Abstract
Purpose of Review
We reviewed the advantages and disadvantages of transperineal prostate biopsy (TP-bx) to evaluate its potential role as the standard of care for prostate biopsy.
Recent Findings
Studies have suggested no difference in prostate cancer (PCa) detection rate between TP-bx and transrectal biopsy (TR-bx) but have suggested potentially increased detection of anterior prostate tumors. Advances in anesthetic technique have obviated the need for sedation thus allowing TP-bx to become an office-based procedure, which in turn can decrease the overall cost of TP-bx. Furthermore, given the low rate of infectious complications after TP-bx, some have foregone peri-procedural antibiotics without a change in the rate of infectious complications.
Summary
Recent procedural advances have made TP-bx a tolerable, office-based procedure. Given the similar diagnostic performance and the benefits for the patient and community, TP-bx should become the standard of care for prostate biopsy for most patients. Future efforts should address the barriers for more universal adoption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate biopsy remains necessary for the diagnosis and treatment of prostate cancer. Biopsies are currently most often performed using a transrectal approach. However, prostate biopsy techniques have changed since inception, adapting to new technologies and discoveries, which now include the re-introduction of the transperineal biopsy (TP-bx). Currently, there is debate over whether transrectal biopsy (TR-bx) or TP-bx should be the standard of care.
Historically, the first prostate biopsies were performed using a transperineal approach, initially as an open surgery and then, after 1922, percutaneously [1]. Up until the 1950s, digitally guided TP-bx was most commonly performed, which was then replaced by digitally guided TR-bx, an approach that persisted into the 1990s [1, 2]. Transrectal ultrasonography (TRUS) was first used to augment the prostate biopsy technique in 1989; its use allowed for more accurate and less morbid procedures [1, 3]. TRUS remains indispensable for office-based biopsies. Next in the prostate biopsy continuum, urologists discovered that a 12-core sextant biopsy led to improved cancer detection rates when compared to a reduced number of cores, which ultimately lead the field to adopt biopsy templates that employ a minimum of 10–12 cores [4, 5]. The next step in prostate biopsy innovation came after the PROMIS trial supported multiparametric MRI (mpMRI) as a tool to increase the detection of clinically significant prostate cancer (CS-PCa) while decreasing the diagnosis of clinically insignificant prostate cancer (CI-PCa) [6]. With each of these innovations and discoveries, urologists were required to learn new skills to better patient care. Most recently, some urologists have abandoned TR-bx while adopting the transperineal route.
In this review, we will address the benefits and disadvantages of TP-bx. Specifically, we will focus on PCa detection rates, infectious and non-infectious complications, antibiotic stewardship, patient experience, the learning curve, and the associated costs; the synthesis of the above will support TP-bx as the new standard of care for most patients undergoing prostate biopsies.
Cancer Detection Rates
Much of the data supports equivalent cancer detection rates between TP-bx and TR-bx, with some early data suggesting higher rates of clinically significant prostate cancer detection with TP-MRI fusion when compared to TR-MRI fusion biopsy [7, 8, 9•, 10••, 11••]. Combined PCa detection rates (CS-PCa and CI-PCa) for systematic, and when indicated, targeted TR-bx range from 30.7 to 71.0% [6, 8, 12, 13]. Similarly, for TP-bx, PCa detection rates range from 42 to 67% [7, 12, 14,15,16]. The lower bound originates from a 2008 study suggesting that the total cancer detection rate with TP-bx is inferior (42.1% vs 48.3%, p = 0.323); however, this was not statistically significant [12]. In comparing systematic TR-bx and TP-bx, multiple studies including randomized controlled trials have found no differences in PCa detection rates between the two approaches (most recently TP-bx 50.4% vs TR-bx 47.3%, p = 0.424) [7, 8]. These data were further confirmed in a meta-analysis (relative risk (RR) of any cancer detection 0.94, 95% confidence interval (CI) 0.81–1.10) [9•]. In contrast to the studies that found no differences in cancer detection rates, a meta-analysis specifically addressing the detection of CS-PCa with MRI-fusion TP-bx and MRI-fusion TR-bx found higher cancer detection rates via the TP approach (RR 1.28, 95% CI 1.03–1.60) [10••]. Furthermore, a large multi-institutional retrospective study comparing MRI-targeted TP-bx and MRI-targeted TR-bx (MRI-fusion and cognitive fusion) found higher cancer detection rates with TP-bx (odds ratio (OR) of CS-PCa 1.19, 95% CI 1.12–1.50) [11••].
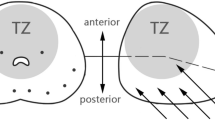
While overall cancer detection rates are similar, there may be some benefit to TP-bx in detecting anterior tumors, especially with the advent of multiparametric prostate MRI [10••, 11••, 17]. A study in 2012 determined a higher proportion of anterior tumors were detected by TP-bx compared to TR-bx (16.2% of exclusively anterior tumors vs 12.0%, p = 0.046), and these were detected at a smaller size and stage [17]. Similarly, a systematic review and meta-analysis of MRI-fusion TP-bx versus MRI-fusion TR-bx determined that anterior CS-PCa detection rates were higher with TP-bx (RR 2.46, 95% CI 1.22–4.98) [10••]. Most recently, a 2022 study showed that MRI-targeted TP-bx, when compared to TR-bx, detected more CS-PCa at the apex (OR 4.81, 95% CI 1.03–6.27), in the anterior prostate (OR 5.62, 95% CI 1.74–8.13), and in the transition/central zone (OR 2.67, 95% CI 1.42–5.0) [11••]. Despite the possible benefit in diagnosing anterior tumors using TP-bx, some urologists report difficulties using the TP approach in patients with a larger prostate and/or a narrow pelvis. With recent technical developments, however, some have anecdotally suggested that using a transperineal access system as opposed to a grid may help overcome these challenges.
