiac dysfunction [21–23]. Conversely, the inhibition of Mst1 provided beneficial effects after myocardial infarction (MI), presumably through the downregulation of pro-inflammatory cytokines [22]. Furthermore, Yap was shown to be critical for adult cardiomyocyte homeostasis in studies showing that Yap loss of function exacerbated functional decline and scar formation after chronic MI [24]. Moreover, it was shown that active Yap reduced cardiac scar formation and improved heart function in chronic MI [17••]. It is conceivable that Yap acts to promote the proliferation of adult cardiomyocytes through the intersection of Wnt and IGF signaling, as seen in the embryonic heart [2••, 4•, 17••, 25]. In addition, loss of function of upstream Hippo pathway signaling provided similar beneficial effects after chronic MI [19], whereby the genetic knockout of Salv and Lats1/2 enhanced adult cardiomyocyte proliferation and improved cardiac function after MI. Recently, Tian et al. [26] showed that the microRNA cluster miR302-367 represses the major Hippo signaling components Mob1b, Mst1, and Lats2. In miR302-367 conditional knockout mouse hearts, Yap becomes active following the suppression of upstream Hippo signaling, improving heart function after MI via increased cardiomyocyte proliferation. These studies support the idea that adult cardiomyocytes can be driven through the cell cycle to promote the regeneration of lost cardiac tissue.
Although the studies discussed above have been groundbreaking, key questions still remain. For example, is scar tissue formed and, if so, how is the scar tissue removed in the various genetic models? Additionally, how do “new” cardiomyocytes repopulate the scar zone, and what downstream genetic pathways are responsible for the improvement in cardiac function? These questions are difficult to answer with in vivo studies. Nonetheless, recent studies have provided new insights.
In a study by Lin et al., the activation of Yap via the viral delivery of a Yap gain-of-function allele into adult mouse hearts was examined as a feasible therapeutic strategy for improving outcome after MI [27]. Based on the results of their gene profiling experiments, Lin et al. proposed an explanation for Yap-activated regeneration; they hypothesized that Yap induced cardiomyocyte-autonomous proliferation and adaptive metabolic changes. In addition, Yap alters the inflammatory response within the heart that may mediate a non-autonomous effect to promote regeneration. Although a variety of chemokines are involved in inflammation, it remains unclear exactly which chemokine(s) promotes regeneration. Nevertheless, this study provides interesting ideas about how cardiac scar tissue may be removed and replaced with functional cardiac tissue.
Previously, Yap was shown to activate Akt signaling to alter the cellular stress response through an unknown mechanism [4•]. Lin at al. [28] recently showed that Yap directly modulated expression of the PI3K subunit Pik3cb and that Pik3cb was required for Yap-mediated regeneration. Furthermore, heart failure induced by the knockout of Yap was mitigated by Pik3cb overexpression.
Cell cycle progression and repopulation of the myocardial scar tissue with functional cardiomyocytes require coordinated cytoskeletal rearrangements. Using a combination of chromatin immunoprecipitation sequencing and RNA profiling, Morikawa et al. [29•] determined that Hippo signaling and Yap regulate multiple cytoskeletal components. Although these data do not definitively show that adult cardiomyocytes migrate to repopulate the scar, they suggest that cytoskeleton remodeling is required in heart regeneration. These authors found that Yap target genes are the major components of the dystrophin glycoprotein complex (DGC) and the talin-vinculin-integrin complex, which connects extracellular matrix with the actin cytoskeleton. Knocking out Salv in adult cardiomyocytes showed extended protrusion into the scar region and expanded focal adhesion molecules. This cellular protrusion was shown to be required for heart regeneration in Mdx mice with disrupted DGC, which exhibited impaired heart repair capability.
Hippo signaling in cardiac cell differentiation and reprogramming
Replenishing cardiac stem/progenitor cells has been studied as another approach to facilitating heart repair. Both exogenous progenitor cells [30] and resident mesenchymal stem cells have been shown to have some potential therapeutic value [31], but understanding the mechanisms involved and the applicability of these cells to heart repair requires further investigation.
