Abstract
Purpose of Review
Eosinophilic granulomatosis with polyangiitis (EGPA) represents a rare clinical entity, which is getting increasing attention and relevance in view of our better understanding and newer insights into its pathogenesis. Concomitantly better recognition and understanding of the immune pathophysiologic role of eosinophils provide a solid ground of their role on systemic inflammatory disorders and defense against infectious triggers, especially parasites. This review will focus on describing the physiopathology of eosinophils, as well as providing an in depth description of the natural history, clinical spectrum, and therapy of EGPA.
Recent Findings
Several studies have aimed at finding useful biomarkers to monitor disease activity, and reported data have shown that eotaxin 3, IL25, IL33, and some eicosanoids to be promising options. Regarding therapeutic advances, recently published studies have revealed the efficacy of mepolizumab during induction and maintenance of EGPA. Recently published data confirmed earlier studies that the use of azathioprine during the induction phase is of no benefit during long-term follow-up. In addition, data from the REOVAS study, which uses rituximab, is still ongoing and apparently with promising results.
Summary
Eosinophils are involved in several systemic inflammatory disorders, and recent gathered data provide support for their role in triggering EGPA. Better understanding of its pathophysiology should generate newer insights into the pathogenesis, biomarkers of disease activity, and therapeutic targets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eosinophils are polymorphonuclear cells that contain a multitude of granules that house proteins, cytokines, chemokines, and growth factors, which following release into the circulation and tissues mediate a variety of immune responses, especially against parasitic infections and allergic reactions, and are also responsible for tumor surveillance [1••, 2••]. Their function, however, appear to be much broader than those recognized thus far, and they have been shown to play important role in tissue repair and remodeling, maintenance of plasma cells in the bone marrow and activated macrophages in adipose tissue [3,4,5].
Eosinophils have also been shown to play a leading role in the causation of several inflammatory systemic disorders, including vasculitis. Within the clinical spectrum of vasculitides, eosinophils play a distinct role in the pathogenesis of eosinophilic granulomatosis with polyangiitis (EGPA), classified within the ANCA-associated group of vasculitis (AAV) [6].
This review will focus on a brief description of the biology and pathophysiology of the eosinophils, their role in the pathogenesis of EGPA, as well as a review of the clinical spectrum of EGPA, and newer insights into its pathogenesis, biomarkers, and therapeutic developments. In addition, brief review of other entities in which eosinophils also play a role will be discussed as part of the differential diagnosis.
Eosinophils: Nature, Biology, and Functions
Eosinophils were first described by Paul Ehrlich in 1879, and are characterized by their bilobed nucleus and the presence of granules, primary and secondary, in their cytoplasm that stain with eosin red. The primary granules contain crystalline Charcot-Leyden proteins. Lipid bodies are responsible for the synthesis of paracrine eicosanoid inflammatory mediators such as leukotrienes, thromboxane B2, prostaglandins, and platelet-activating factor. Secondary granules contained cationic proteins such as major basic protein (MBP), cationic eosinophilic protein (ECP), eosinophilic peroxidase (EPO), and eosinophilic-derived neurotoxin (EDN), as well as a variety of cytokines, all of which play key roles during cell activation. They have a half-life in tissues of 2 to 3 days, but in vitro may last up to 14 days under stimulation by IL-5, IL-3, and granulocytic-macrophage colony-stimulating factor (GM-CSF) [7,8,9] (Fig. 1).
The eosinophil. Abbreviations: 15-HETE, 15-hydroxyeicosatetraenoic acid; APRIL, a proliferation-inducing ligand; CCL, CC-chemokine ligand; CCR, CC-chemokine receptor; CXCL, CXC-chemokine ligand; CXCR, CXC-chemokine receptor; ECP, eosinophil cationic protein; EDN, eosinophil-derived neurotoxin; EPO, eosinophil peroxidase; GM-CSF, granulocyte-macrophage colony-stimulating factor; GRO-α, growth regulated-α protein; LIF, leukemia inhibitory factor; LT, leukotriene; MBP, major basic protein; NGF, nerve growth factor; PAF, platelet-activating factor; PDGF, platelet-derived growth factor; SCF, stem cell factor; TF, tissue factor; TGF, transforming growth factor; VEGF, vascular endothelial growth factor. (Adapted from Khoury P, Grayson PC, Klion AD. Eosinophils in vasculitis: characteristics and roles in pathogenesis. Nat Rev. Rheumatol. 2014 August; 10(8): 474–483.)
Greater than 90% of eosinophils are present in tissues throughout the body, particularly in the gastrointestinal tract, lymph nodes, spleen, thymus, mammary glands, and uterus [2]. Eosinophils represent only 1–3% of leukocytes in peripheral blood, with up to 500 cells per mm3 of blood, but their number increases in the bloodstream during inflammatory conditions induced by distinct chemokines to reach the affected area. More than 500 cells per mm3 in peripheral blood represents mild eosinophilia, and when present over 1500 cells per mm3 or greater than 10% is denominated hyper eosinophilia, and a number above 5000 cells per mm3 is known as massive eosinophilia.