Infectious Complications and Antibiotic Stewardship
Infectious complications of prostate biopsy with standard prophylactic antibiotics have risen, leading urologists to develop new approaches to prophylaxis [18,19,20,21,22]. The rise in infectious complications is likely secondary to antibiotic resistance as the higher infectious complication rate is associated with an increase in antibiotic-resistant Enterobacteriaceae, including fluoroquinolone-resistant E. coli [21, 23,24,25]. In fact, the degree of fluoroquinolone-resistant E. coli between 2003 and 2006 was associated with specialty-specific prescription patterns at one Dutch hospital, with urology having the highest antibiotic prescription rate and resistance to fluoroquinolones [25].
In response to rising rates of infectious complications, two main prophylactic strategies have emerged: targeted prophylaxis and augmented prophylaxis. Targeted prophylaxis involves collecting a preprocedural rectal culture and targeting the prophylactic antibiotic to the culture results. An initial study evaluating the efficacy of targeted prophylaxis demonstrated that despite 19.6% of patients harboring fluoroquinolone-resistant organisms, targeted prophylaxis decreased post-biopsy infectious complications, although this was not statistically significant (targeted prophylaxis 0% vs control 2.6%, p = 0.12) [26]. This finding prompted later studies and a meta-analysis, which demonstrated a statistically significant higher rate of infectious complications when using empiric as opposed to targeted prophylaxis (RR 1.81, 95% CI 1.28–2.55) [27•, 28,29,30,31,32,33]. In contrast to targeted prophylaxis, augmented prophylaxis typically adds a second, often more broad-spectrum antimicrobial to the peri-procedural antibiotic regimen. Bloomfield et al. demonstrated augmented standard ciprofloxacin prophylaxis with a single dose of ertapenem and showed a decrease in all infectious complications (from 2.65 to 0.34%; risk ratio 0.13, 95% CI 0.06–0.27) and bacteremia (from 1.14 to 0.04%; risk ratio 0.04, 95% CI 0.01–0.22) [34]. Similarly, augmenting standard ciprofloxacin with ceftriaxone decreased hospitalizations for post-biopsy infections (from 0.6 to 0.0%, p < .0001) [35]. The efficacy of these two strategies was further supported by implementing them across the Michigan Urological Surgery Improvement Collaborative’s participating practices (individual practices chose which strategy to follow), which resulted in a decrease in post-biopsy infection-related hospitalizations (from 1.19 to 0.56%, p = 0.002) [36].
In contrast to the TR-bx technique, which requires antibiotics for acceptable levels of infectious complications, the reintroduction of TP-bx has been accompanied by an often lower rate of infectious complications when compared to TR-bx, even without antibiotics. TP-bx distinguishes itself from TR-bx from an infectious standpoint given the very nature of avoiding the rectum, which reduces the bacterial load on the biopsy needle as it pierces the prostate [37, 38]. While one early meta-analysis demonstrated no difference in sepsis rates between TP-bx and TR-bx (2/497 vs 2/472, respectively, p = 0.936) nor a significant difference in rates of fever (1/447 vs 7/435, respectively, p = 0.073), this study remains an outlier in its findings [39]. Many other large series demonstrated reduced infectious complications after TP-bx when compared to TR-bx [39,40,41]. One large analysis of 73,630 biopsies determined that TP-bx was associated with a lower rate of readmission secondary to sepsis (1.0% vs 1.4%; adjusted risk difference −0.4%, 95% CI −0.6 to −0.2) [41]. A similar outcome was described in another large study (n = 4233 biopsies) where TP-bx resulted in no patients with bacteremia, no patients requiring hospitalization for complications, and was associated with a lower risk of all infectious complications when compared to TR-bx (adjusted odds ratio 0.28, 95% CI 0.08–0.68) [40]. Separating urinary tract infection (UTI) from sepsis, a 2022 study determined that TR-bx had a higher risk of both UTI and sepsis when compared to TP-bx (RR of sepsis 3.65, 95% CI 1.21–11.03; RR of UTI 3.04, 95% CI 1.07−8.66) [7]. The lower incidence of infectious complications and the nature of the TP-bx not going through the rectum has driven some to omit prophylactic antibiotics with the resultant infectious complications still nearly non-existent. In one retrospective cohort of 184 patients undergoing TP-bx without prophylactic antibiotics, there were no cases of sepsis and only two cases of afebrile UTIs [42]. In another, antibiotic prophylaxis prior to TP-bx was not associated with a lower risk of sepsis (RR 0.78, 95% CI 0.13−4.63) or UTI (RR 1.17, 95% CI 0.24−5.74) [7]. Similarly, two recent systematic reviews and meta-analyses comparing TP-bx with and without antibiotic prophylaxis found no difference in rates of sepsis (with antibiotics 0.05% and 0.13% versus no antibiotics 0.08% and 0.09%, p > 0.05 for both reviews) or overall infections (RR 2.09, 95% CI 0.54–8.10 and RR 1.11, 95% CI 0.84–1.46) [43, 44••]. Recently, a randomized controlled trial (RCT) of 555 patients undergoing TP-bx with or without the use of prophylactic antibiotics demonstrated no hospitalizations for sepsis or UTI in either group. There was, however, a non-significantly higher risk of UTI in the group without prophylaxis (1/277 receiving antibiotics vs 3/276; absolute difference 0.73%, 95% CI −1.08 to 2.81) [45••].
