Abstract
Purpose of Review
Erectile dysfunction (ED) is a common and disabling impairment in ability to attain and maintain erections for sexual activity. Currently approved medical treatments for ED mitigate the organic elements of the disease on a short-term basis but do not address the underlying physiological issues; ergo, they do not “cure” so much as “manage” ED. Shockwave therapy (SWT) has recently attracted a great deal of interest as a potential means to resolve the physiological circumstances that lead to organic ED. In this review, we investigate the mechanisms by which SWT may help resolve ED and explore the existing evidence on this modality for management of ED.
Recent Findings
Recent studies have revealed membrane receptors capable of converting mechanical deformation into molecular signaling in cells. Subsequent signal transduction via these pathways may explain the beneficial effects of SWT including (but not limited to) vasodilation, enhanced nitric oxide synthase (NOS) activity, angiogenesis, neuro-regeneration, activation of progenitor cells, tissue remodeling, and anti-inflammatory effects. A limited body of evidence supports a role for SWT in restoration of erectile function in men with ED. These data are hampered by short-term follow-up and ambiguity about optimal administration protocols. Pulse wave and SWT may be conflated by patients and some providers; however, these modalities are not equivalent in terms of energy transfer nor evidence basis for efficacy in ED.
Summary
Shockwave therapy is an intriguing and counterintuitive approach to the problem of ED. Evidence from other organ systems and a limited body of direct evidence from animal models and human men with ED suggest that this modality may improve erectile function without the need for supplemental or adjuvant treatments. Additional mechanistic data will be informative. Optimal treatment protocols remain unclear. The long-term durability of erection improvement and potential need for repeat treatment with SWT remains unknown.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Erectile dysfunction (ED) is defined as the inability to attain and/or maintain an erection sufficient for satisfactory sexual activity [1•]. Approximately 42 million men are affected in the USA alone, with the international incidence expected to reach 332 million men by the year 2025 [2, 3]. ED can be a source of substantial intrapersonal and interpersonal stress.
The etiology of ED is often multifactorial. Common causes include neurogenic, medication-induced, hormonal, psychogenic, and vascular [4, 5]. Vascular disease is among the more common and important causes of ED, in part because ED may be the sentinel event that warns of incipient vascular disease that could be life-threatening; ED has been associated with increased risk of all-cause mortality. Intensive lifestyle change may reverse ED from vascular causes. While compelling and worthy of recommendation, many men are incapable of sustained and substantial lifestyle change [6, 7].
Shockwave therapy (SWT) has been touted for decades as a management strategy for various medical conditions. Most recently, low-intensity SWT has been investigated for efficacy in management of vascular disease [8]. Although counterintuitive, there is a growing body of evidence that energy transfer by this means could yield benefit in patients with a variety of vascular conditions including coronary artery disease, ischemic myocardial dysfunction, peripheral vascular disease, chronic wound healing, and peripheral neuropathy [9,10,11].
The emerging role of SWT in vascular disease makes SWT an interesting and novel option for management of ED. The penis is a protuberant external organ and hence easily accessible for application of shockwave energy. A number of studies have been conducted on SWT for ED. Evidence to date has been encouraging, but substantial ambiguity remains as to whether SWT will be an effective therapy for ED.
In this review, we will highlight the energy properties of SWT, distinguishing between SWT and other means of energy transfer. We will discuss the physiological rationale by which SWT may enhance penile erectile responses. We will draw data from basic vascular mechanistic studies as well as several publications specifically investigating SWT in the context of penile hemodynamics. We will review existing studies on application of SWT in men with ED. We will conclude with a consideration of the ethics of SWT, gaps in the existing literature, and directions for future research.
Methods
A comprehensive search of PubMed, Medline, and Cochrane databases was performed by using the keywords “erectile dysfunction” OR “penis” AND “shockwave” OR “SWT”. There were no restrictions regarding date of publication. Additional publications on mechanisms by which shockwave energy may exert beneficial effects were collected by review of reference lists. Selected articles were critically reviewed and summarized.
History of Shockwave Therapy in Medicine
Extracorporeal shockwave therapy is defined as the passage of energy waves through tissues, with a point of convergence which optimizes energy transfer to a target tissue or lesion. The amount of energy transferred to the tissue at the focal point is measured by the energy flux density (EFD), expressed in millijoules (mJ) over the surface area in millimeters squared (mm2).
Extracorporeal shockwave therapy has been used for various medical indications over many decades [12]. The first contemporary urological utilization of shockwave therapy was for the destruction of kidney stones. Extracorporeal shockwave lithotripsy has been used since the 1980s [13]. The EFD utilized in treatment of kidney stones is approximately 0.9 mJ/mm2.
Medium-intensity shockwave therapy (0.4–0.6 mJ/mm2) has been utilized since the 1990s for anti-inflammatory effect [14]. Specific clinical applications have included tendon-bone junction neovascularization and other uses in orthopedics [14,15,16,17,18,19,20,21].
Low-intensity SWT was first described in the 2000s to stimulate cardiac angiogenesis. Since that time, it has been adopted and studied in treatment of various pathologies, including treatment of refractory angina, ischemic heart disease, tendinitis, and non-healing bone fractures. SWT has been noted to have angiogenic and anti-inflammatory effects [22,23,24,25].
