Abstract
Purpose of Review
Women have an estimated 12.6% lifetime risk of undergoing surgery for pelvic organ prolapse in the USA (Wu et al. in Obstet Gynecol 123(6): 1201–6, 2014). Surgical repair of uterovaginal prolapse most commonly includes hysterectomy and vaginal vault suspension; however, the value of concomitant hysterectomy is uncertain, and there appears to be growing interest in uterine conservation. Multiple procedures have evolved using a variety of approaches. The aim of this paper is to review uterine sparing (hysteropexy) prolapse repair techniques and outcomes.
Recent Findings
Several randomized controlled trials (RCT) have shown comparable success rates for apical compartment support with sacrospinous hysteropexy as compared to vaginal hysterectomy with uterosacral ligament suspension, with shorter hospitalization and quicker return to work. (Detollenaere et al. in BMJ 351: h3717, 2015); (Dietz et al. in Int Urogynecol J Pelvic Floor Dysfunct 21(2): 209–16, 2010). Available data suggest vaginal mesh hysteropexy is as effective as vaginal mesh with hysterectomy, with lower rates of mesh exposure. (Maher et al., 2017) To date, no RCTs have been published comparing sacral hysteropexy to hysterectomy with sacral colpopexy. Overall, there is a higher reoperation rate for sacral hysteropexy and a higher mesh exposure rate for hysterectomy with sacral colpopexy. (Maher et al., 2017) No RCTs have been published comparing hysteropexy surgical approaches.
Summary
Although hysteropexy data is expanding, there is a need for more information regarding long-term surgical durability, appropriate patient selection, and whether one approach is superior to another.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is estimated that women have nearly a 13% lifetime risk of undergoing surgery for prolapse [1]. Surgical repair of uterovaginal prolapse most commonly involves vaginal vault suspension utilizing native tissues or mesh with concurrent hysterectomy. Despite this, it is unknown whether hysterectomy aids in the success of prolapse repair. There appears to be a growing interest in uterine conservation and hysteropexy procedures. These procedures were first described over a century ago and have gained attention over the past decade.
Cross-sectional studies have shown 36–60% of women with symptomatic prolapse would decline hysterectomy for prolapse repair if given an equally efficacious uterine preserving technique. Reasons for avoiding hysterectomy include a desire for future fertility, a belief that the uterus may affect sexual function, the presence of the uterus relating to a sense of identity, and the surgical risks of hysterectomy itself (see Table 1) [3, 4].
It is generally accepted that the uterus plays a passive role in the development of prolapse [5]. Therefore, hysterectomy may not be necessary to correct the underlying defect in the apical vaginal support structures of the uterosacral cardinal ligament complex. Uterine-sparing procedures have been shown to decrease operative time and estimated blood loss [6]. Despite these benefits, pelvic surgeons may not consider uterine conservation as a viable option at the time of prolapse repair. This may be due to a lack of training in uterine-sparing procedures, a belief that concomitant hysterectomy results in better surgical outcomes, and concern that future hysterectomy for any indication may be more technically challenging.
Our aim is to review the variety of uterine-sparing (hysteropexy) prolapse repair techniques including surgical outcomes and appropriate patient selection.
Patient Selection
For patients considering a uterine-sparing prolapse repair, careful counseling and selection by the provider is critical, as there are certain patients in whom uterine preservation is contraindicated. Patients who have an increased risk for uterine, ovarian, or cervical malignancy are not good candidates for uterine preservation. Overall lifetime risk of cervical (0.6%), uterine (2.7%), and ovarian (1.4%) cancers should be reviewed with patients, and screening for factors that would further increase these risks is critical [7]. Obesity, the largest modifiable risk factor for endometrial cancer, should be considered a relative contraindication for uterine-sparing procedures [8]. Genetic risk factors that increase endometrial, fallopian tube, or ovarian carcinoma are human non-polyposis colorectal cancer (Lynch syndrome) and BRCA 1 or 2. Women with a personal history of estrogen receptor positive breast cancer should consider hysterectomy with bilateral salpingo-oophorectomy. Finally, all women, regardless of risk factors, should be counseled that bilateral salpingo-oophorectomy decreases the risk of ovarian cancer tenfold and may be considered at the time of prolapse repair [9].