In addition to regulating cell proliferation, Hippo signaling and Yap also direct cell differentiation. Because Yap and Taz do not have DNA-binding domains and therefore require transcription factors to perform their activities, their binding partners in certain cellular contexts can largely affect their biologic roles. For example, Yap and Taz can interact with TEADs to promote the proliferation of intestinal stem/progenitor cells. Yap and Taz can also bind to Klf4 to initiate the differentiation of stem/progenitor cells into goblet cells [32]. The binding of Taz to Runx2 switches the differentiation of mesenchymal stem cells from adipocytes to osteoblasts [33]. Yap acts with Smad1 to maintain the non-neurogenic identity of mouse embryonic stem cells [34].
During development and regeneration, to form the correct organ shape or repair the injury, respectively, cells must undergo a series of complex processes including proliferation, adhesion, and migration. These processes place cells under different mechanical tension. Nearly a decade ago, Engler et al. [35] found that mechanical stress is essential for the determination of cell differentiation. Recent studies have suggested that Hippo/Yap act as mechanical mediators connecting extracellular mechanical tension to intracellular biologic output [6, 9]. Another study showed the presence of Yap/Taz activity in Sca1+ cardiac progenitor cells [36]. Matrix stiffness determined the cellular localization of Yap/Taz. Furthermore, physical stiffness (10 KPa) of the heart drove Yap/Taz to the nucleus to perform its co-transcriptional activity and induced progenitor cells to express cardiac differentiation markers. The silencing of Yap/Taz reverted this cardiac differentiation and resulted in endothelial gene expression.
As mentioned earlier, after MI, the myocardial matrix undergoes remodeling, which alters the mechanical properties of the heart [37]. Understanding the role of the Hippo signaling/Yap pathway in cardiac progenitor cells warrants further investigation, but, undoubtedly, it has therapeutic potential. This approach may serve to optimize ongoing cell therapy studies, such as by directing cell differentiation or providing a more supportive mechanical environment for cell relocation.
Another therapeutic approach of interest has been to reprogram cardiac fibroblasts into cardiomyocytes, with a goal of turning malfunctioning fibrotic scar tissue into a well-integrated myocardium [38]. However, these studies have been limited by the low percentage of cardiomyocytes derived from reprogrammed fibroblasts [39]. One study showed that Lats2 acts as a barrier to the generation of human-induced pluripotent cells (iPSCs) [40]. This finding indicates that Hippo signaling may be one of the barriers that prevent the efficient reprogramming of fibroblasts into cardiomyocytes. Furthermore, given what has been learned about the role of Hippo/Yap in progenitor cell differentiation, it is conceivable that cell fate may be redirected by the manipulation of mechanical conditions.
Hippo signaling and cardiac fibrosis
Along with the limited proliferative capacity of the heart, another important factor that impedes heart repair is the pathologic fibrosis that leads to progressive deterioration of ventricular wall compliance and cardiac function. In addition to cytokines and growth factors, such as angiotensin II (AngII), tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and IL-6, the disruption of the mechanical environment in the heart after injury is a driving factor of fibrosis [41]. To date, only one study has described Hippo signaling in cardiac fibroblasts [42]. Using a germline knockout mouse model and cultured primary cardiac fibroblasts, Del Re et al. showed that Rassf1a/Mst1 repressed nuclear factor (NF)-κb and TNF-α, the key mediators of fibrosis and hypertrophy. Knocking out Rassf1a/Mst1 would be expected to give a phenotype of exacerbated fibrosis, which is opposite of its role in cardiomyocytes. Additional in vivo studies of Hippo signaling in cardiac fibroblasts are warranted.