The angiogenic potential of LSWT makes it of great interest as a potential treatment for ED. In the management of ED, the typical EFD is less than 0.25 mJ/mm2. The first contemporary report of SWT for the management of ED was in 2010 [12]. This initial study described a protocol involving two rounds of EFD of 0.09 mJ/mm2 at 120 Hz for 300 pulses biweekly for 3 weeks, with an intervening 3 week no-treatment period. This study found durable improvements in International Index of Erectile Function (IIEF-ED) domain scores in 20 middle-aged men over 6-month follow-up, with an average increase of 7.4 points. The authors also demonstrated increased duration and rigidity of erections. Approximately half of these ED patients did not require PDE5-I therapy at 6 months follow-up. These results prompted the authors to conduct the first prospective, randomized, double-blind sham-controlled trial in 67 men with ED, which demonstrated significant increases in IIEF-ED domain scores (mean increase 6.7 vs 3), ability to achieve erections sufficient for penetration (19 men in the treatment group with baseline erectile hardness score ≤ 2 before treatment and > 2 after treatment vs none in the sham group), and maximal post-ischemic penile blood flow (8.2 vs 0.1 ml/min/dl in the treatment and sham group, respectively) at 1-month follow-up in treated men compared to sham-treated men [26].
Not All Energy Is Created Equal
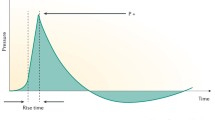
A variety of energy transfer technologies exist. Shockwaves and radial pulse (aka acoustic) waves are the two forms that have been mostly widely promulgated for use in ED. These technologies differ in several important ways, which are described below and summarized in Fig. 1.
Graphical representation of the difference between shockwaves (a) and radial pulse waves (b) with pressure on the y-axis (mPa) and time on x-axis. Reproduced from Edmonton Shockwave Center [27]
Shockwaves have a much higher amplitude and involve a sharp spike of energy delivered to the tissue. This is characterized by a fast pressure rise (10 ns), a high peak pressure (around 10–100 mPa), and a short lifecycle (around 10 ms). In contrast, radial pulse waves are generally delivered at longer pressure rise (0.5–2 ms), lower amplitude (1 mPa), and with a sinusoidal pattern of energy delivery over a longer lifecycle. Radial pulse waves are pressure waves rather than true shockwaves and deliver gradual energy with lower stress at tissue interfaces when compared to shockwaves. The depth of tissue penetration is less with radial pulse waves compared to true shockwaves [27, 28].
Radial pulse waves are widely used and studied in sports medicine and orthopedic practice and literature [29, 30]. All randomized controlled trials of energy transfer in the treatment of ED thus far have focused on shockwave therapies [31•]. Devices that use radial pulse waves are available and widely promoted for the treatment of ED despite a dearth of peer-reviewed evidence on efficacy.
Mechanical Stress as a Mediator of Cellular Responses
Delivery of shockwave energy to cells leads to conformational changes. On a broad, cellular level, this cycle is characterized by three phases:
-
1)
The resting phase, which is the cellular conformation prior to delivery of the shockwave
-
2)
The compressive phase, which is the cellular confirmation during the delivery of the initial energy delivery, characterized by a 2% contraction of the overall size of the cell compared to baseline
-
3)
The tensile phase, which occurs immediately following the delivery of the shockwave pulse energy, characterized by a 10% expansion of the overall size of the cell compared to baseline [32]
Cellular deformation and membrane stress mediate cellular response through modulation of a family of membrane-bound ion channels known as Piezo channels [33]. Piezo channels are membrane-bound ion channels that convert mechanical signals into molecular pathways within the cell and are stimulated during the delivery of shockwaves to the tissue. Mechanical stress leads to cation influx through these channels which propagates both membrane depolarization and ATP/NO release through Ca-dependent signaling pathways. The same signaling pathway has been shown to stimulate progenitor cells, most notably in the digestive tract of Drosophila [34].
Cellular and Tissue Effects of SWT
The cellular and tissue effects of SWT are numerous and have been reported in a number of studies, characterized by the upregulation and downregulation of various mechanisms. These are summarized in Table 1.
Vasodilation
Neuronal nitric oxide synthase (nNOS) and endothelial nitric oxide synthase (eNOS) play critical roles in erectile function through modulation of the cGMP pathway resulting in smooth muscle relaxation, vasodilation, and subsequent penile erection [35, 36]. SWT enhances expression of eNOS and nNOS in both penile and non-penile tissues. Both NOS isoforms lead to production of nitric oxide (NO). NO in turn activates guanylate cyclase, which produces cyclic guanosine monophosphate (cGMP) using guanosine triphosphate (GTP) as a substrate. cGMP produces downstream signals resulting in intracellular calcium regulation, subsequent smooth muscle relaxation, and, ultimately, vasodilation [37]. Animal studies have demonstrated increased nNOS and eNOS expression in penile tissue in rats following SWT, including the dorsal nerves and sinusoids. This biochemical pathway is also enhanced downstream, manifesting in increased cGMP levels. These effects have been demonstrated also in rats treated with SWT after cavernous nerve injury [38,39,40,41,42].
Application of SWT to human umbilical vein endothelial cell (HUVEC) cultures stimulates increased eNOS activity, with a peak eNOS activity at SWT 1000 shocks. This effect was shown to be mediated by increased tyrosine dephosphorylation of eNOS, a successive increase in nitric oxide (NO) production, and suppression of NF-kB activation [36].
SWT has also been associated with increased alpha-2 receptor expression with concomitant decreased alpha-1 receptor expression in the penile tissue of aged rats. The modulation of alpha-1 and alpha-2 receptors leads to overall decreased smooth muscle tone and vasodilation [35, 43].