The most common symptom of uterine cancer is post-menopausal bleeding (PMB). Uterine-sparing procedures should not be recommended for patients who are followed for endometrial hyperplasia (with or without atypia) given a 5–25% risk of developing endometrial cancer [8]. For women with recent PMB and no known uterine pathology, it is important to recognize that there is a 13% risk of unanticipated endometrial cancer or hyperplasia even with a prior negative endometrial biopsy [8]. These risks should be reviewed for patients with recent PMB, and consideration given to performing concomitant hysterectomy at the time of prolapse repair.
Another factor that surgeons should consider and evaluate is cervical elongation because of the potential negative impact on outcomes. One study demonstrated an 11-fold increased risk of failure in patients with cervical elongation undergoing sacrospinous hysteropexy [10]. Partial trachelectomy, surgical removal of the distal elongated portion of the cervix, has been shown to improve success rates up to 96–99% [10].
Hysterectomy Risks
When considering surgical treatment options for uterovaginal prolapse, it is important to consider the risks and benefits of each part of the procedure, including hysterectomy. While many patients are concerned about the risks of hysterectomy, the procedure is relatively low-risk in skilled hands. Excessive bleeding (2.5%), bladder injury (2%), ureteral injury (0.1–0.5%), and bowel injury (0.4%) are the most common complications with vaginal hysterectomy [11]. These intraoperative risks may be even lower in a prolapse patient with a small, postmenopausal uterus that descends, allowing easier access to vascular pedicles.
There are also postoperative risks of hysterectomy to consider. Hysterectomy in premenopausal women may negatively impact ovarian function, resulting in earlier menopause. Compared to matched controls, premenopausal women who underwent hysterectomy with ovarian conservation had an earlier onset of menopause in two large prospective trials [12]. The 5-year risk of menopause was twofold higher in women undergoing hysterectomy and increased to threefold higher in those with unilateral oophorectomy and hysterectomy [12].
Mesh risks are also increased when hysterectomy is performed at the time of vaginal mesh repair or sacral colpopexy, as compared to hysteropexy. The 2017 International Consultation on Incontinence reviewed studies that described mesh exposure as an outcome [13••]. Those that underwent total hysterectomy at the time of laparoscopic or open sacral colpopexy had a 3.5-fold higher risk of mesh exposure compared to hysteropexy (7.2 vs 2.2%, p < 0.0001; 19 studies, n = 1149 hysterectomy, n = 1661 hysteropexy). Mesh exposure was also significantly lower in those that underwent supracervical hysterectomy (0.7%, n = 541, 9 studies). Similar increased rates of mesh exposure were seen with vaginal mesh repairs when concomitant hysterectomy was performed comparing vaginal mesh repairs with hysteropexy (14 vs 6%, p = 0.02; 3 studies, n = 131 hysterectomy, n = 218 hysteropexy) [13••]. Notably, most studies included in this analysis did not independently find significantly higher erosion rates in their analyses [13••].
Historical Perspective
Hysteropexy procedures for prolapse repair were popularized in the late nineteenth century to mitigate the intraoperative risks of hysterectomy, which were significantly higher at that time. The Manchester procedure, first performed in 1888 by Dr. Archibald Donald in Manchester England, involved surgical removal of the cervix (trachelectomy) followed by plication of the cardinal and uterosacral ligaments in the midline [2]. This was primarily used for patients with cervical elongation in an era where no antibiotic prophylaxis existed, and rates of postoperative infection were high with total hysterectomy.
In the late twentieth century, as surgical techniques evolved and antibiotics became available, hysterectomy became safer and was widely accepted as a primary treatment for uterine prolapse. More recently, with recognition that the uterus plays a passive role in the development of prolapse and growing patient interest in uterine conservation, hysteropexy is once again gaining popularity.
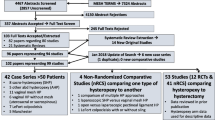
There are now a variety of hysteropexy procedures performed for the repair of uterovaginal prolapse. These procedures can be separated into vaginal and abdominal approaches with or without the use of mesh. Hysteropexy procedures traditionally performed abdominally are increasingly being performed via a laparoscopic or robotic approach. There are no published randomized controlled trials (RCTs) comparing efficacy and/or safety between hysteropexy procedures to recommend one approach over another. However, there are studies with high quality evidence comparing similar types of prolapse repairs with and without hysterectomy. The following sections review the various procedures and their outcomes.