Therapeutics application of hippo signaling
The current state of knowledge regarding Hippo signaling in heart regeneration, as outlined in Fig. 1, suggest that efforts to manipulate the Hippo pathway for therapeutic benefit are warranted. Hippo inactivation can promote cardiomyocyte proliferation and also increase cardiomyocyte resistance to injury. While it is plausible to target the Hippo pathway to treat heart disease, any intervention will have to be proven safe as well as effective since Hippo pathway has important homeostatic and physiologic functions in other organs such as the liver.
Hippo signaling in cardiac development and disease. From top to bottom: Hippo signaling during development, homeostasis, and cardiac injury acts to inhibit cellular proliferation in cardiomyocytes and fibroblasts. Yap/Taz drives progenitor cells to differentiate into cardiomyocytes and inhibits endothelial cell generation. During cardiac regeneration, Hippo signaling prevents essential protrusion formation and cellular remodeling. In cardiac fibroblasts, Hippo inhibits the expression and secretion of paracrine factors that drive cardiac hypertrophy.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Edgar BA. From cell structure to transcription: Hippo forges a new path. Cell. 2006;124(2):267–73.
Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332(6028):458–61. Pioneer work demonstrated Hippo signaling interacting with Wnt signaling suppresses cardiomyocyte proliferation.
von Gise A, Lin Z, Schlegelmilch K, Honor LB, Pan GM, Buck JN, et al. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc Natl Acad Sci U S A. 2012;109(7):2394–9. Follow-up study of Heallen et al Science 2011 demonstrated Hippo signaling effect Yap acting through TEAD is sufficient to stimulate neonatal cardiomyocyte proliferation.
Xin M, Kim Y, Sutherland LB, Qi X, McAnally J, Schwartz RJ, et al. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal. 2011;4(196):ra70. Follow-up study of Heallen et al Science 2011, showed Yap connects to β-catenin through activating IGF signaling in cardiac growth regulation.
Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16(5):425–38.
Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–83.
Fernandez BG, Gaspar P, Bras-Pereira C, Jezowska B, Rebelo SR, Janody F. Actin-capping protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development. 2011;138(11):2337–46.
Sansores-Garcia L, Bossuyt W, Wada K, Yonemura S, Tao C, Sasaki H, et al. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 2011;30(12):2325–35.
Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138(18):3907–14.
Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15(6):637–46.
Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, et al. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464(7288):601–5.
Poss KD. Getting to the heart of regeneration in zebrafish. Semin Cell Dev Biol. 2007;18(1):36–45.
Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98–102.
Kajstura J, Rota M, Cappetta D, Ogorek B, Arranto C, Bai Y, et al. Cardiomyogenesis in the aging and failing human heart. Circulation. 2012;126(15):1869–81.
Mollova M, Bersell K, Walsh S, Savla J, Das LT, Park SY, et al. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci U S A. 2013;110(4):1446–51.
Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493(7432):433–6.
Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci U S A. 2013;110(34):13839–44. First time illustrated activating Yap promotes adult mammalian heart regeneration.
Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331(6020):1078–80. First time shown that newborn mice have heart regeneration ability within the first week after birth, which aroused wide interests in the mechanism study.
Heallen T, Morikawa Y, Leach J, Tao G, Willerson JT, Johnson RL, et al. Hippo signaling impedes adult heart regeneration. Development. 2013;140(23):4683–90.
Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13(8):877–83.
Matsui Y, Nakano N, Shao D, Gao S, Luo W, Hong C, et al. Lats2 is a negative regulator of myocyte size in the heart. Circ Res. 2008;103(11):1309–18.
Odashima M, Usui S, Takagi H, Hong C, Liu J, Yokota M, et al. Inhibition of endogenous Mst1 prevents apoptosis and cardiac dysfunction without affecting cardiac hypertrophy after myocardial infarction. Circ Res. 2007;100(9):1344–52.
Yamamoto S, Yang G, Zablocki D, Liu J, Hong C, Kim SJ, et al. Activation of Mst1 causes dilated cardiomyopathy by stimulating apoptosis without compensatory ventricular myocyte hypertrophy. J Clin Invest. 2003;111(10):1463–74.
