Abstract
Purpose of Review
There is a significant rise in the interest in genital gender affirmation surgery (gGAS), which is increasingly offered and performed throughout the world. While gGAS is not new, the expansion of gGAS is associated with progressive societal acceptance of transgender and gender non-conforming individuals. There is a clear role for gGAS in the management of gender dysphoria, and with the prevalance of gGAS it is important for physicians to be familiar with the altered anatomy and potential complications of gGAS. In this review, we summarize the literature on the outcomes and complications associated with gGAS.
Recent Findings
Fifty-five studies were utilized in this review, encompassing meta-analyses, literature reviews, retrospective primary studies, and case reports.
Summary
gGAS is a complex procedure with a variety of techniques that each carry their own strengths and weaknesses. Current gGAS procedures deliver predictable results with high patient satisfaction despite high complication rates for gGAS. Further research is needed to refine gGAS techniques in order to to minimize complication rates and to improve the management of complications when they do occur.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gender dysphoria (GD) is a psychological conflict that arises from discordance between a person’s gender identity and their assigned sex at birth [1]. Furthermore, it is important to understand that gender identity is distinct from sexual orientation, which helps to understand the in caring for patient with GD. The management of GD is multi-disciplinary and includes psychological, medical and surgical treatments [2]. Genital gender affirmation surgery (gGAS) helps to resolve the discrepancy between the patient’s gender identity and sexual phenotype; however, not all patients with GD will pursue gGAS [2]. To proceed with gGAS, patients are required to meet World Professional Association for Transgender Health (WPATH) criteria, with the goal of selecting patients that will be most likely to benefit from gGAS and minimize the potential for regret. [2]. Herein, we review the contemporary outcomes and complications of feminizing and masculinizing gGAS.
Methods
A comprehensive review of the literature, using MEDLINE and Pubmed, was performed to identify contemporary articles detailing the surgical experience and complications after gGAS. We included articles published between 2018–2021 and published in English and including patients at least 18 years; however, older articles were included if there was a lack of contemporary data. We excluded articles that included patients under 18 years old or presented incomplete data. A total of 394 abstracts were reviewed, 64 manuscripts read in their entirety, and 55 articles were included in this review.
Feminizing Genital Gender Affirmation Surgery
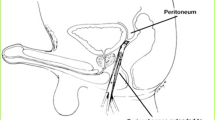
Feminizing gGAS includes the creation of phenotypic female genitalia, in which vaginoplasty or vulvoplasty enables alignment of gender identity with their genitalia [3••]. Penile inversion vaginoplasty (PIV) is the most common technique to create a functional neo-vagina with sufficient depth. The PIV technique also has low rates of intraoperative and major postoperative complications, and provides high patient satisfaction [3••]. This procedure involves reconstructing male anatomy into homologous female phenotypic anatomy, including a functional neo-vaginal canal and sensate neo-clitoris, enabling satisfactory penetrative intercourse and orgasm and normal urinary function with creation of a perineal urethrostomy [4]. Alternatively, intestinal vaginoplasty and peritoneal vaginoplasty are less commonly performed as primary reconstruction, and are techniques that may be utilized during revisional surgery [5].
Vulvoplasty, or “0zero depth” neo-vagina, is the creation of phenotypic female genitalia without a vaginal canal. This may be done for patients who do not desire penetrative intercourse or are risk averse in the creation of the neovaginal canal. Furthermore, vulvoplasty is also recommended for patients with a history of pelvic surgery or pelvic radiation, which significantly increases risks associated with neovaginal canal dissection and risks of poor graft take and healing [6]. In patients undergoing full depth vaginoplasty, patients are required to perform lifelong routine vaginal dilation to prevent complications such as neovaginal stenosis or shortening [7].
A high percentage of patients report improvement in their GD following vaginoplasty and 94% of patients in one study reported they would choose to do the procedure again [3••]. In a systematic review, overall satisfaction was 92%, with 86% satisfaction regarding function and 90% with aesthetic outcomes [8]. Among reported series, regret about feminizing gGAS was found to be rare (0%-3%) [8].
PIV is the most common technique for gGAS in transgender patients due to its low rate of major complications [3••]. Intestinal vaginoplasty and peritoneal vaginoplasty includes additional risks associated with abdominal and bowel resection and anastomosis, which alters the potential complications of reconstruction. Certainly, there is a learning curve for gGAS and, with increasing experience, there should be improved outcomes and reduced complication rates. Ferrando performed a retrospective review on 76 consecutive vaginoplasty cases completed by one surgeon and found that a high volume surgeon, defined by the author as performing more than 50 vaginoplasties, leads to more favorable outcomes [9]. When compared to before and after the 50-case threshold, there was a statistically significant decrease in rates of any delayed postoperative event (18% before; 4% after), abnormal urinary stream (8% before; 2% after), introital stenosis (6% before; 1% after), and need for revision surgery (22% before; 5% after) [9].
There is conflicting evidence on patient demographics that correlate to higher rates of postoperative complications. Massie et al. found that patients with hypertension or a history of a bleeding disorder were significantly correlated to postoperative complications [3••]. Conversely, Levy et al. did not find a statistically significant difference in terms of hypertension, but did have a statistically significant value for noncompliance with the dilation regimen or sexual activity restrictions [10•].