Anti-inflammatory
SWT is associated with reduction of pro-inflammatory mediators such as TNF-α, TGF-β1, IL-1, IFN-γ, and advanced glycation end products (AGEs) [44]. Additionally, HUVEC cells stimulated by SWT generate eNOS in vitro [36]. Exposure to lipopolysaccharides (a mediator of infection induced inflammation) and the inflammatory cytokine IFN-gamma reduces eNOS expression, an effect which is ameliorated by exposure to SWT [44, 45].
In rat models of coronary ischemia, SWT induces decreased inflammatory macrophage infiltration. While overall macrophage infiltration declined, an enhanced population of the M2 “wound healing” isotype of macrophage was noted [44]. The implication from this finding is that inflammatory cells overall are reduced and immune cells that are present are more likely to induce healing as opposed to fibrosis. STZ-induced diabetic rats treated with SWT exhibit decreased penile expression of receptor for AGE in a dose-dependent fashion [45].
Angiogenesis
Angiogenesis was among the earliest identified effects of SWT on tissue. Endothelial cell and capillary density increase following SWT in rats, an effect that appears to be mediated by upregulation of VEGF and angiopoietin [38, 45]. These cytokines are known to stimulate angiogenesis. In animal models of wound healing, SWT has been associated with increased flap survival, enhanced wound healing, and improved tissue perfusion in non-penile tissues [11, 46]. Data on penile tissues are limited, but evidence suggests that corporal tissues treated with SWT show enhancement of von Willebrand factor (vWF) expression in cavernous nerve injured rats and endothelial antigens in diabetic rats [39, 45]. Both of these are markers for more robust endothelial tissues/function.
Recruitment and Activation of Progenitor Cells
SWT is associated with upregulation of progenitor cells in a variety of tissues. SWT leads to enhanced recruitment of endothelial progenitor cells (EPC), increased stromal cell-derived factor 1, and improved blood flow in rodent hind leg ischemia models [47]. Increased proliferating cell nuclear antigen expression has been demonstrated with SWT administration in studies of diabetic wound healing in rats [11]. Enrichment in Edu-labeled stem cells localized to the penis and cavernous nerves has been shown in Zucker diabetic fatty rats, cavernous nerve injured rats, and middle-aged rats after SWT exposure [42, 48, 49]. This response has implications in generation of new smooth muscle and endothelial cells, regeneration of nervous tissue, and angiogenesis within the penile tissue.
Nerve Regeneration
SWT-induced nerve regeneration is hypothesized to be regulated by VEGF and other factors. SWT treatment enhances recovery in the setting of sciatic nerve interposition after nerve transection in rats. Specifically, SWT treatment leads to improved functional recovery, reduced phagocyte infiltration, and greater nerve myelination at 3 weeks. There was however little functional difference between groups at 3 months post-injury. This treatment effect is thought to be due to enhanced Wallerian degeneration of axonal tissue distal to the transection site, allowing for more rapid reconnection of relevant nerves [50].
This observation suggests possible neurotrophic effects in increased erectile function in addition to enhanced endothelial and vasodilatory function. This response is hypothesized to contribute to the enhanced erectile function observed in rats that are both treated with SWT and human adipose–derived stem cell (h-ADSC) treatment, observed specifically in cavernous nerve injury rats [38, 42].
Tissue Remodeling
A final proposed effect of SWT is enhanced tissue remodeling. Diabetic rat models of ED have decreased smooth muscle and endothelial content of the penis on histological examination when compared to controls [38]. Pulsed ultrasound or SWT in STZ-induced diabetic rats reduces TGF/Smad activity as well as preserves elastin fibers [40]. Additionally, studies have demonstrated increased density and content of endothelial and smooth muscle cells within the diabetic rat penis following treatment with SWT [38,39,40,41]. This tends to correlate with greater improvements in penile hemodynamics.
Rats treated with SWT after cavernous nerve injury demonstrate increased smooth muscle actin as well as reduced apoptosis within the nerve tissue and increased collagen I/III ratios [38,39,40, 42, 45].
Many of the above cellular and tissue effects are associated with enhanced erectile function and penile hemodynamic responses in diabetic and cavernous nerve injury rat models. It has been suggested that these effects occur in a dose-dependent manner, with the optimal dose being around 300 shocks [45]. These functional data are summarized in greater detail the following section.
Penile Hemodynamic Effects of SWT in Rat Models of ED
Many rat models are used to study ED. Rats that model diabetes are frequently studied as these strains are easily attainable and well studied and mimic human disease processes associated with diabetes, including diminished erectile function [51].
Some rodent models of ED include:
-
1.
STZ-induced diabetes – Type 1 diabetes model of nonobese rats with diabetes induced by injection of streptozotocin, which obliterates islet cells in the rat pancreas.
-
2.
GK (Goto-Kakizaki) rat – Nonobese Wistar substrain that is a model for type 2 diabetes.
-
3.
ZDF (Zucker diabetic fatty) rat – Generally used to study type 2 diabetes associated with obesity. This strain develops type 2 diabetes with 100% incidence by 20 weeks of age.
-
4.
Cavernous nerve injury – Genetically normal rats who undergo surgical damage or destruction of the cavernous nerves which innervate the penis. This model is used to simulate ED related to radical pelvic surgery in humans [52].
Rat studies generally focus on changes in penile hemodynamics before and after intervention, as well as histopathologic changes following treatment. Penile hemodynamic effects are assessed by measurement of intracavernosal pressure (ICP), either directly or as a ratio compared to mean arterial pressure (MAP). ICP is measured during stimulated erection (most often by electrical stimulation of the cavernous nerves) via a pressure transducer in the corpora cavernosa. MAP is measured via a pressure transducer placed in an artery such as the internal carotid [52].
