Opinion statement
Human epidermal growth factor receptor 2-positive (HER2+) breast cancer is associated with aggressive disease and a poor prognosis. There are a number of anti-HER2 therapies currently available and more in development. Cardiac concerns raised by early experience with HER2 inhibitor trastuzumab had led to subsequent trial designs focused on a more selective patient population with a lower cardiac risk, generally with a median age of 50–55 years old, with a normal left ventricular ejection fraction (LVEF) at study entry, and with no clinical cardiovascular disease or uncontrolled cardiac risk factors. Within this context, the cardiac safety profile of the agents outlined in this review appears to be quite acceptable and thus the risk to benefit ratio is favorable. Given the theoretical cardiac concerns of this class of cancer therapeutics that prompted the stringent patient selection and eligibility criteria in clinical trials, clinicians should exercise reasonable caution and monitor patients carefully for cardiac sequelae when using these agents outside of the controlled conditions applied in the clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cause of cancer-related death in women worldwide [1]. HER2+ tumors account for approximately 20 % of breast cancers and are associated with a more aggressive cancer that has a high risk of recurrence and an early propensity for metastasis resulting in a poorer prognosis [2–4]. The HER2 receptor belongs to a family of tyrosine kinase receptors that also include other HER receptors namely, HER1, HER 3, and HER4. These receptors work together to activate signaling pathways that regulate cell proliferation, apoptosis, angiogenesis, and differentiation among other functions. Each has an extracellular component for ligand binding and an intracellular component that has tyrosine kinase activity. Dimerization of HER2 with itself (homodimerization) or with one of the other 3 HER receptors (heterodimerization), activates the tyrosine kinase via autophosphorylation and results in downstream cell signaling and proliferation. Inhibition of HER2, therefore, is a logical therapeutic target.
At present, there are several approved and investigational biological agents directed against HER2-dependent cell signaling. Among those discussed in this review are trastuzumab, lapatinib, pertuzumab, and trastuzumab emtansine (T-DM1). Trastuzumab was the first humanized monoclonal antibody approved for treatment of HER2+ breast cancer. It binds to the extracellular domain IV of the HER2 receptor. Lapatinib is a reversible HER1 and HER2 tyrosine kinase inhibitor. While trastuzumab binds to the extracellular domain of HER2, lapatinib competes with ATP for intracellular binding of the tyrosine kinase receptor and inhibits phosphorylation. This results in increased cell apoptosis and anti-tumor activity. Pertuzumab binds to domain II of the HER2 receptor. This domain is necessary for dimerization with other HER receptors. Thus, pertuzumab inhibits the cellular pathways that are usually activated by such dimerization. Pertuzumab is believed to wield its greatest effect through its inhibition of HER2-HER3 dimerization. T-DM1 belongs to a class of agents known as antibody-drug conjugants. The concept behind these agents is that the cytotoxic agent is linked to a monoclonal antibody targeted toward a tumor-specific antigen. In the case of T-DM1, the cytotoxic agent is a vinca alkaloid-like maytansine derivative known as DM1. DM1 is linked to the monoclonal antibody trastuzumab via a thioether linker. The stability of this combination is believed to be the reason for its selective antitumor effects.
As a therapeutic group, HER2 inhibitors have significantly altered the prognosis of women with HER2+ breast cancer [5]. However, because many of the affected cellular pathways inhibited by these agents are also involved in the heart’s response to stress, there are cardiac safety considerations for this class of cancer therapeutics. The focus of this review is the cardiac safety profiles of these agents.
Trastuzumab
The most well-studied HER2 inhibitor is trastuzumab, the first approved HER2 receptor inhibitor to become available for use against breast cancer. A landmark trial showed clear benefit from adding trastuzumab to traditional chemotherapy with doxorubicin and cyclophosphamide or paclitaxel [6••, 7]. By 2006, chemotherapy plus trastuzumab became the standard of care for both adjuvant and metastatic breast cancer (MBC). The addition of trastuzumab therapy to sequential anthracycline and taxane led to a nearly 50 % reduction in breast cancer recurrence and a 30 % reduction in risk of death [7–9].