Eosinophils develop within the bone marrow from progenitor myeloid cells following stimulation by several transcription factors such as erythroid transcription factor type 1 (GATA-1), GATA-2, and CCAAT/enhancer-binding protein (c/EBP) [1, 2]. Regulation of their clonal expansion is mediated by IL-3, IL-5, and GM-CSF. IL-5, also known as eosinophilic differentiation factor, is the most specific, and is also responsible for facilitating their release into the blood stream. They subsequently will pass through the blood vessels into the tissues by stimulating chemokines and mediators produced by endothelial and epithelial cells, and this process involves the interaction of several mediators, including adhesion molecules (integrins and selectins) and the eosinophil chemokine receptor (CCR3) through its chemokine gradient or eotaxins (CC-chemokine ligand 11 or CCL11 eotaxin, CCL24 or eotaxin 2, CCL26 or eotaxin 3, CCL5, CCL28, among others) and cytokines such as IL-4, IL-5, and IL-13. Both IL-4 and IL-13 are synergic promoters of eotaxin synthesis. In addition, other non-chemokine factors such as complement factor C5a and platelet-activating factor also participate. CCL17 eotaxin facilitates amplification of inflammatory lesions by induction of Th2 lymphocytes [10,11,12] (Fig. 1).
Eosinophils exert their cytotoxic functions by degranulation either by cytolysis or by exocytosis. Granules released by cytolysis may remain viable outside the cell, functioning as secretory organelles, while stimulated by cysteinyl leukotriene receptors or cytokines. By exocytosis, the cellular contents are released by fusion of intracellular granules or hat vesicles with the plasma membrane. The cationic proteins are released from secondary granules, including MBP, ECP, EPO, EDN, and derivatives of arachidonic acid: leukotriene C4 are toxic to helminths, protozoa, bacteria, fungi, viruses, and neurons, stimulate the release of histamine from mast cells and basophils, and activate neutrophils and platelets, and also have bronchoconstrictor and vasodilator activities [13] (Fig. 1).
In addition, to their cytotoxic effect, eosinophils also recruit and activate other inflammatory cells, and these two mechanisms are responsible for tissue damage. Their accumulation and activation results in tissue fibrosis, thrombosis, allergic inflammation, and also neural damage [1, 2].
Tissue fibrosis is mediated by ECP stimulation of collagen contraction, IL-1B, and TGF-B. In vitro studies showed that eosinophils promote collagen synthesis and fibroblast proliferation [14].
Thrombosis is a manifestation of a variety of eosinophilic disorders, and its pathophysiology appears secondary to initiation of the coagulation cascade due to tissue factors released during degranulation, endothelial dysfunction, inhibition of the endothelial vascular thrombomodulin by MBP, and platelet activation by MBP and EPO [15, 16].
Allergic inflammation as seen in several allergic disorders, such as atopic dermatitis, rhinitis, eosinophilic esophagitis, and more importantly asthma, is associated with peripheral eosinophilia. The finding of ECP in bronchoalveolar lavage of patients with severe asthma and MBP in pulmonary epithelium of patients with fatal asthma supports the notion that these inflammatory mediators could be used as biomarkers [2].
Neural damage, especially axonal neuropathy, has been seen in hyper eosinophilic syndromes, and also in patients with eosinophilic vasculitis. In vitro studies have shown that eosinophils induce neuronal retraction through a dependent contact mechanism [17, 18]. But further studies are needed to confirm this observation.
Eosinophilia-Associated Vasculitis
The presence of eosinophilia and/or eosinophils in either peripheral blood and/or tissue of patients with a variety of vasculitic disorders, including vasculitis of small vessels such as granulomatosis with polyangiitis (GPA), EGPA, microscopic polyangiitis (MPA), Kawasaki disease, provides support for a role of these cells in their pathogenesis. But EGPA is the prototype of eosinophilia-associated vasculitis, since unlike the other disorders, hyper eosinophilia is part of its diagnostic criteria [19,20,21].
Eosinophilic Granulomatosis with Polyangiitis (EGPA)
In 1951, Jacob Churg and Lotte Strauss (Churg-Strauss syndrome) described the presence of tissue eosinophilia, necrotizing granulomatous vascular lesions, and extravascular granulomas in the majority of organs of 13 patients exhibiting asthma, fever, and eosinophilia [22]. In 2012, following a change in the nomenclature, and to promote the nomenclature that evidences the real pathology of the disease, the name was changed to EGPA [6].
EGPA is a vasculitis of the small and medium vessels characterized by eosinophilia and eosinophilic infiltrates, and can affect any major organ system in the body [19]. The presence of ANCA antibodies facilitates its diagnosis, although their presence are present in 30–40% of patients. In patients with negative-ANCA antibodies, the clinicopathological picture in the presence of vasculitis provides support for its definite diagnosis.
Epidemiology
EGPA usually peaks between the ages of 40 and 60 years, but without predominance for sex, ethnicity, or family history [23]. Its prevalence has been estimated at 2–22 cases per million people, with an annual incidence of 0.5–3.7 cases per million. It is 10 times less common than GPA or MPA [24]. Genetic predisposition appears to be linked to the presence of HLA-DRB1* 07 [25, 26]. Certain anti-leukotriene drugs, vaccines, and other environmental factors might trigger EGPA [27,28,29].