Vaginal Native Tissue Hysteropexy
LeFort Colpocleisis
While LeFort (partial) colpocleisis may not be viewed in the same category as other native tissue hysteropexies, it achieves prolapse repair by inverting the vaginal sidewalls and obliterating the vaginal canal, leaving the uterus in situ. Since its inception in the late 1800s, this procedure continues to be one of the best options due to high success rates in cases of advanced prolapse, low morbidity, and short operative time. This is the preferred procedure for older women or those with multiple medical comorbidities who are no longer sexually active. Colpocleisis procedure can also be performed with concomitant hysterectomy. While there are no studies directly comparing LeFort colpocleisis to vaginal hysterectomy with total colpocleisis, hysterectomy should be performed for those with known uterine abnormalities or risk factors for future endometrial cancer, as the LeFort procedure will preclude future endometrial sampling [13••]. Retrospective analyses have shown shorter operative time, lower blood loss, and similar anatomic success with LeFort compared to total colpocleisis with concomitant hysterectomy [14, 15].
Sacrospinous Hysteropexy
Sacrospinous hysteropexy (SSHP) is performed by transfixing the cervix or uterosacral ligament complex to the sacrospinous ligament. This is performed using permanent or delayed absorbable suture with the utilization of a suture ligature carrier such as the Deschamps or more modern Capio™ device. Because the SSHP is performed in an extraperitoneal fashion, it has advantages in patients with risk factors for pelvic adhesive disease. Like the traditional SSLF, the SSHP deflects the vagina posteriorly, which may contribute to anterior vaginal wall prolapse recurrence.
Several RCTs have compared SSHP to total vaginal hysterectomy (TVH) with native tissue repair (either SSLF or uterosacral ligament suspension (USLS)), the largest and most recent study compared SSHP (n = 103) to TVH/USLS (n = 105) in 2015. The primary outcome was a composite of apex <stage 2, no prolapse symptoms, and no reoperation. Outcomes at 12 months were similar with 100% success in SSHP compared to 96% with TVH/USLS. Most recurrences occurred in the anterior compartment and were not included in this primary outcome. They concluded that SSHP was non-inferior to TVH/USLS [16]. A similar RCT by Dietz et al. in 2010 compared SSHP (n = 37) to TVH/USLS (n = 34), with primary outcome of success at 12 months defined as apex <stage 2 only [17]. They found success 79% SSHP vs 97% TVH/USLS with 4 (11%) reoperations in SSHP vs 2 (6%) reoperations TVH/USLS. Hysteropexy had a significantly higher apical failure rate by this definition (p = 0.03). Both had similar anterior failure rates (51% SSHP vs 64% TVH/USLS). The hysteropexy group was found to have shorter hospitalization and more rapid return to work [17].
The only RCT comparing SSHP to TVH/SSLF had shorter follow-up (6 months) and primary outcome assessed sexual function using validated questionnaires, rather than anatomic outcomes. No differences in sexual function scores were found [18]. Four cohort trials (two retrospective and two prospective) comparing SSHP vs TVH/SSLF showed no significant differences in anatomic or symptomatic failure rates with follow-up ranging from 19 to 57 months [19,20,21,22].
In summary, most studies have shown comparable success rates for apical compartment support with SSHP as compared to TVH/USLS, with shorter hospitalization and quicker return to work. Like the traditional SSLF, the SSHP deflects the vaginal axis posteriorly, which may be a cause for the high recurrent anterior wall prolapse seen with this procedure despite concomitant cystocele repairs [23, 24].
Uterosacral Hysteropexy
Multiple methods of uterosacral hysteropexy (USH) have been described involving plication or shortening of the uterosacral ligaments with uterine preservation. Approaches include vaginal (VUSH), abdominal (AUSH), and laparoscopic (LUSH) uterosacral hysteropexy. Currently, there are no RCTs comparing any method of uterosacral hysteropexy to hysterectomy with USLS. There are four cohort studies that compare differing approaches of uterosacral hysteropexy to TVH/USLS [25,26,27,28]. The only prospective cohort of the four compared LUSH (n = 28) to TLH/laparoscopic USLS (n = 27) [25]. Success was measured objectively as <stage 2 prolapse at 24 months. Success rates were similar among hysteropexy versus hysterectomy groups (79 vs 78%, p = 0.746). The largest and most recent retrospective cohort found lower anatomic success rates (47 vs 63%, p = 0.019) and higher reoperation for prolapse (28 vs 21%, p > 0.05) after LUSH (n = 104) when compared to laparoscopic hysterectomy with USLS (n = 160). The median follow-up was 2.5 years. The apical failure rate was significantly higher in hysteropexy group (24 vs 13%, p = 0.034) [27]. Conversely, the second largest retrospective cohort found no difference in outcomes for apical success (defined as <stage 2) for VUSH (n = 100) and TVH/USLS (n = 100), (96 vs 97%, p = 0.90). There was also no difference in anterior or posterior compartment objective outcomes at 24 months (p = 0.31 and p = 0.16, respectively) [28].