The association between cardiotoxicity and trastuzumab therapy was first realized in the early 2000s when results from one of the earliest trials in MBC reported that cardiac dysfunction occurred in 27 % of patients receiving combined adjuvant anthracycline and trastuzumab compared to 8 % of patients receiving anthracycline and cyclophosphamide alone and 13 % of the group given paclitaxel and trastuzumab. In this seminal study, 16 % of those receiving adjuvant therapy with anthracyclines and trastuzumab developed NYHA class III or IV heart failure versus 3 % of patients who received anthracycline and cyclophosphamide alone and 2 % of those treated with combination trastuzumab and paclitaxel [6••, 10]. Because of these results, concomitant adjuvant treatment with anthracycline and trastuzumab was generally avoided in clinical practice. This report however was followed by subsequent studies with trastuzumab as an adjuvant agent in which patients were required to have normal baseline cardiac function to be eligible, and cardiac function was more closely monitored with regularly scheduled cardiac imaging and clinical assessments while in the study. These interventions resulted in lower symptomatic cardiotoxicity rates ranging from 2 to 4 % [7, 11, 12].
The majority of the large trials have looked at cardiotoxicity associated with adjuvant trastuzumab therapy. Trastuzumab, however, is also used in the neoadjuvant setting in HER2+ patients with locally advanced disease or to decrease the tumor burden and improve outcomes after surgical resection [13, 14•, 15, 16].
In the neoadjuvant setting, trastuzumab may be given concurrently with anthracyclines. In the GeparQuattro study, 445 patients with operable or locally advanced HER2+ tumors were treated with anthracycline and cyclophosphamide followed by taxane with or without capecitabine and trastuzumab every 3 weeks with all chemotherapy cycles. Pathologic complete response was twice as high as the reference group (n = 1058, HER negative tumors), which received the same chemotherapy regimen without trastuzumab [15]. Similar impressive results were achieved by combining anthracyclines with trastuzumab in the neoadjuvant setting in HER2+ tumors in other studies [13, 14•, 15]. Symptomatic decline in LVEF in GeparQuattro was low, with 2 patients reporting a decrease in LVEF of ≥10 % from baseline in the trastuzumab group (2/445 = 0.4 %). Follow-up for LVEF decline, however, was short; at best 36 weeks in the group that received trastuzumab. Another study, the NOAH study, conducted in patients with locally advanced or inflammatory breast cancer compared anthracycline-based neoadjuvant chemotherapy alone to the same neoadjuvant chemotherapy combined with 1 year of trastuzumab [13, 14•]. A NHYA class III heart failure rate of 2 % was reported in the trastuzumab group with no symptomatic events reported in the group that did not receive trastuzumab. Of note, an asymptomatic drop in LVEF of ≥10 % occurred in 24 % the trastuzumab group versus 17 % in the non-trastuzumab group.
Long-term cardiotoxicity appears to be less problematic. Late follow-up data (median 8 years) from the HERA trial in which patients received adjuvant trastuzumab for either 1 or 2 years demonstrated an incidence of severe congestive heart failure of 0.8 % in both the 2-year and 1-year groups [17••]. A significant drop in LVEF, defined as a decline of ≥10 % points to a value <50 %, was seen in ∼7 % for the 2-year trastuzumab group and ∼4 % for the 1-year trastuzumab group. The authors suggested that the lower rate of cardiotoxicity seen in this study may in part be due to the fact that patients entering the study had to have an LVEF ≥55 % and also due to the relatively longer time interval between the end of anthracycline chemotherapy and the start of trastuzumab compared with other trials, potentially giving the heart longer time to recover. Recovery rates for patients with a confirmed decrease in LVEF in the follow-up of the HERA trial were higher than 80 %.
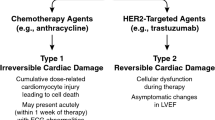
Unlike anthracycline-associated cardiotoxicity, significant ultrastructural changes on myocardial biopsy are not typically seen with trastuzumab. It has been postulated that trastuzumab-associated cardiac dysfunction may be mediated by loss of contractility or myocardial stunning [18]. Also, unlike anthracycline-associated cardiotoxicity, left ventricular (LV) dysfunction from trastuzumab is not related to cumulative dose received. Instead, some of the identified risk factors for toxicity include prior anthracycline therapy, older age, pre-existing impairment in LVEF, presence of coronary artery disease, atrial flutter or atrial fibrillation, high body mass index, and hypertension requiring medical therapy [6••, 10, 19, 20]. Despite the potential for LV dysfunction with trastuzumab as reflected in the above-mentioned studies, cardiotoxicity with this agent is often reversible on its discontinuation and re-challenge is well-tolerated if recovery has occurred in the interim. The mechanism of HER2-mediated cardiotoxicity is believed to be associated with the additional, inadvertent blocking of HER2 receptors on cardiac myocytes which have a physiological role in the maintenance of cardiac myocyte integrity and the prevention of LV dysfunction. In fact, both HER2 and HER4 expression on cardiac myocytes are significantly depressed in heart failure. This would explain why stopping HER2 inhibitor therapy may result in normalization of cardiac function in patients with breast cancer being treated with trastuzumab.