Body mass index (BMI) in prospective vaginoplasty patients is included in many surgeons’ criteria for surgical candidacy [11]. Patients may be refused gGAS based on high BMIs, due to a perceived risk of higher surgical complications [12]. Ives et al. did not find a significant increase in complications for patients with increased BMI undergoing PIV, while Massie et al. found a significant association between BMI and risk for granulation tissue and neovaginal prolapse [3••, 11]. Further studies should be performed to determine if a strict cutoff for vaginoplasty based on BMI is indicated.
The rate of any complication after full depth vaginoplasty is 49–70% [3••, 9]. Table 1 details the rates of complications in recently published studies. The rate for any complication with vulvoplasty is approximately 35% [13]. Common postoperative complications seen with PIV are skin necrosis (1.5%- 23.3%), wound dehiscence (9.6%—10.6%), and hematomas (1.06%– 16%) [3••, 9, 10•, 14, 15•, 16•]. Image 1 shows infection, dehiscence, and a labial hematoma two weeks after undergoing PIV. Necrosis commonly occurs along the inferior wound edge and can often be managed with dressing changes [3••]. This results in need for reoperation in 0.6% of patients [17]. Intraoperative and postoperative transfusion rates vary across series ranging between 1.3%-15% and need for emergent unplanned reoperation is 2.6%-6% in reported series [3••, 9, 14, 15•, 16•]. Unplanned reoperations were for postoperative bleeding, neovaginal fistula, neovaginal prolapse, bowel obstruction, or abscess [9, 16•].
Rectal injury and development of a rectoneovaginal fistula is a potential devastating complication, with reported rates 0.5 – 2% [3••, 6, 9, 10•, 14, 15•, 16•]. Typically, this is the result of an unrecognized intraoperative rectal injury or a recognized and repaired injury that healed as a fistula [18]. A bowel preparation is routine for surgery to avoid fecal spillage in the setting of an intraoperative rectal injury [19]. Fecal spillage increases the risk of wound infection and failure of rectal repair that may result in a recto-neovaginal fistula [19]. The post-operative pathway following repair of a rectal injury includes keeping the vaginal packing in place for two weeks with exchange of the packing under anesthesia one-week after surgery [20]. The acute management includes fecal diversion with colostomy or loop ileostomy. Management of this complication often includes a low-residue diet and a variety of reconstructive techniques including use of buccal mucosal grafting and gracilis flap [18, 21] The success rate of this reconstruction is 100% in selected studies for patients that were not lost to follow-up, and does not seem to affect long-term sexual function [18, 21].
Neovaginal vault prolapse is a rare complication of vaginoplasty, reported in 0.4%-2% of contemporary series [3••, 10•]. The management of prolapse can be challenging, as the penile skin flap/scrotal skin composite is much thinner than cis-female vaginal mucosa and significantly less elastic [22]. Kavvadias et al. reported their experience with sacrocolpopexy for one patient with recurrent neo-vaginal prolapse following vaginopexy to the Denonvilliers fascia [22]. There are several techniques that formally fix the apex of the neovagina to prevent prolapse; as in robotic assisted PIV the neovaginal canal is sutured to the peritoneal refelection and with perineal PIV sacrospinus ligamnet fixation has been described [16•] [23].
Urinary complaints are a relatively common minor postoperative complaint. Frequently encountered urinary complaints include urinary retention (2.6–15.5%), urethral stenosis (0–1.06%), urinary tract infections (7%), and various other urinary issues such as incontinence and a disrupted stream (13.2–15%) [3••, 9, 14, 15•].
Preservation of erogenous tissue to the neo-clitoris is crucial for proper sexual function [7]. Recent literature finds that loss of sensation occurs in approximately 3% of patients and is negatively associated with patient satisfaction [3••]. Necrosis of the neo-clitoris is a potential post-operative complication, though rates in recent literature (0.5%) are lower when compared to rates of necrosis in the neo-vagina (1–15%) and the neo-labia (8.3%) [10•, 15•].
Other commonly encountered postoperative complications associated with vaginoplasty are granulation tissue (13.2 – 26%) and minor wound dehiscence (9.6 – 10.6%), which can often be managed conservatively [3••, 9, 10•, 15•]. Neovaginal introital stenosis and inadequate depth are suboptimal outcomes in patients undergoing feminizing gGAS and may prevent patients from participating in penetrative intercourse [7]. These complications may occur due to noncompliance with the postoperative dilation regimen, infection, or tissue retraction [7]. The rates of introital and vaginal stenosis are 1.5 – 9.2% and 2.6 – 6.38%, respectively [3••, 6, 9, 10•, 14, 15•]. The average neo-vaginal depth reported in the literature is 13.7 – 14.2 cm [6, 24]. The rates for suboptimal neovaginal depth are 21% for depths of 8–12 cm and 3.7% for depths of less than 8 cm [15•]. A full-thickness skin graft can add additional depth if the penile skin is insufficient to achieve optimal depth [17]. Shoureshi et al. reported in their series of 200 PIV a median depth of 14 cm with 4% (8 patients) with vaginal stenosis and 1.5% (3 patients) with introital stenosis, and 7 of these patients undergoing revisional surgery [6]. Massie et al. evaluated patient satisfaction after feminizing gGAS and found that minor complications were more associated with poor satisfaction compared to major complications [3••]. The authors propose that this may be a result of the prolonged convalescence related to minor complications.