In summary, there is conflicting data and a lack of RCTs comparing success rates of hysteropexy versus hysterectomy with uterosacral ligament suspension. Studies utilize different approaches to uterosacral ligament suspension (laparoscopic or vaginal) which may also account for these differences.
Vaginal Mesh Hysteropexy
Vaginal mesh hysteropexy is performed with graft placement in the anterior vaginal wall with concurrent apical support procedure, most commonly a sacrospinous ligament suspension. Many kits previously on the market required trocars for placement and included mesh in the anterior and posterior compartments with a concomitant apical procedure. The latest Cochrane database review offers level 1 evidence for improved success rates for anterior vaginal wall support with mesh when compared to without mesh [29]. This same review also reported that there is no evidence to support improved efficacy using mesh in the posterior wall. This, combined with the United States Food and Drug Administration’s (FDA) warning in 2008 and safety communication in 2011 regarding the complications of surgical mesh, has resulted in a sharp decline in the use of these products [30]. Although initially there were over 40 vaginal mesh products on the market, this number has declined to less than a handful. Remaining products for vaginal mesh hysteropexy aim to combine the apical success of the SSHP procedure, with the added support that vaginal mesh can bring to the anterior wall. It is important to note that these procedures can be safe and effective when selecting the right surgical candidate. Several organizations (American Urogynecologic Society (AUGS), Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction (SUFU), and American Congress of Obstetricians and Gynecoogists (ACOG)) have published guidelines for the use of transvaginal mesh for pelvic organ prolapse repair.
A large, RCT (n = 180) currently in the follow-up phase from the Pelvic Floor Disorders Network (SUPeR Trial) is comparing vaginal mesh hysteropexy (Uphold™) to TVH/USLS repair. This study will compare anatomic, subjective, quality of life outcomes at 3–4 years and is projected to complete in 2018. There are three retrospective cohorts comparing vaginal mesh hysteropexy to TVH with vaginal mesh repair. All used different mesh devices (Perigree/Apogree, Total Prolift, posterior intravaginal slingplasty) [31,32,33]. No differences in anatomic success rates were noted between groups, and there was no significant difference in mesh erosion rates. Notably, these devices are no longer commercially available. The only published report with a product currently on the market is a retrospective case series reviewing hysteropexy or hysterectomy with Uphold™ with an average follow-up of 12 months [34]. They reviewed cases performed by the surgeon-inventor of Uphold showing no significant differences in success rates (98 vs 96%) between mesh hysteropexy (n = 43) and hysterectomy (n = 24). There was one mesh exposure in the hysteropexy group (2%) and two in the hysterectomy group (8%). One of the two exposures in the hysterectomy group occurred after subsequent vaginal cuff radiation from incidental endometrial cancer at the time of hysterectomy. It is important to consider that these studies showed a higher rate of mesh exposure in hysterectomy group although not statistically significant. Uterine conservation at the time of vaginal mesh repair for prolapse appears to decrease the risk of mesh exposure (14 vs 6%, p = 0.02) [13••].
Lastly, some surgeons prefer to cut and shape their own graft from a larger section of material. There are no comparative studies that review this technique. At this point, available data suggests vaginal mesh hysteropexy is as effective as vaginal mesh with hysterectomy, with lower rates of mesh exposure.
Sacral Mesh Hysteropexy
Sacral hysteropexy is performed by attachment of mesh graft material to the cervix and uterus to the anterior longitudinal ligament overlying the sacrum. Various approaches have been described attaching the graft to either anterior, posterior or both sides of the uterus/cervix. The procedure can be accomplished abdominally or laparoscopically with or without robotic assistance. Most commonly, this is performed laparoscopically using a mesh graft that bifurcates into a Y-shape, where the long stem of the graft is attached to the anterior longitudinal ligament, and the split arms are brought posteriorly through an avascular portion of the broad ligament, wrapped around, then attached to the anterior cervix.
While vaginally-placed grafts have decreased since the FDA changes, laparoscopic sacral colpopexy both with and without hysterectomy is gaining popularity as many consider it to be a more durable procedure. Long-term data is limited for laparoscopic mesh hysteropexy. Most available evidence compares sacral hysteropexy to hysterectomy with sacral colpopexy. Unfortunately, the method of sacral hysteropexy varies considerably among these trials, making comparison between trials difficult. No prospective trials have compared efficacy of sacral hysteropexy methods. Few studies compare sacral hysteropexy with native tissue hysteropexy.