Concept of trastuzumab resistance
Sixteen to 22 % of women with HER2+ breast cancer given trastuzumab in the adjuvant setting will relapse, raising concern for the possibility of trastuzumab resistance [21, 22].
This has led to the exploration of dual-HER receptor inhibition as a mechanism that may allow improved disease control. However, this potential approach also raises theoretical concern that there may be amplification of the cardiotoxic impact. The relevant safety data on lapatinib and pertuzumab, both agents that are employed in dual-HER inhibition, are reviewed in the sections that follow.
Lapatinib
Lapatinib is a small molecule dual tyrosine kinase inhibitor of HER1 and HER2 that has been approved for treatment of MBC. Lapatinib has shown success as single-agent therapy in patients who have progressed on trastuzumab. It has also been used in combination with capecitabine, docetaxel, and paclitaxel and has been combined with trastuzumab with the intent of dual HER-receptor blockade in patients with MBC who have progressed on trastuzumab [23–26]. The combination of trastuzumab and lapatinib has shown improved overall survival when compared with lapatinib alone [27•].
One of the earlier supporting trials of lapatinib compared its use with capecitabine versus capecitabine alone in patients with either locally advanced or MBC [23]. These patients had already received an anthracycline, a taxane, and trastuzumab. There were no reports of symptomatic cardiac events. Only 4 patients in the combination therapy group showed an asymptomatic drop in LVEF (defined as a decline of ≥20 % from baseline). Of note, in this study, only women with normal LVEF after trastuzumab therapy were enrolled and follow-up was limited to the study duration. A later trial compared lapatinib monotherapy with lapatinib in combination with trastuzumab, dual-HER2 inhibition, in patients with HER2+ MBC [27•, 28]. These patients had also already received trastuzumab-based therapy and had normal LVEF on study entry. The investigators found a 7 % incidence of serious cardiac events in the combination arm and 2 % in the monotherapy arm. In this study, cardiotoxicity was defined as symptomatic LV systolic dysfunction or an asymptomatic decrease in LVEF ≥20 % relative to the baseline value.
A more stringent criterion for cardiotoxicity was used in the NeoALTTO trial where lapatinib was used as neoadjuvant therapy [29•]. Patients with early stage breast cancer and normal LVEF were randomized to receive lapatinib, trastuzumab, or lapatinib plus trastuzumab for 6 weeks, followed by 12 weeks of the anti-HER2 therapy with weekly paclitaxel. Patients then underwent surgical resection of cancer followed by adjuvant chemotherapy with an anthracycline-containing (epirubicin) regimen and 34 weeks of the same anti-HER2 therapy. LVEF was assessed at baseline and serially up to 10 years. A primary cardiac event was defined as a cardiac death or symptomatic NYHA III or IV heart failure. A secondary cardiac event was described as an asymptomatic or mildly symptomatic drop in LVEF by more than 10 % from baseline to a value below 50 %. The overall incidence of primary and secondary cardiac events was 5 % with dual HER2 blockade and ∼1 % in the other two groups.
Cardiac safety data from the ALTTO trial (NCT00490139), designed to look at treatment with lapatinib and/or trastuzumab in over 8000 patients with early stage breast cancer in the adjuvant setting, will add to our understanding of any differences in cardiac toxicity between lapatinib and trastuzumab as well as any incremental toxicity from their combination.
Pertuzumab
Pertuzumab is a recombinant humanized monoclonal antibody against domain II of the HER2 receptor that prevents dimerization with other HER receptors. When combined with trastuzumab, there is conceptually a broader inhibition of HER2-mediated cell signaling, and hence greater cytotoxic potential. Initial phase II safety data on the use of pertuzumab combined with trastuzumab in HER2+ MBC patients who progressed on trastuzumab were somewhat disconcerting; but, a larger subsequent phase II study that excluded patients with a prior decline in LVEF on trastuzumab and patients with a history of hypertension was more reassuring [30, 31].