While technically considered a minor complication, a suboptimal aesthetic outcome of feminizing gGAS frequently results in revisional procedures to correct scars and improve the cosmesis. The reported rates of revision surgery for patients undergoing feminizing gGAS is (3.8—54%) [3••, 9, 10•, 15•, 25]. Revisions typically involve labiaplasty, revision of the mons, revision of the clitoral hood, or adjacent tissue transfer at the neovaginal introitus. A functional and aesthetic outcome is important for patients and is needed to alleviate GD. However, it is similarly important to recognize that no two patients are alike and there are many different anatomic variations of phenotypic female anatomy, and to set expectations accordingly. There are a variety of different techniques for feminizing gGAS that have been used successfully [19]. Certainly there is no high quality data to support one technique over another with regard to aesthetic outcomes and complications rates in feminizing gGAS. Furthermore, there is lack of validated instruments to measure patient-reported outcome measures in transgender and gender non-conforming individuals, which hinders the ability to assess these outcomes [3••].
Vaginoplasty is an effective intervention to improve or resolve GD [3••]. The high likelihood of improving GD associated with the low incidence of major complications makes PIV a practical surgical option for transgender patients who choose to undergo surgical intervention.
Masculinizing Genital Gender Affirmation Surgery
Masculinizing gGAS involves creation of a phallus, which is performed in a variety of different techniques, depending on each individual’s goals and risk tolerance for complications. Metiodioplasty and phalloplasty are complex reconstructions and are associated with significantly higher complication rates relative to feminizing gGAS. Metoidioplasty is the lengthening and straightening of hormonally enlarged clitoris to form a phallus and phalloplasty is the construction of the neophallus using tubed skin flaps or pedicled myocutaneous flaps [26, 27]. Both techniques have the option to include urethral lengthening (UL) for the purposed of voiding while standing [27,28,29]. However, there are a variety of options for patients. Decision points regarding techniques for masculinizing gGAS include the individual’s body habitus and anatomy, considerations regarding donor site morbidity and cosmesis, and, from a functional perspective, the individuals’ desire to stand to void, desire to have a functional phallus for penetrative intercourse, and overall risk tolerance for a complex reconstruction [30].
Metoidioplasty
The foundation of metoidioplasty is the lengthening and straightening of hormonally enlarged clitoris [26]. Unlike the phalloplasty, the goal of metoidioplasty is not so much to achieve the function of a phallus but to achieve the appearance and erogenous sensation of one [31]. Metoidioplasty does not promise the ability to engage in penetrative intercourse. However, metoidioplasty does offer preserved erogeneity and tactile sensation, provides a natural erection, and minimizes procedures and complications [26]. Metoidioplasty offers an alternative for transmale patients who desire a masculinizing gGAS with lower risks of complications and less donor site morbidity compared with phalloplasty. UL enables most patients the ability to stand to void; however, variations in patient anatomy and body habitus may restrict this outcome. In metoidioplasty, UL relies on labia minora pedicled skin flaps and, occasionally, is in combination with anterior vaginal mucosa or buccal mucosal grafting [29, 32••, 33]. Metoidioplasty can also be performed without UL based on the individual’s goals and thus further minimizes complications of gGAS [32••].
Stojanovic et al. reported high rates of satisfaction, with 69 patients (82.3%) conveying complete satisfaction [29]. All 79 patients were able to void while standing and were satisfied with erogenous sensation and sexual arousal of the phallus. Overall sexual satisfaction was achieved in 87.3% of patients, with 86% reporting ability to orgasm during masturbation.
In summarizing the experience in the literature, five studies and a total of 269 patients were reported, with median follow-up of 36 months (range: 3.4–90 months) [29, 32••, 33]. Supplementary Table 2 summarizes the outcomes of metoidioplasty series. In the reported series, the complication rate is between 8.9–65.6% and some patients experience more than one complication following reconstruction [29, 32••, 33, 34••, 35••]. The most common complications are related to urethral reconstruction and the rates of urethral fistula are 2.5%-40.6% (Image 2) and urethral strictures (Image 3) occur in 2.5%-18.8%, and can occur together [29, 32••, 33, 34••, 35••]. In the early post-operative setting, urethrocutaneous fistula can be managed conservatively with continued catheter and/or suprapubic catheter drainage, which can be successful in 36% of patients within 2 months [36]. However, urethral complications may require secondary reconstruction to correct [29, 34••, 36]. Fistulas large size or persisting more that 3 months may require surgical repair [36].Image 2Urethrovaginal fistulaImage 2Urethrovaginal fistulaImage 2Urethrovaginal fistula
Despite the benefits of metoidioplasty, patient satisfaction, functional and cosmetic outcomes, and quality of life must be taken into account [33]. For some patients, metoidioplasty is not sufficient to meet their goals, and metoidioplasty can be successfully converted to a full phalloplasty if desired [34••]. There is a need, however, for more investigation into patient satisfaction and outcomes after metoidioplasty. For patients unsure of which procedure is best for them, phalloplasty after metoidioplasty is a safe and feasible option for patients [28, 34••].