Immunopathogenesis
The exact immunopathogenesis of EGPA remains to be defined. In contrast to GPA and MPA, a direct role of ANCA antibodies has not been demonstrated, and animal models of EGPA are lacking. A prominent role appears to be played by TH2 type lymphocytes, with their respective clonal expansion, and activation of the humoral system, and release of large amounts of IL-4, IL-5, and IL-13, which eventually stimulate the humoral response of B cells resulting in the secretion of IgE and IgG4 [30,31,32]. Periods of active disease are characterized by large release of IL-5 and IL-17A (secreted by TH1 and Th17 cells), and IFNγ (responsible for the formation of granulomas), and lower concentrations of CD4+ CD25 high FOXP3+ regulatory T cells [33]. In addition, predominance of Th29 marker CD294 lymphocytes in affected tissue has been demonstrated [11].
Eotaxin3 release generates the stimulus needed for eosinophils to extravasate and infiltrate the different tissues in the inflamed areas [34, 35]. Once in the tissues, eosinophils initiate the degranulation process, through which secreted proteins and inflammation mediators lead to cell damage, among which IL-25 is responsible for enhancing the stimulation and perpetuation of the Th2 response. Data presented by Terrier et al. provides support since they have shown that levels of eosinophilia are associated with disease activity [36].
As previously discussed, a clear role for ANCA in EGPA has not been shown, but its presence or absence has allowed the recognition of two clinical phenotypes. Those who are positive ANCA (ANCA+) have a higher frequency of constitutional symptoms, peripheral neuropathy, and glomerulonephritis, as well a predisposition to relapse, while negative ANCA (ANCA−) have a higher frequency of pulmonary, myocardial, and gastrointestinal involvement, and higher mortality [37, 38]. Of great interest is the presence of high concentrations of IgG4 and IgG4:IgG ratio in some patients with active EGPA [39,40,41].
Histopathology
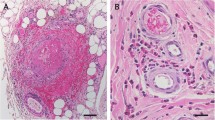
The pathological triad composed of eosinophilic infiltration, necrotizing vasculitis, and extravascular granuloma formation is rarely present in the same patient, but eosinophils are found in all stages. Pathological findings depend on the stage of the disease, but the most important changes will occur during the third stage, the vasculitic stage in which it is possible to observe the infiltration of the eosinophil in the tissue, arteries, and small veins, and formation of extravascular granulomas, although in some cases they could also affect arteries. Vasculitis is characterized by fibrinoid necrosis, and eosinophilic infiltration of vessel walls, while granulomas have a central area of eosinophilic necrosis surrounded by palisade histiocytes and multinucleated giant cells. Involved organs exhibit different changes that occur secondary to direct eosinophilic infiltration and ischemic damage caused by occlusion of small arteries. In general, upper airway biopsies are not of diagnostic help, while best chances of observing vasculitic changes and granuloma formation will be in the lung, peripheral nerves, skin, gastrointestinal tract, and heart. The presence of segmental glomerulonephritis, pauci-immune with or without crescents, and rarely eosinophilic infiltration will be found in kidney tissue.
Clinical Spectrum: Stages of Disease
EGPA is a systemic disorder that impacts on several organs. The typical clinical picture is the presence of asthma in an adult with chronic rhinosinusitis in the presence of more than 10% eosinophilia and pulmonary infiltrates. Three distinct phases are well recognized: allergic or prodromal, eosinophilic, and vasculitic (Fig. 2).
Organ Involvement
Upper Airway
This is present in 47 to 93% of patients, occurs during the prodromal phase, and usually in the presence of nasal polyps, allergic rhinitis, and/or chronic sinusitis. Secretory otitis media, chronic ear drainage, sensorineural hearing loss, and facial nerve paralysis may also occur, especially in children.
Lower Airway
Involvement of lung parenchyma may occur in two thirds of patients. Common reported findings include peripheral migratory infiltrates (x-ray or CT scan), ground glass opacities (86%), bronchial thickening, and bronchiectasis (66%). Alveolar hemorrhage may also occur in 3 to 8% of patients. It should be kept in mind, however, that none of these findings are specific for EGPA, and they may occur in other eosinophilic lung disorders.
Cardiac
Endomyocarditis with an increased risk of thrombus formation, pericarditis, valvular defects, and arrhythmias has also been described.
Gastrointestinal
Abdominal pain, gastrointestinal bleeding with a high possibility of perforation, and small bowel involvement may occur. Rare cases of cholecystitis have been reported.
Peripheral nerves
Peripheral neuropathy, either mononeuritis multiplex or mixed peripheral motor sensory neuropathy, may occur in up to 70% of patients. Droopy wrist or foot is the most common manifestation, which may progress to asymmetric polyneuropathy. Central nervous system (CNS) involvement may rarely occur with the presence of infarctions or cerebral hemorrhage. Neuropathic pain and sensory deficit are also frequent clinical manifestations.
Renal
Kidneys are affected in up to 25% of cases, but it is clinically less severe than in other types of AAV. It may occur as isolated abnormalities in the urinary sediment to rapid progressive glomerulonephritis, and more rarely some patients may exhibit chronic kidney disease at presentation.
