Abstract
Purpose of Review
Enthesitis is a cardinal feature of spondyloarthritis (SpA). Despite increasing available treatments, challenges remain in adequately controlling inflammation and subsequent new bone formation (NBF) in entheses; thus, a better understanding of the immunopathogenesis is warranted.
Recent Findings
Increasing evidence has identified immune cells playing key roles in enthesitis such as γδ T cells and group 3 innate lymphoid cells (ILC3), possibly with site-specific regulatory systems. The presence of T cells producing interleukin (IL)-17 independent of IL-23 in human spinal entheses was recently reported, which may corroborate the discrepancy between recent clinical trials and pre-clinical studies. In addition, the contribution of myeloid cells has also been focused in both human and pre-clinical SpA models. Moreover, not only the IL-23/IL-17 signaling, but other key type 3 immunity mediators, such as IL-22 and granulocyte-macrophage colony-stimulating factor (GM-CSF), have been reported as pivotal cytokines in inflammation and NBF of entheses.
Summary
Immune cells demonstrating distinct features orchestrate entheses, leading to the complex landscape of enthesitis. However, recent advances in understanding the immunopathogenesis may provide new therapeutic targets and future research directions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The hallmark of spondyloarthritis (SpA) is enthesitis, the inflammation of enthesis where tendon, ligament, or joint capsules attach to the bone [1]. While enthesitis is also seen in non-SpA-related conditions such as lateral epicondylitis (tennis elbow) and planter fasciitis due to repetitive mechanical stress or injuries, the inflammation is spontaneously resolved in general [2]. In contrast, SpA-related enthesitis persists and often affects multiple sites, indicating underlying chronic immune responses occurring in the inflamed enthesis, subchondral bones, and neighboring tissues [1]. Traditionally, enthesitis had not been paid considerable attention, primarily because it was considered to be a focal inflammation within the uniformed tissue consisting of fibrochondrocytes and tenocytes; however, since the “enthesis-organ” concept was introduced [3], the complexity of enthesitis has been well-recognized and profound progress in research has been made. In particular, recent studies have revealed tissue residential immune cells that play key pathological roles in enthesitis and thus they are regarded as potential therapeutic targets in enthesitis [4•, 5••, 6, 7•].
Enthesitis is not only inflamed tissue but also induces abnormal new bone formation (NBF), especially when uncontrolled inflammation persists [8]. NBF is often referred to an enthesophyte, or ankylosis if it completely bridges the bones in the spine (bamboo spine) or sacroiliac joints. In addition to prolonged inflammation, mechanical stress also plays a critical role in the development of NBF [9]. For instance, recent studies have demonstrated altered local immune responses in mechanical stressing entheses of pre-clinical SpA animal models [10]. These findings substantiate the concept of “mechano-inflammation” in the pathogenesis of NBF associated with SpA.
To date, treatments for enthesitis have targeted key inflammatory mediators involved in the pathogenesis of enthesitis. As prostaglandin E2 (PGE2) is one of the central mediators synthesized by cyclooxygenase (COX)-1 and COX-2 in enthesitis [1], non-steroidal anti-inflammatory drugs (NSAIDs) are considered as cornerstones in enthesitis treatment and generally show satisfactory efficacies [11]. However, uncontrolled inflammation and ongoing NBF with unresolved symptoms have also occurred in a considerable number of cases [12]. Since interleukin (IL)-23 has been identified as a critical initiator in enthesitis [13], treatments targeting the IL-23 signaling have evidenced the involvement of type 3 immunity, especially in peripheral enthesitis. However, recent clinical trials with monoclonal antibodies (mAb) of IL-23 continue to fail in axial SpA [14], demonstrating the elaborate immune pathogenesis of enthesitis with potential site-specific regulatory systems. Therefore, better understanding of the immunopathogenesis of enthesitis is crucial to identify new treatment targets.
In this review, we first introduce anatomical features of enthesis and main contributing factors to SpA-related enthesitis. Next, we focus on recent advances in the immunopathogenesis of enthesitis and discuss potential treatment options. We also highlight myeloid cells that may play indispensable roles in enthesitis and NBF.