On this background, the final analysis of the phase III CLEOPATRA study was published [32••]. In this trial, combination of pertuzumab and trastuzumab was used as first-line treatment of HER2+ MBC in 808 patients who had not received prior chemotherapy or biologic therapy for their metastatic disease. Patients were randomized to received placebo plus trastuzumab plus docetaxel chemotherapy, or pertuzumab plus trastuzumab plus docetaxel chemotherapy. Prior adjuvant or neoadjuvant chemotherapy included anthracycline in 37.3 % of patients in the pertuzumab arm and 40.4 % in the placebo arm. Prior trastuzumab was given in 11.7 % and 10.1 % of pertuzumab and placebo arms, respectively. LVEF was followed serially up to 3 years after discontinuation of therapy. LV dysfunction, defined as a decline in LVEF of ≥10 % to a value <50 % occurred in 24 of 394 patients (6.6 %) in the pertuzumab group and in 28 of 378 patient (8.6 %) in the placebo combination. There was only 1 patient with symptomatic LV dysfunction in the pertuzumab group that occurred after 40 months and resolved 3 months following pertuzumab and trastuzumab discontinuation. Treatment outcomes were extremely favorable with the combination of pertuzumab to trastuzumab and docetaxel, resulting in a 56.6-month medial overall survival compared to 40.8 months in the placebo combination group and an improvement in progression free survival of 6.3 months. Given the cardiac outcomes reported in this selected population, maintained over the several interim analyses, the efficacy of this combination appears achievable without significantly compromising cardiac safety [32••, 33, 34].
Although the above data pertain to patients with metastatic HER2+ disease, there is also data to support the use of pertuzumab and trastuzumab in the neoadjuvant setting. The NeoSphere trial was a phase II open label trial randomizing 417 patients with locally advanced, inflammatory, or early HER2+ breast cancer into 1 of 4 arms: trastuzumab plus docetaxel (group A), pertuzumab plus trastuzumab plus docetaxel (group B), pertuzumab and trastuzumab (group C), or pertuzumab plus docetaxel [35•]. After completion of 4 cycles in the given arm, patients underwent surgery followed by 3 cycles of an anthracycline-containing (epirubicin) chemotherapy regimen and concomitant trastuzumab for 1 year. Hormonal and radiotherapy were prescribed based on local criteria. There were no cardiac deaths. Focusing just on the neoadjuvant safety results, there was 1 instance of symptomatic heart failure that occurred in the pertuzumab plus trastuzumab arm (group C) leading to discontinuation of treatment. This patient was a protocol violation due to a history of hypertension, angina, and coronary artery disease with an intracoronary stent. The patient was also on digoxin. There were 4 patients with a 10–15 % decline in LVEF to a value <50 % and 2 patients with a decline of ≥ 15 % to a value of <50 %. No patient had a decline in LVEF to <40 %. All patients with asymptomatic LVEF declines had improvement in LVEF by cycle 4 resulting in an LVEF >50 %. Although longer-term follow-up of this cohort is needed to also evaluate the impact of the anthracycline-containing adjuvant portion of the protocol, the addition of pertuzumab to trastuzumab and docetaxel resulted in the greatest tumor eradication among the treatment arms with a reasonable cardiac safety profile.
Because of prior studies demonstrating an increase in cardiac toxicity when giving trastuzumab in combination with an anthracycline-based chemotherapy regimen [10, 36], the TRYPHAENA study sought to evaluate the safety of pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free (carboplatin-based) chemotherapy regimens in 225 patients with HER2+ early breast cancer [37•]. Following this neoadjuvant therapy, patients underwent surgery followed by 1 year of adjuvant trastuzumab with or without radiotherapy and hormonal treatment in accordance with local practice. There were 3 cases of symptomatic LV dysfunction in the study population that resolved with its treatment: none while on trastuzumab and pertuzumab concurrent with anthracycline-containing chemotherapy, 2 patients in whom trastuzumab and pertuzumab were given sequentially after anthracycline-containing chemotherapy (only one of which occurred during the trastuzumab-pertuzumab part of the treatment), and 1 in the non-anthracycline-containing chemotherapy group but during the adjuvant portion of the protocol while receiving trastuzumab. Overall, the rate of symptomatic heart failure with trastuzumab and pertuzumab in combination with standard chemotherapy was 0.4 %. An asymptomatic decline in LVEF ≥10 % to a value <50 % occurred in 11 patients during neoadjuvant therapy (4.9 %) and was similar across groups. In all cases, LVEF subsequently improved to a value of >50 %. During the adjuvant part of the protocol, 15 patients (6.7 %) had a reversible decline in LVEF ≥10 % to a value <50 %, with the greatest number occurring in patients who received the pertuzumab-trastuzumab combination following anthracycline-containing chemotherapy. During the follow-up period, 9 patients (4 %) developed a similar reversible decline in LVEF to <50 %. Overall, the pathological response rate was favorable, ranging from 57–66 % across treatment arms, without apparent increase in toxicity whether neoadjuvant pertuzumab and trastuzumab were combined concomitantly or sequentially with anthracycline-containing chemotherapy or non-anthracycline chemotherapy. Results from the APHINITY study (NCT01358877) assessing the safety of trastuzumab and pertuzumab combined with standard chemotherapy regimens in the adjuvant setting are awaited.