Phalloplasty
The goal of phalloplasty is to create a neophallus that successfully achieves both cosmetic functional objectives of a phallus, i.e., full tactile and erogenous sensation, ability to urinate while standing, and penetrative intercourse. There are multiple techniques of phalloplasty including the use of pedicled flaps (anterolateral thigh flap, abdominal flap) or free flaps (radial forearm free flap, latissimus dorsi flap). Phalloplasty flap choice depends on patient’s anatomy, desires regarding function of the phallus, and acceptance of visible donor site scars [37•]. In contemporary practice, the radial forearm free flap (RFFF) is the most commonly performed and preferred technique [27, 38]. It is important to understand an individual’s goals of phalloplasty with regard to future urinary function, sexual function, and acceptance of potential complications. The most common complications of masculinizing gGAS are associated with urethral reconstruction and UL from the orthotopic female meatus to the end of the phallus, this is called the pars fixa [39]. There are multiple techniques that enable UL including the use of labia minora pedicle flaps, buccal mucosal grafting, double flaps, skin grafting and occasionally use of gracilis muscle flap as an adjunct [40]. These urethral complications of metoidioplasty and phalloplasty may be mitigated if the individual is not interested in voiding through the phallus [41, 42].
Masculinizing gGAS also includes colpectomy, scrotoplasty, clitoral burying in phalloplasty, and glans sculpting, and can be completed in multiple stages or as a single stage operation. Vaginal preservation or refusal of UL can also shift the procedural goals and choice of procedure, and, as a result, the outcomes and complications are dependent on a multitude of factors [41, 43]. If the individual is interested in sexual function, a penile prosthesis is placed 6–12 months after completion of the gGAS and ensuring no urethral complications. In this review, we will not address complications associated with penile prosthesis placement [38].
We identified a total of 18 studies that reported on phalloplasty outcomes. The outcomes and complications are summarized in Supplementary Table 3. Phalloplasty flaps used included suprapubic pedicle (SPP), RFFF, anterolateral thigh (ALT), superficial circumflex iliac artery (SCIP), groin flap, and musculocutaneous latissimus dorsi (MLD), and, occasionally, a composite of two flaps have been described [37•, 38, 43,44,45]. Patient outcomes of phalloplasty are heterogenous as a result of the multitude of variables, a variety of flaps types, whether it is single-stage or planned multi-stage reconstruction, and patient factors including co-morbidities and goals of gGAS. Table 3 details the published series on phalloplasty outcomes. Among the included studies, the mean follow-up was 38.2 months (7.5–88 months) [28, 37•, 38, 41, 42, 46,47,48,49]. Overall, the reported rates of phalloplasty satisfaction across the literature are 63–98%, based on non-validated questionnaires to assess patient-reported outcome measurement (PROM), patient self-report, and physician-recorded information in the medical records used as a proxy [35••, 41, 48, 50]. Additionally, the range of achieving orgasm with penetrative intercourse with penile prosthesis was 66–100% [48, 50]. Anastomosis of the clitoral nerve to the radial nerve is often seen in RFFF with the goal of combining erogenous and tactile sensation to the neophallus [51]. However, a majority of patients reported achieving orgasm by direct stimulation of the clitoris at the transposed site by moving the neophallus, while a minority reported orgasm through touching the phallic skin [52, 53]. Among phalloplasty series that included UL, 56%-92% were able to stand to void [28, 35••, 44, 54]. D’Arpa et al. compared a series of ALT phalloplasty with different UL techniques [54]. The authors reported lower rates of voiding for phalloplasty with a SCIP flap used for UL (86.8%) in comparison to the “tube-in-tube” method (100%). Despite the high rates of patient satisfaction after undergoing phalloplasty, the minority of transmale patients pursue gGAS due to the high rates of potential complications [55].
Phalloplasty reconstruction is associated with high rates of perioperative complications. The average complication rate among phalloplasty series range from 2.2%—84%, with many patients having more than one complication in 15.8%—61.1% [34••, 47]. Urologic complications are the most common complication after phalloplasty, this includes urethral strictures, urethrocutaneous fistula, and voiding dysfunction. As a result of the high rates of urethral complications, some individuals will elect to omit UL and urethral reconstruction as part of gGAS. The reported rates of urethral complications vary among series and are reported to be between 8.6%-64% for urethral stricture and 3.2%-56% for urethral fistula [28, 34••, 35••, 37•, 43, 44, 47, 54].
Many options for UL exist in the literatur, while no single technique has been shown to have superior outcomes. The tube-in-tube technique, used most commonly with RFFF, combines the creation of the neophallus with that of the neourethra by using the same donor tissue for both [37•, 45]. The procedure allows for a single stage phalloplasty; however, due to the nature of the donor tissue, fistulas and strictures are common and the presence of hair in the neourethra can lead to further complications [28]. Pedicled labia minora flaps are becoming a popular option due to good vascularization of the flap, hairless donor tissue, and elimination of the need for additional donor tissue [28]. However, patient-specific anatomy does not make this option viable for all patients [56]. Shaft-only phalloplasty is becoming an increasingly popular option among physicians and patients, as it foregoes complications and excessive post-operative care associated with neourethra creation [41, 42].