Enthesitis: a Central Feature in SpA
SpA is an umbrella term that encompasses ankylosing spondylitis (AS), psoriatic arthritis (PsA), reactive arthritis (ReA), arthropathy associated with inflammatory bowel diseases (IBD; Crohn’s disease and ulcerative colitis), and a group of undifferentiated SpA (USpA) [15]. In 1971, Ball proposed enthesitis as a central feature of SpA based on histological findings of unique inflammation in entheses, which was not observed in those from patients with rheumatoid arthritis (RA) [16]. Despite that synovitis is also seen in SpA akin to RA, the synovitis associated with SpA seems to attribute to an intrinsic dysregulation of the enthesitis that provokes inflammation in the adjacent tissues [17]. This is a distinct feature from RA-synovitis where autoantibody-mediated immune response plays pivotal roles. Furthermore, SpA is a disease entity that has strong association with HLA-B*27, a major histocompatibility complex (MHC)–encoded class I molecule. It has been reported that an allele of HLA-B*27 (B*27:05:02) has positive association with enthesitis in PsA [18]. Moreover, the same study has shown that in 282 patients with PsA, the highest propensity score for severe PsA (based on frequency of enthesitis, joint fusion, deformities, and dactylitis) was with B*27:05:02-C*02:02:02 haplotype [18]. This genetic contribution may also support that enthesitis is one of the unique manifestations of PsA. However, a full picture as to why PsA patients are much more susceptible to enthesitis than other inflammatory arthritis has not fully been elucidated. Although several hypotheses have been proposed in PsA, such as a low threshold for inflammation, dysfunction of epithelial barriers in skin and gut, and deep Koebner phenomenon [19, 20], the exact reason still remains unclear.
Anatomical Features of Enthesis: Enthesitis Organ Concept
As mechanical stress loads on the enthesis that bridges soft fibrotic tissues and hard bones, entheseal tissues possess unique anatomical characteristics to sufficiently resist loading and the repetitive mechanical stress. The mixture of distinct cell populations and the deposition of extracellular matrices, such as calcium, phosphate, type I/II collagen, and aggrecan, maintain the robustness of the insertion point by providing both stiffness and elasticity [21]. The cooperative function by a group of tissues including fibrocartilage, bursa, fat pad, adjacent trabecular bone networks, deeper fascia, and enthesis led to the concept of “enthesis organ,” in which the tissues collectively carry out a common function to reduce mechanical stress and minimize tissue damage at the bony interface and neighboring tissues [3, 22].
Akin to articular cartilage, the “enthesis organ” contains synovial tissues providing nourishment, lubrication, and micro-debris removal [23]. Although the tissue of fibrocartilage is avascular, this synovial membrane (often referred to “synovio-entheseal complex”) accommodates blood vessels and plenty of macrophages, which induce inflammatory response in entheses. This is particularly seen in peripheral entheses, but uncommon in axial entheses [23]. Furthermore, bone marrow also supplies nutrition through porous and thin bone adjacent to the enthesis [3]. Thus, the concept of “enthesis organ” and “synovio-entheseal complex” is implicated both in the homeostasis of entheses as well as the pathogenesis of enthesitis, further substantiating the immune-therapeutic strategy for treating enthesitis.
Recent Updates in Immunopathogenesis of Enthesitis
-
i)
Roles of IL-23 in enthesitis
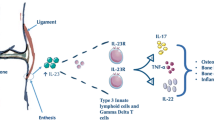
The key immune response involved in SpA is type 3 immunity in which IL-23, IL-17, IL-22, and granulocyte-macrophage colony-stimulating factor (GM-CSF) play central roles [24, 25]. A genome-wide association study (GWAS) has found various IL-23 receptor (IL-23R) single-nucleotide polymorphisms (SNPs) being strongly associated with SpA spectrum diseases such as AS, PsA, and IBD [26]. Since IL-17-producing CD3+CD4−CD8−IL-23R+ T cells were found in the enthesis of mice, the IL-23/IL-17 signaling pathway has further been the focus of attention. Indeed, IL-23 is increased in serum, cell culture supernatant, and gut tissues in SpA patients, and resident type 3 immune cells including IL-23R-positive cells have been identified in enthesis, aortic valve, eye ciliary body, skin, and ileum [27,28,29]. Thus, IL-23-producing cells and IL-23R-bearing cells are extensively present in SpA-related anatomical sites (Fig. 1).
Schematic of type 3 immunity-mediated enthesitis in axial spondyloarthritis. Triggers (e.g., mechanical stress and DAMP) increase the production of prostaglandin E2 (PGE2), which causes the infiltration of immune cells from circulating blood and sub-enthesis bone marrow. The triggers also activate tissue residential CD14+ myeloid cells that release IL-23. The IL-23 stimulates IL-23-receptor (IL-23R)-bearing tissue residential immune cells (e.g., γδ T cells and ILC3) that produce pathogenic cytokines IL-17A, IL-17F, IL-22, and granulocyte-macrophage colony-stimulating factor (GM-CSF). γδ T cells not expressing IL-23R also have an ability to produce IL-17A and IL-17F independent of IL-23. In addition, conventional CD4+ and CD8+ T cells produce IL-17A and IL-17F in entheses. These cytokines further amplify the recruitment of immune cells, and provoke inflammation and subsequent new bone formation (NBF) in axial entheses. DAMP, damage-associated molecular patterns
IL-23 is a heterodimeric cytokine, chiefly released by myeloid cells such as monocytes, macrophages, and dendritic cells, following stimuli such as danger-associated molecular patterns (DAMPS) [28]. IL-23 is a well-established cytokine to induce IL-17 from Th17 cells, γδ T cells, CD8+ T cells, and other innate lymphoid cells [29, 30]. In addition, IL-23 contributes to the expansion and stabilization of Th17 cells [31, 32].