A number of studies have investigated application of both shockwave and radial pulse wave energy to rat models of ED, including diabetic, cavernous-nerve-injured, hypertensive, and aged rats [35, 42, 48, 53,54,55,56,57]. In several of these studies, SWT was used as an adjunct to administration of stem cells [58, 59] or herbal therapies [60]. The bulk of these data have shown significant and positive effects of SWT in terms of ICP/MAP ratios compared to sham-treated animals.
In rat models for diabetes, increases in ICP have been demonstrated following SWT [39, 45, 56,57,58,59,60] as well as after radial pulse ultrasound [40]. This effect was seen in all studies save for one, which demonstrated diminished ICP/MAP ratios following treatments consisting of either 1000 or 2000 shocks for 1, 2, or 3 sessions [61]. This protocol varied notably from most other such studies, which typically utilize dosages of 300 shocks 2 or 3 times per week for several weeks. This study found decreased ICP/MAP, thought to be attributable to increased apoptosis and collagen deposition in smooth muscle. In the remaining studies, observed increases in ICP/MAP ratios were thought to be secondary to a multitude of observed effects during histopathological examination of the penis, as outlined above.
Additional data have shown that SWT in combination with stem cell treatment improves ICP and markers for erectile function in diabetic rats. These effects are noted to be synergistic when compared to either intervention alone. SWT improves survival of stem cells in the penis and increases stem cell expression of multiple factors including VEGF, NGF, BDNF, and stromal cell-derived factor 1, all of which help stimulate angiogenesis and vasodilation, as discussed previously [58, 59].
Other animal studies have focused on SWT effects in non-diabetic rats. Most notably, nerve injury rat models simulating radical pelvic surgery and resultant impaired penile hemodynamics have been studied in detail [42, 48, 54]. Application of SWT in these rats demonstrates increased ICP both when applied immediately after nerve injury [42, 48] or 4 weeks after injury [54]. Other observed benefits include increased progenitor cell presence, angiogenesis, nerve regeneration, Schwann cell proliferation, and expression of nNOS and VEGF.
Other nondiabetic models have examined improvement in penile hemodynamics after SWT in hypertensive [53] and aged rats [35]. Findings in these studies echo those of the majority of the other rat studies, which demonstrate increased ICP in comparison to controls, via similar mechanisms previously noted.
Human Data on SWT in ED
Several human studies of SWT have been reported, with varying protocols and quality of evidence. There have been two primary meta-analyses on SWT for the treatment of ED in humans by Campbell et al. [31•] and Clavijo et al. [62]. These studies each analyzed seven RCTs and a total of 607 and 602 men with ED, respectively. Among the seven RCTs analyzed by Campbell et al. [26, 63,64,65,66,67,68], mean International Index of Erectile Function: Erectile function (IIEF-EF) scores at follow-up posttreatment and the change in baseline IIEF-EF domain scores at follow-up were significantly better in those patients randomized to SWT as compared to control groups receiving sham treatment. Absolute mean difference in change of IIEF-EF scores between groups was 4.13 (95% CI 0.8–7.47) at 1 month follow-up. These findings were echoed by those studies assessed by Clavijo et al. [26, 64,65,66,67,68,69], who analyzed questionnaire scores at a mean follow-up of 19.2 weeks. The difference in IIEF-EF persisted at this interval, with a mean difference between groups of 4.75. These data are demonstrated in Fig. 1, first presented by Clavijo et al. [62]. Secondary findings from Campbell et al. included significantly better odds of increased erectile hardness score (EHS) to 3 or higher (relative risk 6.63, 95% CI 1.59–27.71) and improvement of IIEF-EF by at least 5 points (relative risk 1.94, 95% CI 0.97–3.85) relative to sham-treated men.
Interestingly, one study included in these meta-analyses [68] found no difference between treatment and sham-treated men. The protocol for shock administration utilized in this study differed significantly from the other studies included in the analyses. The protocol presented by Fojecki et al. included 600 shocks per session to only 3 locations, as opposed to the other analyzed protocols which ranged from 1500 to 3000 shocks per treatment at anywhere from 3 to 6 locations. Additionally, the treatments in Fojecki et al.’s study were provided in two rounds of weekly treatment for 5 weeks with a 4-week interval, whereas the majority of the other studies followed a more rigorous protocol of two rounds of twice weekly treatments for 3 weeks with a 3-week interval of nontreatment. The outlying protocol culminated in only 6000 shocks throughout the treatment, in contrast to 18,000 total shocks in the remaining studies [62]. This discrepancy supports previous data suggesting a dose-dependent response to SWT [45].
Five of 7 studies in the Campbell et al. meta-analysis reported that there were no adverse events with the other two studies reporting mild burning or local irritation immediately following treatment [31•].
Future Directions
A distinguishing feature of SWT for ED is the favorable side effect profile. The tolerability of the therapy makes it very appealing to both patients and providers. The safety profile does however create the potential for an ethical conundrum; since risks seem minimal, should SWT be utilized even without robust efficacy data?