Two RCTs of sacral hysteropexy to native tissue controls are available. Roovers (2004) randomized abdominal sacral hysteropexy (n = 41) to TVH/USLS (n = 41) [35]. The primary endpoint was UDI-6 scores; one secondary outcome was pelvic examination with prolapse stages by compartment. At 12 months follow-up, significantly higher scores in the UDI-6 domains of discomfort/pain, overactive bladder, and obstructive micturition were found in the abdominal hysteropexy group but there were no significant differences in genital prolapse and urinary incontinence. There were no significant differences in anatomic outcomes in the anterior, posterior, and apical compartments.
A recent pilot RCT of laparoscopic sacral hysteropexy (n = 40) to TVH/USLS (n = 39) demonstrated superior apical support in the hysteropexy group [36]. At 1-year follow-up, there was a higher chance of repeat apical repair in the vaginal hysterectomy group (14 vs 6%, p = 0.185). However, there was a significantly higher chance of repeat anterior repair in the hysteropexy group (4 vs 0%, p = 0.022). The authors noted that significantly more women undergoing TVH/ULSLS had a concurrent anterior repair compared to sacral hysteropexy (38 vs 18%, p = <0.001), which perhaps explained the higher anterior failure rate at 12 months. Drop-out after randomization was a limitation, and 80% of the 31 women who dropped out did so due to preference of hysteropexy over hysterectomy [36].
No RCTs have been published comparing sacral hysteropexy to hysterectomy with sacral colpopexy. Two prospective cohorts are available that showed similar success rates; both used the open abdominal approach. Constantini (2005) found 91% success rate with abdominal sacral hysteropexy (n = 34) versus 92% with abdominal hysterectomy and sacral colpopexy (n = 38) at 51 months. Success was defined as <stage 2 and apex < − 6 by POP-Q. Notably, there was an 8% mesh exposure rate in the hysterectomy group compared to no mesh exposure in the hysteropexy group [37]. After this study’s publication, the same author followed 32 sacral hysteropexy and 36 hysterectomy with sacral colpopexy patients for 12 months with 100% objective success rate in both groups (apex < − 6) [38]. Retrospectively, there are several cohort studies comparing laparoscopic mesh sacral hysteropexy to hysterectomy with mesh sacral colpopexy. The largest study reviewed outcomes at a mean follow-up of 33 months (n = 65 hysteropexy and n = 34 hysterectomy) [39]. No mesh erosions were noted in either group. Success was higher in hysterectomy group, although it did not reach statistical significance (88 vs 72%, p = 0.07). The subjective satisfaction rate, although high in both groups, was significantly higher in the hysterectomy with sacral colpopexy group (92 vs 100%, p < 0.001). Ten women required retreatment with either pessary (n = 9) or reoperation (n = 1) in the hysteropexy group compared to no retreatment in the hysterectomy group [39].
Overall, there is inadequate data to compare anatomic outcomes of sacral hysteropexy to sacral colpopexy. In a review of available data, there was a higher reoperation rate for hysteropexy and a higher mesh exposure rate in the hysterectomy group [13••].
Hysteropexy Comparisons
As previously stated, there are no RCTs comparing hysteropexy procedures to date. The largest retrospective comparative study reviewed a total of 240 hysteropexies at one institution [40]. Included were vaginal mesh (n = 61), laparoscopic sacral (n = 43), robotic sacral (n = 27), abdominal sacral (n = 15), and native tissue (n = 99) hysteropexies. There were differences between groups including age, baseline exams, and follow-up (6–22 months). Prolapse recurrence overall was 12%, defined as >stage 1 and bulge symptoms. They compared recurrence rates of vaginal and laparoscopic approaches with mesh versus native tissue. No significant differences were noted between vaginal native tissue and vaginal mesh (12 vs 10%, p = 0.71) or laparoscopic non-mesh versus mesh repairs (10 vs 23%, p = 0.07). Additionally, recurrence rates were similar among all groups (abdominal 13.3%, vaginal 14.7%, laparoscopic 11.6%, robotic 3.6%, p = 0.39). Mesh exposure rates were similar between vaginal and laparoscopic approaches (2 vs 2.4%) [40].