Skin
Palpable purpura and nodules are seen in up to 25% of cases, predominantly in the scalp and lower limbs. Other findings include livido reticularis, vesicles, aseptic pustules, petechiae, ecchymosis, and urticarial lesions. Papular nodular lesions may become necrotic ulcers.
Diagnosis
A gold standard test to establish a diagnosis of EGPA is not available. Diagnosis is based on the clinical characteristics found, and the application of the 2012 Chapel Hill diagnostic classification criteria has proven to be a useful tool during daily clinical practice (Table 1). Certain laboratory findings may assist in those cases where the clinical picture is not clear, and some findings may allow early identification of organ involvement in asymptomatic patients. The presence of peripheral eosinophilia, at least 10% or more than 1500 cells/μL, elevated ESR and CRP, are of relevance to establish a diagnosis of EGPA. Degree of eosinophilia, as well as CRP, correlates well with disease activity. On the other hand, ANCA positivity or negativity does not influence a final diagnosis. It is essential, however, to confirm diagnosis by biopsy findings.
Imaging studies, from conventional radiographs to CT scan and cardiac MRI of different involved organs are very helpful to establish degree and severity of involvement in affected organs. Pulmonary function tests, bronchoscopy, and echocardiographic studies will also provide useful information.
Biomarkers
To date, the pathophysiological mechanisms involved in EGPA have not been fully elucidated, but interleukins and eotaxins are thought to play key roles during eosinophil activation, as well as triggering inflammatory responses in vessels and tissues. During the past few years, several group of investigators have attempted to evaluate a series of potential serum biomarkers of disease activity in EGPA and other related vasculitis disorders with varying degrees of success [42,43,44]. Polzer at al. found that levels of eotaxin 3 (CCL26) correlated well with eosinophil counts, serum IgE levels, and acute phase reactants only in patients with EGPA but not in patients with other eosinophilic disorders [45]. A recent study performed in patients with newly diagnosed EGPA, in relapse and in remission, revealed that the concentration of ILC2 (innate lymphoid cells type 2) and IL-33 correlated with disease activity [46]. Other studies have shown that leukotrienes E4 present in the urine correlates with EGPA and asthma, and elevated levels of 12-hydroxyleicosatetraenoic acid (12-HETE) are present in exhaled breath of patients with EGPA but not in asthmatic or patients with hyper eosinophilic syndrome, and their levels were not affected by steroid therapy [47, 48]. Indirect evidence about the role of leukotrienes in EGPA is provided by the association between anti-leukotriene receptor antagonists (LTRAs) and ANCA positivity [49].
Rodriguez-Pla et al. recently reported their experience following evaluation of potential biomarkers of disease activity in giant cell arteritis (GCA), Takayasu’s arteritis (TA), polyarteritis nodosa (PAN), and EGPA (Churg-Strauss). A panel of 22 serum proteins was tested, and in EGPA, G-CSF, GM-CSF, IL-6, IL-15, and sIL-2Rα exhibited significant increases during active disease, as did BCA-1/CXCL13 but only after adjustment for treatment [50•]. Authors concluded that further studies are needed to define their potential for clinical use in distinguishing active disease from remission or in predicting long-term outcomes.
In contrast, Pagnoux et al. tested the levels of 54 cytokines and chemokines in the sera of 40 patients with active EGPA, 10 during inactive disease, 6 with hyper eosinophilic syndrome (HES), 8 with asthma, and 10 healthy controls. Significant lower levels were observed only in serum levels of MDC, IL-8, MIP-1a and 1b, TNF-α, in patients with active EGPA than in healthy controls. No clear difference in serum levels of measured cytokines and chemokines was allowed to differentiate between active or inactive EGPA, or other disease or control groups [51•].
Differential Diagnosis with Other Vasculitides
EGPA remains a challenging disorder with great difficulties for its correct classification and diagnosis. There are several disorders characterized by hyper eosinophilia, and the Working Conference on Eosinophilic Disorders and Syndromes held in Vienna in 2011 proposed a classification that included hyper eosinophilic syndrome with its three variants and other conditions associated with eosinophilia [21, 52,53,54] (Table 2).
Therapeutic Management
There is no established treatment for EGPA, and most recommended therapeutic approaches are based on extrapolation of the management of other types of vasculitis. A practical way of making a decision regarding the therapy to follow is to stratify the patient according to their prognostic factors at the time of their initial diagnosis.
Patients with FFS > − 1 have a mortality of 25.9%, and with FFS > − 2 factors a mortality of 46% at 5 years [55].
Glucocorticoids remain the cornerstone of EGPA treatment, and can be used as monotherapy or in combination with other immunosuppressive agents depending on the severity of organ involvement [56, 57]. Cyclophosphamide is commonly used during the induction phase, and is considered by many the first-line medication for severe cases [58]. Methotrexate, azathioprine, cyclosporine, mofetil mycophenolate, intravenous immunoglobulin (IVIG), and others can be used to induce or maintain remission, depending on the severity of the clinical presentation [59]. However, it has been shown that 1-year combination of azathioprine to glucocorticoids (GC) for patients with non-severe, newly diagnosed EGPA failed to lower remission failure, vasculitis relapse, and isolated asthma/rhinosinusitis exacerbation rates, or cumulative GC use at month 24 or longer [60•, 61•]. There is great need for prospective, controlled studies comparing the use of immunosuppressive agents during induction of maintenance therapy in patients with EGPA.