The rates of stricture vary across studies and differ between surgical techniques and flap type for phalloplasty and performing single or multi-stage reconstructions. Among RFFF and ALT series, the rates of urethral strictures and fistulas are 10.1% to 14.1% and 5.4% 47.4%, respectively (Image 4) [37•, 47, 54]. Ascha et al. reported in their comparison of ALT and RFFF cohorts, patients with ALT phalloplasty had 2.50 greater odds of urethral fistula formation than those with RFFF [37•]. The rates among single staged procedures are 10.1% to 21.9% [37•]. Flap combination phalloplasty, in which two separate flaps were used in the construction of the neourethra and neophallus, had rates of 2.6% to 47.4% [46, 47, 54]. The most common sites of urethral strictures and fistula are associated with the UL from the native meatus to the phalloplasty, this may also be associated with persistent vaginal cavity; however, the incidence of this is unknown. Urethral strictures and fistulas are difficult to reconstruct and require a variety of techniques depending on the length and location and on the type of phalloplasty, this may require buccal mucosa grafting, skin graft, secondary flap or combination thereof to reconstruct the urethra to enable normal voiding function [36]. Other complications of phalloplasty include venous thrombosis (1.5–23.1%), flap loss (1.2–7.5%), early reoperation, wound dehiscence (5.6%-39.4%), infection (2.8%-21.1%), and partial flap necrosis (2.8%-19.5%) [28, 33, 34••, 35••, 37•, 38, 41,42,43, 45,46,47,48,49,50, 54, 57]. The incidence in revision surgery for patients undergoing phalloplasty is 16–55% [34••, 38, 44, 57]. Vascular complications are of considerable concern due to the risk of complete flap loss [50, 57]. Venous complications are the leading cause of complete flap loss, leading investigators to believe that venous anastomosis may be the key to limiting postoperative complications [38, 57]. Wirthmann et al. reported that six of the nine patients with either partial or complete flap necrosis were heavy smokers; however, this is controversial, as a clear association between smoking habits and vascular complications has yet to be made [38, 45].Image 423 year old ALT phalloplasty with multiple urethrocutaneous fistula and urethral stricture (Photo source from author CRB, informed consent obtained for use of photo)Image 423 year old ALT phalloplasty with multiple urethrocutaneous fistula and urethral stricture (Photo source from author CRB, informed consent obtained for use of photo)Image 423 year old ALT phalloplasty with multiple urethrocutaneous fistula and urethral stricture (Photo source from author CRB, informed consent obtained for use of photo)
Use of the SPP flap resulted in significantly fewer major complications (1.4–5.8%) in comparison to all other forms of phalloplasty. Complete loss of flap was a less common complication (1.2–7.5%); however, it was the most devastating, resulting in complete revision of the phalloplasty and therefore new donor tissue consideration [34••, 35••, 38, 41, 45, 47, 48, 54, 57]. Partial necrosis was often treated with skin grafting (0.9–11.9%). Phalloplasty using the ALT flap resulted in a higher number of partial (2.2–5.3%) and complete (2.9–7.5%) flap necrosis [41, 47, 54]. RFFF had lower rates of partial necrosis (0.9–16%) and comparable rates of complete necrosis (3–4%) [37•, 38, 45].
Urological complications were the most common complications and the most common reason for revisional surgery [34••]. Strictures requiring surgical management were repaired by excising the stenotic area with direct anastomosis or augmentation with new oral mucosa graft [48]. Fistulas were equally as prevalent and were managed with urethrocutaneous fistula excision [34••]. Most minor fistulas and stricture are managed conservatively [34••]. Despite high complication rates in phalloplasty, patient choosing to undergo phalloplasty recontrution remain largely satisfied (63–98%) [35••, 41, 48, 50, 54].
Conclusion
gGAS is a technically complex procedure that is effective at alleviating GD. Prospective patients are required to go through rigorous screening and counseling regarding options and goals and the potential risks of complications. Despite the risks associated with gGAS, there are high rates of patient satisfaction and, clearly, gGAS plays an important role for transgender and gender non-conforming individuals with GD. As the world experience in gGAS continues to grow and evolve, further research is needed to better define and optimize reconstructive techniques that will improve patient outcomes and satisfaction.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Am. Psychiatr. Assoc. Arlington, VA; 2013.
World Professional Association for Transgender Health. Stand. Care Heal. Transsexual, Transgender, Gender-Conforming People [7th Version]. 2012.
Massie JP, Morrison SD, Van Maasdam J, Satterwhite T. Predictors of Patient Satisfaction and Postoperative Complications in Penile Inversion Vaginoplasty. Plast Reconstr Surg United States. 2018;141:911e–21e. This study retrospectively investigates 117 penile-inversion vaginoplasties performed by a single surgeon. This study uniquely reports both postoperative complications and patient-reported outcomes.