Despite the enormous contribution, anatomical origins of IL-23 in enthesitis remain to be imperfectly determined. Clinical manifestations involve psoriasis (PsC) and gut inflammation in SpA. Human tissues and animal models evidenced that IL-23 are produced in gut tissues and psoriatic skins [13, 33, 34]. Thus, it is very conceivable that increased serum levels of IL-23 in SpA patients originate from gut and skin, which systemically circulates and eventually affects IL-23R-bearing immune cells in entheses. Indeed, IL-23-overexpressing mice injected with IL-23 mini-circle caused severe enthesitis, which supports that systemic overexpression of IL-23 is adequate to induce enthesitis [13, 27]. Furthermore, a recent study has shown that IL-23-induced skin inflammation drives enthesitis, dactylitis, and bone destruction in mice [33]. However, it had not been fully determined as to whether IL-23 is locally produced in human entheses. Recently, Bridgewood et al. reported that cells capable of producing IL-23 are present at non-inflammed human spinal enthesis and that those cells are primarily CD14+ myeloid cells [7]. The same study also showed that monocytes isolated from peripheral blood of SpA patients with enthesitis have an increased IL-23 secretion when stimulated, compared to those from healthy individuals. This study underpins the possibility that IL-23 is a locally produced by residential immune cells in human spinal entheses. However, further studies that explore immune cell infiltrating into entheses from blood circulation are warranted to better understand the source of IL-23 producing cells, potentially by latest technologies such as single-cell RNA sequencing and mass cytometry.
-
ii)
IL-23 targeting therapy
It has now been well-established that IL-23 blockers, such as ustekinumab (anti-IL12/IL23 p40 monoclonal antibody) and risankizumab (anti-IL-23 p19 monoclonal antibody), are effective to SpA-related conditions such as PsC, PsA, and IBD [35,36,37].
In axial SpA, pre-clinical animal models with IL-23 blockers have shown positive results to suppress clinical symptoms [38]. In addition, a human GWAS study identified IL-23 SNPs and related gene SNPs, which are implicated in patients with AS. Furthermore, increased expression of IL-23 was found in AS patients [39, 40]. Thus, therapeutic strategies targeting IL-23 were anticipated to be effective for patients with AS. However, unexpectedly, ustekinumab and risankizumab failed to meet the primary outcomes to adequately show their efficacies in AS [14, 41]. These studies have posted critical questions over the importance and role of IL-23 in axial enthesitis, represented by AS.
Although there are numbers of potential reasons that could be considered, we discuss potential reasons from the aspect of immunopathology to explain the ineffectiveness of IL-23 blockers in axial SpA. First, the anatomical difference between spinal entheses and peripheral entheses is conceivable. The peripheral skeleton accommodates abundant synovio-entheseal complexes that contain numbers of myeloid cells [23], whereas these cells are scarce in axial entheses where more peri-entheseal osteitis is observed compared to peripheral entheses. Although tissue residential myeloid cells capable of producing IL-23 have recently been identified in human spinal entheses [7•], the production of IL-23 seem to be less than that of peripheral entheses encompassed by profound synovial tissues. Thus, targeting IL-23 may not be the best approach for patients with axial SpA.
Second, given a IL-17A blocker secukinumab being the promising therapy in axial SpA, there may be immune cells producing IL-17 independent of IL-23. Indeed, Cuthbert et al. recently reported that human spinal entheses accommodate γδ T cells, specifically Vδ1 T cells, which do not possess IL-23R but produce IL-17A/ IL-17F independent of IL-23 [5••]. This finding is certainly able to account for the reason for unresponsiveness to IL-23 blockers in AS patients. Of note, it is important to describe that this data does not indicate that the γδ T cells are not activated by any factors. Future investigation to seek for undiscovered triggers that potentially drive the γδ T cells producing IL-17 is warranted. Moreover, other cell populations such as invariant natural killer (iNK) T cells and mucosal-associated invariant T (MAIT) cells have been reported to produce IL-17A independent of IL-23 in arthritis mouse models and synovial fluids from AS patients [42, 43]. Thus, the role of these cells in entheses also needs to be investigated.
Finally, IL-23 may not be an essential mediator once enthesitis is established. Pre-clinical SpA mouse models showed that overexpression of IL-23 is sufficient to provoke SpA phenotypes including arthritis, enthesitis, and ileitis, while inhibition of IL-17 or IL-22 predominantly suppressed enthesitis compared to anti-IL-23 treatment. These data suggest that IL-17 and IL-22 are more prominent than IL-23 for persistency of enthesitis [44].