TP-bx has the potential to decrease and nearly eliminate the use of prophylactic antibiotics for prostate biopsies, thus reducing the contribution of prostate biopsies to antibiotic resistance [46, 47]. While the targeted and augmented prophylactic antibiotic approaches have decreased post-biopsy infectious complications, there is concern over the contribution of continued antibiotic use prior to prostate biopsy on the emergence of resistant organisms and their subsequent infections, especially if using broader-spectrum antibiotics [22, 48,49,50]. Antibiotic resistance develops in association with microbial exposure to antibiotics and, while necessary for TR-bx, likely contributes to increased resistance [48,49,50]. The suggested use of carbapenems to augment prophylaxis furthermore runs contrary to the Center for Disease Control recommendation to avoid carbapenems when possible given the urgent threat level (highest) of carbapenem-resistant Enterobacteriaceae, which cost 1100 lives and $130,000,000 in 2017 in the USA [51].
Non-Infectious Complications
While infectious complications remain the most distinctive difference between TP-bx and TR-bx, urologists should consider other complications of prostate biopsy including acute urinary retention (AUR), rectal bleeding, hematuria, erectile dysfunction, and vasovagal/syncopal events. Some suggest increased rates of AUR with TP-bx when compared to TR-bx [52,53,54]. However, this increased rate may be an artifact of the number of biopsy cores rather than the method of biopsy. Other recent studies, in which the number of cores is comparable to TR-bx, have reported similar rates of AUR (2.15–5.0% for TP-bx vs 2.46–6.3% after TR-Bx, p > 0.05 in all studies) [7, 10••, 55]. TP-bx has lower rates of rectal bleeding (RR 0.02, 95% CI 0.01–0.06) and similar rates of hematuria (RR 0.79, 95% CI 0.63–1.01) [8, 9•, 55]. The impact on erectile function also appears similar between both approaches. After both TR-bx and TP-bx, there is a significant decrease in erectile function, as measured by the International Index of Erectile Function score, without a difference between the two approaches; the reduced erectile dysfunction appears to resolve in 3 months [56,57,58]. Furthermore, some suggest an increased risk of syncopal/vasovagal events after TP-bx; however, published data support a similar range between both approaches, from 0.6 to 0.9% after TP-bx compared to 0.05% to 1.2% after TR-bx [8, 59, 60].
Patient Experience and Pain
Many studies demonstrate that when comparing TP-bx and TR-bx under local anesthesia, patients undergoing TP-bx are more likely to experience pain, and that the pain is worse. However, techniques to improve analgesia during TP-bx have been introduced and continue to evolve. These new techniques have contributed to a significant reduction in pain, resulting in the TP-bx now being fairly well-tolerated with a similar impact on quality of life (QOL) as TR-bx [61]. An early (2015) study comparing pain during TP-bx and TR-bx performed under local anesthesia determined that in the TP-bx group, the pain intensity was twice that of the TR-bx group, albeit the reported pain was mild (median visual analogue scale [VAS] score 4.0 vs 2.0, p < 0.001); the difference was driven by the infiltration with local anesthesia [8]. A review also concluded that there was a higher chance of feeling any pain during TP-bx (RR 1.83, 95% CI 1.27–2.65) [9•]. Similar pain scores between TP-bx and TR-bx in another study underline that with good anesthetic technique, the difference in pain can be substantially decreased and possibly even eliminated (mean VAS score 1.56 vs 1.42, respectively, p = 0.591) [62]. In fact, in a prospective study of 1218 patients who were surveyed on their experience with TP-bx, only 5.6% believed it caused significant enough pain to necessitate general anesthesia [16].
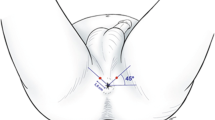
Techniques to improve analgesia during TP-bx continue to evolve [8, 9•, 61]. The pain generated during TR and TP biopsies shares some similarities given the use of the TR ultrasound probe and piercing of the prostatic capsule but is differentiated by the TP-bx uniquely causing pain through the sensors at the perineal skin and the structures superficial to the prostatic capsule (innervated by the pudendal nerve) [63]. These pelvic innervation patterns differentially contribute to the discomfort patients feel during the TP-bx procedure and have led to the development of directed anesthetic techniques [8, 64, 65••, 66]. Understanding which parts of the biopsy the patient finds most uncomfortable allows urologists to address them with new techniques. Multiple studies of TP-bx performed under local anesthesia have demonstrated that patients report the most pain during the administration of local anesthesia and the least during probe placement [15, 64, 66]. The unique pain of local anesthesia raises the possibility of decreasing the maximum pain level by buffering the local anesthetic with sodium bicarbonate, which has been shown to decrease pain levels in both breast biopsies and hand surgery [67,68,69]. One study performing a peri-prostatic block and skin infiltration of buffered local anesthesia found excellent pain control comparable to that reported for TR-bx and the most effective techniques for TP-bx (median VAS score 2) [15]. In a RCT comparing three anesthesia methods, the best pain control was provided with local infiltration of the skin and a pelvic plexus block (mean VAS score 2.1) [65••]. Regardless, most studies have found that with current analgesic techniques, most men have adequate pain control during TP-bx with pain scores in the mild range [14, 64, 66]. Furthermore, the use of a transperineal access system avoids multiple skin punctions (compared to one puncture per biopsy with a grid) and has also been associated with decreased pain scores (mean whole procedure VAS score 2.20 vs 2.90, respectively, p < 0.01) [70]. Studies further suggest that patient characteristics, such as anxiety level, are associated with pain level during both TP-bx and TR-bx [64, 71]. Despite the widespread tolerability of TP-bx, just as with TR-bx, there are patients that will require sedation to undergo the procedure. Contemporary data, however, support that both TR-bx and TP-bx are well-tolerated under local anesthesia, obviating the need for sedation for most, regardless of the technique.
Additional research into novel techniques for pain control will only further improve the patient experience.
Learning Curve
Some contend that the learning curve may be longer with TP-bx when compared to TR-bx. However, the difference might not be significant. Prior work suggests that it takes roughly 12 procedures to perform a high-quality systematic TR-bx [72]. Similarly, initial data suggests that high-quality TP-biopsies are achieved after 15 procedures [73].