Gentile G, Martino A, Nadalin D, Masetti M, Marta BL, Palmisano F, et al. 2020 Penile-scrotal flap vaginoplasty versus inverted penile skin flap expanded with spatulated urethra: A multidisciplinary single-centre analysis. Arch Ital di Urol Androl organo Uff [di] Soc Ital di Ecogr Urol e Nefrol. Italy 92.
Dy GW, Blasdel G, Shakir NA, Bluebond-Langner R, Zhao LC. 2021 Robotic Peritoneal Flap Revision Vaginoplasty in Transgender Women: a Novel Technique for Treating Neovaginal Stenosis. Urology [Internet]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/33823174
Shoureshi P, Dy GW, Dugi D. Neovaginal Canal Dissection in Gender-Affirming Vaginoplasty. J Urol. 2021;205:1110–8.
Scahrdein JN, Zhao LC, Nikolavsky D. Management of Vaginoplasty and Phalloplasty Complications. Urol Clin North Am. 2019;46:605–18. https://doi.org/10.1016/j.ucl.2019.07.012.
Manrique OJ, Adabi K, Martinez-Jorge J, Ciudad P, Nicoli F, Kiranantawat K. Complications and Patient-Reported Outcomes in Male-to-Female Vaginoplasty-Where We Are Today: A Systematic Review and Meta-Analysis. Ann Plast Surg. 2018;80:684–91.
Ferrando CA. Adverse events associated with gender affirming vaginoplasty surgery. Am J Obstet Gynecol United States. 2020;223:267.e1-267.e6.
Levy JA, Edwards DC, Cutruzzula-Dreher P, McGreen BH, Akanda S, Tarry S, et al. Male-to-Female Gender Reassignment Surgery: An Institutional Analysis of Outcomes, Short-term Complications, and Risk Factors for 240 Patients Undergoing Penile-Inversion Vaginoplasty. Urology United States. 2019;131:228–33. This study is a retrospective study of 240 penile-inversion vaginoplasties performed by a single surgeon. It analyzes outcomes, complications, and risk factors in a large sample size.
Ives GC, Fein LA, Finch L, Sluiter EC, Lane M, Kuzon WM, et al. Evaluation of BMI as a Risk Factor for Complications following Gender-affirming Penile Inversion Vaginoplasty. Plast Reconstr surgery Glob open. 2019;7:e2097.
Mañero Vazquez I, García-Senosiain O, Labanca T, Gómez GE. Aesthetic Refinement in the Creation of the Clitoris, Its Preputial Hood, and Labia Minora in Male-to-Female Transsexual Patients. Ann Plast Surg United States. 2018;81:393–7.
van der Sluis WB, Steensma TD, Timmermans FW, Smit JM, de Haseth K, Özer M, et al. Gender-Confirming Vulvoplasty in Transgender Women in the Netherlands: Incidence, Motivation Analysis, and Surgical Outcomes. J Sex Med. 2020;17:1566–73. https://doi.org/10.1016/j.jsxm.2020.04.007.
Cocci A, Rosi F, Frediani D, Rizzo M, Cito G, Trombetta C, et al. 2019 Male-to-Female (MtoF) gender affirming surgery: Modified surgical approach for the glans reconfiguration in the neoclitoris (M-shape neoclitorolabioplasty). Arch Ital di Urol Androl organo Uff [di] Soc Ital di Ecogr Urol e Nefrol. Italy 91.
Cristofari S, Bertrand B, Leuzzi S, Rem K, Rausky J, Revol M, et al. Postoperative complications of male to female sex reassignment surgery: A 10-year French retrospective study. Ann Chir Plast Esthet France. 2019;64:24–32. This study evaluates postoperative complications in 189 patients who underwent feminizing gGAS by a single surgeon.
Dy GW, Jun MS, Blasdel G, Bluebond-Langner R, Zhao LC. Outcomes of Gender Affirming Peritoneal Flap Vaginoplasty Using the Da Vinci Single Port Versus Xi Robotic Systems. Eur Urol [Internet]. European Association of Urology; 2021;79:676–83. Available from: https://doi.org/10.1016/j.eururo.2020.06.040
Buncamper ME, Van Der Sluis WB, Van Der Pas RSD, Özer M, Smit JM, Witte BI, et al. Surgical Outcome after Penile Inversion Vaginoplasty: A Retrospective Study of 475 Transgender Women. Plast Reconstr Surg. 2016;138:999–1007.
Elmer-DeWitt MA, Wood HM, Hull T, Unger CA. Rectoneovaginal Fistula in a Transgender Woman Successfully Repaired Using a Buccal Mucosa Graft. Female Pelvic Med Reconstr Surg United States. 2019;25:e43-4.
Safa B, Lin WC, Salim AM, Deschamps-Braly JC, Poh MM. Current Concepts in Feminizing Gender Surgery. Plast Reconstr Surg. 2019;143:1081e–91e.
Chi AC, Poh MM. 2021 Urological Care for the Transgender Patient. Urol Care Transgender Patient 83–97.
Van Der Sluis WB, Bouman MB, Buncamper ME, Pigot GLS, Mullender MG, Meijerink WJHJ. Clinical characteristics and management of neovaginal fistulas after vaginoplasty in transgender women. Obstet Gynecol. 2016;127:1118–26.