It is our opinion that even procedures without apparent risk require demonstration of efficacy and long-term safety before widespread adoption. There is a possibility, albeit a slim one, that SWT has a negative long-term effect on penile hemodynamics. Setting aside concerns for occult major pathology, a very serious financial and patient autonomy argument can be made for a guarded approach to SWT therapy. Energy-based therapies may be promoted/utilized by individuals (both patients and doctors) who may not have the sophistication to identify what is evidence-based and what is not [1•]. Providers may charge patients large sums for these unproven therapies. Aside from the direct financial cost, there is an intangible expense in terms of lost opportunity to utilize effective therapy earlier on and/or frustration with treatment failure that may cause some men to give up before trialing therapies known to be efficacious.
There is a strong possibility that SWT or any similar technology will exert a placebo effect in the management of ED. It may hence benefit even patients with psychogenic ED. Despite this, we believe that SWT is a sub-optimal treatment for primarily psychogenic ED, even if it may yield benefit. Invasive/medical treatments should be given to patients who will experience biological effects, not a purely placebo-driven benefit.
We are strongly supportive of further research on SWT. We believe that this research must be conducted at minimal cost to patients and only in the context of an institutional review board sanctioned clinical trial [1•]. We are not supportive of charging patients for SWT treatments and treating this technology as a standard of care at this time. Furthermore, we do not believe that clinicians should make unsubstantiated claims about efficacy of any therapy, including SWT.
At the time of this writing, SWT has not been formally approved in the USA specifically for the management of ED. The 2018 American Urological Association Guidelines on ED recommend that SWT should only be utilized in the context of a clinical trial with IRB approval, documented informed consent, and minimal cost transfer to the patient [1•]. Patients who are interested in SWT for ED should be referred to a center conducting IRB-approved studies. Clinicaltrials.gov is a clearing house for IRB approved studies and is one resource that could be used to locate reputable clinical trials of SWT. Prior guidance on SWT for management of Peyronie’s disease (PD, a condition of scarification and deformity of the penis) also recommended against SWT for management of deformity but did offer that it may have utility in pain management [70].
Additional long-term data are required to determine if and under what circumstances SWT is appropriate for men with ED [71]. The durability of therapy is unclear; it seems likely that barring substantial changes to underlying risk factors the underlying disease process will recur. Men with ED should be advised that positive lifestyle changes be part of their long-term management plan as these approaches have been shown to help restore/preserve erectile function [7]. Given the challenges inherent in lifestyle changes, it is likely that repeat administration of SWT at some point posttreatment may be required.
The ideal SWT protocol for ED has yet to be elucidated. Questions remain regarding optimal EFD, number of shocks, and treatment interval. The bulk of studies to date have focused on shockwaves, not acoustic waves. It is our opinion that SWT data is advanced, and at this time, it is difficult to conclude that any particular shockwave generator or manufacturer is superior to another. Whether acoustic wave therapy has potential for benefit remains a fertile area for further research, but the state of these data lags far behind that of shockwave data.
Additional considerations should be given to whether a standardized protocol would be sufficient for all patients or if alterations should be made to account for certain factors such as penile size, vascular integrity, underlying etiology, and presence of fibrosis. Varying energy flux and number of shocks may bring about different dose-dependent changes in a variety of end effects, including angiogenesis, progenitor cell recruitment, tissue remodeling, or anti-inflammation. The limited existing data does suggest that protocols utilizing relatively few shocks (e.g., Fojecki et al. [68]) have markedly less efficacy than protocols with over 10,000 shocks administered.
The magnitude of mean change in IIEF-EF is clinically significant but may not be sufficient for restoration of satisfactory erectile function in all men. Specifically, men with severe ED require (on average) a 7-point improvement in IIEF-EF domain score for clinically noticeable benefit [72]. Hence, men with a more severe ED phenotype (e.g., men with severe diabetes or status post-radical pelvic surgery) may be less likely to benefit from SWT. SWT may have a role to play in severe ED phenotypes, but expectations should be set accordingly, and it is likely that such men will still require adjuvant ED therapy. For the time being, the index patient for SWT should be a man with mild to moderate ED thought to be related to organic, ideally vascular, etiologies.
Conclusions
Shockwave-based therapies are an exciting development that may be useful for restoration of erectile function without the need for on-demand supplemental therapy. Existing data are interesting, but data remain limited. Rigorous long-term studies are required before this approach can be considered a standard of care; until that time, shockwave therapy is most appropriately used only in a clinical trial setting. Care must be taken to distinguish between energy modalities that have robust body of evidence and those that do not.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
• Burnett AL, Nehra A, Breau RH, Culkin DJ, Faraday MM, Hakim LS, et al. Erectile Dysfunction: AUA Guideline. J Urol. 2018;200(3):633–41. The 2018 AUA GUideline on ED is the most up to date resource for information on the medical standard of care for ED. These guidelines articulate that SWT is a promising technology but should not be utilized outside of a clinical trial setting at this time.
Goldstein I, Goren A, Li VW, Tang WY, Hassan TA. Epidemiology update of erectile dysfunction in eight countries with high burden. Sex Med Rev. 2020;8(1):48–58.
Aytaç IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84(1):50–6.
Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013;381:153–65.
Lasker GF, Maley JH, Kadowitz PJ. A review of the pathophysiology and novel treatments for erectile dysfunction. Adv Pharmacol Sci. 2010. https://doi.org/10.1155/2010/730861.
Fan Y, Hu B, Man C, Cui F. Erectile dysfunction and risk of cardiovascular and all-cause mortality in the general population: a meta-analysis of cohort studies. World J Urol. Springer Verlag. 2018;36:1681–9.
Esposito K, Ciotola M, Giugliano F, Maiorino MI, Autorino R, De Sio M, et al. Effects of intensive lifestyle changes on erectile dysfunction in men. J Sex Med. 2009;6(1):243–50.