-
iii)
IL-17: an essential mediator in enthesitis
As IL-17-targeting treatments are effective, IL-17 is a pathological cytokine in axial SpA. Among the IL-17 cytokine superfamily being consisted of six ligands from IL-17A to IL-17F, IL-17A is the most potent pro-inflammatory cytokine and shares 50% homology with IL-17F. In contrast, although much less is known, IL-17D may act as an anti-inflammatory mediator in SpA [45].
IL-17A and IL-17F exist as homodimers but also form an IL-17A-IL-17F heterodimer. The distinct feature of IL-17F from IL-17A has not been fully clarified; however, it likely acts in a similar manner to IL-17A in inducing pro-inflammatory cytokines such as TNF [46, 47]. IL-17A is produced by various immune cells including Th17 cells, CD8+ T cells, γδ T cells, ILC3, and NK cells. Despite staining positive for IL-17 in neutrophils and mast cells, they do not seem to produce IL-17, instead absorbing exogenous IL-17 into their cytoplasm [48, 49].
In addition to increased levels of Th17 cells producing IL-17A in serum of AS patients [50,51,52,53], local production of IL-17A by ILC3 and γδ T cells in human entheses has also been reported [5••, 6]. Interestingly, Vδ1 T cells, a subset of γδ T cells producing IL-17A independent of IL-23, are more abundant in spinal entheses compared to circulating peripheral blood mononuclear cells (PBMCs) in human [5••]. Moreover, conventional CD4+ and CD8+ T cells resident in human entheses also have an ability to produce IL-17A when stimulated [4•]. These data suggest that IL-17A is locally produced by tissue residential immune cells and drives enthesitis. In this regard, the role of IL-17A in enthesitis has been reported as an amplifier rather than initiator. Pre-clinical mouse model showed that systemic overexpression of IL-23 was sufficient to induce enthesitis, but this did not occur when IL-17A was overexpressed. Instead, IL-23-induced enthesitis was substantially controlled by IL-17A inhibition [13].
Recent studies have shown that the IL-17 family is also essential in NBF associated with AS. A 4-year observation study showed that inhibition of IL-17A with secukinumab resulted in stopping radiographic progressions in around 80% of AS patients [54]. Spinal tissue–derived osteoprogenitor cells from AS patients also showed the enhancement of osteoblast differentiation with increased mineralization and the alkaline phosphatase (ALP) activity in response to IL-17A stimulation [55]. Interesting, dual inhibition of IL-17A and IL-17F appears to possess a greater efficacy to block NBF compared to individual neutralization of IL-17A or IL-17F in human osteoprogenitor cells (peri-osteal cells) in vitro [56]. This data suggests that IL-17F also promotes NBF along with IL-17A. Of note, pre-clinical studies have shown conflicting data on the role of IL-17A in NBF. While IL-17A has been reported to promote NBF in an AS rat model [57], several studies showed that genetic knockout of IL-17A promotes NBF by suppressing osteoblast differentiation [58, 59]. This discrepancy may indicate potential distinct roles of IL-17A on enthesitis and NBF between humans and pre-clinical animal models.
-
iv)
IL-22: a critical cytokine in enthesitis
Besides IL-17, IL-22 is also a critical cytokine produced by type 3 immune cells such as Th17, γδ T cells, CD8+ T cells, and ILC3. Enthesis-resident T cells that produce IL-22 have been identified in IL-23-overexpressing mice [13]. Similar to IL-17, IL-22 has been considered, at least in entheses, to be one of the downstream mediators of IL-23. Indeed, IL-23-induced enthesitis was attenuated by IL-22 inhibition [13]. Interestingly, IL-22 promotes NBF in periosteal areas through upregulation of signal transducer and activator of transcription 3 (STAT3) and NBF-related gene such as Wnt family members [13]. Given that human entheses accommodate osteoprogenitor cell populations [60], IL-22 may stimulate those cells and drives NBFs. To support this idea, El-ayadi et al. have shown that IL-22 promotes proliferation and migration of human bone marrow–derived mesenchymal stem cells (MSCs) [61]. Furthermore, IL-22 increases genes related to osteogenic, bone formation, bone matrix, such as RUNX2, ALP, and COL1A1 [61]. Together, IL-22 may be one of the essential mediators in promoting NBF in entheses and need to be targeted in the treatment of enthesitis.