Del Re DP, Yang Y, Nakano N, Cho J, Zhai P, Yamamoto T, et al. Yes-associated protein isoform 1 (Yap1) promotes cardiomyocyte survival and growth to protect against myocardial ischemic injury. J Biol Chem. 2013;288(6):3977–88.
Imajo M, Miyatake K, Iimura A, Miyamoto A, Nishida E. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/beta-catenin signalling. The EMBO journal. 2012;31(5):1109–22.
Tian Y, Liu Y, Wang T, Zhou N, Kong J, Chen L, et al. A microRNA-Hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice. Sci Transl Med. 2015;7(279):279ra238.
Lin Z, von Gise A, Zhou P, Gu F, Ma Q, Jiang J, et al. Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model. Circ Res. 2014;115(3):354–63.
Lin Z, Zhou P, von Gise A, Gu F, Ma Q, Chen J, et al. Pi3kcb links Hippo-YAP and PI3K-AKT signaling pathways to promote cardiomyocyte proliferation and survival. Circ Res. 2015;116(1):35–45.
Morikawa Y, Zhang M, Heallen T, Leach J, Tao G, Xiao Y, et al. Actin cytoskeletal remodeling with protrusion formation is essential for heart regeneration in Hippo-deficient mice. Sci Signal. 2015;8(375):ra41. This paper discovered Hippo signaling suppresses cytoskeleton remodeling and cell protrusion, which are required for heart regeneration.
Hong KU, Bolli R. Cardiac stem cell therapy for cardiac repair. Curr Treat Options Cardiovasc Med. 2014;16(7):324.
Chong JJ, Chandrakanthan V, Xaymardan M, Asli NS, Li J, Ahmed I, et al. Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell. 2011;9(6):527–40.
Imajo M, Ebisuya M, Nishida E. Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat Cell Biol. 2015;17(1):7–19.
Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309(5737):1074–8.
Alarcon C, Zaromytidou AI, Xi Q, Gao S, Yu J, Fujisawa S, et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell. 2009;139(4):757–69.
Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89.
Mosqueira D, Pagliari S, Uto K, Ebara M, Romanazzo S, Escobedo-Lucea C, et al. Hippo pathway effectors control cardiac progenitor cell fate by acting as dynamic sensors of substrate mechanics and nanostructure. ACS Nano. 2014;8(3):2033–47.
Jacot JG, Martin JC, Hunt DL. Mechanobiology of cardiomyocyte development. J Biomech. 2010;43(1):93–8.
Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485(7400):599–604.
Sadahiro T, Yamanaka S, Ieda M. Direct cardiac reprogramming: progress and challenges in basic biology and clinical applications. Circ Res. 2015;116(8):1378–91.
Qin H, Blaschke K, Wei G, Ohi Y, Blouin L, Qi Z, et al. Transcriptional analysis of pluripotency reveals the Hippo pathway as a barrier to reprogramming. Hum Mol Genet. 2012;21(9):2054–67.
Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123(2):255–78.
Del Re DP, Matsuda T, Zhai P, Gao S, Clark GJ, Van Der Weyden L, et al. Proapoptotic Rassf1A/Mst1 signaling in cardiac fibroblasts is protective against pressure overload in mice. J Clin Invest. 2010;120(10):3555–67.
Acknowledgments
J.F.M was supported by the Transatlantic Network of Excellence Award LeDucq Foundation Transatlantic Networks of Excellence in Cardiovascular Research 14CVD01: “Defining the genomic topology of atrial fibrillation,” NIH Grants DE 023177 and HL 118761, and the Vivian L. Smith Foundation (J.F.M.)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Regenerative Medicine and Stem-cell Therapy
Rights and permissions
About this article
Cite this article
Xiao, Y., Leach, J., Wang, J. et al. Hippo/Yap Signaling in Cardiac Development and Regeneration. Curr Treat Options Cardio Med 18, 38 (2016). https://doi.org/10.1007/s11936-016-0461-y
Published:
DOI: https://doi.org/10.1007/s11936-016-0461-y