Raza A, Harwood A, Totty J, Smith G, Chetter I. Extracorporeal shockwave therapy for peripheral arterial disease: a review of the potential mechanisms of action. Ann Vasc Surg. Elsevier Inc. 2017;45:294–8.
Fu M, Sun CK, Lin YC, Wang CJ, Wu CJ, Ko SF, et al. Extracorporeal shock wave therapy reverses ischemiarelated left ventricular dysfunction and remodeling: molecular-cellular and functional assessment. PloS one. 2011;6(9):e24342.
Nishida T, Shimokawa H, Oi K, Tatewaki H, Uwatoku T, Abe K, et al. Extracorporeal cardiac shock wave therapy markedly ameliorates ischemia-induced myocardial dysfunction in pigs in vivo. Circulation. 2004;110(19):3055–61.
Kuo YR, Wang CT, Wang FS, Chiang YC, Wang CJ. Extracorporeal shock-wave therapy enhanced wound healing via increasing topical blood perfusion and tissue regeneration in a rat model of STZ-induced diabetes. Wound Repair Regen. 2009;17(4):522–30.
Vardi Y, Appel B, Jacob G, Massarwi O, Gruenwald I. Can low-intensity extracorporeal shockwave therapy improve erectile function? A 6-month follow-up pilot study in patients with organic erectile dysfunction. Eur Urol. 2010;58(2):243–8.
Chaussy C, Brendel W, Schmiedt E. Extracorporeally induced destruction of kidney stones by shock waves. Lancet. 1980;316(8207):1265–8.
Birnbaum K, Wirtz DC, Siebert CH, Heller KD. Use of extracorporeal shock-wave therapy (ESWT) in the treatment of non-unions. A review of the literature. Arch Orthop Trauma Surg. 2002;122(6):324–30.
Wang CJ, Wang FS, Yang KD, Weng LH, Hsu CC, Huang CS, et al. Shock wave therapy induces neovascularization at the tendon-bone junction. A study in rabbits. J Orthop Res. 2003;21(6):984–9.
Valchanou VD, Michailov P. High energy shock waves in the treatment of delayed and nonunion of fractures. Int Orthop. 1991;15(3):181–4.
Rompe JD, Zoellner J, Nafe B. Shock wave therapy versus conventional surgery in the treatment of calcifying tendinitis of the shoulder. Clin Orthop Relat Res. 2001;72–82.
Loew M, Daecke W, Kusnierczak D, Rahmanzadeh M, Ewerbeck V. Shock-wave therapy is effective for chronic calcifying tendinitis of the shoulder. J Bone Joint Surg Br. 1999;81(5):863–7.
Rompe JD, Hopf C, Küllmer K, Heine J, Bürger R. Analgesic effect of extracorporeal shock-wave therapy on chronic tennis elbow. J Bone Joint Surg Br. 1996;78(2):233–7.
Rompe JD, Decking J, Schoellner C, Nafe B. Shock wave application for chronic plantar fasciitis in running athletes: a prospective, randomized, placebo-controlled trial. Am J Sports Med. 2003;31(2):268–75.
Cacchio A, Rompe JD, Furia JP, Susi P, Santilli V, De Paulis F. Shockwave therapy for the treatment of chronic proximal hamstring tendinopathy in professional athletes. Am J Sports Med. 2011;39(1):146–53.
Alunni G, Marra S, Meynet I, D’amico M, Elisa P, Fanelli A, et al. The beneficial effect of extracorporeal shockwave myocardial revascularization in patients with refractory angina. Cardiovasc Revasc Med. 2015;16(1):6–11.
Yang P, Guo T, Wang W, Peng YZ, Wang Y, Zhou P, et al. Randomized and double-blind controlled clinical trial of extracorporeal cardiac shock wave therapy for coronary heart disease. Heart Vessel. 2013;28(3):284–91.
Zuoziene G, Leibowitz D, Celutkiene J, Burneikaite G, Ivaskeviciene L, Kalinauskas G, et al. Multimodality imaging of myocardial revascularization using cardiac shock wave therapy. Int J Cardiol. 2015;187(1):229–30.
Wang CJ, Cheng JH, Kuo YR, Schaden W, Mittermayr R. Extracorporeal shockwave therapy in diabetic foot ulcers. Int J Surg. 2015;24. Elsevier Ltd.:207–9.
Vardi Y, Appel B, Kilchevsky A, Gruenwald I. Does low intensity extracorporeal shock wave therapy have a physiological effect on erectile function? Short-term results of a randomized, double-blind, sham controlled study. J Urol. 2012;187(5):1769–75.
Shockwave therapy versus radial shockwave therapy. Edmonton shockwave therapy centre. https://edmontonshockwavetherapycentre.com/shockwave-therapy/shockwave-therapyversus-radial-shockwavetherapy/. Accessed 25 Aug 2020.
Basic physical principles. In: Physical prinicples of ESWT. International society for medical shockwave treatment. https://www.shockwavetherapy.org/about-eswt/physical-principles-ofeswt/. Accessed 25 Aug 2020.
Speed C. A systematic review of shockwave therapies in soft tissue conditions: Focusing on the evidence. Br J Sports Med. 2014;48(21):1538–42.
Kertzman P, Császár NBM, Furia JP, Schmitz C. Radial extracorporeal shock wave therapy is efficient and safe in the treatment of fracture nonunions of superficial bones: a retrospective case series. J Orthop Surg Res. 2017;12(1):164.