-
v)
GM-CSF: a potential key mediator of enthesitis
GM-CSF is a pathological cytokine primarily produced by CD4+ T cells (Th1/Th17), CD8+ T cells, γδ T cells, NK cells, ILCs, and mast cells [62,63,64]. Compared to patients with RA or healthy controls, the frequency of GM-CSF+ T cells is significantly increased in PBMCs of patients with SpA [63]. The same study also showed that the percentage of IL-17A+GM-CSF+ double-positive cells, a well-known pathogenic Th17 phenotype [65, 66], is substantially increased in PBMCs of patients with SpA, with further increase in the ex vivo synovial fluid [63]. Interestingly, the transcriptional profile of GM-CSF+CD4+ T cells was distinct from that of IL-17A+CD4+ T cells. Indeed, a previous study showed that inhibition of RORγt (a transcription factor of Th17 lineage cells) in human Th17 cells led to the suppression of IL-17A+CD4+ T cells, but not GM-CSF+CD4+ T cells in vitro [67], suggesting that GM-CSF is regulated by factors independent of RORγt. One of the potential key mediators is GPR65 [63], a G-protein-coupled receptor with an extracellular proton sensing domain, which is also shown the association with AS by GWAS [26]. Both human GM-CSF+CD4+ T cells and GM-CSF+ Th17 cells highly express GPR65, and silencing the expression of GPR65 reduced the secretion of GM-CSF, but not IL-17A in vitro [63]. These data demonstrate that GPR65 may be one of the therapeutic targets in SpA.
Monoclonal antibodies against GM-CSF, namilumab and gimsilumab, are currently under clinical trials in axial SpA (NCT03622658 and NCT04205851). A recent pre-clinical study showed that enthesitis was successfully attenuated by anti-GM-CSF in an SpA mouse model [64]. In this study, the production of GM-CSF was enhanced by IL-33 through the activation of mast cells expressing IL-33R [64]. Interestingly, increased levels of serum IL-33 and high proportion of mast cells were reported in SpA [68, 69], demonstrating that GM-CSF may be a potential therapeutic target in enthesitis associated with SpA. Further studies are warranted to clarify the specific roles of GM-CSF in enthesitis, including the potential of promoting NBF.
-
vi)
Importance of myeloid cells in type 3 immunity-mediated enthesitis
Pivotal roles of myeloid cells are represented by the production of IL-23 in dendritic cells and macrophages, which subsequently activate lymphoid lineage cells such as IL-17 producing T cells in entheses [7•, 8, 25]. Thus, myeloid cells are critical initiators of enthesitis. Furthermore, increased production of PGE2 in enthesitis causes vasodilation in local blood vessels, which allows neutrophils and macrophages to infiltrate into entheses and release further IL-23, proteases, and reactive oxygen species, resulting in augmentation of inflammation in enthesitis. To support this, the infiltration of neutrophils that potentially produce IL-23 in both human and mouse spinal entheses has recently been reported [70].
Pre-clinical studies have also highlighted the importance of myeloid cells in SpA. A recent study showed that enthesitis is triggered by dysregulated activation of STAT1 in myeloid cells, which results in promoting cytokine production [71]. In contrast, the absence of A20 protein, a negative regulator of STAT1, induced enthesitis spontaneously [71]. Nevertheless, as research focusing on the role of myeloid cells during the occurrence and progression of enthesitis is limited, future studies to decipher the role of myeloid cells are warranted and may serve new treatment avenues in SpA-related enthesitis.
Conclusion
Recent clinical and pre-clinical observations have expanded our understandings on the pathogenesis of enthesitis in SpA. Type 3 immunity plays a central role in the pathogenesis of enthesitis, yet the difference in the response to IL-23 inhibitors between PsA and AS may offer distinct treatment strategies between peripheral and axial SpA. Myeloid cells such as neutrophils and macrophages have been reported to exist in the human spinal tissues and their pathological roles have recently been recognized. However, there are still considerable limitations in understanding the whole picture of enthesitis pathologies. Future studies need to address remaining questions, such as the identification of key factors that activate tissue residential innate and adoptive T cells in axial SpA, and how immune cells interact each other to provoke and sustain inflammation in entheses.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Schett G, Lories RJ, D’Agostino M-A, Elewaut D, Kirkham B, Soriano ER, et al. Enthesitis: from pathophysiology to treatment. Nat Rev Rheumatol. 2017;13:731–41.
Vaquero-Picado A, Barco R, Antuña SA. Lateral epicondylitis of the elbow. EFORT Open Rev. 2016;1:391–7.
Benjamin M, Moriggl B, Brenner E, Emery P, McGonagle D, Redman S. The “enthesis organ” concept: why enthesopathies may not present as focal insertional disorders. Arthritis Rheum. 2004;50:3306–13.
Watad A, Rowe H, Russell T, et al (2020) Normal human enthesis harbours conventional CD4+ and CD8+ T cells with regulatory features and inducible IL-17A and TNF expression. Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2020-217309. This study shows that conventional CD4+ and CD8+ T cells exist in human anxial entheses and have an ability to produce IL-17A and TNF.
Cuthbert RJ, Watad A, Fragkakis EM, et al. Evidence that tissue resident human enthesis γδT-cells can produce IL-17A independently of IL-23R transcript expression. Ann Rheum Dis. 2019;78:1559 LP–1565 This study provides the first evidence of IL-17 producing γδ T cells independent of IL-23 in human axial entheses, which may be the reason for the ineffectiveness of IL-23 monoclonal antibodies in patients with ankylosing spondylitis.