The largest prospective study is a multicenter cohort of vaginal mesh hysteropexy versus laparoscopic sacral hysteropexy [41•]. Women with >stage 2 prolapse who desired surgical repair with uterine conservation were offered enrollment. Patients with cervical elongation were excluded. Primary outcome was success at 1 year using a composite definition of symptomatic success (not seeing/feeling bulge) and anatomic success (no prolapse beyond hymen, no reoperation or pessary use, and cervix above mid-vagina) [41•].
Composite cure rate was not significantly different between groups (72% laparoscopic vs 74% vaginal, OR 0.58, 95% CI 0.2–1.5). There were no differences in anatomic cure (77 vs 80%, OR 0.48 95% CI 0.2–1.5) or symptomatic cure (90vs 95% OR 0.4 95% CI 0.7–1.8) between laparoscopic and vaginal approaches, respectively. There was high patient satisfaction with both procedures (95% overall). No significant difference in mesh exposure rates was found (2.7% laparoscopic vs 6.6% vaginal, p = 0.44) [41•].
This is the only prospective study comparing two hysteropexy techniques. While there were some baseline differences between the groups due to lack of randomization, it provides insight that both hysteropexy procedures offer overall safe, effective management of uterovaginal prolapse at 1-year follow-up [41•].
Conclusions
Uterine preservation is a reasonable option in the surgical management of pelvic organ prolapse. It is becoming increasingly common for women to request uterine preservation for a variety of reasons: maintenance of fertility, sense of identity, concern on impact of sexual function, and risk of hysterectomy. It is imperative for pelvic surgeons to understand who is a candidate for these procedures, as certain patients have contraindications to uterine conservation. Although a variety of surgical approaches exist, robust evidence on uterine sparing prolapse repairs is lacking. Future research, with level 1 quality data, is needed to compare the efficacy of various hysteropexy procedures.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Wu JM, Matthews CA, Conover MM, Pate V, Jonsson FM. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol. 2014;123(6):1201–6. https://doi.org/10.1097/AOG.0000000000000286.
Ridgeway BM, Frick AC. Chapter 26: uterine conservation for the surgical treatment of uterovaginal prolapse. In: Walters MD, Karram M, editors. Urogynecology and reconstructive pelvic surgery. Philadelphia: Saunders; 2014. p. 383–99.
Korbly NB, Kassis NC, Good MM, Richardson ML, Book NM, Yip S, et al. Patient preferences for uterine preservation and hysterectomy in women with pelvic organ prolapse. Am J Obstet Gynecol. 2013;209(5):470.e1–6. https://doi.org/10.1016/j.ajog.2013.08.003.
Frick AC, Barber MD, Paraiso MF, Ridgeway B, Jelovsek JE, Walters MD. Attitudes toward hysterectomy in women undergoing evaluation for uterovaginal prolapse. Female Pelvic Med Reconstr Surg. 2013;19(2):103–9. https://doi.org/10.1097/SPV.0b013e31827d8667.
DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 Pt 1):1717–24. https://doi.org/10.1016/0002-9378(92)91562-O.
Ridgeway BM. Does prolapse equal hysterectomy? The role of uterine conservation in women with uterovaginal prolapse. Am J Obstet Gynecol. 2015;213(6):802–9. https://doi.org/10.1016/j.ajog.2015.07.035.
Pearce CL, Stram DO, Ness RB, Stram DA, Roman LD, Templeman C, et al. Population distribution of lifetime risk of ovarian cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2015;24(4):671–6. https://doi.org/10.1158/1055-9965.EPI-14-1128.
Frick AC, Walters MD, Larkin KS, Barber MD. Risk of unanticipated abnormal gynecologic pathology at the time of hysterectomy for uterovaginal prolapse. Am J Obstet Gynecol. 2010;202(5):507 e1–4. https://doi.org/10.1016/j.ajog.2010.01.077.
Parker WH, Feskanich D, Broder MS, Chang E, Shoupe D, Farquhar CM, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet Gynecol. 2013;121(4):709–16. https://doi.org/10.1097/AOG.0b013e3182864350.
Lin TY, Su TH, Wang YL, Lee MY, Hsieh CH, Wang KG, et al. Risk factors for failure of transvaginal sacrospinous uterine suspension in the treatment of uterovaginal prolapse. J Formos Med Assoc = Taiwanyizhi. 2005;104(4):249–53.
Harris WJ. Early complications of abdominal and vaginal hysterectomy. Obstet Gynecol Surv. 1995;50(11):795–805. https://doi.org/10.1097/00006254-199511000-00019.
Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112(7):956–62. https://doi.org/10.1111/j.1471-0528.2005.00696.x.