There are more published data on the learning curve for MRI-fusion TP and TR-bx techniques. These data suggest that the learning curve for the TP-MRI fusion technique is not prohibitive and can be augmented by structured training protocols. The learning curve for MRI-fusion TR-bx ranges from 82 to 109 biopsies, depending on the outcome measure used to evaluate the learning curve [74,75,76]. Formalized training shortens the learning curve for MRI-fusion TP-biopsies. In one urologist’s experience with no formalized didactics, the procedure time and PCa detection rate were optimized after 119 and 124 biopsies, respectively [76]. In a study assessing junior residents’ performance after a 2-week training period followed by approximately 84 independently performed biopsies, there was no difference in PCa detection or patient pain when compared to the attending physician; the residents, however, did take longer to perform the procedure (16 min versus 19.7–20.1 min, p < 0.001) [77]. In another study of residents undergoing a rigorous MRI-fusion TP-bx and systematic TP-bx training program, the residents achieved PCa detection rates comparable to published norms after 10 training biopsies and approximately 37 independently performed biopsies [78•]. While concerns over the learning curve persist, structured training protocols might hasten urologists’ progression and learning and render the learning curves for TP-bx and TR-bx similar.
Procedure duration is also referenced when discussing learning curves. Published procedure durations for MRI-fusion TR-bx have ranged from 14.73 to 24.0 min, and for MRI-fusion TP-bx, they have ranged from 14.4 to 22.5 min [8, 75,76,77, 78•, 79]. The considerable overlap between the methods likely reflects the heterogeneity of the biopsy technique, anesthetic method, and operator characteristics, raising questions about the generalizability of published values. Ultimately, to better analyze the learning curve and procedure times, newer studies are needed using modern techniques and training programs.
Associated Costs
Three distinct cost categories must be considered regarding TP-bx in comparison to TR-bx: capital costs to be able to perform TP-bx, procedural costs, and downstream costs.
Capital costs include the non-consumables required to perform TP-bx. At a minimum, the facility needs an exam bed and stirrups to achieve lithotomy position, a transrectal ultrasound probe adequate for TP-bx, and an ultrasound machine. In order to perform software-based MRI-fusion TP-bx (currently performed by approximately 58% of urologists in the USA), the facility also requires the necessary commercial hardware, which can be purchased or rented [80]. Furthermore, costs related to lost productivity and training must be considered.
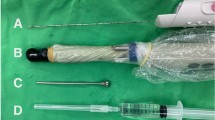
Procedural costs include personnel and physician fees and consumables, some of which are specific to TP or TR biopsy. Performing TP-bx under general anesthesia/sedation has traditionally kept the TP-bx procedural costs higher than TR-bx; however, the recent introduction of performing TP-bx under local anesthesia removes these anesthesia-related costs [81, 82]. There are other procedural costs which are specific to TP-bxs, such as a transperineal access unit (e.g., the FDA-approved PrecisionPoint™ Transperineal Access System, which retails for an estimated $200) or a biopsy grid. However, some clinicians are using a lower-cost alternative (e.g., a 14-g peripheral IV catheter), and other potentially lower-cost options including non-disposable guides are being developed (e.g., the Cambridge Prostate Biopsy Device, SureFire™). Cost savings for TP-bxs potentially lie in omitting prophylactic antibiotics and stool cultures.
Finally, downstream costs of prostate biopsy should include costs of complications, which, as previously described, vary in magnitude by the biopsy approach. In one example, the cost of treating post-biopsy sepsis has been estimated between $8672 and $19100 per episode in the USA [83]. One study found that when including complications in their cost analysis, TP-bxs were associated with a lower cost for the health system when compared to TR-bx [55]. Treating non-infectious complications incurs other costs (e.g., with associated unplanned clinic or emergency department visits) and should be included in calculations.
Cost calculations are complicated given the immediate and potential downstream impacts of cost. Furthermore, costs may have differential impacts on various stakeholders. For practices not yet equipped to transition to TP-bx, up-front capital costs can be significant; these costs are not reimbursed and could disincentivize and/or limit a practice’s ability to transition to performing TP biopsies. On the other hand, costs of complications currently impact healthcare payers and patients (through copayments and premiums), yet they do not currently directly affect the proceduralist. The interplay of these costs provides an opportunity to implement system-based changes that incentivize involved parties to adopt TP-bx whether it be by increasing reimbursements for TP-bx or bundling prostate biopsies to their complications.
Conclusion
Recent data and advancements in technique suggest that TP-bx may now be superior to TR-bx for most patients undergoing prostate biopsy. The data comparing TP-bx present a similar cancer detection rate to TR-bx, with the potential of TP-bx allowing for a better sampling of the anterior prostate. Infectious complications are nearly avoided with TP-bx, and one can safely omit antibiotics in many patients thus not further contributing to antibiotic resistance. TP-bx has a non-infectious complication profile that is similar to TR-bx. While many studies report worse pain scores with TP-bx under local anesthesia when compared to TR-bx, with the introduction of new anesthetic techniques, this pain level is tolerable, and the procedure is frequently performed in a clinic using local anesthetic. The learning curve for TP-bx should not preclude implementation given its similarity to TR-bx. Once the initial capital costs required to perform TP-bx are surpassed, the procedural costs are similar with modern techniques, and there are potential savings at a system level by avoiding costly complications. On the road to better diagnose prostate cancer, urologists have adapted new technologies and protocols to better serve our patients; recent data support a return to TP-bx as the next step in this prostate biopsy evolution.
The question remains, “should TP-bx be the standard of care?” The answer is “yes.” TP-bx should be the standard of care for most patients. This does not discredit, however, the significant capital investment and training which will be required to make this become the standard of care, and there will still be some patients for whom TP-bx is not optimal. Additional research should evaluate methods to improve patient tolerability and identify barriers and facilitators to universal adoption (e.g., capital costs, cost of consumables, education).