Cuthbert RJ, Fragkakis EM, Dunsmuir R, Li Z, Coles M, Marzo-Ortega H, et al. Brief report: group 3 innate lymphoid cells in human enthesis. Arthritis Rheumatol (HobokenNJ). 2017;69:1816–22.
Bridgewood C, Watad A, Russell T, et al. Identification of myeloid cells in the human enthesis as the main source of local IL-23 production. Ann Rheum Dis. 2019;78:929–33 This study demonstrates that IL-23 is produced by CD14+ myeloid cells in human axial entheses, which highlights the importance of locally produced IL-23 in the pathogenesis of axial spondyloarthritis.
Nakamura A, Talukdar A, Nakamura S, Pathan E, Haroon N. Bone formation in axial spondyloarthritis: is disease modification possible? Best Pract Res Clin Rheumatol. 2019;33:101491.
Jacques P, Lambrecht S, Verheugen E, et al. Proof of concept: enthesitis and new bone formation in spondyloarthritis are driven by mechanical strain and stromal cells. Ann Rheum Dis. 2014;73:437–45.
Cambre I, Gaublomme D, Schryvers N, Lambrecht S, Lories R, Venken K, et al. Running promotes chronicity of arthritis by local modulation of complement activators and impairing T regulatory feedback loops. Ann Rheum Dis. 2019;78:787–95.
Kroon F, Landewé R, Dougados M, van der Heijde D. Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis. Ann Rheum Dis. 2012;71:1623–9.
Sieper J. New treatment targets for axial spondyloarthritis. Rheumatology (Oxford). 2016;55:ii38–42.
Sherlock JP, Joyce-Shaikh B, Turner SP, et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4-CD8- entheseal resident T cells. Nat Med. 2012;18:1069–76.
Baeten D, Østergaard M, Wei JC-C, et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann Rheum Dis. 2018;77:1295–302.
Dougados M, Baeten D. Spondyloarthritis. Lancet (London, England). 2011;377:2127–37.
Ball J. Enthesopathy of rheumatoid and ankylosing spondylitis. Ann Rheum Dis. 1971;30:213–23.
McGonagle D, Gibbon W, Emery P. Classification of inflammatory arthritis by enthesitis. Lancet (London, England). 1998;352:1137–40.
Haroon M, Winchester R, Giles JT, Heffernan E, FitzGerald O. Certain class I HLA alleles and haplotypes implicated in susceptibility play a role in determining specific features of the psoriatic arthritis phenotype. Ann Rheum Dis. 2016;75:155–62.
Tinazzi I, McGonagle D, Aydin SZ, Chessa D, Marchetta A, Macchioni P. “Deep Koebner” phenomenon of the flexor tendon-associated accessory pulleys as a novel factor in tenosynovitis and dactylitis in psoriatic arthritis. Ann Rheum Dis. 2018;77:922–5.
Gracey E, Dumas E, Yerushalmi M, Qaiyum Z, Inman RD, Elewaut D. The ties that bind: skin, gut and spondyloarthritis. Curr Opin Rheumatol. 2019;31:62–9.
Benjamin M, Evans EJ, Copp L. The histology of tendon attachments to bone in man. J Anat. 1986;149:89–100.
Hems T, Tillmann B. Tendon entheses of the human masticatory muscles. Anat Embryol (Berl). 2000;202:201–8.
McGonagle D, Aydin SZ, Tan AL. The synovio-entheseal complex and its role in tendon and capsular associated inflammation. J Rheumatol Suppl. 2012;89:11–4.
Gracey E, Hromadova D, Lim M, et al. TYK2 inhibition reduces type 3 immunity and modifies disease progression in murine spondyloarthritis. J Clin Invest. 2020. https://doi.org/10.1172/JCI126567.
Pedersen SJ, Maksymowych WP. The pathogenesis of ankylosing spondylitis: an update. Curr Rheumatol Rep. 2019;21:58.
Cortes A, Hadler J, Pointon JP, et al. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat Genet. 2013;45:730–8.
Reinhardt A, Yevsa T, Worbs T, Lienenklaus S, Sandrock I, Oberdörfer L, et al. Interleukin-23-dependent γ/δ T cells produce interleukin-17 and accumulate in the enthesis, aortic valve, and ciliary body in mice. Arthritis Rheumatol (HobokenNJ). 2016;68:2476–86.
Shichita T, Hasegawa E, Kimura A, et al. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat Med. 2012;18:911–7.
Aggarwal S, Ghilardi N, Xie M-H, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–4.
Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32.