Kavvadias T, Seifert HH, Ebbing J, Garcia DN, Kind AB. Robotic sacrocolpopexy for recurrent vaginal vault prolapse after sex reassignment surgery in a trans-woman. J Obstet Gynaecol J Inst Obstet Gynaecol England. 2019;39:569–70.
Stanojevic DS, Djordjevic ML, Milosevic A, Sansalone S, Slavkovic Z, Ducic S, et al. Sacrospinous Ligament Fixation for Neovaginal Prolapse Prevention in Male-to-Female Surgery. Urology. 2007;70:767–71.
Jacoby A, Maliha S, Granieri MA, Cohen O, Dy GW, Bluebond-Langner R, et al. Robotic Davydov Peritoneal Flap Vaginoplasty for Augmentation of Vaginal Depth in Feminizing Vaginoplasty. J Urol. 2019;201:1171–5.
Amend B, Seibold J, Toomey P, Stenzl A, Sievert KD. Surgical reconstruction for male-to-female sex reassignment. Eur Urol. 2013;64:141–9.
Bizic M, Stojanovic B, Bencic M, Bordás N, Djordjevic M. 2020 Overview on metoidioplasty: variants of the technique. Int J Impot Res. England
Monstrey S, Hoebeke P, Selvaggi G, Ceulemans P, Van Landuyt K, Blondeel P, et al. Penile reconstruction: is the radial forearm flap really the standard technique? Plast Reconstr Surg United States. 2009;124:510–8.
Al-Tamimi M, Pigot GL, Ronkes B, de Haseth KB, van de Grift TC, van Moorselaar RJA, et al. The First Experience of Using the Pedicled Labia Minora Flap for Urethral Lengthening in Transgender Men Undergoing Anterolateral Thigh and Superficial Circumflex Iliac Artery Perforator Flap Phalloplasty: A Multicenter Study on Clinical Outcomes. Urology United States. 2020;138:179–87.
Stojanovic B, Bizic M, Bencic M, Kojovic V, Majstorovic M, Jeftovic M, et al. One-Stage Gender-Confirmation Surgery as a Viable Surgical Procedure for Female-to-Male Transsexuals. J Sex Med Netherlands. 2017;14:741–6.
Meyer R, Daverio PJ, Dequesne J. One-stage phalloplasty in transsexuals. Ann Plast Surg United States. 1986;16:472–9.
Jolly D, Wu CA, Boskey ER, Taghinia AH, Diamond DA, Ganor O. Is Clitoral Release Another Term for Metoidioplasty? A Systematic Review and Meta-Analysis of Metoidioplasty Surgical Technique and Outcomes. Sex Med. 2021;9:100294.
Lin-Brande M, Clennon E, Sajadi KP, Djordjevic ML, Dy GW, Dugi D. Metoidioplasty With Urethral Lengthening: A Stepwise Approach. Urology. United States; 2021. p. 319–22. This study compares outcomes and complications of metoidioplasty with an without urethral lengthening at a single-center.
Kjölhede A, Cornelius F, Huss F, Kratz G. Metoidioplasty and groin flap phalloplasty as two surgical methods for the creation of a neophallus in female-to-male gender-confirming surgery: A retrospective study comprising 123 operated patients. JPRAS open. 2019;22:1–8.
Robinson IS, Blasdel G, Cohen O, Zhao LC, Bluebond-Langner R. Surgical Outcomes Following Gender Affirming Penile Reconstruction: Patient-Reported Outcomes From a Multi-Center, International Survey of 129 Transmasculine Patients. J Sex Med. Netherlands; 2021. This study compares outcomes and complication from phalloplasty, metoidioplasty, and phalloplasty secondary to metoidioplasty at a single-center.
Al-Tamimi M, Pigot GL, van der Sluis WB, van de Grift TC, van Moorselaar RJA, Mullender MG, et al. The Surgical Techniques and Outcomes of Secondary Phalloplasty After Metoidioplasty in Transgender Men: An International, Multi-Center Case Series. J Sex Med Netherlands. 2019;16:1849–59.
Schardein J, Weinberg AC, Zhao LC, Nikolavsky D. 2020 Management of Urethral Complications Following Metoidioplasty and Phalloplasty BT - Gender Confirmation Surgery: Principles and Techniques for an Emerging Field. In: Schechter LS, editor. Cham: Springer International Publishing. p. 201–13.
Ascha M, Massie JP, Morrison SD, Crane CN, Chen ML. Outcomes of Single Stage Phalloplasty by Pedicled Anterolateral Thigh Flap versus Radial Forearm Free Flap in Gender Confirming Surgery. J Urol United States. 2018;199:206–14. This study compares complications of single-stage ALT and single-stage RFFF at a single-center.
Falcone M, Preto M, Timpano M, Ciclamini D, Crosio A, Giacalone F, et al. 2021 The surgical outcomes of radial artery forearm free-flap phalloplasty in transgender men: single-centre experience and systematic review of the current literature. Int J Impot Res. England
Bouman FG. The first step in phalloplasty in female transsexuals. Plast. Reconstr. Surg. United States; 1987. p. 662–4.