•• Maher C, et al. Surgical Treatment of Uterovaginal Prolapse, IV. Committee 15: Pelvic Organ Prolapse Surgery. Incontinence, 6th ed 2017, pages 1874–1886. Editors Abrams, P, Cardoza, L, et al. International Continence Society. Summary of all available paper for all modalities of hysteropexy Tables and references of all prior studies along with critical findings Critical evaluation of the literature with recommendations for best practice based on level of evidence available at the end of the summary.
Fitzgerald MP, Richter HE, Bradley CS, et al. Pelvic support, pelvic symptoms, and patient satisfaction after colpocleisis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(12):1603–9. https://doi.org/10.1007/s00192-008-0696-6.
Bochenska K, Leader-Cramer A, Mueller M, et al. Perioperative complications following colpocleisis with and without concomitant vaginal hysterectomy. Int Urogynecol J. 2017;28(11):1671–5.
Detollenaere RJ, den Boon J, Stekelenburg J, IntHout J, Vierhout ME, Kluivers KB, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with suspension of the uterosacral ligaments in women with uterine prolapse stage 2 or higher: multicentre randomised non-inferiority trial. BMJ. 2015;351:h3717.
Dietz V, van der Vaart CH, van der Graaf Y, Heintz P, Schraffordt Koops SE. One-year follow- up after sacrospinous hysteropexy and vaginal hysterectomy for uterine descent: a randomized study. Int Urogynecol J Pelvic Floor Dysfunct. 2010;21(2):209–16. https://doi.org/10.1007/s00192-009-1014-7.
Jeng CJ, Yang YC, Tzeng CR, Shen J, Wang LR. Sexual functioning after vaginal hysterectomy or transvaginal sacrospinous uterine suspension for uterine prolapse: a comparison. J Reprod Med. 2005;50(9):669–74.
Hefni M, El-Toukhy T, Bhaumik J, Katsimanis E. Sacrospinous cervicocolpopexy with uterine conservation for uterovaginal prolapse in elderly women: an evolving concept. Am J Obstet Gynecol. 2003;188(3):645–50. https://doi.org/10.1067/mob.2003.75.
Maher CF, Cary MP, Slack MC, Murray CJ, Milligan M, Schluter P. Uterine preservation or hysterectomy at sacrospinous colpopexy for uterovaginal prolapse? Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(6):381–4. https://doi.org/10.1007/s001920170017.
van Brummen HJ, van de Pol G, Aalders CI, Heintz AP, van der Vaart CH. Sacrospinous hysteropexy compared to vaginal hysterectomy as primary surgical treatment for a descensus uteri: effects on urinary symptoms. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14(5):350–5; discussion 5. https://doi.org/10.1007/s00192-003-1084-x.
Hefni MA, El-Toukhy TA. Long-term outcome of vaginal sacrospinous colpopexy for marked uterovaginal and vault prolapse. Eur J Obstet Gynecol Reprod Biol. 2006;127(2):257–63.
Morley GW, DeLancey JO. Sacrospinous ligament fixation for eversion of the vagina. Am J Obstet Gynecol. 1988;158(4):872–81. https://doi.org/10.1016/0002-9378(88)90088-9.
Morgan DM, Rogers MAM, Huebner M, Wei JT, DeLancey JO. Heterogeneity in anatomic outcome of sacrospinous ligament fixation for prolapse: a systematic review. Obstet Gynecol. 2007;109(6):1424–33. https://doi.org/10.1097/01.AOG.0000264066.89094.21.
Rosen DMSA, Cario GM, Carlton MA, Chou D. Is hysterectomy necessary for laparoscopic pelvic floor repair? J Minim Invasive Gynecol. 2008;15(6):729–34. https://doi.org/10.1016/j.jmig.2008.08.010.
Diwan ARC, Strohsnitter WC, Weld A, Rosenblatt P, Kohli N. Laparoscopic uterosacral ligament uterine suspension compared with vaginal hysterectomy with vaginal vault suspension for uterovaginal prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(1):79–83. https://doi.org/10.1007/s00192-005-1346-x.
Bedford ND, Seman EI, O'Shea RT, Keirse MJ. Effect of uterine preservation on outcome of laparoscopic uterosacral suspension. J Minim Invasive Gynecol. 2013;20(2):172–7. https://doi.org/10.1016/j.jmig.2012.10.014.
Romanzi LJ, Tyagi R. Hysteropexy compared to hysterectomy for uterine prolapse surgery: does durability differ? Int Urogynecol J. 2012;23(5):625–31. https://doi.org/10.1007/s00192-011-1635-5.