Abbreviations
- AUR:
-
Acute urinary retention
- CI:
-
Confidence interval
- CI-PCa:
-
Clinically insignificant prostate cancer
- CS-PCa:
-
Clinically significant prostate cancer
- mpMRI:
-
Multiparametric magnetic resonance imaging
- OR:
-
Odds ratio
- PCa:
-
Prostate cancer
- QOL:
-
Quality of life
- RCT:
-
Randomized controlled trial
- RR:
-
Relative risk
- TP-bx:
-
Transperineal prostate biopsy
- TR-bx:
-
Transrectal prostate biopsy
- TRUS:
-
Transrectal ultrasound
- UTI:
-
Urinary tract infection
- VAS:
-
Visual analogue scale
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Yeo L, Patel D, Bach C, Papatsoris A, Buchholz N, Junaid I, et al. The development of the modern prostate biopsy. In: Bissada NK, editor. Prostate Biopsy. InTech; 2011 [cited 2022 Aug 9]. Available from: http://www.intechopen.com/books/prostate-biopsy/the-development-of-the-modern-prostate-biopsy.
Sathianathen NJ, Konety BR, Crook J, Saad F, Lawrentschuk N. Landmarks in prostate cancer. Nat Rev Urol. 2018;15:627–42.
Hodge KK, McNeal JE, Stamey TA. Ultrasound guided transrectal core biopsies of the palpably abnormal prostate. J Urol. 1989;142:66–70.
Levine MA, Ittman M, Melamed J, Lepor H. Two consecutive sets of transrectal ultrasound guided sextant biopsies of the prostate for the detection of prostate cancer. J Urol. 1998;159:471–6.
Taneja SS, Bjurlin MA, Carter HB, Cookson MS, Gomella LG, Penson DF, et al. American urological association optimal techniques of prostate biopsy and specimen handling [white paper]. 2015. https://www.auanet.org/guidelines-and-quality/quality-and-measurement/quality-improvement/clinical-consensus-statement-and-quality-improvement-issue-brief-(ccs-and-qiib)/prostate-biopsy-and-specimen-handling.
Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. The Lancet. 2017;389:815–22.
He J, Guo Z, Huang Y, Wang Z, Huang L, Li B, et al. Comparisons of efficacy and complications between transrectal and transperineal prostate biopsy with or without antibiotic prophylaxis. Urol Oncol Semin Orig Investig. 2022;40:191.e9-191.e14.
Guo L-H, Wu R, Xu H-X, Xu J-M, Wu J, Wang S, et al. Comparison between ultrasound guided transperineal and transrectal prostate biopsy: a prospective, randomized and controlled trial. Sci Rep. 2015;5:16089.
• Xiang J, Yan H, Li J, Wang X, Chen H, Zheng X. Transperineal versus transrectal prostate biopsy in the diagnosis of prostate cancer: a systematic review and meta-analysis. World J Surg Oncol. 2019;17:31. Review including 6609 patients, which found a five-fold reduction in post-prostate biopsy sepsis after TP-bx compared to TR-bx.
•• Rai BP, Mayerhofer C, Somani BK, Kallidonis P, Nagele U, Tokas T. Magnetic resonance imaging/ultrasound fusion-guided transperineal versus magnetic resonance imaging/ultrasound fusion-guided transrectal prostate biopsy—a systematic review. Eur Urol Oncol. 2021;4:904–13. Review which found that TP-bx had a higher PCa detection rate of anterior CS-PCa and a lower complication rate when correcting for number of cores compared to TR-bx.
•• Zattoni F, Marra G, Kasivisvanathan V, Grummet J, Nandurkar R, Ploussard G, et al. The detection of prostate cancer with magnetic resonance imaging-targeted prostate biopsies is superior with the transperineal vs the transrectal approach. A European Association of Urology-Young Academic Urologists Prostate Cancer Working Group Multi-Institutional Study. J Urol. 2022;208:830–7. A large, multi-institution retrospective study demonstrating MRI-targeted TP-bx had a higher detection rate of all PCa and CS-PCa compared to TR-bx. MRI-targed TP-bx also had a higher detection rate of anterior, apical, and transition-zone PCa.
Hara R, Jo Y, Fujii T, Kondo N, Yokoyoma T, Miyaji Y, et al. Optimal approach for prostate cancer detection as initial biopsy: prospective randomized study comparing transperineal versus transrectal systematic 12-core biopsy. Urology. 2008;71:191–5.
Takenaka A, Hara R, Ishimura T, Fujii T, Jo Y, Nagai A, et al. A prospective randomized comparison of diagnostic efficacy between transperineal and transrectal 12-core prostate biopsy. Prostate Cancer Prostatic Dis. 2008;11:134–8.
Immerzeel J, Israël B, Bomers J, Schoots IG, van Basten J-P, Kurth K-H, et al. Multiparametric magnetic resonance imaging for the detection of clinically significant prostate cancer: what urologists need to know. Part 4: Transperineal Magnetic Resonance–Ultrasound Fusion Guided Biopsy Using Local Anesthesia. Eur Urol. 2022;81:110–7.
Jacewicz M, Günzel K, Rud E, Lauritzen PM, Galtung KF, Hinz S, et al. Multicenter transperineal MRI-TRUS fusion guided outpatient clinic prostate biopsies under local anesthesia. Urol Oncol Semin Orig Investig. 2021;39:432.e1-432.e7.
Lopez JF, Campbell A, Omer A, Stroman L, Bondad J, Austin T, et al. Local anaesthetic transperineal (LATP) prostate biopsy using a probe-mounted transperineal access system: a multicentre prospective outcome analysis. BJU Int. 2021;128:311–8.
Hossack T, Patel MI, Huo A, Brenner P, Yuen C, Spernat D, et al. Location and pathological characteristics of cancers in radical prostatectomy specimens identified by transperineal biopsy compared to transrectal biopsy. J Urol. 2012;188:781–5.