Biological agents can be used in patients’ refractory to conventional therapy, or with severe organ, i.e., kidney, involvement. In such a situation, the use of rituximab has resulted in successful remission of both disease activity and renal involvement, and also resulted in normalization of the eosinophil cell count and IL-5 serum level [62,63,64,65]. In this regard, the REOVAS study in progress is a phase 3 (https://clinicaltrials.gov/ct2/show/NCT02807103), and it represents the first large randomized study that evaluates the use of rituximab in patients with EGPA. Rituximab also leads to a reduction in prednisolone requirement, but asthma and upper airway involvement relapse rates are high despite continued treatment. In addition, the ANCA-positive subgroup exhibits a more sustained response [66, 67•].
Mepolizumab, which is a humanized monoclonal antibody against IL-5, has been shown effective during maintenance therapy in EGPA [68]. The MIRRA phase 3 study showed that patients who received 300 mg monthly for 1 year with stable doses of prednisone had more weeks of remission and a greater proportion of participants in remission than placebo, which allowed significant decrease in the use of glucocorticoids [69•]. A recently published post hoc analysis of the MIRRA trial in which a comprehensive definition of clinical benefit was applied revealed that 78% to 87% of patients with EGPA exhibited benefit with mepolizumab [70•]. Moosig et al. attempted to use mepolizumab during induction therapy, achieving favorable results, but serious relapses were seen when trying to discontinue it [71, 72].
Omalizumab, alone or in combination with rituximab, has also shown promising results in patients with EGPA. But prospective, control randomized clinical trials are needed to confirm results from small uncontrolled, pilot studies [73, 74].
Other agents used in EGPA, especially in refractory situations, include IFN-α, IVGG, and plasma exchange in combination with GC and cyclophosphamide with the latter approach exhibiting efficacy and achieving remission in all patients, with relapses of 11% during the 36-month follow-up [75]. Newer therapeutic approaches including the use of tyrosine kinase inhibitors that have a key role among targeted therapies of hypereosinophilic syndromes appear to be promising [76, 77].
Conclusions
Evidence is presented for a clear role of eosinophils in the pathogenesis of a wide clinical spectrum of systemic clinical disorders that might be associated with severe organ damage, including vasculitis. Diagnosis remains difficult to establish due to lack of diagnostic and/or classification criteria and serum biomarkers, and a definite consensus on therapy does not exist at the present time. Progress, however, has been made with the use of biological therapy, especially rituximab. Better understanding of the role played by eosinophils might eventually lead to development of newer biologic agents and therapeutic strategies.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Wen T, Rothenberg ME. The regulatory function of eosinophils. Microbiol Spectr. 2016;4. https://doi.org/10.1128/microbiolspec.MCHD-0020-2015 Very good review of the regulatory functions of eosinophils.
•• Khoury P, Grayson PC, Klion AD. Eosinophils in vasculitis: characteristics and roles in pathogenesis. Nat Rev Rheumatol. 2014;10(8):474–83 Very good review of the structural characteristics, and potential role of eosinophils in disease-related pathogenesis.
Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy. 2010;40:563–75.
Chu VT, Fröhlich A, Steinhauser G, Scheel T, Roch T, Fillatreau S, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol. 2011;12:151–9.
Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–7.
Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11.
Tefferi A, Patnaik MM, Pardanani A. Eosinophilia: secondary, clonal and idiopathic. Br J Haematol. 2006;133(5):468–92.
Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709–50.
Melo RC, Paganoti GF, Dvorak AM, Weller PF. The internal architecture of leukocyte lipid body organelles captured by three-dimensional electron microscopy tomography. PLoS One. 2013;8:e59578.
John AE, Thomas MS, Berlin AA, Lukacs NW. Temporal production of CCL28 corresponds to eosinophil accumulation and airway hyperreactivity in allergic airway inflammation. Am J Pathol. 2005;166:345–53.
Dallos T, Heiland GR, Strehl J, Karonitsch T, Gross WL, Moosig F, et al. CCL17/thymus and activation-related chemokine in Churg-Strauss syndrome. Arthritis Rheum. 2010;62:3496–503.
Nussbaum JC, van Dyken S, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–8.
Melo RC, Spencer LA, Perez SA, Neves JS, Bafford SP, Morgan ES, et al. Vesicle-mediated secretion of human eosinophil granule-derived major basic protein. Lab Investig. 2009;89:769–81.
Zagai U, Dadfar E, Lundahl J, Venge P, Skold CM. Eosinophil cationic protein stimulates TGF-β1 release by human lung fibroblasts in vitro. Inflammation. 2007;30:153–60.
Kanno K, Minami-Hori M, Honma < Ishida-Yamamoto A. Histopathological findings and increased D-dimer are predictive factors of systemic thromboses in eosinophilic granulomatosis with polyangiitis. Am J Dermatopathol 2018; 40: 879–883.
Pacholczak R, Bazan-Socha S, Iwaniec T, Zaręba L, Kielczewski S, Walocha JA, et al. Endothelial dysfunction in patients with eosinophilic granulomatosis with polyangiitis. Clin Rheumatol. 2019;38:417–24.