Maher C, Schmid C, Baessler K, Feiner B. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;(4):CD004014. https://doi.org/10.1002/14651858.CD004014.pub5.
Haya N, Baessler K, Christmann-Schmid C, de Tayrac R, Dietz V, Guldberg R, et al. Prolapse and continence surgery in countries of the Organization for Economic Cooperation and Development in 2012. Am J Obstet Gynecol. 2015;212(6):755 e1–e27. https://doi.org/10.1016/j.ajog.2015.02.017.
Huang KHCF, Fu HC, Kung FT. Polypropylene mesh as an alternative option for uterine preservation in pelvic reconstruction in patients with uterine prolapse. J Obstet Gynaecol Res. 2012;38(1):97–101. https://doi.org/10.1111/j.1447-0756.2011.01647.x.
Neuman M, Lavy Y. Conservation of the prolapsed uterus is a valid option: medium term results of a prospective comparative study with the posterior intravaginal slingoplasty operation. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(8):889–93. https://doi.org/10.1007/s00192-006-0262-z.
Chu LC, Chuang FC, Kung FT, Huang KH. Comparison of short-term outcomes following pelvic reconstruction with perigee and apogee systems: hysterectomy or not? Int Urogynecol J. 2012;23(1):79–84. https://doi.org/10.1007/s00192-011-1513-1.
Vu MK, Letko J, Jirschele K, Gafni-Kane A, Nguyen A, Du H, et al. Minimal mesh repair for apical and anterior prolapse: initial anatomical and subjective outcomes. Int Urogynecol J. 2012;23(12):1753–61. https://doi.org/10.1007/s00192-012-1780-5.
Roovers J, van der Vaart C, van der Bom J, van Leeuwen J, Scholten P, Heintz A. A randomized controlled trial comparing abdominal and vaginal prolapse surgery: effects on urogenital function. BJOG. 2004;111(1):50–6. https://doi.org/10.1111/j.1471-0528.2004.00001.x.
Rahmanou P, Price N, Jackson SR. Laparoscopic hysteropexy versus vaginal hysterectomy for the treatment of uterovaginal prolapse: a prospective randomized pilot study. Int Urogynecol J. 2015;26(11):1687–94. https://doi.org/10.1007/s00192-015-2761-2.
Costantini E, Mearini L, Bini V, Zucchi A, Mearini E, Porena M. Uterus preservation in surgical correction of urogenital prolapse. Eur Urol. 2005;48(4):642–9. https://doi.org/10.1016/j.eururo.2005.04.022.
Costantini E, Porena M, Lazzeri M, Mearini L, Bini V, Zucchi A. Changes in female sexual function after pelvic organ prolapse repair: role of hysterectomy. Int Urogynecol J. 2013;24(9):1481–7. https://doi.org/10.1007/s00192-012-2041-3.
Pan K, Cao L, Ryan NA, Wang Y, Xu H. Laparoscopic sacral hysteropexy versus laparoscopic sacrocolpopexy with hysterectomy for pelvic organ prolapse. Int Urogynecol J. 2016;27(1):93–101. https://doi.org/10.1007/s00192-015-2775-9.
Kow N, Goldman HB, Ridgeway B. Uterine conservation during prolapse repair: 9-year experience at a single institution. Female Pelvic Med Reconstruct Surg. 2016;22(3):126–31. https://doi.org/10.1097/SPV.0000000000000221.
• Gutman RE, Rardin CR, Sokol ER, Matthews C, Park AJ, Iglesia CB, et al. Vaginal and laparoscopic mesh hysteropexy for uterovaginal prolapse: a parallel cohort study. Am J Obstet Gynecol. 2017;216(1):38.e1–11. Well-designed cohort study and the only prospective study comparing efficacy and adverse effects of two hysteropexy approaches outcomes at 12 months of subjective, objective, and composite criteria. https://doi.org/10.1016/j.ajog.2016.08.035.
Author information
Authors and Affiliations
Ethics declarations
Conflict of Interest
Sarah Bradley, Robert E. Gutman, and Lee A. Richter each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Female Urology
Rights and permissions
About this article
Cite this article
Bradley, S., Gutman, R.E. & Richter, L.A. Hysteropexy: an Option for the Repair of Pelvic Organ Prolapse. Curr Urol Rep 19, 15 (2018). https://doi.org/10.1007/s11934-018-0765-4
Published:
DOI: https://doi.org/10.1007/s11934-018-0765-4