Trastuzumab emtansine
Trastuzumab emtansine (T-DM1) is an antibody-drug conjugate whose efficacy is derived from the combination of the HER2 targeting of trastuzumab with the intracellular cytotoxicity of DM1. There were 2 phase II studies in which T-DM1 was given to heavily pre-treated patients with HER2+ MBC who had prior HER2-directed therapy. No cardiac deaths and no symptomatic heart failure occurred. There was no patient who had a decline in LVEF to <40% [38, 39].
The phase III TH3RESA trial was a randomized open-label study of 602 patients with HER2+-advanced breast cancer that received 2 or more HER2-directed regimens including trastuzumab and lapatinib and a previous taxane. Patients were randomized to T-DM1 versus a regimen of the physician’s choice [40•]. The latter included trastuzumab (with chemotherapy, with lapatinib, or with hormonal therapy), lapatinib plus chemotherapy, or a single-agent chemotherapy. Anthracyclines were not among the agents selected in the physician’s choice group. Two patients (1 %) in the physician’s choice group and 6 patients (1 %) in the T-DM1 group had a decline in LVEF ≤ 50 % and ≥15 % decline from baseline; however, no patient had an LVEF <40 %. There was 1 death in the physician’s choice group (1 %) from non-cardiogenic pulmonary edema. There was no death attributed to cardiac cause in the T-DM1 group. Treatment with T-DM1 resulted in a significantly greater progression free survival compared to physician’s choice in this heavily pre-treated HER2+ MBC population, thus validating the above-mentioned phase II studies.
The EMILIA trial was another important phase III trial that focused on the use of T-DM1 as a second-line therapy in 991 patients who had progressed despite trastuzumab treatment [41••]. In this study, investigators compared T-DM1 as a single agent to lapatinib plus capecitabine, the standard combination for HER2+ MBC patients who progressed on prior trastuzumab. A total of 61 % of patients had prior anthracycline therapy. Treatment with T-DM1 resulted in a greater overall survival and progression free survival compared to lapatinib and capecitabine. LVEF was maintained over 45 % in 97.1 % of patients in the T-DM1 group and 93 % in the lapatinib-capecitabine group. Three patients in each group had a decline in LVEF from baseline to a value <40 %. LVEF fell to a value <50 % and at least 15 % points from baseline in 8 (1.7 %) patients in the T-DM1 group and 7 (1.6 %) in the lapatinib-capecitabine group. Symptomatic LV dysfunction occurred in 1 patient in the T-DM1 group. Four of 5 deaths that were considered adverse events occurring within 30 days of last drug dose occurred in the lapatinib-capecitabine group, one of which was due to a coronary artery disease. There was 1 non-cardiac death in the T-DM1 group. T-DM1 is now FDA-approved for use in HER2+ MBC patients who progressed with trastuzumab and a taxane.
Moving even earlier into the treatment of advanced breast cancer, Hurvitz et al were the first to publish data on the use of T-DM1 compared to trastuzumab plus docetaxel as the first-line therapy for patients with HER2+ MBC [42]. This was a phase II international study of 137 patients. Prior neoadjuvant or adjuvant trastuzumab was received in 27.1 % and 17.9 % of patients in the trastuzumab-docetaxel group and T-DM1 group, respectively. In the trastuzumab-docetaxel group, 48.6 % had prior anthracycline exposure and 44.8 % in the T-DM1 group. T-DM1 resulted in a 40 % reduction in the relative risk of disease progression compared to standard therapy with trastuzumab-docetaxel. From the cardiac perspective, 3 patients in each arm had a reduction in LVEF. Two patients in the control arm had a post-baseline LVEF ≤40 % and both had prior anthracycline in the adjuvant setting. One patient who received T-DM1 had an LVEF ≤40 % without prior anthracycline exposure and had no prior cardiac history. The patient was asymptomatic despite lack of medical intervention. There was 1 cardiac death in each group (cardiopulmonary failure in the control arm and sudden death in the T-DM1 arm) that was deemed not attributable to the study treatment. The results from this trial suggest that T-DM1 may be more potent as a first-line therapy in this patient population, a benefit accompanied by a favorable cardiac safety profile.