Carignan A, Valiquette L, Sabbagh R, Pepin J. Increasing risk of infectious complications after transrectal ultrasound–guided prostate biopsies: time to reassess antimicrobial prophylaxis? Eur Urol. 2012;7.
Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830–4.
Nam RK, Saskin R, Lee Y, Liu Y, Law C, Klotz LH, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol. 2010;183:963–9.
Karlowsky JA, Adam HJ, Desjardins M, Lagace-Wiens PRS, Hoban DJ, Zhanel GG, et al. Changes in fluoroquinolone resistance over 5 years (CANWARD 2007–11) in bacterial pathogens isolated in Canadian hospitals. J Antimicrob Chemother. 2013;68:i39-46.
Roberts MJ, Bennett HY, Harris PN, Holmes M, Grummet J, Naber K, et al. Prostate biopsy-related infection: a systematic review of risk factors, prevention strategies, and management approaches. Urology. 2017;104:11–21.
Spellberg B, Doi Y. The rise of fluoroquinolone-resistant Escherichia coli in the community: scarier than we thought. J Infect Dis. 2015;212:1853–5.
Johnson JR, Porter S, Thuras P, Castanheira M. Epidemic emergence in the United States of Escherichia coli sequence type 131- H 30 (ST131- H 30), 2000 to 2009. Antimicrob Agents Chemother. 2017;61:e00732-17.
Willemsen I, Bogaers-Hofman D, Winters M, Kluytmans J. Correlation between antibiotic use and resistance in a hospital: temporary and ward-specific observations. Infection. 2009;37:432–7.
Taylor AK, Zembower TR, Nadler RB, Scheetz MH, Cashy JP, Bowen D, et al. Targeted antimicrobial prophylaxis using rectal swab cultures in men undergoing transrectal ultrasound guided prostate biopsy is associated with reduced incidence of postoperative infectious complications and cost of care. J Urol. 2012;187:1275–9.
• Pilatz A, Dimitropoulos K, Veeratterapillay R, Yuan Y, Omar MI, MacLennan S, et al. Antibiotic prophylaxis for the prevention of infectious complications following prostate biopsy: a systematic review and meta-analysis. J Urol. 2020;204:224–30. Review that shows the impact of targeted prophylaxis on decreasing infectious complications after TR-bx.
Elshal AM, Atwa AM, El-Nahas AR, El-Ghar MA, Gaber A, Elsawy E, et al. Chemoprophylaxis during transrectal prostate needle biopsy: critical analysis through randomized clinical trial. World J Urol. 2018;36:1845–52.
Fahmy A, Rhashad H, Mohi M, Elabbadie A, Kotb A. Optimizing prophylactic antibiotic regimen in patients admitted for transrectal ultrasound-guided prostate biopsies: a prospective randomized study. Prostate Int. 2016;4:113–7.
Ozgur A, Asif Y, Gokhan A, Berrin T, Cenk G, Bulent E, et al. Prevalence of antibiotic resistance in fecal flora before transrectal ultrasound-guided prostate biopsy and the clinical impact of targeted antibiotic prophylaxis. Arch Esp Urol. 2017;70(10):852–858.
Liss MA, Sherrill A, Barney S, Yunes A, Sokurenko E, Wickes B. Prospective implementation of a point-of-care PCR-based detection method to guide antibiotic use prior to prostate biopsy compared to targeted prophylaxis and physician choice. Urology. 2019;129:87–91.
Van Besien J, Uvin P, Weyne E, Van Praet C, Merckx L, De Graeve N, et al. Use of fosfomycin as targeted antibiotic prophylaxis before prostate biopsy: a prospective randomized study. Int J Urol. 2019;26:391–7.
Doherty AF, Ikuerowo SO, Jeje EA, Ibrahim NA, Ojongbede OL, Mutiu WB, et al. A prospective randomized comparative study of targeted versus empirical prophylactic antibiotics in the prevention of infective complications following transrectal ultrasound- guided prostate biopsy. Ann Afr Med. 2019;18:7.
Bloomfield MG, Wilson AD, Studd RC, Blackmore TK. Highly effective prophylaxis with ertapenem for transrectal ultrasound-guided prostate biopsy: effects on overall antibiotic use and inpatient hospital exposure. J Hosp Infect. 2020;106:483–9.
Luong B, Danforth T, Visnjevac O, Suraf M, Duff M, Chevli KK. Reduction in hospital admissions with the addition of prophylactic intramuscular ceftriaxone before transrectal ultrasonography–guided prostate biopsies. Urology. 2015;85:511–6.
Womble PR, Linsell SM, Gao Y, Ye Z, Montie JE, Gandhi TN, et al. A statewide intervention to reduce hospitalizations after prostate biopsy. J Urol. 2015;194:403–9.
Lightner DJ, Wymer K, Sanchez J, Kavoussi L. Best practice statement on urologic procedures and antimicrobial prophylaxis. J Urol. 2020;203:351–6.
Werneburg GT, Adler A, Zhang A, Mukherjee SD, Haywood S, Miller AW, et al. Transperineal prostate biopsy is associated with lower tissue core pathogen burden relative to transrectal biopsy: mechanistic underpinnings for lower infection risk in the transperineal approach. Urology. 2022;165:1–8.
Xue J, Qin Z, Cai H, Zhang C, Li X, Xu W, et al. Comparison between transrectal and transperineal prostate biopsy for detection of prostate cancer: a meta-analysis and trial sequential analysis. Oncotarget. 2017;8:23322–36.
Tops SCM, Grootenhuis JGA, Derksen AM, Giardina F, Kolwijck E, Wertheim HFL, et al. The effect of different types of prostate biopsy techniques on post-biopsy infectious complications. J Urol. 2022;208:109–18.