Kingham PJ, McLean W, Walsh MT, Fryer AD, Gleich GJ, Costello RW. Effects of eosinophils on nerve cell morphology and development: the role of reactive oxygen species and p38 MAP kinase. Am J Physiol Lung Cell Mol Physiol. 2003;285:L915–24.
Kephart GM, et al. Marked deposition of eosinophil-derived neurotoxin in adult patients with eosinophilic esophagitis. Am J Gastroenterol. 2010;105:298–307.
Comarmond C, et al. Eosinophilic granulomatosis with polyangiitis (Churg–Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis study group cohort. Arthritis Rheum. 2013;65:270–81.
Wilkins JH, et al. Hypereosinophilic syndrome: an update. Am J Hematol. 2005;80:148–57.
Simon HU, Rothenberg ME, Bochner BS, Weller PF, Wardlaw AJ, Wechsler ME, et al. Refining the definition of hypereosinophilic syndrome. J Allergy Clin Immunol. 2010;126:45–9.
Churg J, Strauss L. Allergic granulomatosis, allergic angiitis, and periarteritis nodosa. Am J Pathol. 1951;27:277–301.
Piram M, Maldini C, Mahr A. Effect of race/ethnicity on risk, presentation and course of connective tissue diseases and primary systemic vasculitides. Curr Opin Rheumatol. 2012;24:193–200.
Berti A, Cornec D, Crowson CS, Specks U, Matteson EL. The epidemiology of antineutrophil cytoplasmic autoantibody-associated vasculitis in Olmsted County, Minnesota: a twenty-year US population-based study. Arthritis Rheumatol. 2017;69:2338–50.
Vaglio A, Martorana D, Maggiore U, Grasselli C, Zanetti A, Pesci A, et al. HLA-DRB4 as a genetic risk factor for Churg-Strauss syndrome. Arthritis Rheum. 2007;56:3159–66.
Wieczorek S, Hellmich B, Gross WL, Epplen JT. Associations of Churg- Strauss syndrome with the HLA-DRB1 locus, and relationship to the genetics of antineutrophil cytoplasmic antibody-associated vasculitides: comment on the article by Vaglio et al. Arthritis Rheum. 2008;58:329–30.
Harrold LR, Patterson MK, Andrade SE, Dube T, Go AS, Buist AS, et al. Asthma drug use and the development of Churg-Strauss syndrome (CSS). Pharmacoepidemiol Drug Saf. 2007;16:620–6.
Hauser T, Mahr A, Metzler C, et al. The leucotriene receptor antagonist montelukast and the risk of Churg-Strauss syndrome: a case-crossover study. Thorax. 2008;63:677–82.
Kostianovsky A, Charles P, Alves JF, Goulet M, Pagnoux C, le Guern V, et al. Immunogenicity and safety of seasonal and 2009 pandemic a/H1N1 influenza vaccines for patients with autoimmune diseases: a prospective, monocentre trial on 199 patients. Clin Exp Rheumatol. 2012;30:S83–9.
Vaglio A, Buzio C, Zwerina J. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): state of the art. Allergy. 2013;68:261–73.
Ramentol-Sintas M, Martínez-Valle F, Solans-Laqué R. Churg–Strauss syndrome: an evolving paradigm. Autoimmun Rev. 2012;12:235–40.
Izquierdo-Domínguez A, Cordero A, Alobid I, Mullol J. Churg-Strauss syndrome or eosinophilic granulomatosis with polyangiitis. Sinusitis. 2016;1:24–43. https://doi.org/10.3390/sinusitis1010024.
Jakiela B, Sanak M, Szczeklik W, Sokolowska B, Plutecka H, Mastalerz L, et al. Both Th2 and Th17 responses are involved in the pathogenesis of Churg-Strauss syndrome. Clin Exp Rheumatol. 2011;29:S23–34.
Zwerina J, Bach C, Martorana D, Jatzwauk M, Hegasy G, Moosig F, et al. Eotaxin-3 in Churg–Strauss syndrome: a clinical and immunogenetic study. Rheumatology (Oxford). 2011;50:1823–7.
Saito H, Tsurikisawa N, Tsuburai T, Akiyama K. Involvement of regulatory T cells in the pathogenesis of Churg–Strauss syndrome. Int Arch Allergy Immunol. 2008;146(Suppl 1):73–6.
Terrier B, Bieche I, Maisonobe T, Laurendeau I, Rosenzwajg M, Kahn JE, et al. Interleukin-25: a cytokine linking eosinophils and adaptive immunity in Churg–Strauss syndrome. Blood. 2010;116:4523–31.
Sinico RA, Bottero P, Guillevin L. Antineutrophil cytoplasmic autoantibodies and clinical phenotype in patients with Churg–Strauss syndrome. J Allergy Clin Immunol. 2012;130:1440.
Moosig F, et al. A vasculitis Centre based management strategy leads to improved outcome in eosinophilic granulomatosis and polyangiitis (Churg–Strauss, EGPA): monocentric experiences in 150 patients. Ann Rheum Dis. 2013;72:1011–7.