This is concordant with a recently published integrated safety analysis of the use of T-DM1 in HER2+ MBC studies [43]. In 884 patients, there was only 1 case where T-DM1 infusion was prematurely discontinued. The reason was HTN. Four patients (0.45 %) discontinued T-DM1 therapy for cardiac reasons that included atrial fibrillation (1 patient), LV dysfunction (1 patient), and a decline in LVEF (2 patients). Of the patients who had a decline in LVEF, 4 patients (0.5 %) had a decline to a value ≤40 % while 16 (1.8 %) had a decline of ≥15 % from baseline to a value <50 %. Given this cumulative safety experience and the demonstrated efficacy of T-DM1 in MBC trials, Krop et al sought to examine the feasibility and cardiac safety of using T-DM1 to treat early stage breast cancer, in the neoadjuvant or adjuvant setting following anthracycline chemotherapy [44]. Patients received 4 cycles of an anthracycline-containing regimen, followed by 4 cycles of T-DM1 with the option to receive 3–4 cycles of docetaxel and trastuzumab for 9–12 weeks. Thereafter, patients had the option to receive radiotherapy with or without trastuzumab followed sequentially by T-DM1 or radiotherapy concurrent with T-DM1. A history of prior cardiotoxic chemotherapy or history of radiotherapy was not permitted. A total of 153 patients were studied, 99 in the adjuvant group and 54 in the neoadjuvant group. There were no episodes of CHF. There were 4 patients (2.7 %) with an asymptomatic decline in LVEF of ≥10 % points to a value <50 % but none had an LVEF <40 %. In 3 of the 4 patients, the LV dysfunction was deemed unrelated to T-DM1, and 2 of those 3 occurred during treatment with the optional trastuzumab-docetaxel therapy. In the fourth patient with a decline in LVEF, the patient had already had an LVEF of 50 % after the anthracycline therapy and ultimately discontinued T-DM1 when the LVEF was 45 %. The LVEF recovered to 50 % by 14 months after the last dose. In only 1 patient (0.7 %) was T-DM1 discontinued due to cardiac dysfunction. If a similarly low incidence of cardiac events is found with phase III clinical trials of T-DM1 in early breast cancer, then it is conceivable that longer use of this targeted agent relative to conventional cytotoxic regimens will be tolerated in this patient population in whom reasonably good long-term survival is expected and in whom minimizing cardiac sequelae is especially relevant.
Conclusions
The cumulative data collected thus far on the use of anti-HER2 therapies has been insightful and has changed the natural history of HER2+ breast cancer. Much has been learned about the importance of cardiac monitoring and patient selection to enhance the risk-benefit profile of these agents. There are, however, still a number of knowledge gaps. For instance, elderly patients are more likely to have pre-existing cardiac risk factors or underlying clinical or subclinical cardiovascular disease; yet, they may still need cancer therapy that is potentially cardiotoxic. Data on cardiac consequences of anti-HER2 therapy in this population is scant [45, 46]. Additionally, there is little ethnic diversity among the trial populations cited in this review. Therefore, any ethnic variation in the cardiac tolerability of these agents is unknown.
In the majority of studies described in this review, the cardiac events were asymptomatic LV dysfunction. There is now much interest on the detection of subclinical cardiovascular dysfunction with the aim of identifying patients at risk for cardiotoxicity before an overt decline in LVEF occurs [47–49]. Whether the patients enrolled in the above trials would have had evidence of subclinical LV dysfunction at study entry or during treatment as detected by myocardial strain imaging or biomarkers is unknown and, therefore, the impact of such circumstances on the likelihood of cardiac events is uncertain. Little is known about what treatment, if any, was received by patients who did develop asymptomatic LV dysfunction while on the anti-HER2 therapies described above; nor is it known whether the decline in LVEF could have been prevented with prophylactic angiotensin converting enzyme inhibitors or beta-blockers. Studies are underway to examine this issue [50, 51]. The answers to some of these questions will further inform the use of these agents in a “real world” setting. However, in carefully selected patients with preserved LVEF who have no significant cardiovascular disease or uncontrolled cardiac risk factors, the anti-HER2 therapies described herein appear to be reasonably safe and greatly improve outcomes for patients with HER2+ breast cancer.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Tevaarwerk AJ, Gray RJ, Schneider BP, Smith ML, Wagner LI, Fetting JH, et al. Survival in patients with metastatic recurrent breast cancer after adjuvant chemotherapy: little evidence of improvement over the past 30 years. Cancer. 2013;119:1140–8.
Pegram M, Slamon D. Biological rationale for HER2/neu (c-erbB2) as a target for monoclonal antibody therapy. Semin Oncol. 2000;27:13–9.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82.