Valmori D, Raffin C, Raimbaud I, Ayyoub M. Human RORγt+ TH17 cells preferentially differentiate from naive FOXP3+Treg in the presence of lineage-specific polarizing factors. Proc Natl Acad Sci U S A. 2010;107:19402–7.
Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupé P, Barillot E, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–7.
Yawalkar N, Tscharner GG, Hunger RE, Hassan AS. Increased expression of IL-12p70 and IL-23 by multiple dendritic cell and macrophage subsets in plaque psoriasis. J Dermatol Sci. 2009;54:99–105.
Uhlig HH, McKenzie BS, Hue S, et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–18.
Kerschbaumer A, Smolen JS, Dougados M, de Wit M, Primdahl J, McInnes I, et al. Pharmacological treatment of psoriatic arthritis: a systematic literature research for the 2019 update of the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis. 2020;79:778–86.
Feagan BG, Panés J, Ferrante M, et al. Risankizumab in patients with moderate to severe Crohn’s disease: an open-label extension study. Lancet Gastroenterol Hepatol. 2018;3:671–80.
McInnes IB, Kavanaugh A, Gottlieb AB, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet (London, England). 2013;382:780–9.
Rehaume LM, Matigian N, Mehdi AM, Lachner N, Bowerman KL, Daly J, et al. IL-23 favours outgrowth of spondyloarthritis-associated pathobionts and suppresses host support for homeostatic microbiota. Ann Rheum Dis. 2019;78:494–503.
Ciccia F, Bombardieri M, Principato A, et al. Overexpression of interleukin-23, but not interleukin-17, as an immunologic signature of subclinical intestinal inflammation in ankylosing spondylitis. Arthritis Rheum. 2009;60:955–65.
Ciccia F, Accardo-Palumbo A, Rizzo A, Guggino G, Raimondo S, Giardina A, et al. Evidence that autophagy, but not the unfolded protein response, regulates the expression of IL-23 in the gut of patients with ankylosing spondylitis and subclinical gut inflammation. Ann Rheum Dis. 2014;73:1566–74.
Deodhar A, Gensler LS, Sieper J, et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol (Hoboken, NJ). 2019;71:258–70.
Yoshiga Y, Goto D, Segawa S, Ohnishi Y, Matsumoto I, Ito S, et al. Invariant NKT cells produce IL-17 through IL-23-dependent and -independent pathways with potential modulation of Th17 response in collagen-induced arthritis. Int J Mol Med. 2008;22:369–74.
Gracey E, Qaiyum Z, Almaghlouth I, et al. IL-7 primes IL-17 in mucosal-associated invariant T (MAIT) cells, which contribute to the Th17-axis in ankylosing spondylitis. Ann Rheum Dis. 2016;75:2124–32.
Benham H, Rehaume LM, Hasnain SZ, et al. Interleukin-23 mediates the intestinal response to microbial β-1,3-glucan and the development of spondyloarthritis pathology in SKG mice. Arthritis Rheumatol (Hoboken NJ). 2014;66:1755–67.
Chen S, Manning C, van Tok M, Maeda Y, Montoro D, Kim J, et al. Interleukin-17D, a cytokine derived from stromal cells, attenuates joint inflammation [abstract]. Arthritis Rheum. 2020;72:supple10.
Taams LS, Steel KJA, Srenathan U, Burns LA, Kirkham BW. IL-17 in the immunopathogenesis of spondyloarthritis. Nat Rev Rheumatol. 2018;14:453–66.
Zrioual S, Ecochard R, Tournadre A, Lenief V, Cazalis M-A, Miossec P. Genome-wide comparison between IL-17A- and IL-17F-induced effects in human rheumatoid arthritis synoviocytes. J Immunol. 2009;182:3112–20.
Noordenbos T, Blijdorp I, Chen S, Stap J, Mul E, Cañete JD, et al. Human mast cells capture, store, and release bioactive, exogenous IL-17A. J Leukoc Biol. 2016;100:453–62.
Tamassia N, Arruda-Silva F, Calzetti F, et al. A reappraisal on the potential ability of human neutrophils to express and produce IL-17 family members in vitro: failure to reproducibly detect it. Front Immunol. 2018;9:795.
Taylan A, Sari I, Kozaci DL, Yuksel A, Bilge S, Yildiz Y, et al. Evaluation of the T helper 17 axis in ankylosing spondylitis. Rheumatol Int. 2012;32:2511–5.
Mei Y, Pan F, Gao J, et al. Increased serum IL-17 and IL-23 in the patient with ankylosing spondylitis. Clin Rheumatol. 2011;30:269–73.
Jandus C, Bioley G, Rivals J-P, Dudler J, Speiser D, Romero P. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008;58:2307–17.
Wang C, Liao Q, Hu Y, Zhong DA. T lymphocyte subset imbalances in patients contribute to ankylosing spondylitis. Exp Ther Med. 2015;9:250–6.