Cohen O, Stranix JT, Zhao L, Levine J, Bluebond-Langner R. Use of a Split Pedicled Gracilis Muscle Flap in Robotically Assisted Vaginectomy and Urethral Lengthening for Phalloplasty: A Novel Technique for Female-to-Male Genital Reconstruction. Plast Reconstr Surg United States. 2020;145:1512–5.
Pigot GLS, Al-Tamimi M, Nieuwenhuijzen JA, van der Sluis WB, van Moorselaar RJA, Mullender MG, et al. Genital Gender-Affirming Surgery Without Urethral Lengthening in Transgender Men-A Clinical Follow-Up Study on the Surgical and Urological Outcomes and Patient Satisfaction. J Sex Med Netherlands. 2020;17:2478–87.
Chen W, Cylinder I, Najafian A, Dugi DD 3rd, Berli JU. An Option for Shaft-Only Gender-Affirming Phalloplasty: Vaginal Preservation and Vulvoscrotoplasty. A Technical Description. Plast Reconstr Surg United States. 2021;147:480–3.
Massie JP, Morrison SD, Wilson SC, Crane CN, Chen ML. Phalloplasty with Urethral Lengthening: Addition of a Vascularized Bulbospongiosus Flap from Vaginectomy Reduces Postoperative Urethral Complications. Plast Reconstr Surg United States. 2017;140:551e–8e.
Veerman H, de Rooij FPW, Al-Tamimi M, Ronkes BL, Mullender MG, Bouman BM, et al. Functional Outcomes and Urological Complications after Genital Gender Affirming Surgery with Urethral Lengthening in Transgender Men. J Urol United States. 2020;204:104–9.
Wirthmann AE, Majenka P, Kaufmann MC, Wellenbrock SV, Kasper L, Hüttinger S, et al. Phalloplasty in Female-to-Male Transsexuals by Gottlieb and Levine’s Free Radial Forearm Flap Technique-A Long-Term Single-Center Experience Over More than Two Decades. J Reconstr Microsurg United States. 2018;34:235–41.
Namba Y, Watanabe T, Kimata Y. Flap Combination Phalloplasty in Female-to-Male Transsexuals. J Sex Med Netherlands. 2019;16:934–41.
van der Sluis WB, Smit JM, Pigot GLS, Buncamper ME, Winters HAH, Mullender MG, et al. Double flap phalloplasty in transgender men: Surgical technique and outcome of pedicled anterolateral thigh flap phalloplasty combined with radial forearm free flap urethral reconstruction. Microsurgery. 2017;37:917–23.
Djordjevic ML, Bencic M, Kojovic V, Stojanovic B, Bizic M, Kojic S, et al. Musculocutaneous latissimus dorsi flap for phalloplasty in female to male gender affirmation surgery. World J Urol Germany. 2019;37:631–7.
Terrier M, Morel-Journel N, Carnicelli D, Ruffion A, Terrier J-E, Maucort-Boulch D, et al. 2021 Suprapubic phalloplasty in transmen: surgical results and critical review. Int J Impot Res. England
Falcone M, Timpano M, Oderda M, Cocci A, Morelli G, Preto M, et al. 2020 Suprapubic pedicled phalloplasty in transgender men: a multicentric retrospective cohort analysis. Int J Impot Res. England
Terrier J-É, Courtois F, Ruffion A, Morel JN. Surgical outcomes and patients’ satisfaction with suprapubic phalloplasty. J Sex Med Netherlands. 2014;11:288–98.
Garcia MM, Christopher NA, De Luca F, Spilotros M, Ralph DJ. Overall satisfaction, sexual function, and the durability of neophallus dimensions following staged female to male genital gender confirming surgery: the Institute of Urology, London U.K. experience. Transl Androl Urol. 2014;3:156–62.
Küenzlen L, Nasim S, van Neerven S, Kühn S, Burger AE, Sohn M, et al. Multimodal Evaluation of Functional Nerve Regeneration in Transgender Individuals After Phalloplasty With a Free Radial Forearm Flap. J Sex Med Netherlands. 2020;17:1012–24.
D’Arpa S, Claes K, Lumen N, Oieni S, Hoebeke P, Monstrey S. Urethral Reconstruction in Anterolateral Thigh Flap Phalloplasty: A 93-Case Experience. Plast Reconstr Surg United States. 2019;143:382e–92e.
Nolan IT, Kuhner CJ, Dy GW. Demographic and temporal trends in transgender identities and gender confirming surgery. Transl Androl Urol. 2019;8:184–90.
Takamatsu A, Harashina T. Labial ring flap: a new flap for metaidoioplasty in female-to-male transsexuals. J Plast Reconstr Aesthet Surg Netherlands. 2009;62:318–25.
Danker S, Annen AW, Cylinder I, Esmonde NO, Berli JU. Technical Description and Microsurgical Outcomes in Phalloplasty Using the Deep Inferior Epigastric Artery and Locoregional Veins. Plast Reconstr Surg United States. 2020;146:196e–204e.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topical Collection on Sexual Orientation and Identity
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Melancon, D.M., Walton, A.B., Mundinger, G. et al. Surgical Outcomes and Complications of Genital Gender Affirmation Surgery. Curr Sex Health Rep 13, 107–116 (2021). https://doi.org/10.1007/s11930-021-00318-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11930-021-00318-3