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12.
Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28:92–8.
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. This was the first study to draw attention to the cardiotoxic effects of trastuzumab. In this study concurrent treatment with anthracycline and trastuzumab significantly increased the risk of cardiac toxicity.
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84.
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72.
Moja L, Tagliabue L, Balduzzi S, Parmelli E, Pistotti V, Guarneri V, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012;4:CD006243.
Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–21.
Russell SD, Blackwell KL, Lawrence J, Pippen JE, Roe MT, Wood F, et al. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the National Surgical Adjuvant breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol. 2010;28:3416–21.
Procter M, Suter TM, de Azambuja E, Dafni U, van Dooren V, Muehlbauer S, et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol. 2010;28:3422–8.
Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–85.
Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–84. In this study, trastuzumab was given concurrently with anthracyclines in the neoadjuvant setting, improving event-free survival with a low incidence of symptomatic heart failure that responded to therapy. This study renewed hope in the concurrent treatment with anthracyclines and trastuzumab that had previously been discouraged.
Untch M, Fasching PA, Konecny GE, Hasmüller S, Lebeau A, Kreienberg R, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29:3351–7.
Aebi S, Davidson T, Gruber G, Cardoso F. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22 Suppl 6:vi12–24.
De Azambuja E, Procter MJ, van Veldhuisen DJ, Agbor-Tarh D, Metzger-Filho O, Steinseifer J, et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin Adjuvant Trial (BIG 1-01). J Clin Oncol. 2014;32:2159–65. This was a long-term follow-up of the HERA trial that randomized patients with early-stage breast cancer to observation, 1-year or 2-years of Trastuzumab. After a median follow-up of 8 years, cardiac toxicity was tolerable and the probability of recovery was shown to be high.
Ewer MS, Vooletich MT, Durand J-BB, Woods ML, Davis JR, Valero V, et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23:7820–6.
Perez EA, Suman VJ, Davidson NE, Sledge GW, Kaufman PA, Hudis CA, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26:1231–8.
Ezaz G, Long JB, Gross CP, Chen J. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Heart Assoc. 2014;3:e000472.
Rimawi MF, Schiff R, Osborne CK. Targeting HER2 for the treatment of breast cancer. Annu Rev Med. 2015;66:111–28.
Jelovac D, Wolff AC. The adjuvant treatment of HER2-positive breast cancer. Curr Treat Options Oncol. 2012;13:230–9.
Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–43.
Burstein HJ, Storniolo AM, Franco S, Forster J, Stein S, Rubin S, et al. A phase II study of lapatinib monotherapy in chemotherapy-refractory HER2-positive and HER2-negative advanced or metastatic breast cancer. Ann Oncol. 2008;19:1068–74.
Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112:533–43.
Guan Z, Xu B, DeSilvio ML, Shen Z, Arpornwirat W, Tong Z, et al. Randomized trial of lapatinib versus placebo added to paclitaxel in the treatment of human epidermal growth factor receptor 2-overexpressing metastatic breast cancer. J Clin Oncol. 2013;31:1947–53.
Blackwell KL, Burstein HJ, Storniolo AM, Rugo HS, Sledge G, Aktan G, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol. 2012;30:2585–92. HER2 positive metastatic breast cancer patients whose disease progressed on trastuzumab were assigned to lapatinib monotherapy or lapatinib with trastuzumab. Results showed that the combination therapy was superior in terms of progression-free survival. Cardiac event rate was low in both groups suggesting that aggressive therapy is an option in these patients.
Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–30.
De Azambuja E, Holmes AP, Piccart-Gebhart M, Holmes E, Di Cosimo S, Swaby RF, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014;15:1137–46. The NeoALTTO trial showed that combination therapy with lapatinib and trastuzumab in the neoadjuvant setting accomplished higher rates of pathological complete response than either therapy alone in women with early breast cancer with no worsening of cardiotoxicity with combination anti-HER2 therapy.
Portera CC, Walshe JM, Rosing DR, Denduluri N, Berman AW, Vatas U, et al. Cardiac toxicity and efficacy of trastuzumab combined with pertuzumab in patients with [corrected] human epidermal growth factor receptor 2-positive metastatic breast cancer. Clin Cancer Res. 2008;14:2710–6.
Baselga J, Gelmon KA, Verma S, Wardley A, Conte P, Miles D, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28:1138–44.
Swain SM, Baselga J, Kim S-BB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–34. The final analysis of the CLEOPATRA study revealed the survival benefit of dual HER-2 inhibition with pertuzumab and trastuzumab plus docetaxel as first line treatment of metastatic breast cancer.