Vaglio A, et al. IgG4 immune response in Churg–Strauss syndrome. Ann Rheum Dis. 2012;71:390–3.
Perez Alamino R, Martinez C, Espinoza LR. IgG4-associatedd vasculitis. Curr Rheumatrol Rep. 2013;15:348–6. https://doi.org/10.1007/s11926-013-0348-9.
Perez Alamino R, Espinoza LR, Zea AH. The great mimicker: IgG4-related disease. Clin Rheumatol. 2013;32:1267–73.
Khoury P, Zagallo P, Talar-Williams C, Santos CS, Dinerman E, Holland NC, et al. Serum biomarkers are similar in Churg-Strauss syndrome and hypereosinophilic syndrome. Allergy. 2012;67:1149–56.
Vega LE, Espinoza LR. Predictors of poor outcome in ANCA-associated vasculitis (AAV). Curr Rheumatol Rep. 2016;18:70. https://doi.org/10.1007/s11926-016-0619-3.
Saku A, Furuta S, Hiraguri M, Ikeda K, Kobayashi Y, Kagami SI, et al. Longterm outcomes of 188 Japanese patients with eosinophilic granulomatosis with polyangiitis. J Rheumatol. 2018;45:1159–66.
Polzer K, Karonitsch T, Neumann T, Eger G, Haberler C, Soleiman A, et al. Eotaxin-3 is involved in Churg–Strauss syndrome-a serum marker closely correlating with disease activity. Rheumatology (Oxford). 2008;47:804–8.
Tsurikisawa N, Oshikata C, Watanabe M, Tsuburai T, Kaneko T, Saito H. Innate immune response reflects disease activity in eosinophilic granulomatosis with polyangiitis. Clin Exp Allergy. 2018;48:1305–16.
Higashi N, Taniguchi M, Mita H, Kawagishi Y, Ishii T, Higashi A, et al. Clinical features of asthmatic patients with increased urinary leukotriene E4 excretion (hyperleukotrienuria): involvement of chronic hyperplastic rhinosinusitis with nasal polyposis. J Allergy Clin Immunol. 2004;113:277–83.
Szczeklik W, Sanak M, Mastalerz L, Sokołowska BM, Gielicz A, Soja J, et al. 12-hydroxy-eicosatetraenoic acid (12-HETE): a biomarker of Churg–Strauss syndrome. Clin Exp Allergy. 2012;42:513–22.
Schroeder JW, Folci M, Losappio LM, Chevallard M, Sinico RA, Mirone C, et al. Anti-neutrophil cytoplasmic antibodies positivity and anti-leukotrienes in eosinophilic granulomatosis with polyangiitis: a retrospective monocentric study on 134 Italian patients. Int Arch Allergy Immunol. 2019;180:64–71.
• Rodriguez-Pla A, Warner RL, Cuthberson D, Carette S, Khalidi NA, Koening CL, et al. Evaluation of potential biomarkers of disease activity in diverse forms of vasculitis. J Rheumatol. 2019. https://doi.org/10.3899/jrheum.190093 Study attempting to assess potential biomarkers of disease activity in a variety of vasculitides including EGPA.
• Pagnoux C, Nair P, Khalidi NA, Carette S, Cuthbertson D, Grayson PC, et al. Serum cytokines and chemokine levels in patients with eosinophilic granulomatosis with polyangiitis, hypereosinophilic syndrome, or eosinophilic asthma. Clin Exp Rheumatol. 2019;37(Suppl 117):40–4 A variety of serum cytokynes and chemokines were evaluated as potential biomarkers in patients with EGPA.
Healy B, Bibby S, Steele R, et al. Antineutrophil cytoplasmic autoantibodies and myeloperoxidase autoantibodies in clinical expression of Churg-Strauss syndrome. J Allergy Clin Immunol 2013; 131:571–576; e1-6.
Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol 2012; 130:607–612; e9.
Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–25.
Guillevin L, Lhote F, Gayraud M, Cohen P, Jarrousse B, Lortholary O, et al. Prognostic factors in polyarteritis nodosa and Churg-Strauss syndrome. A prospective study in 342 patients. Medicine. 1996;75:17–28.
Ribi C, Cohen P, Pagnoux C, Mahr A, Arène JP, Lauque D, et al. Treatment of Churg-Strauss syndrome without poor-prognosis factors: a multicenter, prospective, randomized, open-label study of seventy-two patients. Arthritis Rheum. 2008;58:586–94.
Holle JU, Gross WL. Treatment of ANCA-associated vasculitides (AAV). Autoimmun 733 Rev. 2013;12:483–6.
Cohen P, et al. Churg-Strauss syndrome with poor prognosis factors: a prospective multicenter trial comparing glucocorticoids and six or twelve cyclophosphamide pulses in forty-eight patients. Arthritis Rheum. 2007.
Maritati F, Alberici F, Oliva E, Urban ML, Palmisano A, Santarsia F, et al. Methotrexate versus cyclophosphamide for remission maintenance in ANCA-associated vasculitis: a randomized trial. PLoS One. 2017 October 10;12(10):e0185880.