Braun J, Baraliakos X, Deodhar A, Poddubnyy D, Emery P, Delicha EM, et al. Secukinumab shows sustained efficacy and low structural progression in ankylosing spondylitis: 4-year results from the MEASURE 1 study. Rheumatology (Oxford). 2019;58:859–68.
Jo S, Wang SE, Lee YL, Kang S, Lee B, Han J, et al. IL-17A induces osteoblast differentiation by activating JAK2/STAT3 in ankylosing spondylitis. Arthritis Res Ther. 2018;20:115.
Shah M, Maroof A, Gikas P, Mittal G, Keen R, Baeten D, et al. Dual neutralisation of IL-17F and IL-17A with bimekizumab blocks inflammation-driven osteogenic differentiation of human periosteal cells. RMD Open. 2020. https://doi.org/10.1136/rmdopen-2020-001306.
van Tok MN, van Duivenvoorde LM, Kramer I, et al. Interleukin-17A inhibition diminishes inflammation and new bone formation in experimental spondyloarthritis. Arthritis Rheumatol (Hoboken NJ). 2019;71:612–25.
Shaw AT, Maeda Y, Gravallese EM. IL-17A deficiency promotes periosteal bone formation in a model of inflammatory arthritis. Arthritis Res Ther. 2016;18:104.
Uluçkan Ö, Jimenez M, Karbach S, et al. Chronic skin inflammation leads to bone loss by IL-17-mediated inhibition of Wnt signaling in osteoblasts. Sci Transl Med. 2016;8:330ra37.
Duchamp de Lageneste O, Julien A, Abou-Khalil R, Frangi G, Carvalho C, Cagnard N, et al. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat Commun. 2018;9:773.
El-Zayadi AA, Jones EA, Churchman SM, Baboolal TG, Cuthbert RJ, El-Jawhari JJ, et al. Interleukin-22 drives the proliferation, migration and osteogenic differentiation of mesenchymal stem cells: a novel cytokine that could contribute to new bone formation in spondyloarthropathies. Rheumatology (Oxford). 2017;56:488–93.
McWilliams IL, Rajbhandari R, Nozell S, Benveniste E, Harrington LE. STAT4 controls GM-CSF production by both Th1 and Th17 cells during EAE. J Neuroinflammation. 2015;12:128.
Al-Mossawi MH, Chen L, Fang H, et al. Unique transcriptome signatures and GM-CSF expression in lymphocytes from patients with spondyloarthritis. Nat Commun. 2017;8:1510.
Regan-Komito D, Swann JW, Demetriou P, Cohen ES, Horwood NJ, Sansom SN, et al. GM-CSF drives dysregulated hematopoietic stem cell activity and pathogenic extramedullary myelopoiesis in experimental spondyloarthritis. Nat Commun. 2020;11:155.
Gaublomme JT, Yosef N, Lee Y, et al. Single-cell genomics unveils critical regulators of Th17 cell pathogenicity. Cell. 2015;163:1400–12.
Lee Y, Awasthi A, Yosef N, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–9.
de Wit J, Al-Mossawi MH, Hühn MH, et al. RORγt inhibitors suppress T(H)17 responses in inflammatory arthritis and inflammatory bowel disease. J Allergy Clin Immunol. 2016;137:960–3.
Han G-W, Zeng L-W, Liang C-X, Cheng B-L, Yu B-S, Li H-M, et al. Serum levels of IL-33 is increased in patients with ankylosing spondylitis. Clin Rheumatol. 2011;30:1583–8.
Freemont AJ, Denton J. Disease distribution of synovial fluid mast cells and cytophagocytic mononuclear cells in inflammatory arthritis. Ann Rheum Dis. 1985;44:312–5.
Stavre Z, Bridgewood C, Zhou Q, Maeda Y, Karman J, McGonagle DGE. The role for neutrophils in the early phases of enthesitis in spondyloarthritis [abstract]. Arthritis Rheum. 2020;72:supple10.
De Wilde K, Martens A, Lambrecht S, et al. A20 inhibition of STAT1 expression in myeloid cells: a novel endogenous regulatory mechanism preventing development of enthesitis. Ann Rheum Dis. 2017;76:585–92.
Funding
This article was supported by grants to NH from the Canadian Institute of Health Research (CIHR) and Arthritis Society. AN is a recipient of CIHR fellowship, Spondyloarthritis Research and Treatment Network (SPARTAN) fellowship, Spondyloarthritis Research Consortium of Canda (SPARCC) fellowship, and Krembil fellowship award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This Article is part of the Topical Collection on Spondyloarthritis
Rights and permissions
About this article
Cite this article
Nakamura, A., Haroon, N. Recent Updates in the Immunopathology of Type 3 Immunity-Mediated Enthesitis. Curr Rheumatol Rep 23, 31 (2021). https://doi.org/10.1007/s11926-021-00995-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s11926-021-00995-y