Berry B, Parry MG, Sujenthiran A, Nossiter J, Cowling TE, Aggarwal A, et al. Comparison of complications after transrectal and transperineal prostate biopsy: a national population-based study. BJU Int. 2020;126:97–103.
Sigle A, Suarez-Ibarrola R, Pudimat M, Michaelis J, Jilg CA, Miernik A, et al. Safety and side effects of transperineal prostate biopsy without antibiotic prophylaxis. Urol Oncol Semin Orig Investig. 2021;39:782.e1-782.e5.
Castellani D, Pirola GM, Law YXT, Gubbiotti M, Giulioni C, Scarcella S, et al. Infection Rate after transperineal prostate biopsy with and without prophylactic antibiotics: results from a systematic review and meta-analysis of comparative studies. J Urol. 2022;207:25–34.
•• Basourakos SP, Alshak MN, Lewicki PJ, Cheng E, Tzeng M, DeRosa AP, et al. Role of prophylactic antibiotics in transperineal prostate biopsy: a systematic review and meta-analysis. Eur Urol Open Sci. 2022;37:53–63. Systematic review showing no difference in sepsis or infections after TP-bx with or without pre-biopsy antibiotics.
•• Jacewicz M, Günzel K, Rud E, Sandbæk G, Magheli A, Busch J, et al. Antibiotic prophylaxis versus no antibiotic prophylaxis in transperineal prostate biopsies (NORAPP): a randomised, open-label, non-inferiority trial. Lancet Infect Dis. 2022;S1473309922003735. Randomized controlled trial of pre-biopsy antibiotics or not (555 patients total), which found no septic events in either group.
Fishman N, Society for Healthcare Epidemiology of America, Infectious Diseases Society of America, Pediatric Infectious Diseases Society. Policy Statement on Antimicrobial Stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol. 2012;33:322–7.
Dyar OJ, Huttner B, Schouten J, Pulcini C. What is antimicrobial stewardship? Clin Microbiol Infect. 2017;23:793–8.
Wilcox MH. The tide of antimicrobial resistance and selection. Int J Antimicrob Agents. 2009;34:S6-10.
Kaye KS, Cosgrove S, Harris A, Eliopoulos GM, Carmeli Y. Risk factors for emergence of resistance to broad-spectrum cephalosporins among Enterobacter spp. Antimicrob Agents Chemother. 2001;45:2628–30.
Vestergaard M, Paulander W, Marvig RL, Clasen J, Jochumsen N, Molin S, et al. Antibiotic combination therapy can select for broad-spectrum multidrug resistance in Pseudomonas aeruginosa. Int J Antimicrob Agents. 2016;47:48–55.
Centers for Disease Control and Prevention (U.S.). Antibiotic resistance threats in the United States, 2019. Centers for Disease Control and Prevention (U.S.); 2019. Available from: https://stacks.cdc.gov/view/cdc/82532.
Skouteris VM, Crawford ED, Mouraviev V, Arangua P, Metsinis MP, Skouteris M, et al. Transrectal ultrasound–guided versus transperineal mapping prostate biopsy: complication comparison. Rev Urol. 2018;20(1):19–25.
Borghesi M, Ahmed H, Nam R, Schaeffer E, Schiavina R, Taneja S, et al. Complications after systematic, random, and image-guided prostate biopsy. Eur Urol. 2017;71:353–65.
Miah S, Eldred-Evans D, Simmons LAM, Shah TT, Kanthabalan A, Arya M, et al. Patient reported outcome measures for transperineal template prostate mapping biopsies in the PICTURE study. J Urol. 2018;200:1235–40.
Roberts MJ, Macdonald A, Ranasinghe S, Bennett H, Teloken PE, Harris P, et al. Transrectal versus transperineal prostate biopsy under intravenous anaesthesia: a clinical, microbiological and cost analysis of 2048 cases over 11 years at a tertiary institution. Prostate Cancer Prostatic Dis. 2021;24:169–76.
Fainberg J, Gaffney CD, Pierce H, Aboukhshaba A, Chughtai B, Christos P, et al. Erectile dysfunction is a transient complication of prostate biopsy: a systematic review and meta-analysis. J Urol. 2021;205:664–70.
García Rojo E, García Gómez B, González Padilla DA, Abad López P, García González L, Rodríguez Antolín A, et al. Assessment of the influence of transrectal and transperineal prostate biopsies on erectile function: a prospective observational single-center study. Int J Urol. 2019;26:1054–8.
Mehta A, Kim WC, Aswad KG, Brunckhorst O, Ahmed HU, Ahmed K. Erectile function post prostate biopsy: a systematic review and meta-analysis. Urology. 2021;155:1–8.
Pepe P, Pennisi M. Morbidity following transperineal prostate biopsy: our experience in 8.500 men. Arch Ital Urol E Androl. 2022;94:155–9.
Chiang I-N, Chang S-J, Pu Y-S, Huang K-H, Yu H-J, Huang C-Y. Major complications and associated risk factors of transrectal ultrasound guided prostate needle biopsy: a retrospective study of 1875 cases in Taiwan. J Formos Med Assoc. 2007;106:6.
Shankar PR, Ellimoottil C, George AK, Hadj-Moussa M, Modi PK, Salami S, et al. Testing-related health impact of transrectal and transperineal prostate biopsy as assessed by health utilities. J Urol. 2021;206:1403–10.
Cerruto MA, Vianello F, D’Elia C, Artibani W, Novella G. Transrectal versus transperineal 14-core prostate biopsy in detection of prostate cancer: a comparative evaluation at the same Institution. Arch Ital Urol E Androl. 2014;86:284.
Wang H, Lin H, He B, Guo X, Zhou Y, Xi P, et al. A novel perineal nerve block approach for transperineal prostate biopsy: an anatomical analysis-based randomized single-blind controlled trial. Urology. 2020;146:25–31.