• Puechal X, Pagnoux C, Baron G, Quemeneur T, Neel A, Agard C, et al. Adding azathioprine to remission-induction glucocorticoids for eosinophilic granulomatosis with polyangiitis (Churg-Strauss), microscopic polyangiitis, or polyarteritis nodosa without poor prognostic factors: a randomized, controlled trial. Arthritis Rheumatol. 2017;69:2175–86 Data presented showed that azathioprine is of no value when added to glucocorticoids to maintain remission at 1 year.
• Puechal X, Pagnoux C, Baron G, Lifermann F, Geffray L, Quemeneur T, et al. Non-severe eosinophilic granulomatosis with polyangiitis: long-term outcomes after remission-induction trial. Rheumatology. 2019. https://doi.org/10.1093/rheumatology/kez139 Data obtained revealed lack of efficacy for azathioprine to maintain glucocorticoid-induced remission beyon 1 year.
Pepper RJ, et al. Rituximab is effective in the treatment of refractory Churg–Strauss syndrome and is associated with diminished T-cell interleukin-5 production. Rheumatology (Oxford). 2008;47:1104–5.
Guillevin L, Pagnoux C, Karras A, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014;371:1771–80.
Mohammad AJ, Hot A, Arndt F, et al. Rituximab for the treatment of eosinophilic granulomatosis with polyangiitis (Churg–Strauss). Ann Rheum Dis 2014 in press DOI; https://doi.org/10.1136/annrheumdis-2014-206095.
Thiel J, Troilo A, Salzer U, Schleyer T, Halmschlag K, Rizzi M, et al. Rituximab as induction therapy in eosinophilic granulomatosis with polyangiitis refractory to conventional immunosuppressive treatment: a 36-month follow-up analysis. J Allergy Clin Immunol Pract. 2017;5:1556–63.
Texeira V, Mahammad AJ, Jones RB, Smith R, Jayne D. Efficacy and safety of rituximab in the treatment of eosinophilic granulomatosis with polyangiitis. RMD Open. 2019;5:e000905. https://doi.org/10.1136/rmdopen-2019-000905.
• Emejuaiwe N. Treatment strategies in ANCA-associated vasculitis. Cuu Rheumatol Rep. 2019;21(7):33. https://doi.org/10.1007/s11926-019-0835-8 Good review of treatment strategies to follow in the management of ANCA-associated vasculitis.
Herrmann K, Gross WL, Moosig F. Extended follow-up after stopping mepolizumab in relapsing/refractory Churg–Strauss syndrome. Clin Exp Rheumatol. 2012;30(Suppl 70):S62–5.
• Wechsler ME, Akuthota P, Jayne D, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017;376(20):1921–32. https://doi.org/10.1056/NEJMoa1702079 Very good prospective, controlled study demonstrating the efficacy and safety of mepolizumab in the therapy of EGPA.
• Steinfeld J, Bradford ES, Brown J, Mallett S, Yancey SW, Akuthota P, et al. Evaluation of clinical benefit from treatment with mepolizumab for patients with eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol. 2018. https://doi.org/10.1016/j.jaci.2018.11.041 Post hoc analysis of the MIRRA study demonstrating the efficacy and safety of mepolizumab in primary and secondary outcomes.
Moosig F, Gross WL, Herrmann K, Bremer JP, Hellmich B. Targeting interleukin-5 in refractory and relapsing Churg-Strauss syndrome. Ann Intern Med. 2011;155:341–3.
Ennis D, Lee JK, Pagnoux C. Mepolizumab for the treatment of eosinophilic granulomatosis with polyangiitis. Exp Opin Biol Ther. 2019;19:617–30.
Celebi Sozener Z, Gorgulu B, Mungan D, Sin BA, Misirligil Z, Aydin O, et al. Omalizumab in the treatment of eosinophilic granulomatosdis with polyangiitis (EGPA): single-center experience in 18 cases. World Allergy Organ J. 2018;11:39. https://doi.org/10.1186/s40413-018-0217-0.
Aguirre-Valencia D, Posso-Osorio I, Bravo JC, Bonilla-Abadia F, Tobon GJ, Canas CA. Sequential rituximab and omalizumab for the treatment of eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome). Clin Rheumatol. 2017;36:2159–62.
Danieli MG, Cappelli M, Malcangi G, Logullo F, Salvi A, Danieli G. Long term effectiveness of intravenous immunoglobulin in Churg-Strauss syndrome. Ann Rheum Dis. 2004;63:1649–54.
Iurlo A, Cattaneo D, Gianelli U. Hypereosinophilic syndromes in the precison medicine era: clinical, molecular aspects and therapeutic approaches (targeted therapies). Expert Rev Hematol. 2019. https://doi.org/10.1080/17474086.2019.1677461.
Akuthota P, Neves JS, Ueki S. Editorial: severe eosinophilic disorders: mechanisms and clinical management. Front Immunol. 2019;10:2118. https://doi.org/10.3389/fimmu.2019.02118.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Vasculitis
Rights and permissions
About this article
Cite this article
Vega Villanueva, K.L., Espinoza, L.R. Eosinophilic Vasculitis. Curr Rheumatol Rep 22, 5 (2020). https://doi.org/10.1007/s11926-020-0881-2
Published:
DOI: https://doi.org/10.1007/s11926-020-0881-2