Baselga J, Cortés J, Kim S-BB, Im S-AA, Hegg R, Im Y-HH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–19.
Swain SM, Kim S-BB, Cortés J, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–71.
Gianni L, Pienkowski T, Im Y-HH, Roman L, Tseng L-MM, Liu M-CC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. This study demonstrated the benefit of dual HER2 inhibition with pertuzumab and trastuzumab as neoadjuvant therapy.
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–83.
Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24:2278–84. Combination of neoadjuvant pertuzumab plus trastuzumab given concurrently or sequentially with anthracycline -containing chemotherapy was associated with no greater cardiotoxicity than when given with non-anthracycline chemotherapy.
Burris HA, Rugo HS, Vukelja SJ, Vogel CL, Borson RA, Limentani S, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 2011;29:398–405.
Krop IE, LoRusso P, Miller KD, Modi S, Yardley D, Rodriguez G, et al. A phase II study of trastuzumab emtansine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who were previously treated with trastuzumab, lapatinib, an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2012;30:3234–41.
Krop IE, Kim S-BB, González-Martín A, LoRusso PM, Ferrero J-MM, Smitt M, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:689–99. Treatment with T-DM1 as adjuvant therapy in heavily pretreated patients with advanced breast cancer resulted in greater progression free survival compared to a regimen of physician’s choice with comparable cardiac safety.
Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91. The EMILIA study led to the approval of T-DM1 for HER2+ metastatic breast cancer patients who progressed on trastuzumab because T-DM1 showed greater overall and progression free survival compared to lapatinib and capecitabine as standard therapy.
Hurvitz SA, Dirix L, Kocsis J, Bianchi GV, Lu J, Vinholes J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2013;31:1157–63.
Diéras V, Harbeck N, Budd GT, Greenson JK, Guardino AE, Samant M, et al. Trastuzumab emtansine in human epidermal growth factor receptor 2-positive metastatic breast cancer: an integrated safety analysis. J Clin Oncol. 2014;32:2750–7.
Krop IE, Suter TM, Dang CT, Dirix L, Romieu G, Zamagni C, et al. Feasibility and cardiac safety of trastuzumab emtansine after anthracycline-based chemotherapy as (neo)adjuvant therapy for human epidermal growth factor receptor 2-positive early-stage breast cancer. J Clin Oncol. 2015;33:1136–42.
Vaz-Luis I, Keating NL, Lin NU, Lii H, Winer EP, Freedman RA. Duration and toxicity of adjuvant trastuzumab in older patients with early-stage breast cancer: a population-based study. J Clin Oncol. 2014;32:927–34.
Van Rooijen JM, de Munck L, Teeuwen GM, de Graaf JC, Jansman FG, Boers JE, et al. Use of trastuzumab for HER2-positive metastatic breast cancer in daily practice: a population-based study focusing on the elderly. Anticancer Drugs. 2016;27:127–32.
Thavendiranathan P, Poulin F, Lim K-DD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63:2751–68.
Tan TC, Bouras S, Sawaya H, Sebag IA, Cohen V, Picard MH, et al. Time trends of left ventricular ejection fraction and myocardial deformation indices in a cohort of women with breast cancer treated with anthracyclines, taxanes, and trastuzumab. J Am Soc Echocardiogr. 2015;28:509–14.
Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5:596–603.
Pituskin E, Haykowsky M, Mackey JR, Thompson RB, Ezekowitz J, Koshman S, et al. Rationale and design of the Multidisciplinary Approach to Novel Therapies in Cardiology Oncology Research Trial (MANTICORE 101—Breast): a randomized, placebo-controlled trial to determine if conventional heart failure pharmacotherapy can prevent trastuzumab-mediated left ventricular remodeling among patients with HER2+ early breast cancer using cardiac MRI. BMC Cancer. 2011;11:318.
Heck SL, Gulati G, Ree AH, Schulz-Menger J, Gravdehaug B, Røsjø H, et al. Rationale and design of the prevention of cardiac dysfunction during an Adjuvant Breast Cancer Therapy (PRADA) Trial. Cardiology. 2012;123:240–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Cardio-oncology
Rights and permissions
About this article
Cite this article
Addetia, K., DeCara, J.M. Cardiac Complications of HER2-Targeted Therapies in Breast Cancer. Curr Treat Options Cardio Med 18, 36 (2016). https://doi.org/10.1007/s11936-016-0458-6
Published:
DOI: https://doi.org/10.1007/s11936-016-0458-6