• Campbell JD, Trock BJ, Oppenheim AR, Anusionwu I, Gor RA, Burnett AL. Meta-analysis of randomized controlled trials that assess the efficacy of low-intensity shockwave therapy for the treatment of erectile dysfunction. Ther Adv Urol. 2019;11:175628721983836. This meta-anlsyis summarizes the latest human data on SWT for management of ED. Several studies of limited duration imply superiority of SWT to sham treatment for ED; the durabiltiy of improvement remains an unanswered question.
Li D, Pellegrino A, Hallack A, Petrinic N, Jérusalem A, Cleveland RO. Response of single cells to shock waves and numerically optimized waveforms for cancer therapy. Biophys J. 2018;114(6):1433–9.
Haselwandter CA, Mackinnon R. Piezo’s membrane footprint and its contribution to mechanosensitivity. Elife. 2018;7:e41968.
He L, Si G, Huang J, Samuel ADT, Perrimon N. Mechanical regulation of stem-cell differentiation by the stretch-activated Piezo channel. Nature. 2018;555(7694):103–6.
Sokolakis I, Dimitriadis F, Psalla D, Karakiulakis G, Kalyvianakis D, Hatzichristou D. Effects of low-intensity shock wave therapy (LiST) on the erectile tissue of naturally aged rats. Int J Impot Res. 2019;31(3):162–9.
Mariotto S, Cavalieri E, Amelio E, Ciampa AR, De Prati AC, Marlinghaus E, et al. Extracorporeal shock waves: From lithotripsy to anti-inflammatory action by NO production. Nitric Oxide Biol Chem. 2005;12(2):89–96.
Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32:379–95.
Jeon SH, Shrestha KR, Kim RY, Jung AR, Park YH, Kwon O, et al. Combination therapy using human adipose-derived stem cells on the cavernous nerve and low-energy shockwaves on the corpus cavernosum in a rat model of post-prostatectomy erectile dysfunction. Urology. 2016;88:226.e1–9.
Qiu X, Lin G, Xin Z, Ferretti L, Zhang H, Lue TF, et al. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med. 2013;10(3):738–46.
Lei H, Xin H, Guan R, Xu Y, Li H, Tian W, et al. Low-intensity pulsed ultrasound improves erectile function in streptozotocin-induced type i diabetic rats. Urology. 2015;86(6):1241.e11–8.
Liu T, Shindel AW, Lin G, Lue TF. Cellular signaling pathways modulated by low-intensity extracorporeal shock wave therapy. Int J Impot Res. Nature Publishing Group. 2019;31:170–6.
Li H, Matheu MP, Sun F, Wang L, Sanford MT, Ning H, et al. Low-energy shock wave therapy ameliorates erectile dysfunction in a pelvic neurovascular injuries rat model. J Sex Med. 2016;13(1):22–32.
Traish A, Kim NN, Moreland RB, Goldstein I. Role of alpha adrenergic receptors in erectile function. Int J Impot Res. 2000;12(S1):S48–63.
Abe Y, Ito K, Hao K, Shindo T, Ogata T, Kagaya Y, et al. Extracorporeal low-energy shock-wave therapy exerts anti-inflammatory effects in a rat model of acute myocardial infarction. Circ J. 2014;78(12):2915–25.
Liu J, Zhou F, Li GY, Wang L, Li HX, Bai GY, et al. Evaluation of the effect of different doses of low energy shock wave therapy on the erectile function of streptozotocin (STZ)-induced diabetic rats. Int J Mol Sci. 2013;14(5):10661–73.
Yan X, Zeng B, Chai Y, Luo C, Li X. Improvement of blood flow, expression of nitric oxide, and vascular endothelial growth factor by low-energy shockwave therapy in random-pattern skin flap model. Ann Plast Surg. 2008;61(6):646–53.
Aicher A, Heeschen C, Sasaki KI, Urbich C, Zeiher AM, Dimmeler S. Low-energy shock wave for enhancing recruitment of endothelial progenitor cells: a new modality to increase efficacy of cell therapy in chronic hind limb ischemia. Circulation. 2006;114(25):2823–30.
Lin G, Reed-Maldonado AB, Wang B, Lee YC, Zhou J, Lu Z, et al. In situ activation of penile progenitor cells with low-intensity extracorporeal shockwave therapy. J Sex Med. 2017;14(4):493–501.
Lin G, Alwaal A, Zhang X, Wang J, Wang L, Li H, et al. Presence of stem/progenitor cells in the rat penis. Stem Cells Dev. 2015;24(2):264–70.
Hausner T, Pajer K, Halat G, Hopf R, Schmidhammer R, Redl H, et al. Improved rate of peripheral nerve regeneration induced by extracorporeal shock wave treatment in the rat. Exp Neurol. 2012;236(2):363–70.
Clark JT. Sexual function in altered physiological states: comparison of effects of hypertension, diabetes, hyperprolactinemia, and others to “normal” aging in male rats. Neurosci Biobehav Rev. 1995;19(2):279–302.
Chung E, De Young L, Brock GB. Investigative models in erectile dysfunction: a state-of-the-art review of current animal models. J Sex Med. 2011;8(12):3291–305.
Assaly R, Giuliano F, Clement P, Laurin M, Favier M, Teo P, et al. Extracorporeal shock waves therapy delivered by aries improves erectile dysfunction in spontaneously hypertensive rats through penile tissue remodeling and neovascularization. Sex Med. 2019;7(4):441–50.