Marra G, Zhuang J, Marquis A, Zhao X, Calleris G, Kan Y, et al. Pain in men undergoing transperineal free-hand multiparametric magnetic resonance imaging fusion targeted biopsies under local anesthesia: outcomes and predictors from a multicenter study of 1,008 patients. J Urol. 2020;204:1209–15.
•• Ding X, Huang T, Lu S, Tao H, Ye X, Wang F, et al. Pelvic plexus block to provide better anesthesia in transperineal template-guided prostate biopsy: a randomised controlled trial. BMC Urol. 2019;19:63. Randomized controlled trial introducing anesthetic techniques with low pain scores.
Stefanova V, Buckley R, Flax S, Spevack L, Hajek D, Tunis A, et al. Transperineal prostate biopsies using local anesthesia: experience with 1,287 patients. Prostate Cancer Detection Rate, Complications and Patient Tolerability. J Urol. 2019;201:1121–6.
Vasan A, Baker JA, Shelby RA, Soo MSC. Impact of sodium bicarbonate-buffered lidocaine on patient pain during image-guided breast biopsy. J Am Coll Radiol. 2017;14:1194–201.
Basourakos SP, Allaway MJ, Ross AE, Schaeffer EM, Hu JC, Gorin MA. Local anaesthetic techniques for performing transperineal prostate biopsy. Nat Rev Urol. 2021;18:315–7.
Lee HJ, Cho YJ, Gong HS, Rhee SH, Park HS, Baek GH. The effect of buffered lidocaine in local anesthesia: a prospective, randomized, double-blind study. J Hand Surg. 2013;38:971–5.
Novella G, Ficarra V, Galfano A, Ballario R, Novara G, Cavalleri S, et al. Pain assessment after original transperineal prostate biopsy using a coaxial needle. Urology. 2003;62:689–92.
Saracoglu T, Unsal A, Taskin F, Sevincok L, Karaman CZ. The impact of pre-procedural waiting period and anxiety level on pain perception in patients undergoing transrectal ultrasound guided prostate biopsy. Diagn Interv Radiol. 2011 [cited 2022 Aug 30]; Available from: https://www.dirjournal.org/en/the-impact-of-pre-procedural-waiting-period-and-anxiety-level-on-pain-perception-in-patients-undergoing-transrectal-ultrasound-guided-prostate-biopsy-13668.
Benchikh El Fegoun A, El Atat R, Choudat L, El Helou E, Hermieu J-F, Dominique S, et al. The learning curve of transrectal ultrasound-guided prostate biopsies: implications for training programs. Urology. 2013;81:12–6.
Berkenwald A, Stensland KD, Sebel LE, Moinzadeh A, Faust W. Initial transperineal prostate biopsy experience at a high-volume center. Can J Urol. 2021;28:10692–8.
Kasabwala K, Patel N, Cricco-Lizza E, Shimpi AA, Weng S, Buchmann RM, et al. The learning curve for magnetic resonance imaging/ultrasound fusion-guided prostate biopsy. Eur Urol Oncol. 2019;2:135–40.
Mager R, Brandt MP, Borgmann H, Gust KM, Haferkamp A, Kurosch M. From novice to expert: analyzing the learning curve for MRI-transrectal ultrasonography fusion-guided transrectal prostate biopsy. Int Urol Nephrol. 2017;49:1537–44.
Halstuch D, Baniel J, Lifshitz D, Sela S, Ber Y, Margel D. Characterizing the learning curve of MRI-US fusion prostate biopsies. Prostate Cancer Prostatic Dis. 2019;22:546–51.
Song J, He B, Li H, Yu X, Shi Z, Ren G, et al. A prospective study comparing cancer detection rates of transperineal prostate biopsies performed by junior urologists versus a senior consultant in a real-world setting. Urol Int. 2021;1–7.
• Mantica G, Pacchetti A, Aimar R, Cerasuolo M, Dotta F, Olivero A, et al. Developing a five-step training model for transperineal prostate biopsies in a naïve residents’ group: a prospective observational randomised study of two different techniques. World J Urol. 2019;37:1845–50. The authors describe a structured training program for TP-bx.
Cricco-Lizza E, Wilcox Vanden Berg RN, Laviana A, Pantuck M, Basourakos SP, Salami SS, et al. Comparative effectiveness and tolerability of transperineal mri-targeted prostate biopsy under local versus sedation. Urology. 2021;155:33–8.
Tooker GM, Truong H, Pinto PA, Siddiqui MM. National survey of patterns employing targeted MRI/US guided prostate biopsy in the diagnosis and staging of prostate cancer. Curr Urol. 2019;12:97–103.
Leung AK, Patil D, Howard DH, Filson CP. Payments and patient cost sharing for prostate biopsies according to image guidance, practice site and use of anesthesia. Urol Pract. 2020;7:138–44.
Altok M, Kim B, Patel BB, Shih Y-CT, Ward JF, McRae SE, et al. Cost and efficacy comparison of five prostate biopsy modalities: a platform for integrating cost into novel-platform comparative research. Prostate Cancer Prostatic Dis. 2018;21:524–32.
Gross MD, Alshak MN, Shoag JE, Laviana AA, Gorin MA, Sedrakyan A, et al. Healthcare costs of post-prostate biopsy sepsis. Urology. 2019;133:11–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Wilcox Vanden Berg has nothing to disclose. Dr. George reports Philips Medical—Research agreement. Dr. Kaye reports personal fees from Janssen Pharmaceuticals, outside the submitted work.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors were performed in accordance with all applicable ethical standards including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Prostate Cancer
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wilcox Vanden Berg, R.N., George, A.K. & Kaye, D.R. Should Transperineal Prostate Biopsy Be the Standard of Care?. Curr Urol Rep 24, 135–142 (2023). https://doi.org/10.1007/s11934-022-01139-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11934-022-01139-0