Wang HS, Ruan Y, Banie L, Cui K, Kang N, Peng D, et al. Delayed low-intensity extracorporeal shock wave therapy ameliorates impaired penile hemodynamics in rats subjected to pelvic neurovascular injury. J Sex Med. 2019;16(1):17–26.
Wang B, Ning H, Reed-Maldonado AB, Zhou J, Ruan Y, Zhou T, et al. Low-intensity extracorporeal shock wave therapy enhances brain-derived neurotrophic factor expression through PERK/ATF4 signaling pathway. Int J Mol Sci. 2017;18(2):433.
Ortaç M, Küçükergin C, Salabaş E, Seçkin Ş, Kadıoğlu A. Düşük enerjili şok dalga tedavisinin diyabetik sıçanların penis dokusundaki anjiyogenez faktörlerine etkisi. Turk Urol Derg. 2017;43(2):130–4.
Assaly-Kaddoum R, Giuliano F, Laurin M, Gorny D, Kergoat M, Bernabé J, et al. Low intensity extracorporeal shock wave therapy improves erectile function in a model of type II diabetes independently of NO/cGMP pathway. J Urol. 2016;196(3):950–6.
Zhu GQ, Jeon SH, Bae WJ, Choi SW, Jeong HC, Kim KS, et al. Efficient promotion of autophagy and angiogenesis using mesenchymal stem cell therapy enhanced by the low-energy shock waves in the treatment of erectile dysfunction. Stem Cells Int. 2018;2018:1302672.
Shan HT, Zhang HB, Chen WT, Chen FZ, Wang T, Luo JT, et al. Combination of low-energy shock-wave therapy and bone marrow mesenchymal stem cell transplantation to improve the erectile function of diabetic rats. Asian J Androl. 2017;19(1):26–33.
Jeong HC, Bae WJ, Zhu GQ, Jeon SH, Choi SW, Kim SJ, et al. Synergistic effects of extracorporeal shockwave therapy and modified ojayeonjonghwan on erectile dysfunction in an animal model of diabetes. Investig Clin Urol. 2019;60(4):285–94.
Müller A, Akin-Olugbade Y, Deveci S, Donohue JF, Tal R, Kobylarz KA, et al. The impact of shock wave therapy at varied energy and dose levels on functional and structural changes in erectile tissue. Eur Urol. 2008;53(3):635–43.
Clavijo RI, Kohn TP, Kohn JR, Ramasamy R. Effects of low-intensity extracorporeal shockwave therapy on erectile dysfunction: a systematic review and meta-analysis. J Sex Med. Elsevier B.V. 2017;14:27–35.
Olsen AB, Persiani M, Boie S, Hanna M, Lund L. Can low-intensity extracorporeal shockwave therapy improve erectile dysfunction? A prospective, randomized, double-blind, placebo-controlled study. Scand J Urol. 2015;49(4):329–33.
Yee CH, Chan ESY, Hou SSM, Ng CF. Extracorporeal shockwave therapy in the treatment of erectile dysfunction: a prospective, randomized, double-blinded, placebo controlled study. Int J Urol. 2014;21(10):1041–5.
Kitrey ND, Gruenwald I, Appel B, Shechter A, Massarwa O, Vardi Y. Penile low intensity shock wave treatment is able to shift PDE5i nonresponders to responders: a double-blind, sham controlled study. J Urol. 2016;195(5):1550–5.
Kalyvianakis D, Hatzichristou D. Low-intensity shockwave therapy improves hemodynamic parameters in patients with vasculogenic erectile dysfunction: a triplex ultrasonography-based sham-controlled trial. J Sex Med. 2017;14(7):891–7.
Srini VS, Reddy RK, Shultz T, Denes B, Vs S, Rk R. Low intensity extracorporeal shockwave therapy for erectile dysfunction: a study in an Indian population. Can J Urol. 2015;22:7614–22.
Fojecki GL, Tiessen S, Osther PJS. Effect of linear low-intensity extracorporeal shockwave therapy for erectile dysfunction—12-month follow-up of a randomized, double-blinded, sham-controlled study. Sex Med. 2018;6(1):1–7.
Feldman R, Denes B, Appel B, Vasan SS, Shultz T, Burnett A. PD45–10 the safety and efficacy of li-eswt in patients for erectile dysfunction: summary of current and evolving evidence. J Urol. 2015;193(4S):e905–6.
Nehra A, Alterowitz R, Culkin DJ, Faraday MM, Hakim LS, Heidelbaugh JJ, et al. Peyronie’s disease: AUA guideline. J Urol. 2015;194(3):745–53.
Capogrosso P, Frey A, Jensen CFS, Rastrelli G, Russo GI, Torremade J, et al. Low-intensity shock wave therapy in sexual medicine—clinical recommendations from the European Society of Sexual Medicine (ESSM). J Sex Med. 2019;16(10):1490–505.
Rosen RC, Allen KR, Ni X, Araujo AB. Minimal clinically important differences in the erectile function domain of the international index of erectile function scale. Eur Urol. 2011;60(5):1010–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors report no conflict of interest germane to this manuscript. MB has no financial disclosures. AWS is a board member of the Sexual Medicine Society of North America and has received compensation for work as Editor in Chief of Sexual Medicine: Open Access, the open access journal of the International Society for Sexual Medicine.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Current Controversies
Rights and permissions
About this article
Cite this article
Bowman, M., Shindel, A.W. Low-Intensity Extracorporeal Shockwave Therapy for Erectile Dysfunction. Curr Sex Health Rep 12, 421–430 (2020). https://doi.org/10.1007/s11930-020-00289-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11930-020-00289-x